Abstract
The therapeutic potential of radionuclides that emit α particles, as well as their associated health hazards, have attracted considerable attention. The 224Ra daughters 212Pb and 212Bi, by virtue of their radiation properties which involve emission of α and β particles in their decay to stable 208Pb, have been proposed as candidates for radioimmunotherapy. Using mouse testes as the experimental model and testicular spermhead survival as the biological end point, the present work examines the radiotoxicity of 212Pb and its daughters. When 212Pb, in equilibrium with its daughters 212Bi, 212Po and 208Tl, was administered directly into the testis, the dose required to achieve 37% survival (D37) was 0.143 ± 0.014 Gy and the corresponding RBE of the mixed radiation field was 4.7 when compared to the D37 for acute external 120 kVp X rays. This datum, in conjunction with our earlier results for 210Po, was used to obtain an RBE–LET relationship for α particles emitted by tissue-incorporated radionuclides: RBEα = 4.8 − 6.1 × 10−2 LET + 1.0 × 10−3 LET2. Similarly, the dependence of RBE on α-particle energy Eα was given by . These relationships, based on in vivo experimental data, may be valuable in predicting biological effects of α-particle emitters.
INTRODUCTION
The therapeutic potential of radionuclides that emit α particles of high linear energy transfer (LET), and the potential risks involved when they are ingested, have attracted considerable attention over the years. A comprehensive review of the literature on biological effects of internal α-particle emitters was published recently (1). Substantial effort has been directed toward determining the relative biological effectiveness (RBE) of α particles compared to low-LET radiations (e.g. β, γ, X). However, the preponderance of data concerning the RBE of α particles and other high-LET particles both in vivo and in vitro have been obtained using external beams of radiation (2–9). As pointed out by the National Council on Radiation Protection and Measurements (NCRP) (10), there are only a limited number of in vivo studies with internal α-particle emitters which provide clear RBE information in terms of dose specifically to the target cells because of the microdosimetric complexities inherent in such experiments (11–13). Therefore, with the renewed interest in α-particle emitters for therapeutic applications (14–16), as well as the presence of α-particle emitters in the environment, further studies on the RBE of α-particle emitters in vivo are of interest.
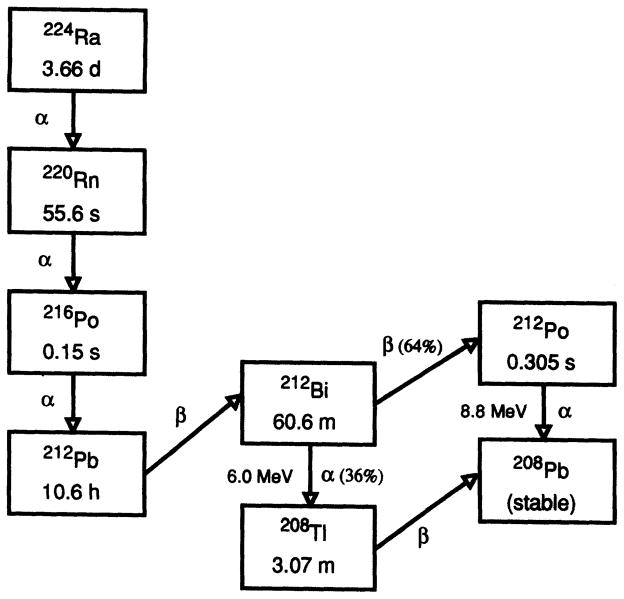
In the present work, the radiotoxicity of 212Pb (Tp = 10.64 h), in equilibrium with its relatively short-lived daughter products 212Bi, 212Po and 208Tl (Fig. 1), is investigated using spermatogonial cell killing in mouse testis as an in vivo model. The former two radionuclides (212Pb, 212Bi), which have been proposed for therapeutic applications (14,15,17), yield a mixed radiation field of photons, β particles and α particles (18). These present results on the radiotoxicity of 212Pb and its daughters, together with our earlier investigations in the same model with the monoenergetic α-particle emitter 210Po (19), are used to establish a relationship between the RBE of α particles emitted by incorporated radionuclides and their energy at low, but deterministic, doses. This relationship, based on in vivo data, may be useful for predicting the biological effects of pure and mixed α-particle fields emitted by incorporated radionuclides.
FIG. 1.
Radium-224 decay series.
MATERIALS AND METHODS
Biological Model
In the present work, spermatogenesis in mouse testes was used as the experimental model and testicular spermhead survival as the biological end point. This model, which is relevant to humans (20, 21), has been employed extensively to study the biological effects of external radiation (20, 22, 23) as well as incorporated radionuclides (19, 24–28). The testis contains a number of different cell populations of varying radiosensitivities. Of these, the spermatogonial cells (types A1–A4, In, B) are the most radiosensitive (LD50 ~ 0.40 Gy in mice). The remaining cell populations are substantially less radiosensitive with LD50 values ranging from 2 to 600 Gy (29). Therefore, irradiation of the testis with low doses results in a reduced testicular spermhead population when assayed 29 days after irradiation, the time required for the spermatogonia to become sonication-resistant spermatids in stages 12–16 (19, 20, 22, 23, 25–28).
