Abstract
The radiochemical dipyrrolidinedithiocarbamato-212Pb(II) [212Pb(PDC)2] is synthesized and its effects on colony formation in cultured Chinese hamster V79 cells are investigated. The cellular uptake, biological retention, subcellular distribution and cytotoxicity of the radiocompound are determined. The 212Pb is taken up quickly by the cells, reaching saturation levels in 1.25 h. When the cells are washed, the intracellular activity is retained with a biological half-life of 11.6 h. Gamma-ray spectroscopy indicates that the 212Pb daughters (212Bi, 212Po and 208Tl) are in secular equilibrium within the cell. About 72% of the cellular activity localizes in the cell nucleus, of which 35% is bound specifically to nuclear DNA. The mean cellular uptake required to achieve 37% survival is 0.35 mBq of 212Pb per cell, which delivers a dose of 1.0 Gy to the cell nucleus when the recoil energy of 212Bi and 212Po decays is ignored and 1.7 Gy when recoil is included. The corresponding RBE values compared to acute external 137Cs γ rays at 37% survival are 4.0 and 2.3, respectively. The chemical Pb(PDC)2 is not chemotoxic at the concentrations used in this study. Because the β-particle emitter 212Pb decays to the α-particle-emitting daughters 212Bi and 212Po, these studies provide information on the biological effects of α-particle decays that occur in the cell nucleus. Our earlier studies with cells of the same cell line using 210Po (emits 5.3 MeV α particle) localized predominantly in the cytoplasm resulted in an RBE of 6. These earlier results for 210Po, along with the present results for 212Pb, suggest that the recoil energy associated with the 212Bi and 212Po daughter nuclei plays little or no role in imparting biological damage to critical targets in the cell nucleus.
INTRODUCTION
The extreme radiotoxicity of radionuclides that emit α particles has long been of concern in radiation protection and more recently has attracted considerable attention for therapeutic applications. The biological effects of external α-particle beams have been well studied in terms of dose response and relative biological effectiveness (RBE) for a variety of end points (1–3). Comparatively less experimental data where RBE values are clearly delineated are available on the effects of tissue-incorporated α-particle emitters (4). A number of studies have been published recently using in vitro techniques where the radionuclides were located in the culture medium, thereby irradiating the cells with a spectrum of α-particle energies (5–10). Other studies have been performed both in vitro and in vivo to elucidate the RBE of α-particle emitters specifically incorporated into cells (10–15). However, to the best of our knowledge no data are available on the RBE of α-particle emitters when most of the cellular activity is incorporated into the nucleus, where the primary radiosensitive targets lie.
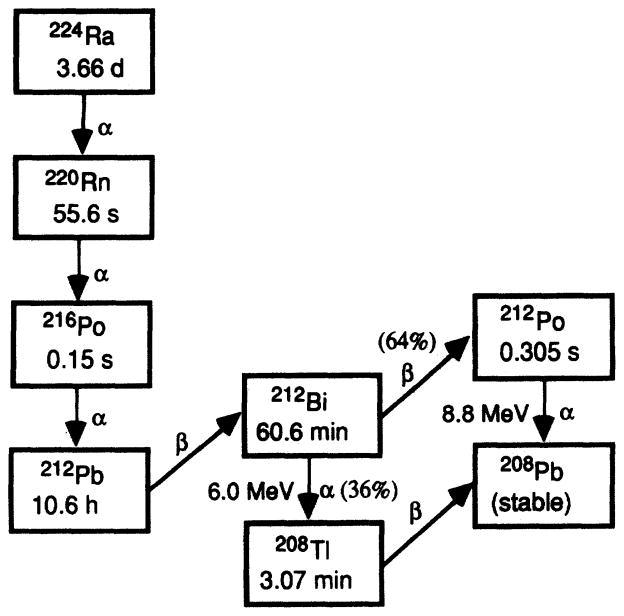
In the present work, the radiochemical dipyrrolidinedithiocarbamato-212Pb(II) [212Pb(PDC)2] is synthesized and its biological effects in cultured Chinese hamster V79 cells are investigated. The β-particle emitter 212Pb decays with a 10.64-h half-life to two α-particle-emitting daughters, 212Bi and 212Po (Fig. 1) (16). The cellular uptake, biological retention, subcellular distribution and cytotoxicity of the radiocompound are determined. The 212Pb(PDC)2 localizes predominantly in the cell nucleus. Therefore, these studies provide information on the biological effects of α-particle decays which occur in the cell nucleus and thereby shed light on the role of recoil energy associated with α-particle decay in causing biological damage to the primary radiosensitive targets.
FIG. 1.
Radium-224 decay series (16).
