Abstract
The biological effects of radionuclides that emit α particles are of considerable interest in view of their potential for therapy and their presence in the environment. The present work is a continuation of our ongoing effort to study the radiotoxicity of α-particle emitters in vivo using the survival of murine testicular sperm heads as the biological end point. Specifically, the relative biological effectiveness (RBE) of very low-energy α particles (3.2 MeV) emitted by 148Gd is investigated and determined to be 7.4 ± 2.4 when compared to the effects of acute external 120 kVp X rays. This datum, in conjunction with our earlier results for 210Po and 212Pb in equilibrium with its daughters, is used to revise and extend the range of validity of our previous RBE–energy relationship for α particles emitted by tissue-incorporated radionuclides. The new empirical relationship is given by RBEα = 9.14 − 0.510 Eα, where 3 < Eα < 9 MeV. The validity of this empirical relationship is tested by determining the RBE of the prolific α-particle emitter 223Ra (in equilibrium with its daughters) experimentally in the same biological model and comparing the value obtained experimentally with the predicted value. The resulting RBE values are 5.4 ± 0.9 and 5.6, respectively. This close agreement strongly supports the adequacy of the empirical RBE-Eα relationship to predict the biological effects of α-particle emitters in Vivo.
INTRODUCTION
Alpha-particle emitters are being explored for use in radioimmunotherapy, and they are present in the environment (i.e. 222Rn). Therefore, there is an increasing need to understand the biological effects caused by internal α-particle emitters. Alpha particles emitted by radionuclides generally have energies that range from about 3 to 9 MeV with corresponding linear energy transfers (LETs) ranging from about 125 to 60 keV/μm. Barendsen (1) has reviewed the relationship between the relative biological effectiveness (RBE) and LET for different types of lethal damage in cultured mammalian cells. For in vitro studies, the RBE is strongly dependent on LET with a maximum at about 120 keV/μm. Similarly, Miller et al. (2) have investigated oncogenic transformation as a function of LET in cultured C3H 10T1/2 cells and found that the RBE for stochastic effects (RBEM) is also maximum at about 120 keV/μm. Although there is a relative abundance of such data obtained in vitro, there are few studies investigating the relationship between RBE and LET in vivo. Given that the biological effects of ionizing radiation are highly dependent on LET (3, 4), and that there are numerous radionuclides of importance to nuclear medicine and radiation protection that emit high-LET α particles, it is essential that the effects of α-particle emitters be investigated in vivo as a function of the emitted α-particle energy (Eα) and LET. In our earlier study (5), empirical RBE–LET and RBE–Eα relationships were established in vivo over a limited range of α-particle energies (5.3–8.8 MeV) using spermatogenesis in mouse testes as the experimental model. In the present study, we extend the RBE–Eα relationship to as low as 3.2 MeV using the radionuclide 148Gd, thereby covering most of the α-particle energies emitted by incorporated radionuclides. This relationship is tested by determining the RBE experimentally for 223Ra in equilibrium with its daughters, a decay series which emits a spectrum of α particles with energies ranging from 5.3 to 7.5 MeV, and comparing the resulting experimental RBE with the calculated RBE based on the empirical RBE–Eα relationship.
MATERIALS AND METHODS
Biological Model
Spermatogenesis in the mouse testis is used as the experimental model with testicular sperm head survival serving as the biological end point. This same model was used in our earlier reports on the biological effects of α-particle emitters (5) and other radionuclides (6–11). The process of spermatogenesis in mouse and man is very similar, except for the time scale: about 5 weeks for mouse and 10 weeks for man (12, 13). This complex process (14) begins with the stem cell (Ais) differentiating to form a pair of cells which further divide to give type A1 spermatogonial cells. The type A1 cells in turn divide repeatedly through several spermatogonial cell stages designated as A2, A3, A4, In and B. For mice, it takes about 7 days for the cells to pass through the spermatogonial cell stages. The type B spermatogonia divide to become spermatocytes which mature over a 14-day period to spermatids. Finally, the spermatids pass through 16 stages during a 14-day process before they become functional sperm. The spermatids are resistant to sonication for about 7 days (stages 12–16).
Oakberg (15) and others (14, 16) have documented the highly differential radiosensitivity of these numerous cell populations in mammalian testes. The spermatogonial cells (types A1–A4, In, B) are the most sensitive to X rays (LD50 ~ 0.40 Gy in mice), while the remaining cell populations are substantially less sensitive, with LD50 values ranging from 2 to 600 Gy (17). Therefore, when the testes are irradiated with low doses which principally affect only the highly radiosensitive spermatogonial cells, a reduced testicular sperm head population is manifested when assayed 29–36 days postirradiation, the time required for the spermatogonia to become sonication-resistant spermatids of stages 12–16 (7–12, 18, 19). Thus we have historically referred to this assay as the sperm head survival assay because the sperm head count is used as an indirect measurement of survival of spermatogonial cells.
Radionuclides and Radiochemistry
Gadolinium-148, a pure α-particle emitter [3.2 MeV, continuously-slowing-down approximation (csda) range in water 20 μm (20)] with a physical half-life (tp) of 75 years, was obtained precalibrated (within 5%) from the Medical Radioisotope Program at Los Alamos National Laboratory as Gd(III) in 0.5 M HCl. The radiochemical 148Gd-citrate was prepared by mixing the stock solution with 1 M sodium citrate (pH 4.7) in accordance with our earlier procedures for 210Po-citrate (11). The 148Gd activity was assayed using a Beckman Model 5500 automatic liquid scintillation counter and Fluorosol® (National Diagnostics, Manville, NJ) cocktail.