Radionuclides and Radiochemistry
A solution of the complex chloro ions of 212Pb, 212Bi, 212Po and 208Tl, all in secular equilibrium, was used as the “radiochemical” in these studies and prepared according to the following procedures. The radionuclide 212Pb, with a 10.64-h half-life, was eluted from a 224Ra (Tp = 3.66 days) generator (Argonne National Laboratory, Argonne, IL) with 3 ml of 2 M HCl. After the initial elution, the resulting eluate was left in the hood for 15 min to allow the 220Rn (Tp = 55.6 s) to dissipate. To remove any traces of 224Ra which may have broken through the column, the eluate was passed through a column plugged with glass wool, packed with Dowex-50X8-100 cation exchange resin (Sigma Chemical Co., St. Louis, MO), which had been cleaned and preconditioned with 10−4M HCl. Subsequently, this eluate was eluted through Dowex 1×10–200 (Sigma) anion exchange resin previously cleaned and preconditioned with 0.9% NaCl. The anion exchange resin was used to expedite separation of the different lead compounds found to be in the solution. The 224Ra generator itself was a cation exchange resin, and over time the constant bombardment by the high-energy α particles causes the resin’s chemical bonds to cleave and fragments to form. These fragments were removed by disposing the eluate from the anion exchange column. This column was then eluted further with 2 M HCl and the eluate containing the 212Pb was collected for additional processing. The 212Bi complex ion remained in the column. The final eluate containing the was centrifuged at 15,000 rpm for 15 min. The supernatant was carefully removed and filtered through a 0.22-μm filter. This purified product was then rotoevaporated to dryness and dissolved in 10−4 M HCl to insure that hydroxide salts would not form and the 212Pb would stay in solution as a complex chloro ion, and then set aside for 4 h for secular equilibrium to be established with the 212Pb daughters 212Bi, 212Po and 208Tl. Hence the final radiochemical solution for injection contained and the complex chloro ions of 212Bi 212Po and 208Tl, all in equilibrium. All chloro ions were in solution as confirmed by centrifuging the product for 10 min at 15,000 rpm and radioassaying an aliquot of the supernatant.
General Procedures
Details of the experimental procedures may be found elsewhere (25–27, 30, 31). Male Swiss Webster mice (Taconic Farms, Germantown, NY) 9–10 weeks old and weighing about 30 g were used in these studies. Animals were maintained in the University animal care facility and provided food and water ad libitum. In keeping with well-established protocols (25, 27, 28), the radiochemical was injected along the long axis of the right testis of anesthetized mice in a standard 3-μl volume. The needle was slowly withdrawn from the organ during the injection so that the radiochemical was initially delivered along a line parallel to the long axis of the organ. Our large database with this model for all classes of radionuclides (α, β, γ, Auger) and numerous radiochemicals provides ample evidence that this “line injection” results in a sufficiently uniform distribution of radioactivity throughout the organ to allow intercomparison of the biological effects of different types of emitters and thus determination of RBE values (see Discussion section entitled RBE of 212Pb in Equilibrium with Daughters) (30,31).
The advantages of the intratesticular mode of administration of the radiopharmaceutical over other modes such as intravenous and intraperitoneal are apparent. First, because the testis is so small (0.1 g), photon radiations emanating from the organ deposit very little of their energy and therefore usually contribute minimally (<10%) to the total absorbed dose to the organ. Second, when the radiochemicals are administered intratesticularly, only very small amounts of radioactivity (a few tens of becquerels to thousands depending on the particles emitted and clearance pattern of the radiochemical) are required to deliver cytotoxic doses to the testis. Hence the testicular absorbed dose from penetrating radiations emitted by radioactivity that has cleared from the testis and entered the body is negligible. Third, unlike other modes of administration, the dose to other organs in the body is negligible for the intratesticular mode. This eliminates potential complications associated with organ toxicity. In short, the intratesticular mode of administration allows clear delineation of the biological effects of the particulate radiations emitted by the radionuclide(s) without significant interference from penetrating low-LET photon radiations (γ, X rays) (19,30).
Clearance of the Radionuclides from the Testis
Intratesticularly administered radioactivity is cleared from the organ into other parts of the body. The clearance pattern of the radionuclides from the testis was determined by administering a fixed amount of radioactivity into the right testis of 30 animals. At various times after injection, animals were sacrificed in groups of three by an overdose of anesthetic. The testicular activity was assayed immediately using a Canberra (Meriden, CT) Model GCW2525 Germanium well detector housed in a Model 747 shield, and a multichannel analyzer. The 238.6, 727.2 and 583.2 keV gamma;-ray peaks were used to quantify the 212Pb, 212Bi and 208T1 activity, respectively. For each time, the fraction of initially injected radioactivity remaining in the testis at the time of sacrifice was calculated, thereby yielding the effective retention of the activity in the organ.
Spermhead Survival Assay
To determine the spermhead survival fraction as a function of the testicular absorbed dose, animals (in groups of three) were injected intratesticularly with various concentrations of the radiochemical to deliver a range of absorbed doses to the testis. On the 29th day after injection, the day the spermhead count reaches a minimum (26, 27), the animals were sacrificed by an overdose of ether, and the right testis of each animal was resected. The testicular spermhead count was obtained by placing the testis in 1 ml deionized water, homogenizing, sonicating and counting the sonication-resistant spermheads in a hemocytometer (25–27). The surviving fraction, S, is the ratio of spermhead counts in the test group to the number of counts in the controls (injected with 10−4N HCl or decayed radiochemical).
Macroscopic Distribution of the Radioactivity in the Testis
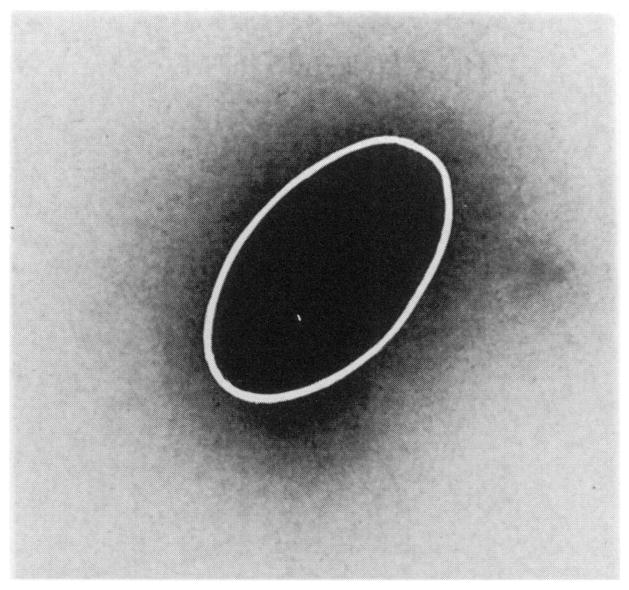
To insure that the intratesticular “line injection” resulted in a fairly uniform distribution of radioactivity throughout the testis at a gross level, the activity distribution was monitored in two ways. Several animals were injected intratesticularly with the radiochemical and sacrificed 12 h after injection, and the testes were removed promptly. The testes were quickly frozen by setting them on a microscope slide placed on a 5-kg lead block previously cooled with liquid nitrogen and sliced into eight sections, and the activity and weight of each slice were determined. The activity per gram of tissue remained relatively constant in the sections, indicating that the radioactivity was distributed in a fairly uniform fashion throughout the organ. Additional frozen testes were sliced in half along the long axis of the organ and placed in a frozen X-ray film cassette (Kodak X-Omatic Regular Lanex) loaded with XAR-5 film and stored at −20°C. After several hours of exposure the film was developed (Fig. 2). Although the geometry of the sample was semi-ellipsoid in nature with a length of ~7.6 mm, width of ~5 mm and thickness up to 2 mm, this technique provides a qualitative view of the gross distribution of activity in the organ. Based on the data above, the intratesticular injection facilitates a reasonably uniform distribution of activity in the organ at a gross level (30).