MATERIALS AND METHODS
Radiochemistry
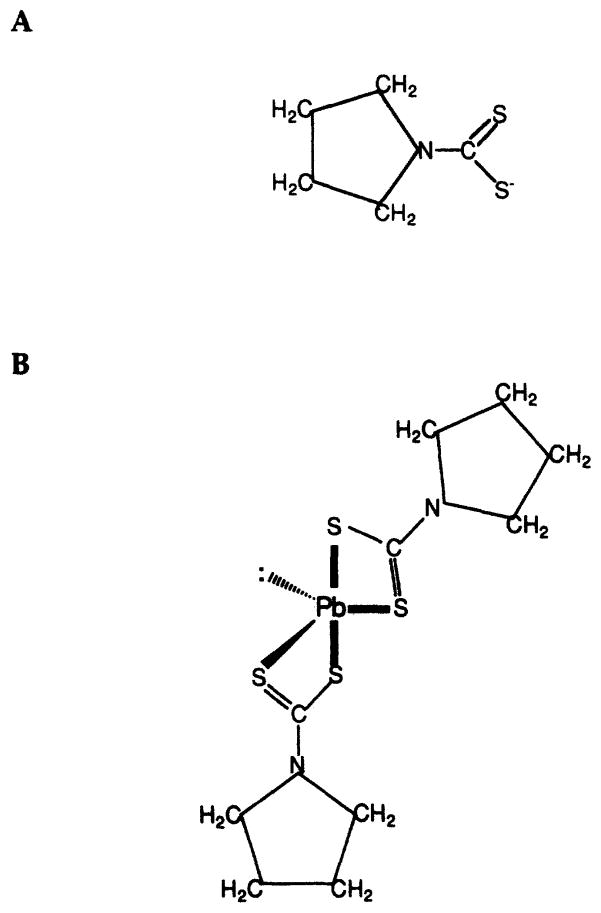
Ammonium pyrrolidinedithiocarbamate (APDC) is an excellent chelating agent for heavy metals (17, 18). The structure of its anion (PDC−) is shown in Fig. 2A. The lead dipyrrolidinedithiocarbamate complex was synthesized with carrier-free 212Pb that was eluted from a 224Ra generator (Argonne National Laboratory, Argonne, IL). The generator, a shielded cation exchange column containing 925 MBq of 224Ra, was eluted with 3 ml of 2 M HCl to obtain the lead complex chloro ion and subsequently flushed with 3 ml of 0.02 M HCl. The eluate containing the 212Pb was then passed through a cation exchange column (Dowex 50W hydrogen form, 1 % crosslinked, packed in 5 ml syringe) preconditioned with 0.02 M HCl to remove breakthrough impurities, if any. In addition, the eluent was passed through an anion exchange column (Dowex™ 1× 10–200 mesh, chloride form, 10% crosslinked, packed in 5 ml syringe) preconditioned with 1 × 10−5 M HCl. The resulting eluate was then allowed to stand until secular equilibrium was established with the 212Pb daughter products (Fig. 1). The 212Pb, 212Bi and 208Tl activities in the eluate were determined by placing the sample 1.73 cm above an energy-calibrated HpGe detector (Canberra, Meriden, CT) and using the 238 [yield = 0.436 (16), efficiency = 0.0325], 727 [yield = 0.0665 (16), efficiency = 0.0113] and 583 keV [yield = 0.867 (16), efficiency = 0.0145] γ-ray photopeaks of 212Pb, 212Bi and 208Tl, respectively. This solution (3 ml) was rotary-evaporated to dryness, dissolved in a minimum of absolute ethanol (2.5 ml) and transferred to a pear-shaped 5 ml flask containing a magnetic stir-bar; the activity was assayed, and the molarity of the 212Pb in solution was calculated.
FIG. 2.
A: Molecular structure of pyrrolidinedithiocarbamate anion used as the ligand in the synthesis of Pb(PDC)2. B: Proposed molecular structure of Pb(PDC)2 in solution. The structure is a pseudo-5-coordinate trigonal bipyramidal structure with a non-bonding electron pair located in the trigonal plane.
The ligand solution was prepared by dissolving the ammonium salt of pyrrolidine dithiocarbamate (MW 164) (Aldrich Chemical Co., Milwaukee, WI) in absolute ethanol. A stoichiometric amount of the ligand was added to the reaction vessel and the solution maintained at 50°C for 1 h. The contents of the flask were transferred to a 12 × 75-mm borosilicate glass tube and rotary-evaporated, and the sample was resuspended in sterile deionized water. Finally, the solution containing 212Pb(PDC)2 was sterilized by filtration through a Gelman 0.22 μm filter and the activity determined using the HpGe detector. It should be noted that bismuth also complexes with PDC to form 212Bi(PDC)3, although, based on half-life considerations, there are about 10 times fewer 212Bi atoms than 212Pb atoms at secular equilibrium (18).
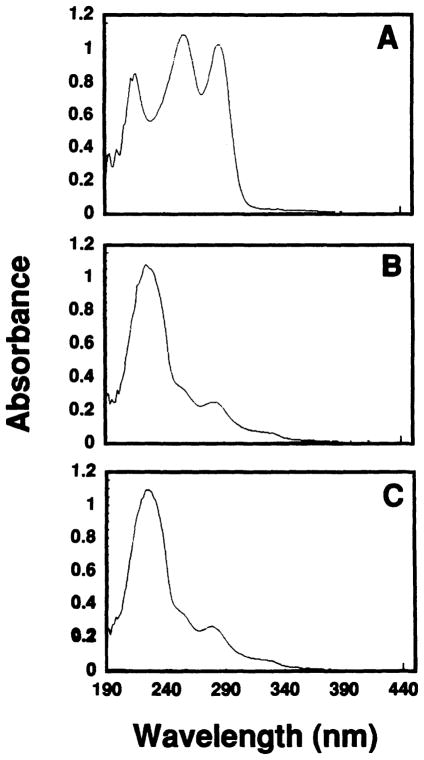
The purity of the radiochemical was ascertained by thin-layer chromatography on aluminum-backed silica gel with fluorescence indicator (0.2 mm DC-Karten SI F 254 nm obtained from Riedel-de Haën AG, D-3016 Seelze 1) with acetone:toluene:H2O serving as the solvent system (70:24:6). A nonradioactive standard of Pb(PDC)2 was prepared with stable lead for which an Rf of 0.26 was obtained using UV visualization. In contrast, Rf values of 0.17 and 0.0 were obtained for PDC and PbCl2, respectively. An autoradiograph of the TLC plate also yielded these Rf values for 212Pb(PDC)2 and 212PbCl2. No spots other than these were observed on the autoradiograph. Ultraviolet absorption spectra for PDC−, Pb(PDC)2 and 212Pb(PDC)2 are shown in Figs. 3A–C. Note that the UV spectra for Pb(PDC)2 and 212Pb(PDC)2 shown in Figs. 3B and 3C are identical. Elemental analysis of the synthesized nonradiolabeled compound is given in Table I. Finally, the proposed structure of Pb(PDC)2 in aqueous solution is shown in Fig. 2B. The structure, based on the chemical analysis data in Table I and valence shell electron pair repulsion (VSEPR) theory, should be a pseudo-5-coordinate trigonal bipyramidal configuration with a non-bonding electron pair located in the trigonal plane.
FIG. 3.
Ultraviolet absorption spectra for (A) ammonium pyrrolidine dithiocarbamate in absolute ethanol, (B) nonradiolabeled Pb(PDC)2 complex and (C) radiolabeled 212Pb(PDC)2.
TABLE I.
Elemental Analysis of Pb(PDC)2
| Element | Theoretical percentage | Experimental percentage |
|---|---|---|
| Lead | 41.6 | 42.0 |
| Carbon | 24.0 | 23.6 |
| Nitrogen | 5.60 | 5.50 |
| Sulfur | 25.6 | 24.8 |
| Hydrogen | 3.20 | 4.10 |
Cell Culture
Chinese hamster lung fibroblasts (V79-513 cells) were used in these studies with clonogenic survival serving as the biological end point. The experimental methods and protocols were the same as in our earlier report (11). The cells were cultured in minimum essential medium (MEM) supplemented with 2 mM L-glutamine, 10 mM Hepes, 10% fetal calf serum, 50 U/ml penicillin, 50 μg/ml streptomycin and 0.1 mM nonessential amino acids. The pH of the culture medium was adjusted to 7.0 with NaHCO3. Media and supplements were from Gibco (Grand Island, NY).