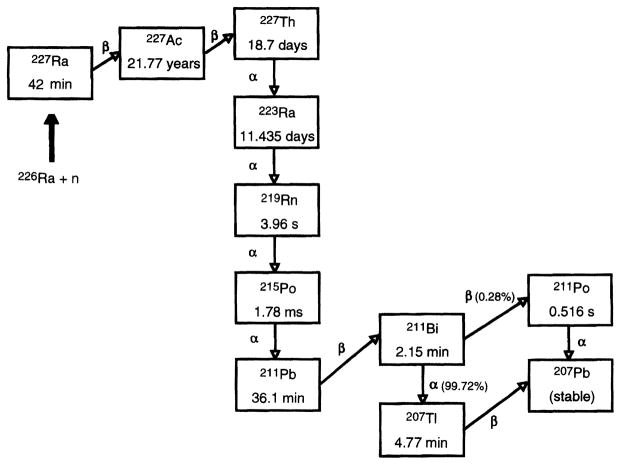
The radionuclide 223Ra (tp = 11.4 days) was produced according to the procedures described by Fisher et al. (21). In brief, a 226Ra target was irradiated with neutrons to produce 227Ra according to the reaction 226Ra (n/γ) 227Ra. As shown in Fig. 1, 227Ra (tp = 42 min) undergoes β-particle decay to 227Ac (tp = 21.77 years), which decays, in turn, to 227Th (tp = 18.7 days). After neutron irradiation of the target, the 227Ac was separated chemically from the target irradiation product mixture and then purified to remove silica solids, actinide contaminants (uranium and plutonium) and iron. The 227Ac, in equilibrium with its decay products (see Fig. 1), was transferred to an anion exchange column and eluted with 0.35 M nitric acid. The 227Th remained alone on the column while the eluate containing the 227Ac and 223Ra was recycled back into the original container for later use. Ten days later, the anion exchange column was eluted again with 0.35 M nitric acid to obtain pure 223Ra. The resulting solution was boiled down three times with HCl to form 223RaCl, the final product.
FIG. 1.
Production of 223Ra and its decay scheme. The radionuclide 223Ra is obtained by chemical separation from the products of the 226Ra (n,γ) 227Ra reaction and the 227Ra daughters 227Ac and 227Th.
The radiochemical 223Ra-citrate was prepared using the same procedures described above for 148Gd-citrate. Radium-223 decays to stable 207Pb via a series of short-lived radioactive daughters (Fig. 1), many of which also emit α particles with csda ranges in water from 35–70 μm (20). The daughter radionuclides were in equilibrium with the parent 223Ra at the time of radiolabeling. The activity of 223Ra and daughters was determined by counting their characteristic γ rays using a Canberra (Meriden, CT) Model GCW2525 HpGe well detector housed in a Model 747 shield, and a Series 100 multichannel analyzer. Gamma-ray yields were taken from Browne and Firestone (22). The efficiency of the detector as a function of photon energy was determined using standards traceable to the National Institute of Standards and Technology.
General Procedures
Male Swiss Webster mice (Taconic Farms, Germantown, NY), 9–10 weeks of age and weighing about 30 g, were maintained in the University animal care facility and provided food and water ad libitum. As in our earlier work (7, 9, 10), the radiochemicals were injected intratesticularly along the long axis of the right testis (standard 3 μl volume) of mice anesthetized with ether. The needle was slowly withdrawn from the organ during the injection to facilitate a reasonably uniform distribution of activity in the organ. Our earlier studies on the macroscopic distribution of radioactivity in the testis show that citrate radiochemicals [114mIn-citrate (8), 111In-citrate (8), 210Po-citrate (11), 55Fe-citrate (23)], and all other radiochemicals that we have studied such as (5), distribute fairly uniformly throughout the testis. These data, in conjunction with the consistency of our database of D37 values (~0.67 Gy) for low-LET radiation effects including those caused by external X rays (8) and intratesticularly injected radiochemicals that emit β particles (8, 24), γ rays (16) and very short-range Auger electrons (cytoplasmically localized Auger emitter) (8, 9, 24, 25), provide evidence that this “line injection” results in a sufficiently uniform distribution of radioactivity throughout the organ to allow intercomparison of the biological effects of different types of emitters based on the mean absorbed dose (16, 24).
There are several advantages to employing the intratesticular mode of administration over intravenous and intraperitoneal: (1) The testis is very small (0.1 g) so that photon radiations emanating from the organ deposit very little of their energy and therefore usually contribute minimally (<10%) to the total absorbed dose to the organ. (2) Only very small amounts of radioactivity (a few becquerels for α-particle emitters) are required to deliver cytotoxic doses to the testis. Hence the testicular absorbed dose from penetrating radiations emitted by radioactivity that has cleared from the testis and entered the body is negligible. (3) The dose to other organs in the body is negligible for the intratesticular mode, thereby eliminating complications due to organ toxicity. In summary, the intratesticular mode of administration allows clear delineation of the biological effects of the particulate radiations emitted by the radionuclide(s) without interference from penetrating low-LET photon radiations (γ rays and X rays) (11, 16).
Clearance of the Radionuclides from the Testis
The clearance of the radionuclides from the testis was determined by administering a fixed amount of radioactivity into the right testis of 30 animals. At various times after injection, animals were sacrificed in groups of three by an overdose of anesthetic. For 223Ra-citrate, the testes were immediately removed and placed in 1-ml airtight tubes, and the testicular activity of parent and daughters was immediately assayed using the HpGe well detector. The 154.2 keV and 269.4 keV, 271.1 keV and 401.7 keV, 427.0 keV, 350.1 keV and 897.2 keV γ-ray peaks were used to quantify the activity of 223Ra, 219Rn, 211Pb, 211Bi and 207Tl, respectively. The γ-ray yields of 211Po and 215Po were too low to monitor. For each time, the fraction of injected radioactivity retained in the testis compared to the control (activity injected into resected testes) was calculated, thereby yielding the biological retention of the activity in the organ.