FIG. 2.
Autoradiograph (5× magnification) of a mouse testis injected along the long axis of the organ with a 3-μl standard volume of solution containing 212Pb in equilibrium with its daughter products. The ellipsoidal geometry of the testis (long axis ~7.6 mm, short axis ~5 mm) is outlined by the white line. The low-density halo around the organ is from crossfire exposure due to the finite thickness of the sample (up to 2 mm at the center).
Subcellular Distribution of the Radioactivity in Testicular Cells
Subcellular distribution of the radionuclides in the testicular cells was determined using a modification of the methods outlined by Rao et al. (32). One day after intratesticular administration of the radiochemical, the animals were sacrificed and the testes were removed, blotted with tissue and placed in 1 ml phosphate-buffered saline (PBS) per testis. The fibrous connective tissue covering each testis (tunica albuginea) was then removed carefully, and the PBS containing the remaining tissue was transferred to a Potter-Elvehjem tissue grinder and homogenized (four strokes) with a Teflon™ pestle. The tissue homogenate was filtered through a double layer of 120-μm nylon mesh and centrifuged at 4°C, 2000 rpm for 12 min. The total volume of the supernatant was noted and an aliquot was assayed for radioactivity. The pellet containing the cells was resuspended in 2 ml PBS and an aliquot was assayed for radioactivity. To the remaining suspension, 100 μl of NP-40 detergent was added and the mixture was homogenized with four or five strokes of the pestle and placed in an ice bath for 12 min. An ice-cold equal volume of 20% Ficoll in normal saline was added, the tube was allowed to stand for 5 min at 0°C, and then centrifuged at 2000 rpm for 15 min. The supernatant (cytoplasmic fraction) was assayed for radioactivity. The pellet containing the cell nuclei was washed once with 2 ml ice-cold hypotonic sucrose buffer (0.25 M, 3 mM CaCl2, 50 mM Tris, pH 7.0) and suspended in an additional 2 ml sucrose buffer, and an aliquot of the nuclear suspension was assayed for radioactivity. Guanidine-HCl-precipitable activity in the cell nucleus was assayed by adding an equal volume of ice-cold 6 M guanidine-HCl and mixing gently with a glass rod. An additional 3 ml of cold ethanol was added while mixing. The entire contents were transferred onto a Gelman Type A–E filter, and the tube, rod and filter were washed three times with 1 ml cold 1:1 guanidine-HCl:ethanol solution. Finally, the activity on the filter was considered to be the DNA-bound fraction.
RESULTS
Clearance of the Radionuclides from the Testis
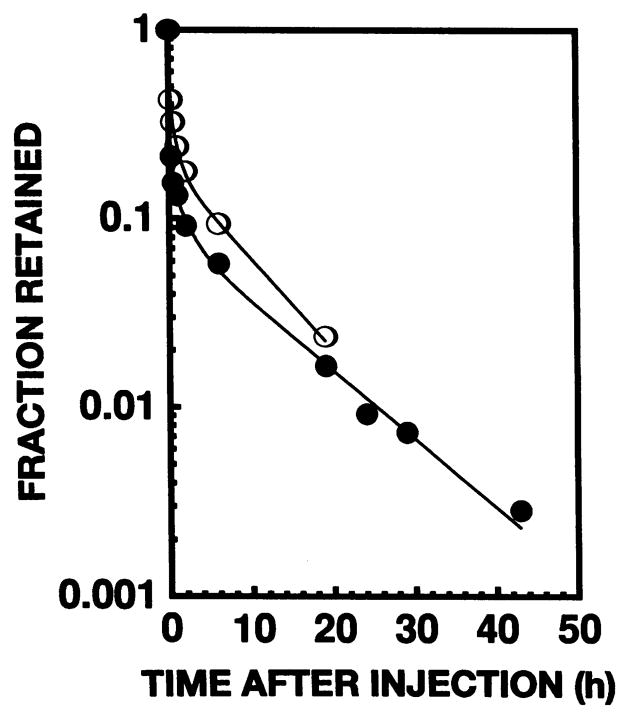
Figure 3 shows the effective clearance of 212Pb and 212Bi from the testis after intratesticular administration. The curve for 208Tl clearance was essentially identical to that for 212Bi. A least-squares fit of the data to a three-component exponential expression yielded the following relationships:
| (1) |
| (2) |
where f is the fraction of initially injected radioactivity remaining in the testis and t is the time after injection in hours.
FIG. 3.
Effective clearance of 212Pb (●) and 212Bi (○) from mouse testis after intratesticular injection of 212Pb in equilibrium with its daughters 212Bi, 212Po and 208T1. The daughter 208T1 followed essentially the same clearance pattern as 212Bi.