Cellular Uptake of Radioactivity
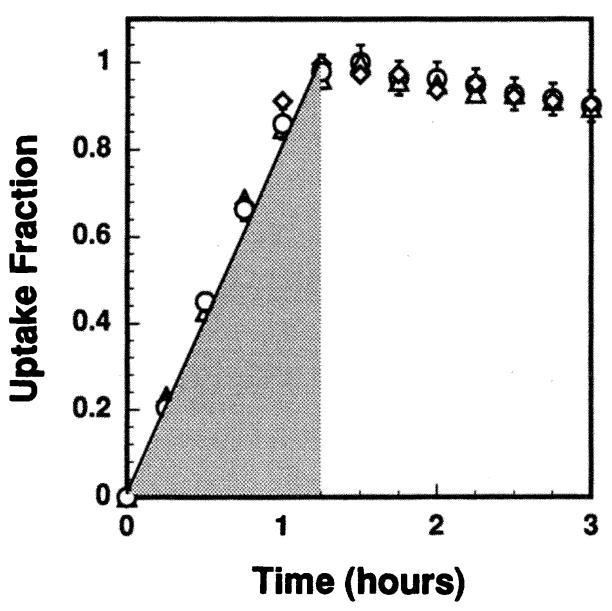
Determination of the kinetics of cellular uptake established the time required to achieve maximum incorporation of the radiochemical into the cells. Chinese hamster V79 cells, in logarithmic growth as monolayers in 75 cm2 flasks, were washed two times with 10 ml of phosphate-buffered saline, trypsinized and suspended at 4 × 105 cells/ml in calcium-free MEM. Aliquots of 1 ml were placed in sterile Falcon 17 × 100 mm polypropylene round-bottom culture tubes and conditioned on a rocker-roller for 4 h at 37°C in an atmosphere of 95% air and 5% CO2. After the conditioning period, 1 ml of calcium-free MEM containing various activities of 212Pb(PDC)2 was added to the tubes (final concentrations 35.3, 16.8 and 9.67 kBq/ml) and the rolling continued. Aliquots of the cell suspension were taken at different times and the cellular uptake of 212Pb, 212Bi and 208Tl was determined using the microfuge method of Kassis and Adelstein (19) and the HpGe detector described earlier. Figure 4 shows the fraction of cellular uptake of radioactivity as a function of incubation time. The cellular uptake of radioactivity saturates within 1.25 h. This pattern was independent of the extracellular concentration of 212Pb(PDC)2 in the culture medium over the range studied.
FIG. 4.
Effective uptake kinetics of 212Pb in V79 cells. The experiment was performed at three different activity concentrations of 212Pb(PDC)2 in the culture medium: 35.3 (○), 16.8 (△), and 9.67 (◇) kBq/ml. Over this range, the kinetics of uptake was independent of concentration. Error bars represent the standard deviation of the mean.
Cellular Retention of Radioactivity
Cells were exposed to various concentrations of 212Pb(PDC)2 (35.3, 16.8 and 9.67 kBq/ml) as described above and the cellular uptake of radioactivity was determined using the microfuge method (19). After an exposure time TI of 1.25 h, the cells were washed four times with MEM and resuspended in calcium-free MEM. Aliquots of the cell suspension were then assayed for cellular uptake of radioactivity (this was designated t = 0). Approximately 8% of the intracellular activity was removed during the washing process. The remaining cells containing 212Pb were distributed among 75 cm2 flasks containing MEM (1–2 × 106 cells per flask) and the flasks were incubated under standard conditions. At various times a flask was removed from the incubator, the cells were washed with two 10-ml aliquots of sterile phosphate-buffered saline, and the cells were removed from the flask with trypsin. The cells were resuspended in MEM and the activity per cell of 212Pb and its daughters was determined.
Clonogenic Survival of V79 Cells
Ten culture tubes of cells were exposed to various concentrations (0–25.0 kBq/ml) of 212Pb(PDC)2 as described above. After an exposure time of TI = 1.25 h, an 0.8-ml aliquot was extracted from each tube and the extracellular activity concentration and cellular uptake of radioactivity were determined using Ecoscint liquid scintillation cocktail (National Diagnostics, Manville, NJ) and an automatic liquid scintillation counter. The remaining cells were washed four times with 10 ml MEM, resuspended in 2 ml of MEM and seeded in triplicate into Falcon 25 cm2 flasks for colony formation, and the flasks were incubated for 1 week (TCF = 7 days) under standard conditions. The resulting colonies were washed three times with normal saline, fixed with methanol and stained with crystal violet. The colonies were then counted; the criterion for cell survival was taken to be its ability to form a colony with 50 or more cells. Additional survival studies were carried out with equimolar concentrations of nonradioactive Pb(PDC)2, freshly prepared from stable lead, to determine whether chemotoxicity contributes to cell death at the concentrations used in the present studies.
Subcellular Distribution of Activity
The procedures described by Kassis (20) were used to determine the subcellular distribution of activity in the cells. Cells were exposed to the radiochemical as described above. After a 1.25-h exposure to the radiochemical, the cells were washed two times with 10 ml of cold calcium-free salt solution composed of 0.4 mM KH2PO4, 0.4 mM Na2HPO4·7H2O, 0.74 mM MgSO4·7H2O, 5 mM KCl and 0.12 M NaCl. The cells were then suspended in 2 ml of cold sucrose buffer (0.25 M sucrose, 3 mM CaCl2, 50 mM Tris, pH 7.0) and placed on ice for 5 min, and aliquots were taken to determine the cellular activity. The cell membrane was ruptured by adding 2 ml of sucrose buffer containing 2% Triton X-100 and vortex mixing. The resulting cell nuclei were placed on ice for an additional 5 min and vortexed vigorously for 30 s, and aliquots of the suspension were assayed for radioactivity. The nuclei were centrifuged at 2000 rpm for 15 min at 4°C and aliquots of the supernatant (cytoplasmic fraction) assayed. The nuclei were washed once with cold sucrose buffer (40.0 ml) and suspended in 2 ml of the same, and the activity in the nucleus was determined. Activity in the nucleus that was associated with RNA, DNA and proteins was precipitated with cold 10% trichloroacetic acid. DNA-bound activity was precipitated with guanidine-HCl by adding 2 ml of cold 6 M guanidine-HCl to 1 ml of the suspension of cell nuclei and mixing gently with a glass rod. Three milliliters of cold 95% ethanol was added followed by further gentle mixing. The contents of the test tube were transferred to a Gelman Type A-E filter and then the test tube, stirring rod and filter were washed three times with 2 ml cold guanidine-HCl:ethanol solution (1:1). The filter paper was transferred to a test tube and the activity assayed.