Sperm Head Survival Assay
The optimal day on which to assay the sperm head survival fraction was determined by injecting the right testis of 30 additional animals with 420 Bq 148Gd-citrate (delivers 7.4 cGy) or 508 Bq 223Ra-citrate (delivers 5.6 cGy). The animals were sacrificed in groups of three by an overdose of ether and the right testis of each animal was resected. The testicular sperm head count was obtained by placing each testis in 1 ml deionized water, homogenizing, sonicating and counting the sonication-resistant sperm heads in a hemocytometer (7–9). The surviving fraction S is the ratio of sperm head counts in the test group to the number of counts in the controls (injected with 1 M sodium citrate, pH 4.7). The optimal day is the day after injection at which the sperm head count is a minimum.
The sperm head survival fraction was then determined as a function of the testicular absorbed dose. Animals (10 groups of 3) were injected intratesticularly with various concentrations of the radiochemical to deliver a range of absorbed doses to the testis. On the optimal day after injection, all of the animals were sacrificed and the sperm head survival fraction was determined for each group as described above (8, 9).
RESULTS AND DISCUSSION
Biokinetics and Optimal Assay Day after Administration of 148Gd
The biological clearance of 148Gd from the testis after intratesticular administration is shown in Fig. 2. A least-squares fit of the data to a two-component exponential expression gives
FIG. 2.
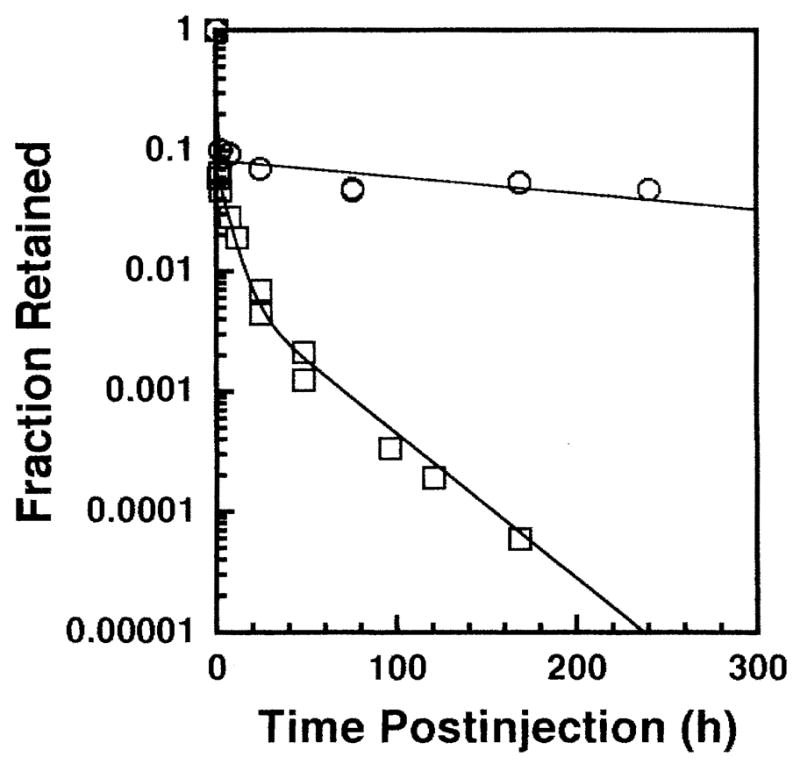
Biological elimination of 148Gd-citrate (○) and 223Ra-citrate (□) after intratesticular administration in mice. The data points represent the average of two independent experiments.
| (1) |
where f is the fraction of initially injected radioactivity remaining in the testis and t is the time after injection in hours. The effective clearance is also essentially given by Eq. (1) because of the 75-year physical half-life of 148Gd. In keeping with our earlier protocols (5, 8), the sperm head survival was monitored as a function of time after injection of a fixed amount of 148Gd-citrate (420 Bq) to determine the day on which the minimum sperm head count is obtained (i.e. optimal day for sperm head survival assay). The optimal day was the 36th day after injection, which was consistent with our earlier observations for long-lived (tp = 138 days) 210Po-citrate(11).
Testicular Dosimetry and Dose–Response Relationship for 148Gd
The testicular absorbed dose from the radionuclides was calculated as described previously (5, 7–10). Briefly, the mean testicular absorbed dose D is given by (26)
| (2) |
where the cumulated activity à is the time integral of the activity in the organ ∫A(t)dt, Δ is the mean energy emitted per nuclear transition, φ is the absorbed fraction, m is the average mass of the testis (0.1 g), and i denotes the ith radiation component emitted by the radionuclide. In keeping with our dosimetry procedures for an optimal assay day of 36 days after injection, the cumulated activity was integrated over 13 days (8, 11). The 13-day integration period corresponds to the time during which the spermatogonial cells were irradiated, and the surviving spermatogonial cells eventually become sonication-resistant spermatids 36 days after injection (8, 11). Substitution of the effective half-lives into Eq. (1), and integration over 13 days, gave à = 17.0 Bq-h per Bq of 148Gd injected. The radionuclide 148Gd emits a single 3.2 MeV α particle per decay (22); hence Δ = 5.12 × 10−13 Gy-kg/Bq-s. With a testicular mass of 0.1 g and φ = 1, the mean testicular absorbed dose per unit cumulated activity is 1.82 × 10−5 Gy/Bq-h.