Testicular Absorbed Dose
The testicular absorbed dose delivered by the radionuclides was calculated in the same manner as in our earlier reports (25–28). Briefly, the mean testicular absorbed dose D is given by (33)
| (3) |
where the cumulated activity à is the time integral of the activity in the organ ∫A(t)dt, Δ is the mean energy emitted per nuclear transition, φ is the absorbed fraction, m is the average mass of the testis (0.1 g), and i denotes the ith radiation component emitted by the radionuclide. The cumulated activity was integrated over the period (7 days) during which the spermatogonial cells, which become spermatids 29 days later, were irradiated (25–28). Integration of the effective clearance (Eqs. 1–2) over the 7-day period yielded à = 1.23 MBq-h and 1.35 MBq-h per MBq injected for 212Pb and 212Bi (208Tl), respectively. It is apparent that, despite the somewhat different clearance patterns observed for these two radionuclides (Fig. 3), their cumulated activities are not markedly different. Because the effective clearance pattern of 208Tl essentially matched that of 212Bi as indicated above, the cumulated activity for both 208Tl and 212Po were taken as 1.35 MBq-h per MBq injected. The values of Δi for all radiations emitted by the radionuclides were taken from Weber et al. (18). Absorbed fractions φ for photons and electrons were calculated using the computer code of Howell et al. (34), whereas φ was taken to be 1 for the 212Bi and 212Po α particles. The resulting mean testicular absorbed dose was 66 Gy/MBq of 212Pb injected in equilibrium with its daughters 212Bi, 212Po and 208Tl. Of the total dose, only 1.8% was from the parent 212Pb radiations. Furthermore, 6.6% of the total dose was from electron and photon contributions, 26.1% from the 6.0 MeV 212Bi α particle, and 67.3% from the 8.8 MeV 212Po α particle. Hence 93.4% of the deposited energy was from the high-LET α particles.
Dose-Response Curves for Spermhead Survival
The fraction of surviving spermheads S/So is shown in Fig. 4 as a function of the average testicular absorbed dose from 212Pb and its daughters. As in our previous studies with internal and external radiation sources (25–28), and as reported by others (35, 36), the survival curve follows the familiar two-component exponential response. A least-squares fit to the expression,
FIG. 4.
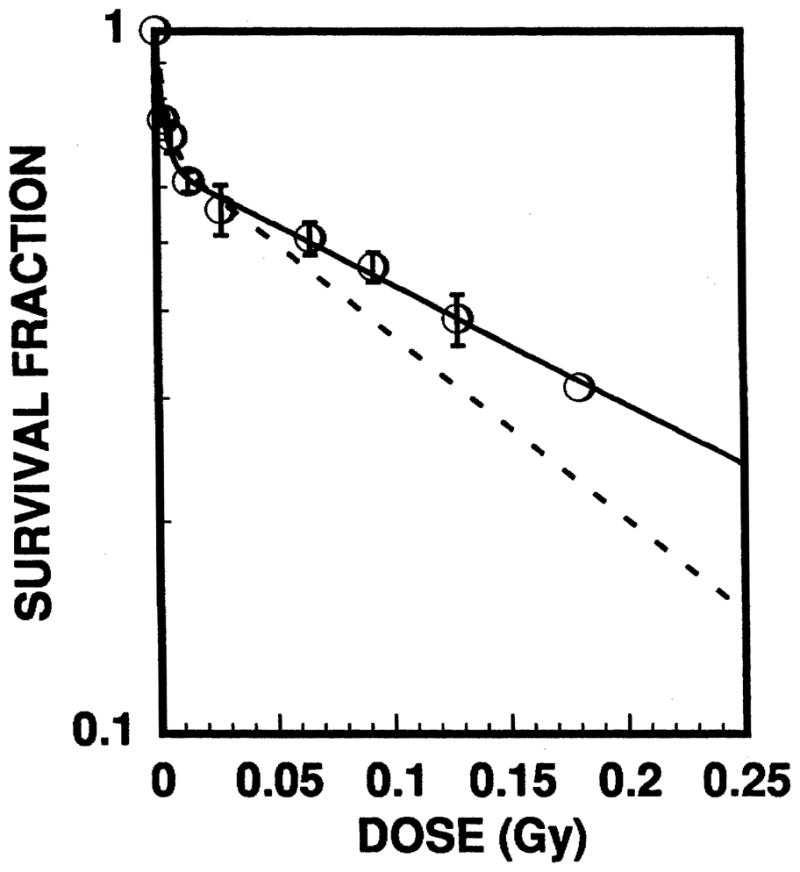
Surviving fraction of spermheads in mouse testis after intratesticular injection of the radiochemical. The open circles represent the data for administration of 212Pb in equilibrium with its daughters 212Bi, 212Po and 208Tl. Probable errors are indicated by the error bars. For comparison, the dotted line is the dose–response curve obtained previously for 210Po which emits 5.3 MeV α particles (19). The two-component exponential nature of the dose–response curves is a familiar feature of the mouse testis model (30, 31, 35, 36).
| (4) |
yields a = 0.65 ± 0.018, D1 = 3.0 × 10−3 ± 5.2 × 10−4 Gy and D2 = 0.25 ± 0.022 Gy. Hence the dose required to achieve 37% survival (D37) is 0.14 ± 0.014 Gy, and the D10 is 0.48 ± 0.048 Gy.
Subcellular Distribution of the Radioactivity
Subcellular distribution studies indicate that 50% of the testicular activity was in the cells of the organ while the remaining 50% was found in the extracellular spaces. Of the cellular activity, 69% was found in the cell nucleus and 31% in the cytoplasm. Finally, of the radioactivity in the cell nucleus, only 6% was bound to the DNA.
DISCUSSION
Selection of a Reference Radiation for Determination of RBE
To calculate RBE values for internal radionuclides from dose–response relationships obtained with the mouse testis model, or any model for that matter, particular attention must be paid to the selection of sources of the reference radiation (28, 30, 37). In our earlier work, the effects of a variety of sources of low-LET radiation were examined including external X rays (26) and intratesticularly administered β-particle and γ-ray emitters (30,31). When testes were irradiated externally with acute 60 or 120 kVp X rays (~0.1 Gy/min), a D37 value of 0.67 ± 0.03 Gy and D10 value 1.9 ± 0.09 Gy were obtained (30, 31). Similarly, when the testes were chronically irradiated with 477 keV γ rays from intratesticularly administered 7Be-chloride (30, 31), or medium-energy β particles (average range ~400 μm) from 131Iododeoxyuridine or H131IPDM (N,N,N′-trimethyl-N′-(2-hydroxyl-3-methyl-5-iodobenzyl)-1,3-propanediamine) (31), essentially the same D37 values were obtained. Based on these data, the chronic vs acute, internal vs external, and photon vs electron nature of the reference radiation are not critical factors in the spermhead survival assay (30). Therefore, in keeping with convention, acute external 120 kVp X rays have been taken as the reference radiation.