RESULTS
Cellular Uptake and Retention of Radioactivity
Figure 4 shows the kinetics of cellular uptake of 212Pb. The same pattern was observed for the 212Pb daughters, indicating that the intracellular daughters were in equilibrium with the parent during the uptake period. The observation of secular equilibrium throughout the uptake period may be due to the presence of 212Bi(PDC)3 in the stock radiochemical solution which in turn is taken up by the cells upon addition to the culture medium. The cellular uptake of radioactivity saturated after about 1.25 h of incubation. A linear least-squares fit of the data in the range 0–1.25 h yields f(t) = 0.831t, where f(t) = A(t)/A(t = 1.25 h) and A(t) is the cellular activity at time t in hours. Integration of f(t) over the exposure time TI = 1.25 h yields the residence time of the activity in the cells during the uptake period τI = 0.65 h. The dependence of cellular uptake of 212Pb radioactivity A(κ) on the activity concentration of 212Pb(PDC)2 in the culture medium κ is shown in Fig. 5. A least-squares fit to a second-order polynomial gives A(κ) = 0.0257κ + 0.00184κ2. Finally, Fig. 6 shows the retention of 212Pb by the V79 cells. The radioactivity was retained with a biological half-life of 11.6 h, which is reasonable given that this was about the same as the cell doubling time for cells of the V79 cell line. The 212Pb daughters cleared with the same biological half-life, indicating that the intracellular daughters were in equilibrium with the parent during the colony-forming period. Using the physical half-life of 212Pb, the effective half-life was 5.5 h, and the residence time during the colony-forming period τCF was 7.9 h.
FIG. 5.
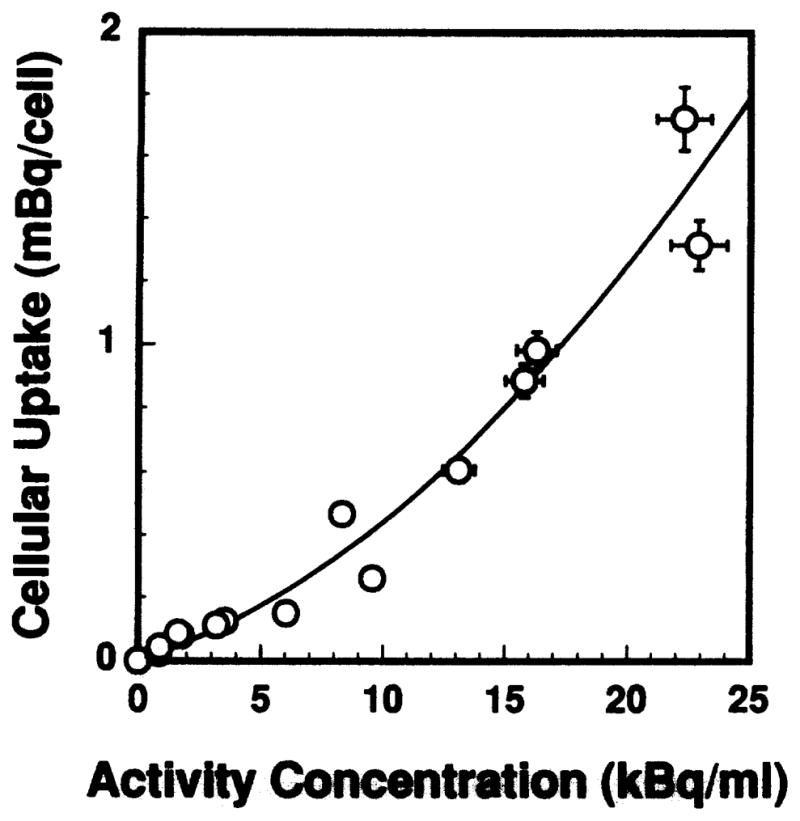
Mean cellular uptake of 212Pb as a function of extracellular activity concentration of 212Pb(PDC)2 in the culture medium. The error bars are the standard deviation of the mean.
FIG. 6.
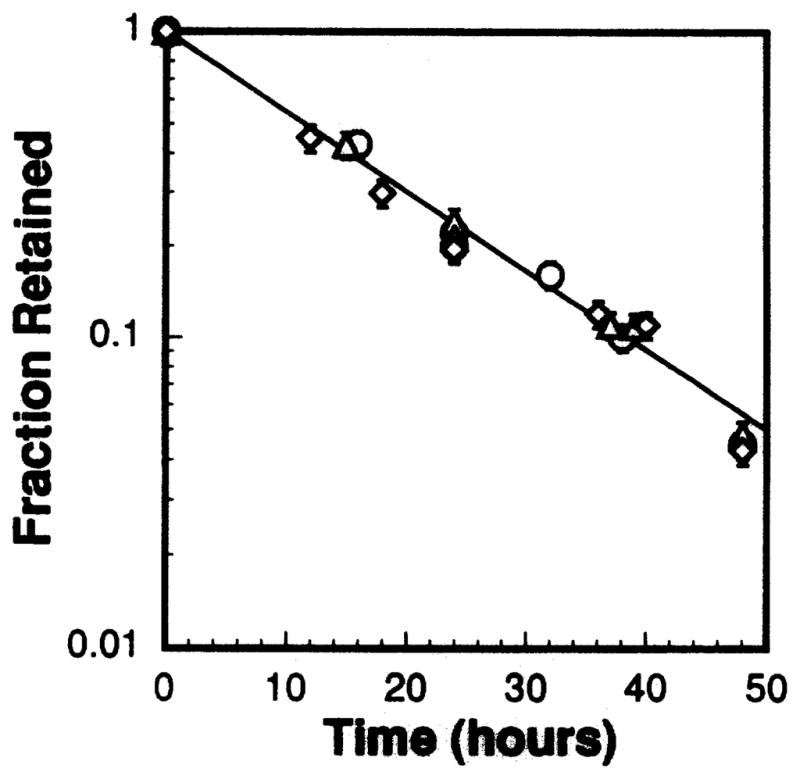
Kinetics of biological retention of 212Pb after exposure of V79 cells to three different activity concentrations of 212Pb(PDC)2 in the culture medium. The kinetics of biological retention was independent of extracellular activity concentration over the range of activities administered: 35.3 (○), 16.8 (△), 9.67 (◇) kBq/ml. The error bars represent the standard deviation of the mean.