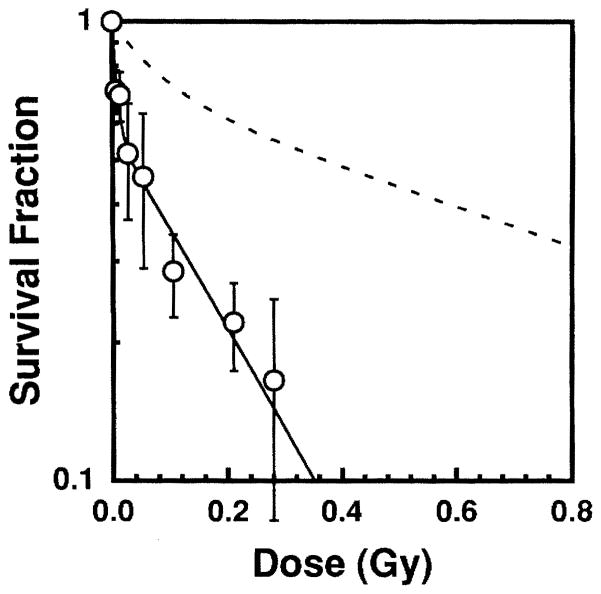
The sperm head survival fraction S/So is shown in Fig. 3 as a function of the average testicular absorbed dose from 148Gd. This two-component exponential dose–response relationship is consistent with our earlier results for internal and external radiation sources (7–10) and with results reported by others (27, 28). A least-squares fit of the data to Eq. (3),
FIG. 3.
Sperm head survival fraction as a function of absorbed dose to mouse testis from intratesticularly administered 148Gd-citrate (○). The data points are the average of two independent experiments. Error bars represent the standard deviation of the mean. The dashed line represents the dose–response curve for acute external 120 kVp X rays (8).
| (3) |
gives a = 0.58 ± 0.065, D1 = 8.28 × 10−3 ± 3.0 × 10−3 Gy, and D2 = 0.20 ± 0.043 Gy. Hence the dose required to achieve 37% survival (D37) is 0.090 ± 0.029 Gy.
Relative Biological Effectiveness as a Function of Alpha-Particle Energy
The RBE is defined, for a specific radiation (A), as
with all physical and biological variables, except radiation quality, being held as constant as possible (29). Therefore, particular attention must be paid to the selection of the reference radiation (5, 10, 16, 30). In our earlier work using the same experimental model, the effects of a variety of sources of low-LET radiation were examined including external X rays (8) and intratesticularly administered β-particle and γ-ray emitters (8, 16, 24). A D37 of 0.67 ± 0.03 Gy was obtained for external irradiation with acute 60 or 120 kVp X rays (8). Similarly, when the testes were irradiated chronically with 477 keV γ rays from intratesticularly administered 7Be-chloride or medium-energy β particles from H131IPDM (24), D37 values of 0.65 ± 0.10 and 0.61 ± 0.06 Gy were obtained, respectively. These and other supporting data are discussed in ref. (16) and show that low-LET radiations (photons, electrons), whether delivered acutely or chronically, externally or internally, yield about the same D37 values. Therefore, any low-LET radiation, delivered acutely or chronically, can be used as the reference radiation for the purpose of calculating RBE values in the sperm head survival assay. In keeping with our past studies (5, 11), the D37 for acute 120 kVp X rays has been used to calculate RBE values for the α-particle emitters used in the present work.
With external X rays serving as the reference radiation, the RBE of the 3.2 MeV α particles emitted by 148Gd is 7.4 ± 2.4 at D37. In other earlier studies, an RBE of 6.7 ± 1.4 was obtained for 210Po-citrate (5.3 MeV) (11), and 212Pb in equilibrium with its daughters 212Bi (6.0 MeV) and 212Po (8.8 MeV) yielded RBE values of 6.0 and 4.6, respectively (5). These earlier data were used to construct a relationship between the RBE and the energy of the α particles (5). The data presented in this work for the 3.2 MeV α particles of 148Gd allow us to revise our earlier RBE–Eα relationship to cover essentially the entire range of α-particle energies emitted by radionuclides in general. Figure 4 illustrates the revised RBE–Eα relationship. A least-squares fit of the data to a linear function yields Eq. (4),
FIG. 4.
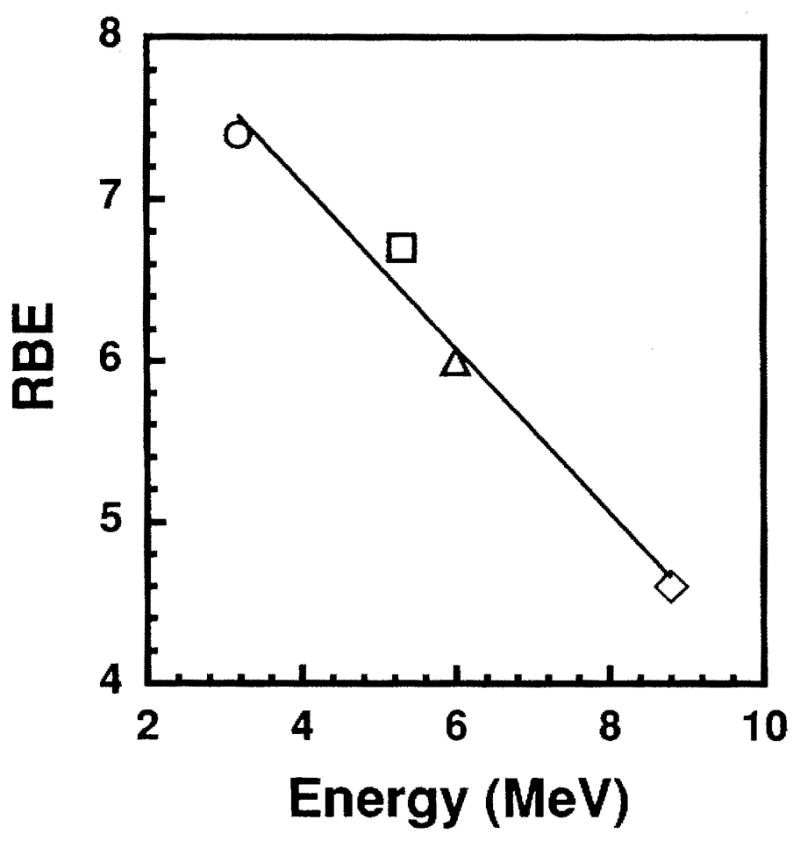
Relative biological effectiveness at 37% survival as a function of α-particle energy emitted by internal emitters. (○) 3.2 MeV from 148Gd, (□) 5.3 MeV from 210Po (11), (△) 6.0 MeV from 212Bi (5), (◇) 8.8 MeVfrom212Po(5).