Influence of Microscopic Distribution of Radioactivity on RBE
It is well known that the microscopic distribution of the radioactivity in the organ may affect the RBE significantly (1, 26–28, 30, 38, 39). For α-particle emitters, this is primarily because the microscopic distribution may be such that some or all of the target cells in the organ do not receive a dose equal to the average organ absorbed dose (39, 40). In addition, unlike cell culture studies with α-particle beams where the energy and LET are precisely controlled, in vivo studies with incorporated radionuclides involve irradiation of the target cells with a polyenergetic α-particle field. Therefore, no single value of energy or LET adequately describes the nature of the α-particle field to which the cells are exposed. In view of these considerations, a full microdosimetric analysis taking into account the finer aspects of target size, source distribution at the cellular level, hit probability, etc., is needed to obtain a better understanding of the RBE of incorporated radionuclides in vivo (39, 41, 42).
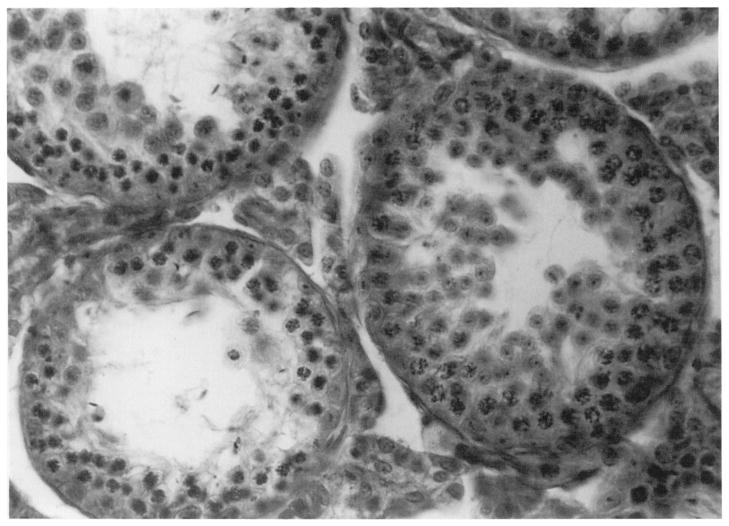
The mouse testis model used in the present studies is not readily amenable to a microdosimetric analysis. Nevertheless, the model has proven itself to be remarkably self-consistent for a host of radionuclides that emit low-LET radiations having maximum ranges both shorter and longer than the a particles encountered in the present work (30,31). A similarly consistent pattern appears to be operational for internal α-particle emitters when our present study is compared with our earlier studies with 210Po (see Discussion section entitled The RBE-LET Relationship for α-Particle Emitters In Vivo). This is perhaps not unexpected given that the range of the α particles is on the order of the diameter (80–100 μm) of the seminiferous tubules (Fig. 5), and that subcellular distribution studies showed that about half of the organ activity was localized within the testicular cells while the remainder was in the intertubular spaces. These considerations suggest that the cells within the testis are irradiated in a sufficiently uniform fashion so that the mean absorbed dose to the organ is a good determinant of the biological response in this experimental model.
FIG. 5.
Seminiferous tubules (large circular structures) in the mouse testis. The tubules are 80–100 μm in diameter. The radiosensitive spermatogonia lie within the peripheral 10–20-μm-thick section of the tubules.
RBE of 212Pb in Equilibrium with Daughters
When acute external X rays are taken as the reference radiation, the RBE of 212Pb in equilibrium with its daughters is 4.7 ± 0.5 and 4.1 ± 0.5 at D37 and D10, respectively. Given that 98.2% of the testicular absorbed dose is delivered by 212Bi and its subsequent daughters, while only 1.8% of the dose is from the parent 212Pb (see Testicular Absorbed Dose section above), it is not unreasonable to compare our results with those obtained for 212Bi by Shadley et al. (43). They irradiated CHO K1 cells in vitro with 212Bi localized extracellularly in the culture medium which resulted in a polyenergetic α-particle field similar to that encountered in the testis. Interestingly, they found an RBE for cell survival of 3.8 at D10, which is close to our value. This close correlation is not unexpected based on our experience in comparing RBE values obtained in vitro with those obtained in vivo with the spermhead survival assay (30).
RBE of the 212Bi and 212Po α Particles
The individual RBE values for both the 6.0 and 8.8 MeV α particles emitted by the 212Pb daughters 212Bi and 212Po, respectively, may be extracted from our data. Our experimentally measured RBE (RBEexp), which reflects the net effect of the mixed radiations emitted by 212Pb and its daughters, may be expressed as a sum of three contributions (see Appendix for derivation of Eq. 5):
| (5) |
where RBE6.0 and RBE8.8 are the RBE values for the 6.0 and 8.8 MeV α particles of 212Bi and 212Po, respectively, and the coefficients f6.0, f8.8 and fother are the fraction of the total testicular absorbed dose delivered by the α particles and the low-LET radiations (β, γ, X, e−), respectively. These coefficients are f8.8 = 0.673, f6.0 = 0.261, fother = 0.066 (see Testicular Absorbed Dose section above). Our earlier studies (27, 30, 31) with radionuclides that emit only low-LET radiations such as γ rays, X rays, conversion electrons and β particles yielded RBE values of ~1.0; hence RBEother may be taken as 1. This leaves only the RBE values for the α particles undefined. According to the data obtained by Barendsen et al. using human cells in tissue culture (4), the RBE of an α particle with LET = 76 keV/μm (energy 6.0 MeV) is ~1.3-fold greater than the RBE of an α particle with LET = 57 keV/μm (energy 8.8 MeV). An analogous comparison between the RBE values for the track-averaged LET values for 6.0 and 8.8 MeV α particles also yields a ratio of ~1.3. Although this ratio was taken from data obtained from cells in culture, a similar value is expected in the mouse testis model based on our experience with both cultured V79 cells and the mouse testis, where similar RBE values have been obtained for a variety of radionuclides including α-particle emitters (30). Hence it is not unreasonable to approximate that in the mouse testis RBE6.0 ~1.3 RBE8.8. It should be noted that a similar ratio may be obtained from the data of Alpen and Powers-Risius using a heavy-ion (12C) beam, where LET values ranging from 11 to 105 keV/μm were investigated (2). Using the above ratio, and RBEexp = 4.7, one obtains RBE6.0 = 6.0 and RBE8.8 = 4.6 when 37% survival is taken as the biological end point.