Clonogenic Survival of V79 Cells
Figure 7 shows the dependence of cell survival fraction on cellular uptake of radioactivity. No chemotoxicity associated with 212Pb(PDC)2 was observed even at the highest concentrations used. The survival curve is exponential, indicative of a high-LET radiation type response. A least-squares fit to the data yields a mean lethal uptake of 0.35 ± 0.02 mBq/cell.
FIG. 7.
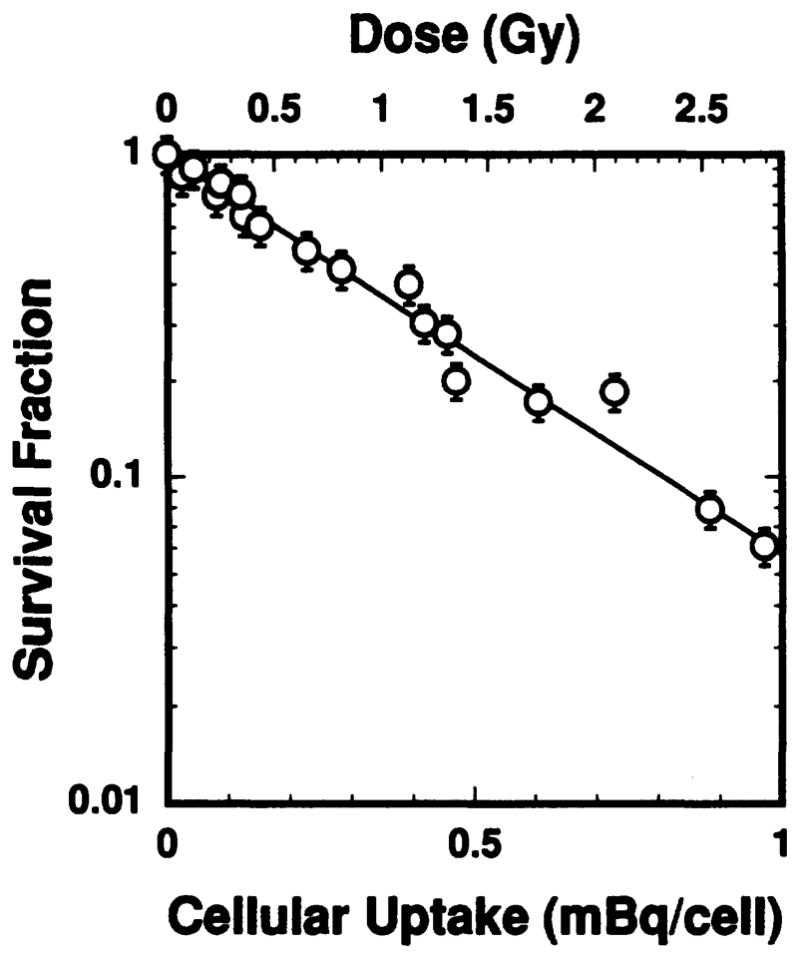
Surviving fraction of V79 cells as a function of mean cellular uptake of radioactivity (lower abscissa) and mean absorbed dose to the cell nucleus (upper abscissa) after exposure to 212Pb(PDC)2. The dose response is exponential with a mean lethal uptake of 0.35 mBq/cell and mean lethal dose of 1.0 Gy when the recoil energy of the 212Pb daughters is ignored.
Subcellular Distribution
Subcellular distribution studies revealed that 72% of the 212Pb was in the nucleus and 28% in the cytoplasm. Approximately 77% of the activity in the nucleus was bound to the RNA, DNA and proteins, and 35% was specifically bound to DNA. Thus 25% of the intracellular activity was bound to DNA in the cell nucleus. The subcellular distribution was independent of the extracellular activity concentration to which the cells were exposed (35.3, 16.8 and 9.67 kBq/ml) and the distribution was the same for the 212Pb daughters.
Cellular Dosimetry
As in our earlier work (11), the absorbed dose to the cell nucleus is calculated. There are four contributions to the mean absorbed dose to the cell nucleus: (1) nontarget-to-target dose from extracellular decays that occur in the culture medium during the exposure period TI, (2) self-dose from intracellular decays during the exposure period TI (3) self-dose from intracellular decays during the colony-forming period TCF, and (4) cross-irradiation from decays occurring in neighboring cells of the colony. The contributions from extracellular decays and cross-irradiation from decays in neighboring cells of the colony (11) are small relative to the self-dose contributions and are ignored.
Calculation of contributions (2) and (3) requires the cumulated activity in the cells during the uptake (ÃI) and colony-forming (ÃCF) periods. The cumulated activity during the uptake period is simply ÃI = AIτI = 2.34 × 103 AI Bq s, where AI is the cellular uptake in becquerels at the end of the 1.25-h incubation period. Similarly, the cumulated activity during the colony-forming period is ÃCF = τCF AI = 2.84 × 104 AI Bq s. Hence the total cumulated 212Pb activity in the cell is 3.07 × 104 AI Bq s, which corresponds to about 11 intracellular decays of 212Pb at 37% survival.