| (4) |
where RBEα is the RBE of an α particle emitted with initial energy Eα in MeV. This empirical expression, which is valid over the range 3 < Eα < 9 MeV, is useful for predicting biological response after internal administration of α-particle emitters. It should be noted that when the microscopic distribution of the α-particle emitter is highly nonuniform (31), the RBE value can be different from that predicted by this relationship.
Test of the RBE–Eα Relationship Using 223Ra
The radionuclide 223Ra has a complex decay series (Fig. 1). There are about five α particles emitted in the series with energies ranging from 5.3 to 7.5 MeV, as well as a host of low-LET radiations. Therefore, 223Ra in equilibrium with its daughters is a good radionuclide to test the adequacy of the empirical RBE–Eα relationship given by Eq. (4).
The biological clearance pattern of 223Ra-citrate after intratesticular administration is shown in Fig. 2. The data were fitted by the least-squares method to the following three-component exponential expression:
| (5) |
where t is hours after injection. The 223Ra and its daughters were found to be in equilibrium in the testis in our experiment. The optimal day for the sperm head survival assay was determined experimentally to be the 29th day post-injection for 223Ra. This optimal day is consistent with our earlier data for radiochemicals with relatively fast effective clearance patterns (5, 7, 32). Using Eq. (5), and the physical half-life of 223Ra (11.43 days), the cumulated activity was calculated by substituting the effective half-times of the three exponential components (0.011, 4.7 and 23.0 h) into Eq. (5) and integrating over 7 days (5, 7, 32). The cumulated activity thus obtained was 0.67 Bq-h per Bq of injected activity. The major radiations emitted by 223Ra and its daughters are shown in Table I with their energies (33), yields (33) and branching ratios (22) shown accordingly. The mean absorbed dose to the testis per unit cumulated activity is calculated for each radiation (34), with the total being 4.33 × 10−8 Gy/Bq-s. The experimental dose–response relationship for 223Ra in equilibrium with its daughters is shown in Fig. 5. A least-squares fit of the data to Eq. (3) gave a = 0.77 ± 0.032, D1 = 0.0012 Gy, D2 = 0.169 ± 0.025 Gy. From this equation, a D31 value of 0.124 ± 0.020 Gy can be calculated. Hence the corresponding RBE for 223Ra in equilibrium with its daughters is 5.4 ± 0.9.
TABLE I.
Testicular Dosimetry Calculations and Dose-Weighted RBE
| Radionuclide | Particle type, energy (MeV)a | Branching ratiob | nia (yield/decay) | sic(Gy/Bq-s) | qid | RBEie | qi × RBEi |
|---|---|---|---|---|---|---|---|
| 223Ra | α, 5.288 | 1 | 0.0015 | 1.26 × 10−11 | 0.000292 | 6.45 | 0.00188 |
| 223Ra | α, 5.340 | 1 | 0.0013 | 1.11 × 10−11 | 0.000256 | 6.42 | 0.00164 |
| 223Ra | α, 5.366 | 1 | 0.0013 | 1.11 × 10−11 | 0.000257 | 6.41 | 0.00164 |
| 223Ra | α, 5.433 | 1 | 0.0226 | 1.97 × 10−10 | 0.00454 | 6.37 | 0.0289 |
| 223Ra | α, 5.502 | 1 | 0.0099 | 8.77 × 10−11 | 0.00203 | 6.34 | 0.0128 |
| 223Ra | α, 5.540 | 1 | 0.0911 | 8.09 × 10−10 | 0.0187 | 6.32 | 0.118 |
| 223Ra | α, 5.607 | 1 | 0.2407 | 2.16 × 10−9 | 0.0499 | 6.28 | 0.314 |
| 223Ra | α, 5.716 | 1 | 0.5223 | 4.78 × 10−9 | 0.110 | 6.23 | 0.688 |
| 223Ra | α, 5.747 | 1 | 0.0945 | 8.70 × 10−10 | 0.0201 | 6.21 | 0.125 |
| 223Ra | α, 5.858 | 1 | 0.0032 | 2.98 × 10−11 | 0.000689 | 6.16 | 0.00424 |
| 223Ra | α, 5.872 | 1 | 0.0085 | 7.95 × 10−11 | 0.00184 | 6.15 | 0.0113 |
| 219Rn | α, 6.425 | 1 | 0.0744 | 7.66 × 10−10 | 0.0177 | 5.87 | 0.104 |
| 219Rn | α, 6.531 | 1 | 0.0012 | 1.25 × 10−11 | 0.000288 | 5.81 | 0.00167 |
| 219Rn | α, 6.553 | 1 | 0.1210 | 1.27 × 10−9 | 0.0293 | 5.80 | 0.170 |
| 219Rn | α, 6.819 | 1 | 0.8025 | 8.77 × 10−9 | 0.203 | 5.67 | 1.15 |
| 215Po | α, 7.386 | 1 | 0.9994 | 1.18 × 10−8 | 0.273 | 5.38 | 1.47 |
| 211Bi | α, 6.279 | 0.9972 | 0.1596 | 1.60 × 10−9 | 0.0370 | 5.94 | 0.220 |
| 211Bi | α, 6.623 | 0.9972 | 0.8377 | 8.86 × 10−9 | 0.205 | 5.77 | 1.18 |
| 211Po | α, 6.569 | 0.0028 | 0.0054 | 1.58 × 10−13 | 0.0000037 | 5.79 | 0.0000212 |
| 211Po | α, 6.891 | 0.0028 | 0.0055 | 1.69 × 10−13 | 0.0000039 | 5.63 | 0.0000219 |
| 211Po | α, 7.450 | 0.0028 | 0.9892 | 3.31 × 10−11 | 0.000764 | 5.34 | 0.00408 |
| Allf | Photons | 5.37 × 10−12 | 0.000124 | 1.00 | 0.000124 | ||
| Allg | Conversion e− | 1.08 × 10−9 | 0.0249 | 1.00 | 0.0249 | ||
| Allh | Auger e− | 1.38 × 10−11 | 0.000319 | 1.00i | 0.000319 | ||
| Totals | 4.33 × 10−8 | 1.00 | 5.63j |
Energies and yields taken from Eckerman et al. (33).