The RBE-LET Relationship for α-Particle Emitters In Vivo
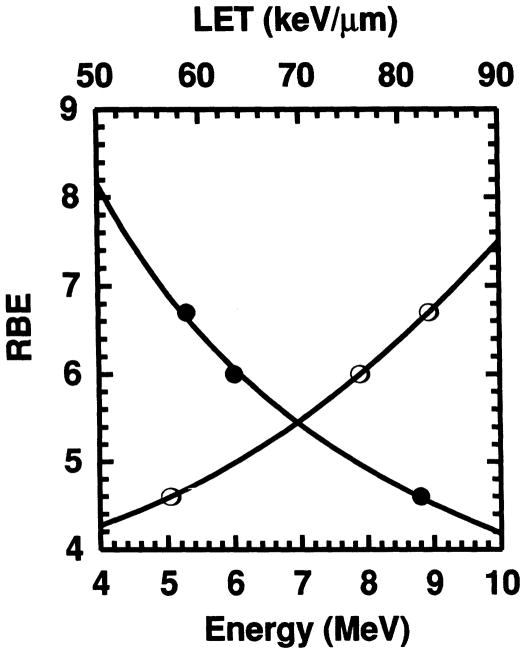
Our earlier studies with 210Po, which emits a single 5.3 MeV α particle, resulted in an RBE of 6.7 in the same experimental model (19). The RBE values obtained in this work for 6.0 and 8.8 MeV α particles, in combination with our previous data for 5.3 MeV α particles, allows one to construct an RBE–LET relationship for α-particle emitters in vivo. Figure 6 graphically shows the relationship between the RBE and the initial LET of the α particle, as well as the RBE and initial α-particle energy Eα. Although perhaps not necessarily the ideal choice, the initial LET has been used here for the sake of simplicity and because the ICRU (44) has indicated that the choice of LET (e.g. track average, etc.) for comparison purposes is somewhat arbitrary. The best fits to the data are provided by a power function for the RBE–energy relationship (i.e. RBE = Ex) and power series for the RBE–LET relationship (i.e. ). A least-squares fit of the data to the above equations gives
FIG. 6.
Relative biological effectiveness of α particles emitted by radionuclides incorporated in mouse testis as a function of particle energy (●) and initial linear energy transfer (○). The data are for the 5.3, 6.0 and 8.8 MeV α particles from incorporated 210Po (19), 212Bi and 212Po, respectively. The least-squares fits to the data indicated by the solid lines cover most of the energies encountered for α-particle emitters.
| (6) |
| (7) |
which should be reasonably valid over the energy range 4–10 MeV and initial LET range 50–90 keV/μm. This range covers most particle energies encountered with α-particle-emitting radionuclides and therefore may be useful in predicting their lethality in vivo. It should be pointed out that the RBE–LET relationship given in Eq. (7) may not be extended to radiations other than α particles. Using heavy-ion beams, Alpen and Powers-Risius (2) have clearly shown that LET alone is not a good predictor of spermatogonial cell killing in mouse testis—other factors such as particle mass play a role in the biological outcome.
CONCLUSIONS
The results presented in this work provide an RBE–LET relationship for α particles at low doses using an in vivo model that is relevant to humans (20,21). It is recognized that the experimental RBE values obtained here are based on deterministic effects and must be used with caution in risk assessment. However, RBE values obtained even at lower doses using induction of spermhead abnormalities in mouse testis as the biological end point (28, 45) may be better suited for this purpose. In any case, recent attempts to use α-particle emitters in radioimmunotherapy of cancer suggest that RBE values based on deterministic effects for α particles of different energies are indeed necessary to assess their therapeutic efficacy. Hence the present results are significant in that they provide some guidance in predicting biological effects when α-particle-emitting radionuclides are administered for therapy or are ingested occupationally or environmentally.
Acknowledgments
The authors wish to thank Ravi Harapanhalli and Kandula Sastry for valuable comments regarding this manuscript. This work was supported in part by USPHS Grants CA54891 and CA32877.
APPENDIX
Derivation of the RBE for Mixed Radiations in the Testis
A derivation for the RBE of mixed radiations based on the linear-quadratic dose dependence for cell survival was presented in Section 6.3 of ICRP Publication 58 (46). Here, a similar relationship is derived for the two-component exponential dose response (Eq. A1) observed in the mouse testis model.
| (A1) |
For the sake of clarity, the logic and definitions used by the ICRP are employed whenever possible. The RBE for the mixed radiation field is defined as
| (A2) |
where D is the total absorbed dose from the mixed field, and De is the equivalent dose from photons (photon dose that produces the same biological effect as D). With q defined as the fraction of the total dose delivered by the high-LET radiation, one may write
| (A3) |
where ae, D1,e, and D2,e are the dose–response parameters for reference photons, aH, D1,H and D2,H are the parameters for the high-LET radiation, and aD is the dose–response parameter for the mixed field. At 37% survival, the D1 terms are negligible compared to the D2 terms. Hence, when R·D is substituted for De, one obtains
| (A4) |
In our recent comprehensive review on the lethality of various radiochemicals in the mouse testis, it may be seen that the coefficient a in the two-component exponential dose–response expression (Eq. A1) is ~0.7 for α-particle emitters, low-LET emitters and external X rays (30). Therefore, ae ≈ aD ≈ aH and RBEH ≈ D2,e/D2,H. Solving for R and substituting in RBEH yields
| (A5) |
Since ae ≈ aD, the last term in Eq. A5 is negligible, and the RBE of the mixed radiation field at 37% survival is then given by
| (A6) |
This is the same result obtained in ICRP Publication 58 (46) for the linear-quadratic dose dependence. For a mixture of multiple high- and low-LET radiations having i different components, Eq. A6 logically extends to the relationship
| (A7) |
where RBEi and qi are the RBE at 37% survival and fraction of total dose delivered by radiation component i, and .