The absorbed dose to the cell nucleus DN from intracellular decays of 212Pb in equilibrium with its daughters is given by
| (1) |
where bj is the branching ratio for the jth radionuclide in the cell (see Table II), and fN and fCy are the fraction of cellular activity in the nucleus and cytoplasm, respectively. The quantities Sj,N←Cy and Sj,N←Cy are the cellular S values (absorbed dose per unit cumulated activity) for the jth radionuclide localized in the nucleus and cytoplasm, respectively. The S values for 212Pb, 212Bi, 212Po and 208Tl are taken from Goddu et al. (21) for our V79 cells (cell diameter = 10 μm, nuclear diameter = 8 μm, ref. 11) and tabulated in Table II. Substituting fN = 0.72, fCy = 0.28, and the branching ratios and S values given in Table II into Eq. (1) yields the total absorbed dose to the cell nucleus DN = 2.87 × 103 AI Gy/Bq, where AI is in becquerels. Accordingly, the survival data in Fig. 7 are replotted as a function of the total absorbed dose to the cell nucleus. The dose–response curve (Fig. 7) is what would be expected after exposure to high-LET radiation with the survival fraction given by SF = exp(−αD), where α = 1.0 ± 0.1 Gy−1. The corresponding dose required to achieve 37% survival (D37) is 1.0 ± 0.1 Gy.
TABLE II.
Cellular Dosimetry for 212Pb and Daughters
| Radionuclide | Branching ratio (16) | SN←N (Gy/Bq s)a,c | SN←Cy (Gy/Bq s)b,c | fNSN←N + fCySN←Cy (Gy/Bq s) | Fraction of total cellular dose (qi) |
|---|---|---|---|---|---|
| 212Pb | — | 3.29 × 10−3 | 9.38 × 10−4 | 2.63 × 10−3 | 0.028 |
| 212Bi | 1 | 4.40 × 10−2 | 1.98 × 10−2 | 3.72 × 10−2 | 0.40 |
| 212Po | 0.64 | 9.83 × 10−2 | 4.45 × 10−2 | 5.33 × 10−2 | 0.57 |
| 208Tl | 0.36 | 4.96 × 10−4 | 2.18 × 10−4 | 1.51 × 10−4 | 0.0016 |
| Total | — | — | — | 9.33 × 10−2 | 1 |
Dose to the V79 cell nucleus per unit cumulated activity in the cell nucleus. Data taken from ref. (21).
Dose to the V79 cell nucleus per unit cumulated activity in the cytoplasm. Data taken from ref. (21).
S values are for V79 cells (diameter of cell = 10 μm, diameter of cell nucleus = 8 μm, ref. 11).
DISCUSSION
RBE of Intracellular 212Pb in Equilibrium with 212Bi, 212Po and 208Tl
Determination of the RBE of intracellular 212Pb(PDC)2 in equilibrium with its daughters requires dose–response relationships for the radiocompound as well as the reference radiation (137Cs γ rays). The dose response for V79-513 cells irradiated with acute external 137Cs γ rays was given in our earlier report as SF = exp(−0.086D −0.040D2), where SF is the survival fraction and D the absorbed dose (11). The corresponding D37 is 4.0 ± 0.5 Gy. Hence the RBE of the intracellular mixed radiation field (212Pb-β, 212Bi-α, 212Po-α, 208Tl-β) compared to acute external 137Cs γ rays is 4.0 ± 0.7 at D37, which is similar to the RBE of 4.7 ± 0.5 obtained previously in vivo for 212Pb in equilibrium with its daughters in the mouse testis model (13). The present RBE of 4.0 is substantially lower than the value of 6 obtained earlier for 5.3 MeV α particles emitted by intracellularly localized 210Po-citrate using the same V79 cell culture model (11). Based on the LETs of the particles, this is expected because in the present case 57% of the dose to the cell nucleus is deposited by the 57 keV/μm (1) (8.8 MeV) α particle emitted by 212Po and 40% of the dose is deposited by the 76 keV/|μm (1) (6.0 MeV) α particle emitted by 212Bi which have substantially lower LETs than the 83 keV/μm (1) of the 5.3 MeV α particle of 210Po.
Given that less than 3% of the dose to the cell nucleus is deposited by the β-particle-emitting parent 212Pb in this model, it is appropriate to compare our RBE values with those in the literature for 212Pb as well as the RBE values for its daughter 212Bi. Charlton et al. (9) obtained a D37 of 1 Gy for V79 cells when 212Bi was distributed uniformly in the culture medium. This corresponds to an RBE of 5.8 when compared to their D37 for 250 kVp X rays (22). Shadley et al. (6) obtained an RBE of 3.8 compared to 250 kVp X rays at D10 for CHO K1 cells when 212Bi was distributed extracellularly in the culture medium. Kurtz-man et al. (10) reported an RBE (at D10) compared to 15 MeV X rays of about 5 for SHAW human pancreatic carcinoma cells under essentially the same irradiation conditions. Our RBE at D10 for V79 cells containing 212Pb in equilibrium with its daughters is similar albeit lower with a value of 2.9 ±0.4.
RBE of the 212Bi and 212Po α Particles
The individual RBE values for the 6.0 MeV and 8.8 MeV α particles of 212Bi and 212Po may be estimated using the expression provided in Section 6.3 of ICRP Publication 58 (23) which relates the RBE of a mixture of high- and low-LET radiations to the RBE values of the individual contributions (23). The RBE of the mixed-field R is given by the ICRP (23) as
| (2) |
where RBEi and qi are the RBE and fraction of total dose delivered by radiation component i, respectively. Hence the RBE of intracellular 212Pb in equilibrium with its daughters is
| (3) |
where the numerical subscripts denote the α-particle energy and “other” denotes all radiations with RBE = 1 (i.e. photons, β particles, etc.). The quantities q6.0, q8.8 and qother are given in Table II as 0.40 (>98% of the dose from 212Bi is from its 6.0 MeV α particle), 0.57 and 0.03, respectively. The quantity R is simply the experimental RBE value of 4.0. Only the RBE values of the 6.0 and 8.8 MeV α particles remain unknown. The data of Barendsen et al. (1) for irradiation of cultured human cells with α-particle beams indicate that the RBE of a 6.0 MeV α particle is about 1.3 times greater than the RBE of an 8.8 MeV α particle. It is not unreasonable to suggest that a similar value would be obtained for V79 cells; hence one may approximate RBE6.0 ~ 1.3 RBE8.8. Using this ratio, one obtains RBE6.0 = 4.7 and RBE8.8 = 3.6 at 37% survival. As expected, based on the LETs of the α particles, these values are lower than the RBE of 6 for monoenergetic 5.3 MeV α particles emitted by intracellularly localized 210Po (11). It should also be noted that these RBE values are similar to those obtained for these radionuclides in vivo (13) and in vitro using external beams of α particles (1–3).