Branching ratios taken from Browne and Firestone (22).
Mean absorbed dose per unit cumulated activity (S value) calculated using computer code of Howell et al. (34).
qi = fraction of total absorbed dose delivered by the ith radiation.
RBE for α particles calculated using Eq. (4).
Photon contribution from 223Ra in equilibrium with its daughters.
Conversion electron contribution from 223Ra in equilibrium with its daughters.
Auger electron contribution from 223Ra in equilibrium with its daughters.
Assumes radionuclides are localized outside the cell nucleus (citrate radiochemicals localize in cytoplasm).
Weighted RBE for 223Ra in equilibrium with its daughters calculated using R = Σ qi RBEt (5).
FIG. 5.
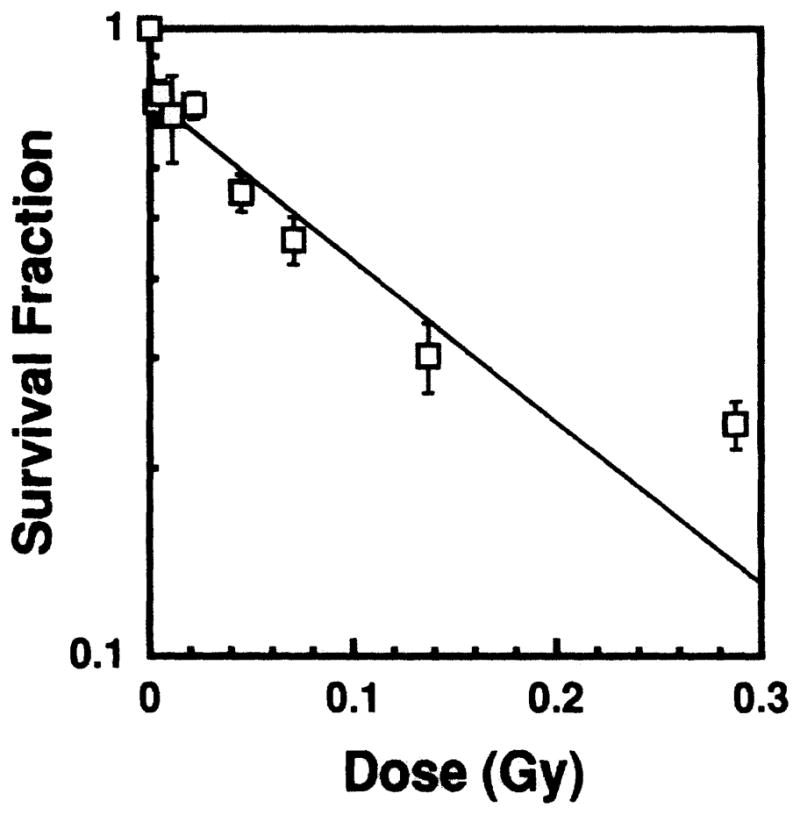
Sperm head survival fraction as a function of absorbed dose to mouse testis from intratesticularly administered 223Ra-citrate. Two experiments were performed, the data points representing the mean. The error bars are the standard deviation of the mean.
Is the experimental RBE of 5.4 for 223Ra in equilibrium with its daughters expected based on our empirical RBE-Eα relationship? It has been shown that when a radionuclide emits a mixed radiation field, the overall RBE can be calculated using , where qi is the fraction of absorbed dose from the ith radiation and RBEt is the relative biological effectiveness of the ith radiation (5). Table I gives the fraction of the total mean absorbed dose (qi) that arises from each α particle emitted in the series, and the corresponding RBEi calculated using Eq. (4). The numerous photons, conversion electrons and Auger electrons emitted by 223Ra and its daughters (33) are all assigned an RBE of 1. It may be noted that the low-LET radiations constitute a negligible fraction of the total absorbed dose to the testis (Table I). The last column of Table I gives the product qi RBEi and the resulting sum , which is the expected overall RBE for 223Ra in equilibrium with its daughters. This RBE value of 5.6 is in excellent agreement with our experimental RBE value of 5.4, thereby verifying the adequacy of the RBE–Eα relationship given by Eq. (4).
CONCLUSIONS
Although there is considerable experimental data on the biological effects of α particles in vitro (35–42), there is a dearth of in vivo data that systematically explore the dependence of RBE on the energy of α particles emitted by incorporated radionuclides. In this work, an empirical RBE–Eα relationship was obtained using spermatogenesis in mouse testes as the experimental model and survival as the biological end point. The range of α-particle energies covered by this relationship is from 3 to 9 MeV, covering the majority of α-particle energies emitted by radionuclides in general. The validity of the empirical relationship was verified using 223Ra (in equilibrium with its daughters), which emits a spectrum of α-particle energies. There was an excellent agreement between the predicted RBE value based on the empirical relationship (Eq. 4) and the value determined experimentally. Therefore, this relationship should be useful for prediction of deterministic effects of internal α-particle emitters.