References
- 1.National Research Council, Committee on the Biological Effects of Ionizing Radiation. Health Risks of Radon and Other Internally Deposited Alpha-Emitters (BEIR IV) National Academy Press; Washington, DC: 1988. [PubMed] [Google Scholar]
- 2.Alpen EL, Powers-Risius P. The relative biological effect of high-Z, high-LET charged particles for spermatogonial killing. Radiat Res. 1981;88:132–143. [PubMed] [Google Scholar]
- 3.Barendsen GW. Dose–survival curves of human cells in tissue culture irradiated with alpha-, beta-, 20-kV. X- and 200-kV. X-radiation. Nature. 1962;193:1153–1155. doi: 10.1038/1931153a0. [DOI] [PubMed] [Google Scholar]
- 4.Barendsen GW, Walter MD, Fowler JF, Bewley DK. Effects of different ionizing radiations on human cells in tissue culture III. Experiments with cyclotron-accelerated alpha-particles and deuterons. Radiat Res. 1963;18:106–119. [PubMed] [Google Scholar]
- 5.Cox R, Thacker J, Goodhead DT, Munson RJ. Mutation and inactivation of mammalian cells by various ionising radiations. Nature. 1977;267:425–427. doi: 10.1038/267425a0. [DOI] [PubMed] [Google Scholar]
- 6.Goodhead DT, Munson RJ, Thacker J, Cox R. Mutation and inactivation of cultured mammalian cells exposed to beams of accelerated heavy ions, IV. Biophysical interpretation. Int J Radiat Biol. 1980;37:135–167. doi: 10.1080/09553008014550201. [DOI] [PubMed] [Google Scholar]
- 7.Goodhead DT, Belli M, Mills AJ, Bance DA, Allen LA, Hall SC, Ianzani F, Simony G, Stevens DL, Stretch A, Tabocchini MA, Wilkinson RE. Direct comparison between protons and alpha-particles of the same LET: I. irradiation methods and inactivation of asynchronous V79, HeLa and C3H 10T1/2 cells. Int J Radiat Biol. 1992;61:611–624. doi: 10.1080/09553009214551421. [DOI] [PubMed] [Google Scholar]
- 8.Lloyd EL, Gemmell MA, Henning CB, Gemmell DS, Zabransky BJ. Cell survival following multiple-track alpha particle irradiation. Int I Radiat Biol. 1979;35:23–31. doi: 10.1080/09553007914550031. [DOI] [PubMed] [Google Scholar]
- 9.Raju MR, Eisen Y, Carpenter S, Inkret WC. Radiobiology of α particles. III. Cell inactivation by a-particle traversals of the cell nucleus. Radi at Res. 1991;128:204–209. [PubMed] [Google Scholar]
- 10.NCRP. The Relative Biological Effectiveness of Radiations of Different Quality. National Council on Radiation Protection and Measurements; Bethesda, MD: 1990. Report No. 104. [Google Scholar]
- 11.Brooks AL. Chromosome damage in liver cells from low dose rate alpha, beta and gamma irradiation: Derivation of RBE. Science. 1975;190:1090–1092. doi: 10.1126/science.1188384. [DOI] [PubMed] [Google Scholar]
- 12.Taylor GN, Mays CW, Lloyd RD, Gardner PA, Talbot LR, McFarland SS, Pollard TA, Atherton DR, VanMoorhem D, Brammer D, Brammer TW, Ayoroa G, Taysum DH. Comparative toxicity of 226Ra, 239Pu, 241Am, 249Cf and 252Cf in C57BL/Do black and albino mice. Radiat Res. 1983;95:584–601. [PubMed] [Google Scholar]
- 13.Mays CW, Finkel MP. RBE of α-particles vs. β-particles in bone sarcoma induction. Proceedings of the 5th International Congress of the IRPA; Jerusalem: Israel Health Physics Society; 1980. pp. 401–405. [Google Scholar]
- 14.Kurtzman SH, Russo A, Mitchell JB, DeGraff W, Sindelar WF, Brechbiel MW, Gansow OA, Friedman AM, Hines JJ, Gamson J, Atcher RW. Bismuth-212 linked to an antipancreatic carcinoma antibody: Model for alpha-particle-emitter radioimmunotherapy. J Natl Cancer Inst. 1988;80:449–452. doi: 10.1093/jnci/80.6.449. [DOI] [PubMed] [Google Scholar]
- 15.Macklis RM, Kinsey BM, Kassis AI, Ferrara JLM, Atcher RW, Hines JJ, Coleman CN, Adelstein SJ, Burakoff SJ. Radioimmunotherapy with alpha-particle-emitting immunoconjugates. Science. 1988;240:1024–1026. doi: 10.1126/science.2897133. [DOI] [PubMed] [Google Scholar]
- 16.Humm JL. A microdosimetric model of astatine-211 labeled anti-bodies for radioimmunotherapy. Int J Radiat Oncol Biol Phys. 1987;13:1767–1773. doi: 10.1016/0360-3016(87)90176-3. [DOI] [PubMed] [Google Scholar]
- 17.Rotmensch J, Atcher RW, Schlenker R, Hines J, Grdina D, Block BS, Press MF, Herbst AL, Weichselbaum RR. The effect of the alpha-emitting radionuclide lead-212 on human ovarian carcinoma: A potential new form of therapy. Gynecol Oncol. 1989;32:236–239. doi: 10.1016/s0090-8258(89)80040-x. [DOI] [PubMed] [Google Scholar]
- 18.Weber DA, Eckerman KF, Dillman LT, Ryman JC. MIRD: Radionuclide Data and Decay Schemes. Society of Nuclear Medicine; New York: 1989. [Google Scholar]
- 19.Rao DV, Narra VR, Howell RW, Govelitz GF, Sastry KSR. In-vivo radiotoxicity of DNA-incorporated 1–125 compared with that of densely ionising alpha-particles. Lancet. 1989;II:650–653. doi: 10.1016/s0140-6736(89)90896-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meistrich ML, Samuels RC. Reduction in sperm levels after testicular irradiation of the mouse: A comparison with man. Radiat Res. 1985;102:138–147. [PubMed] [Google Scholar]
- 21.Gaulden ME. “Biological dosimetry” of radionuclides and radiation hazards. J Nucl Med. 1983;24:160–164. [PubMed] [Google Scholar]