Recoil Energy
The contribution of recoil energy associated with the intracellular 212Bi and 212Po decays has not been considered in the dosimetry calculations above. The recoil energies are 169, 108–117 and 103 keV for 212Po, 212Bi and 210Po, respectively (24). The ranges of these recoiling nuclei are on the order of 100 nm in water with an LET of about 1600–1800 keV/μm (25). Clearly, with such short ranges, recoil energy will have little or no biological impact when the decay occurs in the cytoplasm. However, recoil energy could in principle contribute to the overall biological effect of α-particle emitters when the decay occurs in the cell nucleus, particularly on the DNA. The mean recoil energy released per decay of 212Bi and 212Po is 6.71 × 10−15 and 2.43 × 10−14 Gy kg/Bq s (24). Taking account of the branching ratios (Table II) and that 72% of the cellular activity is localized in the nucleus, the combined recoil energy deposited in the nucleus by the 212Bi and 212Po decays corresponds to an absorbed dose per unit cumulated activity of 0.065 Gy/Bq s. Using this quantity and the total cumulated activity in the cell, one obtains a recoil dose to the cell nucleus of 0.70 Gy at 37% survival. The total dose including recoil energy is then 1.70 Gy and the RBE for 212Pb(PDC)2 in equilibrium with its daughters is 2.3. This translates to RBE values of 2.1 and 2.7 for the 8.8 and 6.0 MeV α particles of 212Po and 212Bi, respectively. This is in contrast to the values 3.6 and 4.7 when recoil energy is ignored.
What are the correct RBE values? Technically, the RBE values which include recoil energy are more appropriate because the doses used to compute the RBE value should take into account all energy deposited in the cell nucleus. However, in the context of assessing the biological effects of the recoil energy, it may be more appropriate to compare both sets of RBE values with our data obtained previously for 5.3 MeV α particles emitted by cytoplasmically localized 210Po-citrate using the same experimental model (11). In the 210Po experiments 28% of the intracellular activity was localized in the cell nucleus, and only about 10% of the nuclear activity was bound to DNA (11). Recoil energy was not included in our reported RBE value of 6 for 210Po-citrate (11). When recoil is included in the calculations, an RBE of about 5 is obtained for 210Po. Hence, when recoil is not included, RBE values of 3.6, 4.7 and 6 are obtained for the 8.8, 6.0 and 5.3 MeV α particles of 212Po, 212Bi and 210Po, respectively. When recoil is included, the corresponding RBE values are 2.1, 2.7 and 5, respectively. As discussed above, the higher RBE observed for the 5.3 MeV α particles is expected based on the LETs of the radiations (13); however, in the case of recoil energy, the factor of nearly two between the RBE for the 6.0 and 5.3 MeV α particles is unreasonably large compared to our earlier data obtained in the mouse testis model (13) and in vitro data in the literature (1). Furthermore, the RBE values of 2.1 and 2.7 at D37 for the 8.8 and 6.0 MeV α particles are very low compared to values obtained for V79 cells under different experimental conditions (9) as well as the values obtained in the mouse testis model (13). In contrast, the RBE values calculated without including recoil energy are in line with expectations (5, 13, 22). These considerations suggest that the recoil energy plays little or no role in the biological damage from intranuclearly localized α-particle emitters, particularly because if the recoil energy was effective and not included in the dose calculations, the RBE should be substantially higher than expected.
There are a number of plausible explanations for the apparent ineffectiveness of the recoil energy. One possible explanation is simply “overkill” owing to the extremely high LET (1600–1800 keV/μm, ref. 25) of the recoiling nucleus. This may be understood better by considering Barendsen’s (26) theoretical calculations which suggest that an α particle will interact with 50 sites on the DNA molecule during its traversal across the cell nucleus. In the present case, about 11 intracellular decays of 212Pb are required to deliver the 37% survival dose of 1 Gy. This corresponds to about 1.7 MeV of energy deposited in the cell nucleus excluding recoil energy. About 40 (0.68 MeV) and 57% (1.02 MeV) of this energy is delivered by the 6.0 and 8.8 MeV α particles, respectively. This is equivalent to about 1 complete traversal across the cell nucleus by the 6.0 MeV α particles plus 2 traversals by the 8.8 MeV particle, for a total of 3 traversals. Thus 3 traversals at 50 interactions per traversal corresponds to about 150 interactions with the DNA. Meanwhile, the 11 disintegrations required to achieve 37% survival result in only about 8 recoil events in the cell nucleus. With 35% of the activity in the cell nucleus bound to DNA, only about 3 of these events occur on the DNA. Therefore, the local damage imparted by these few recoil events may be overshadowed by the damage caused by the 150 interactions between the α particles and the DNA. Although this explanation appears to be most plausible, it is possible that the location of the decay relative to the DNA and mode of binding to the DNA could also be factors. That is, perhaps none of the 212Bi and 212Po α-particle decays occur on the DNA if the ligand is destroyed as a consequence of the parent 212Pb β-particle decay. Under this scenario, the recoil energy may be wasted.
CONCLUSIONS
The lethality of dipyrrolidinedithiocarbamato-212Pb(II) in Chinese hamster V79 cells is investigated. The 212Pb localizes predominantly in the cell nucleus where it is in secular equilibrium with its daughters 212Bi, 212Po and 208Tl. Ninety-seven percent of the absorbed dose to the cell nucleus is delivered by the 212Bi and 212Po α particles when the recoil energy associated with the intracellular 212Bi and 212Po decays is ignored. The mean lethal absorbed dose (D37) to the cell nucleus is 1.0 Gy, and there is no chemotoxicity associated with the radiocompound. The RBE at D37 compared to 137Cs γ rays is 4.0 when recoil energy is ignored and 2.3 when the recoil energy is included. These data, when compared to our data obtained previously for 5.3 MeV α particles from cytoplasmically localized 210Po, suggest that recoil energy does not play a significant role in imparting biological damage when α-particle emitters are localized in the cell nucleus.
Acknowledgments
This work was supported in part by USPHS Grant No. CA-54891.
Footnotes
This work was presented in the Ph.D. Dissertation of Michael T. Azure, Department of Chemistry, University of Massachusetts, Amherst, MA, 1993.