Acknowledgments
This work was supported in part by USPHS Grant No. CA-54891 and the Hanford Isotopes Program, sponsored by the Department of Energy’s Isotope Production and Distribution Program (NE-70) under contract No. DE-AC06-76RLO 1830.
References
- 1.Barendsen GW. The relationship between RBE and LET for different types of lethal damage in mammalian cells: Biophysical and molecular mechanisms. Radiat Res. 1994;139:257–270. [PubMed] [Google Scholar]
- 2.Miller RC, Marino SA, Brenner DJ, Martin SG, Richards M, Randers-Pehrson G, Hall EJ. The biological effectiveness of radon-progeny alpha particles. II. Oncogenic transformation as a function of linear energy transfer. Radiat Res. 1995;142:54–60. [PubMed] [Google Scholar]
- 3.ICRP. International Commission on Radiological Protection, Pergamon Press; Oxford: 1989. RBE for Deterministic Effects. Publication 58. [PubMed] [Google Scholar]
- 4.ICRP. Annals of the ICRP. Vol. 21. Pergamon Press; Oxford: 1991. 1990 Recommendations of the International Commission on Radiological Protection. Publication 60. [PubMed] [Google Scholar]
- 5.Howell RW, Azure MT, Narra VR, Rao DV. Relative biological effectiveness of alpha-particle emitters in vivo at low doses. Radiat Res. 1994;137:352–360. [PMC free article] [PubMed] [Google Scholar]
- 6.Mian TA, Glenn HJ, Haynie TP, Meistrich ML. Radiation dose and biological effects to mouse testis from sodium 32P phosphate. Health Phys. 1982;42:657–664. doi: 10.1097/00004032-198205000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Rao DV, Govelitz GF, Sastry KSR. Radiotoxicity of thallium-201 in mouse testes: Inadequacy of conventional dosimetry. J Nucl Med. 1983;24:145–153. [PubMed] [Google Scholar]
- 8.Rao DV, Sastry KSR, Grimmond HE, Howell RW, Govelitz GF, Lanka VK, Mylavarapu VB. Cytotoxicity of some indium radiopharmaceuticals in mouse testes. J Nucl Med. 1988;29:375–384. [PubMed] [Google Scholar]
- 9.Rao DV, Narra VR, Howell RW, Sastry KSR. Biological consequences of nuclear versus cytoplasmic decays of 125I: Cysteamine as a radioprotector against Auger cascadesin vivo. Radiat Res. 1990;124:188–193. [PubMed] [Google Scholar]
- 10.Rao DV, Narra VR, Howell RW, Lanka VK, Sastry KSR. Induction of spermhead abnormalities by incorporated radionuclides: Dependence on subcellular distribution, type of radiation, dose rate, and presence of radioprotectors. Radiat Res. 1991;125:89–97. [PMC free article] [PubMed] [Google Scholar]
- 11.Rao DV, Narra VR, Howell RW, Govelitz GF, Sastry KSR. In-vivo radiotoxicity of DNA-incorporated 125I compared with that of densely ionising alpha-particles. Lancet. 1989;II:650–653. doi: 10.1016/s0140-6736(89)90896-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meistrich ML, Samuels RC. Reduction in sperm levels after testicular irradiation of the mouse: A comparison with man. Radiat Res. 1985;102:138–147. [PubMed] [Google Scholar]
- 13.Gaulden ME. “Biological dosimetry” of radionuclides and radiation hazards. J Nucl Med. 1983;24:160–164. [PubMed] [Google Scholar]
- 14.Meistrich ML, Hunter NR, Suzuki N, Trostle PK, Withers HR. Gradual regeneration of mouse testicular stem cells after exposure to ionizing radiation. Radiat Res. 1978;74:349–362. [PubMed] [Google Scholar]
- 15.Oakberg EF. Spermatogonial stem-cell renewal in the mouse. Anat Rec. 1971;169:515–532. doi: 10.1002/ar.1091690305. [DOI] [PubMed] [Google Scholar]
- 16.Howell RW, Narra VR, Hou DY, Terrone DA, Harapanhalli RS, Sastry KSR, Rao DV. Relative biological effectiveness of Auger emitters for cell inactivation: In vitro versus in vivo. In: Howell RW, Narra VR, Sastry KSR, Rao DV, editors. Biophysical Aspects of Auger Processes. American Institute of Physics; Woodbury, NY: 1992. pp. 290–318. [Google Scholar]
- 17.Meistrich ML. Critical components of testicular function and sensitivity to disruption. Biol Reprod. 1986;34:17–28. doi: 10.1095/biolreprod34.1.17. [DOI] [PubMed] [Google Scholar]
- 18.Oakberg EF. Sensitivity and time of degeneration of spermatogenic cells irradiated in various stages of maturation in the mouse. Radiat Res. 1955;2:369–391. [PubMed] [Google Scholar]
- 19.Oakberg EF. Duration of spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium. Am J Anat. 1956;99:507–516. doi: 10.1002/aja.1000990307. [DOI] [PubMed] [Google Scholar]
- 20.ICRU. Report 49. International Commission on Radiation Units and Measurements; Bethesda, MD: 1993. Stopping Powers and Ranges for Protons and Alpha Particles. [Google Scholar]
- 21.Fisher DR, Schenter RE, Wester DW. Proceedings, International Isotope Society Symposium on Isotope Production and Applications in Medicine, Science and the Environment. Pacific Northwest Laboratories; Richland, WA: 1993. A new application for old radium: Production of short-lived alpha emitters for radio-immunotherapy. PNL-SA-22060A. [Google Scholar]