- 22.Oakberg EF. Sensitivity and time of degeneration of spermatogenic cells irradiated in various stages of maturation in the mouse. Radiat Res. 1955;2:369–391. [PubMed] [Google Scholar]
- 23.Oakberg EF. Duration of spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium. Am J Anat. 1956;99:507–516. doi: 10.1002/aja.1000990307. [DOI] [PubMed] [Google Scholar]
- 24.Mian TA, Glenn HJ, Haynie TP, Meistrich ML. Radiation dose and biological effects to mouse testis from sodium P-32 phosphate. Health Phys. 1982;42:657–664. doi: 10.1097/00004032-198205000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Rao DV, Govelitz GF, Sastry KSR. Radiotoxicity of thallium-201 in mouse testes: Inadequacy of conventional dosimetry. J Nucl Med. 1983;24:145–153. [PubMed] [Google Scholar]
- 26.Rao DV, Sastry KSR, Grimmond HE, Howell RW, Govelitz GF, Lanka VK, Mylavarapu VB. Cytotoxicity of some indium radiopharmaceuticals in mouse testes. J Nucl Med. 1988;29:375–384. [PubMed] [Google Scholar]
- 27.Rao DV, Narra VR, Howell RW, Sastry KSR. Biological consequence of nuclear versus cytoplasmic decays of 125I: Cysteamine as a radioprotector against Auger cascades in vivo. Radiat Res. 1990;124:188–193. [PubMed] [Google Scholar]
- 28.Rao DV, Narra VR, Howell RW, Lanka VK, Sastry KSR. Induction of spermhead abnormalities by incorporated radionuclides: Dependence on subcellular distribution, type of radiation, dose rate and presence of radioprotectors. Radiat Res. 1991;125:89–97. [PMC free article] [PubMed] [Google Scholar]
- 29.Meistrich ML. Critical components of testicular function and sensitivity to disruption. Biol Rep. 1986;34:17–28. doi: 10.1095/biolreprod34.1.17. [DOI] [PubMed] [Google Scholar]
- 30.Howell RW, Narra VR, Hou DY, Terrone DA, Harapanhalli RS, Sastry KSR, Rao DV. Relative biological effectiveness of Auger emitters for cell inactivation: In vitro versus in vivo. In: Howell RW, Narra VR, Sastry KSR, Rao DV, editors. Biophysical Aspects of Auger Processes. American Institute of Physics; Woodbury, NY: 1992. pp. 290–318. [Google Scholar]
- 31.Narra VR, Howell RW, Harapanhalli RS, Sastry KSR, Rao DV. Radiotoxicity of some I-123, I-125 and I-131 labeled compounds in mouse testes: Implications for radiopharmaceutical design. J Nucl Med. 1992;33:2196–2201. [PubMed] [Google Scholar]
- 32.Rao DV, Sastry KSR, Govelitz GF, Grimmond HE, Hill HZ. In vivo effects of iron-55 and iron-59 on mouse testis: Biophysical dosimetry of Auger electrons. J Nucl Med. 1985;26:1456–1465. [PubMed] [Google Scholar]
- 33.Loevinger R, Budinger TF, Watson EE. MIRD Primer for Absorbed Dose Calculations. The Society of Nuclear Medicine; New York: 1991. [Google Scholar]
- 34.Howell RW, Rao DV, Sastry KSR. Macroscopic dosimetry for radioimmunotherapy: Nonuniform activity distributions in solid tumors. Med Phys. 1989;16:66–74. doi: 10.1118/1.596404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spano M, Pacchierotti F, Mauro F, Quaggia S, Uccelli R. Flow cytometric analysis of the effects of 0.4 MeV fission neutrons on mouse spermatogenesis. Int J Radiat Biol. 1987;51:401–419. doi: 10.1080/09553008714550901. [DOI] [PubMed] [Google Scholar]
- 36.Gasinska A. Mouse testis weight loss and survival of differentiated spermatogonia following irradiation with 250 kV and 5.5 MeV fast neutrons. Neoplasma. 1985;32:443–449. [PubMed] [Google Scholar]
- 37.Howell RW, Rao DV, Hou D-Y, Narra VR, Sastry KSR. The question of relative biological effectiveness and quality factor for Auger emitters incorporated into proliferating mammalian cells. Radiat Res. 1991;128:282–292. [PubMed] [Google Scholar]
- 38.Howell RW, Narra VR, Sastry KSR, Rao DV. On the equivalent dose for Auger electron emitters. Radiat Res. 1993;134:71–78. [PMC free article] [PubMed] [Google Scholar]
- 39.Fisher DR, Frazier ME, Andrews J. Energy distribution and the relative biological effects of internal alpha emitters. Radiat Prot Dosim. 1985;13:223–227. [Google Scholar]
- 40.Green D, Howells GR. Radiation dose to mouse testes from Pu-239. Health Phys. 1980;38:242–243. [PubMed] [Google Scholar]
- 41.Polig E. The localized dosimetry of internally deposited alpha-emitters. Curr Top Radiat Res. 1978;13:189–327. [Google Scholar]
- 42.Roesch WC. Microdosimetry of internal sources. Radiat Res. 1977;70:494–510. [PubMed] [Google Scholar]
- 43.Shadley JD, Whitlock JL, Rotmensch J, Atcher RW, Tang J, Schwartz JL. The effects of radon daughter α-particle irradiation in K1 and xrs-5 CHO cell lines. Mutat Res. 1991;248:73–83. doi: 10.1016/0027-5107(91)90089-7. [DOI] [PubMed] [Google Scholar]
- 44.ICRU. Linear Energy Transfer. International Commission on Radiation Units and Measurements; Bethesda, MD: 1970. Report 16. [Google Scholar]
- 45.Wyrobek AJ, Bruce WR. The induction of sperm-shape abnormalities in mice and humans. In: Hollaender A, de Serres FJ, editors. Chemical Mutagens: Principles and Methods for Their Detection. Plenum; New York: 1978. pp. 257–285. [Google Scholar]
- 46.ICRP. RBE for Deterministic Effects. International Commission on Radiological Protection; Pergamon, Oxford: 1989. Publication 58. [PubMed] [Google Scholar]