References
- 1.Barendsen GW, Walter MD, Fowler JF, Bewley DK. Effects of different ionizing radiations on human cells in tissue culture III. Experiments with cyclotron-accelerated alpha-particles and deuterons. Radiat Res. 1963;18:106–119. [PubMed] [Google Scholar]
- 2.Cox R, Thacker J, Goodhead DT, Munson RJ. Mutation and inactivation of mammalian cells by various ionising radiations. Nature. 1977;267:425–427. doi: 10.1038/267425a0. [DOI] [PubMed] [Google Scholar]
- 3.Raju MR, Eisen Y, Carpenter S, Inkret WC. Radiobiology of α particles. III. Cell inactivation by α-particle traversals of the cell nucleus. Radiat Res. 1991;128:204–209. [PubMed] [Google Scholar]
- 4.NCRP. The Relative Biological Effectiveness of Radiations of Different Quality. National Council on Radiation Protection and Measurements; Bethesda, MD: 1990. Report No. 104. [Google Scholar]
- 5.Kassis AI, Harris CR, Adelstein SJ, Ruth TJ, Lambrecht R, Wolf AP. The in vitro radiobiology of astatine-211 decay. Radiat Res. 1986;105:27–36. [PubMed] [Google Scholar]
- 6.Shadley JD, Whitlock JL, Rotmensch J, Atcher RW, Tang J, Schwartz JL. The effects of radon daughter α-particle irradiation in K1 and xrs-5 CHO cell lines. Mutat Res. 1991;248:73–83. doi: 10.1016/0027-5107(91)90089-7. [DOI] [PubMed] [Google Scholar]
- 7.Jostes RF, Hui TE, James AC, Cross FT, Schwartz JL, Rotmensch J, Atcher RW, Evans HH, Mencl J, Bakale G, Rao PS. In vitro exposure of mammalian cells to radon: Dosimetric considerations. Radiat Res. 1991;127:211–219. [PubMed] [Google Scholar]
- 8.Fisher DR, Frazier ME, Andrews TK., Jr Energy distribution and the relative biological effects of internal alpha emitters. Radiat Prot Dosim. 1985;13:223–227. [Google Scholar]
- 9.Charlton DE, Kassis AI, Adelstein SJ. A comparison of experimental and calculated survival curves for V79 cells grown as monolayers or in suspension exposed to alpha irradiation from 212Bi distributed in the growth medium. Radiat Prot Dosim. 1994;52:311–315. [Google Scholar]
- 10.Kurtzman SH, Russo A, Mitchell JB, DeGraff W, Sindelar WF, Brechbiel MW, Gansow OA, Friedman AM, Hines JJ, Gamson J, Atcher RW. Bismuth-212 linked to an antipancreatic carcinoma antibody: Model for alpha-particle-emitter radioimmunotherapy. J Natl Cancer Inst. 1988;80:449–452. doi: 10.1093/jnci/80.6.449. [DOI] [PubMed] [Google Scholar]
- 11.Howell RW, Rao DV, Hou DY, Narra VR, Sastry KSR. The question of relative biological effectiveness and quality factor for Auger emitters incorporated into proliferating mammalian cells. Radiat Res. 1991;128:282–292. [PubMed] [Google Scholar]
- 12.Macklis RM, Lin JY, Beresford B, Atcher RW, Hines JJ, Humm JL. Cellular kinetics, dosimetry and radiobiology of α-particle radioimmunotherapy: Induction of apoptosis. Radiat Res. 1992;130:220–226. [PubMed] [Google Scholar]
- 13.Howell RW, Azure MT, Narra VR, Rao DV. Relative biological effectiveness of alpha-particle emitters in vivo at low doses. Radiat Res. 1994;137:352–360. [PMC free article] [PubMed] [Google Scholar]
- 14.Rao DV, Narra VR, Howell RW, Govelitz GF, Sastry KSR. In-vivo radiotoxicity of DNA-incorporated I-125 compared with that of densely ionising alpha-particles. Lancet. 1989;II:650–653. doi: 10.1016/s0140-6736(89)90896-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rao DV, Narra VR, Howell RW, Lanka VK, Sastry KSR. Induction of spermhead abnormalities by incorporated radionuclides: Dependence on subcellular distribution, type of radiation, dose rate, and presence of radioprotectors. Radiat Res. 1991;125:89–97. [PMC free article] [PubMed] [Google Scholar]
- 16.Browne E, Firestone RB. Table of Radioactive Isotopes. Wiley; New York: 1986. [Google Scholar]
- 17.Thorn GD, Ludwig KA. The Dithiocarbamates and Related Compounds. Elsevier; New York: 1962. [Google Scholar]
- 18.Lau HKY, Droll HA, Lott PF. Ammonium 1-pyrrolidinedithiocarbamate as a reagent for bismuth. Anal Chim Acta. 1971;56:7–16. [Google Scholar]
- 19.Kassis AI, Adelstein SJ. A rapid and reproducible method for the separation of cells from radioactive media. J Nucl Med. 1980;21:88–90. [PubMed] [Google Scholar]
- 20.Kassis AI, Sastry KSR, Adelstein SJ. Intracellular distribution and radiotoxicity of chromium-51 in mammalian cells: Auger-electron dosimetry. J Nucl Med. 1985;26:59–67. [PubMed] [Google Scholar]
- 21.Goddu SM, Howell RW, Rao DV. Cellular dosimetry: Absorbed fractions for monoenergetic electron and alpha particle sources and S-values for radionuclides uniformly distributed in different cell compartments. J Nucl Med. 1994;35:303–316. [PubMed] [Google Scholar]
- 22.Kassis AI, Adelstein SJ, Haydock C, Sastry KSR. Radiotoxicity of 75Se and 35S: Theory and application to a cellular model. Radiat Res. 1980;84:407–425. [PubMed] [Google Scholar]
- 23.ICRP. RBE for Deterministic Effects. International Commission on Radiological Protection; Pergamon; Oxford: 1989. Publication 58. [PubMed] [Google Scholar]
- 24.Weber DA, Eckerman KF, Dillman LT, Ryman JC. MIRD: Radionuclide Data and Decay Schemes. Society of Nuclear Medicine; New York: 1989. [Google Scholar]
- 25.Ziegler JF. TRIM, The Transport of Ions in Matter. IBM; York-town, NY: 1992. [Google Scholar]
- 26.Barendsen GW. LET dependence of linear and quadratic terms in dose-response relationships for cellular damage: Correlations with the dimensions and structures of biological targets. Radiat Prot Dosim. 1990;31:235–239. [Google Scholar]