- 22.Browne E, Firestone RB. Table of Radioactive Isotopes. Wiley; New York: 1986. [Google Scholar]
- 23.Rao DV, Sastry KSR, Govelitz GF, Grimmond HE, Hill HZ. In vivo effects of iron-55 and iron-59 on mouse testis: Biophysical dosimetry of Auger electrons. J Nucl Med. 1985;26:1456–1465. [PubMed] [Google Scholar]
- 24.Narra VR, Howell RW, Harapanhalli RS, Sastry KSR, Rao DV. Radiotoxicity of some 123I, 125I, and 131I labeled compounds in mouse testes: Implications for radiopharmaceutical design. J Nucl Med. 1992;33:2196–2201. [PubMed] [Google Scholar]
- 25.Howell RW, Narra VR, Sastry KSR, Rao DV. On the equivalent dose for Auger electron emitters. Radiat Res. 1993;134:71–78. [PMC free article] [PubMed] [Google Scholar]
- 26.Loevinger R, Budinger TF, Watson EE. MIRD Primer for Absorbed Dose Calculations. The Society of Nuclear Medicine; New York: 1991. [Google Scholar]
- 27.Spano M, Pacchierotti F, Mauro F, Quaggia S, Uccelli R. Flow cytometric analysis of the effects of 0.4 MeV fission neutrons on mouse spermatogenesis. Int J Radiat Biol. 1987;51:401–419. doi: 10.1080/09553008714550901. [DOI] [PubMed] [Google Scholar]
- 28.Gasinska A. Mouse testis weight loss and survival of differentiated spermatogonia following irradiation with 250 kV and 5.5 MeV fast neutrons. Neoplasma. 1985;32:443–449. [PubMed] [Google Scholar]
- 29.NCRP. Report No 104. National Council on Radiation Protection and Measurements; Bethesda, MD: 1990. The Relative Biological Effectiveness of Radiations of Different Quality. [Google Scholar]
- 30.Howell RW, Rao DV, Hou DY, Narra VR, Sastry KSR. The question of relative biological effectiveness and quality factor for Auger emitters incorporated into proliferating mammalian cells. Radiat Res. 1991;128:282–292. [PubMed] [Google Scholar]
- 31.Green D, Howells GR. Radiation dose to mouse testes from 239Pu. Health Phys. 1980;38:242–243. [PubMed] [Google Scholar]
- 32.Narra VR, Sastry KSR, Goddu SM, Howell RW, Strand SE, Rao DV. Relative biological effectiveness of 99mTc radiopharmaceuticals. Med Phys. 1994;21:1921–1926. doi: 10.1118/1.597230. [DOI] [PubMed] [Google Scholar]
- 33.Eckerman KF, Westfall RJ, Ryman JC, Cristy M Nuclear Decay Data Files of the Dosimetry Research Group. Report ORNL/TM-12350. Oak Ridge National Laboratory; Oak Ridge, TN: 1993. [Google Scholar]
- 34.Howell RW, Rao DV, Sastry KSR. Macroscopic dosimetry for radioimmunotherapy: Nonuniform activity distributions in solid tumors. Med Phys. 1989;16:66–74. doi: 10.1118/1.596404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barendsen GW. Dose–survival curves of human cells in tissue culture irradiated with alpha-, beta-, 20-kV. X- and 200-kV. X-radiation. Nature. 1962;193:1153–1155. doi: 10.1038/1931153a0. [DOI] [PubMed] [Google Scholar]
- 36.Azure MT, Archer RD, Sastry KSR, Rao DV, Howell RW. Biological effect of lead-212 localized in the nucleus of mammalian cells: Role of recoil energy in the radiotoxicity of internal alpha-particle emitters. Radiat Res. 1994;140:276–283. [PMC free article] [PubMed] [Google Scholar]
- 37.Fisher DR, Frazier ME, Andrews TK., Jr Energy distribution and the relative biological effects of internal alpha emitters. Radiat Prot Dosim. 1985;13:223–227. [Google Scholar]
- 38.Goodhead DT, Belli M, Mills AJ, Bance DA, Allen LA, Hall SC, Ianzani F, Simony G, Stevens DL, Stretch A, Tabocchini MA, Wilkinson RE. Direct comparison between protons and alpha-particles of the same LET: I. irradiation methods and inactivation of asynchronous V79, HeLa and C3H 10T1/2 cells. Int J Radiat Biol. 1992;61:611–624. doi: 10.1080/09553009214551421. [DOI] [PubMed] [Google Scholar]
- 39.Jostes RF, Hui TE, James AC, Cross FT, Schwartz JL, Rotmensch J, Atcher RW, Evans HH, Mencl J, Bakale G, Rao PS. In vitro exposure of mammalian cells to radon: Dosimetric considerations. Radiat Res. 1991;127:211–219. [PubMed] [Google Scholar]
- 40.Kassis AI, Harris CR, Adelstein SJ, Ruth TJ, Lambrecht R, Wolf AP. The in vitro radiobiology of astatine–211 decay. Radiat Res. 1986;105:27–36. [PubMed] [Google Scholar]
- 41.Lloyd EL, Gemmell MA, Henning CB, Gemmell DS, Zabransky BJ. Cell survival following multiple-track alpha particle irradiation. Int J Radial Biol. 1979;35:23–31. doi: 10.1080/09553007914550031. [DOI] [PubMed] [Google Scholar]
- 42.Raju MR, Eisen Y, Carpenter S, Inkret WC. Radiobiology of α particles. III. Cell inactivation by α-particle traversals of the cell nucleus. Radiat Res. 1991;128:204–209. [PubMed] [Google Scholar]