Abstract
We have developed clickable active site-directed photoaffinity probes for γ-secretase which incorporate a photoreactive benzophenone group and an alkyne handle for subsequent click chemistry mediated conjugation with azide-linked reporter tags for visualization (e.g., TAMRA-azide) or enrichment (e.g., biotin-azide) of labeled proteins. Specifically, we synthesized clickable analogs of L646 (2) and L505 (3) and validated specific labeling to presenilin 1 N-terminal fragment (PS1-NTF), the active site aspartyl protease component within the γ-secretase complex. Additionally we were able to also identify signal peptide peptidase (SPP) by Western blot analysis. Furthermore, we analyzed the photo-labeled proteins in an unbiased fashion by click chemistry with TAMRA-azide followed by in-gel fluorescence detection. This approach expands the utility of γ-secretase inhibitor (GSI) photoaffinity probes in that labeled proteins can be tagged with any number of azide-linked reporters groups using a single clickable photoaffinity probe for target pull down and/or fluorescent imaging applications.
Keywords: γ-Secretase, Photoaffinity labeling, Click chemistry, Clickable photoprobe, Alzheimer’s disease
Photoaffinity labeling (PAL) is a powerful method to covalently capture the protein targets of small molecules from a variety of matrices.1,2 PAL has enabled critical studies elucidating the mechanism of action of compounds that either inhibit or modulate γ-secretase, an intramembrane aspartyl protease that contributes to forming amyloid-β peptides and is a major target for Alzheimer’s disease therapy.3 γ-Secretase is a complex of four different integral membrane proteins (presenilin, nicastrin, Aph-1 and Pen-2)4 and heterogeneity within the subunit composition has limited the ability of investigators to isolate purified enzyme for subsequent structural biology studies.5 Active site-directed photoreactive hydroxyethylene γ-secretase inhibitors (GSIs) such as L646 (2) and L505 (3) were instrumental in elucidating that the catalytic domain of γ-secretase is contained within presenilin.6 Furthermore, these photoprobes have been used to visually capture active γ-secretase at the plasma membrane.7 Yet, the underlying cell biology of the active γ-secretase complex, including its full complement of physiologically relevant substrates,8 is not completely understood.9
We designed and prepared clickable photoaffinity probes 4 and 5 based on the active site-directed GSIs L458 (1), L646 (2) and L505 (3).6 A benzophenone was incorporated into these probes to effect covalent modification of the target upon irradiation. Rather than incorporate a reporter group into the photoaffinity probe directly, we choose to strategically incorporate a terminal alkyne to allow for click chemistry-mediated conjugation with various azide-reporter tags (e.g., rhodamine-azide for fluorescence detection or biotin-azide for avidin enrichment) after the probe is cross-linked to the target.10 This approach expands the utility of GSI photoaffinity probes in that labeled proteins can be tagged with any number of azide-linked reporter groups using a single clickable photoaffinity probe for target pull down and fluorescent imaging applications. We recently utilized this approach to show that piperidine acetic acid γ-secretase modulators (GSMs) directly bind to PS1-NTF.11
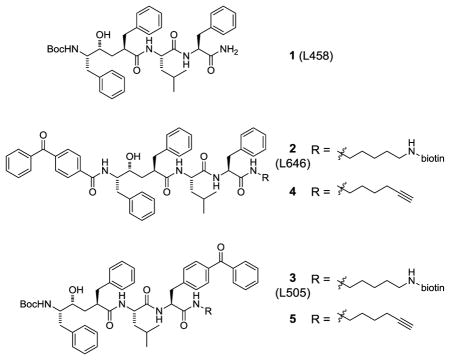
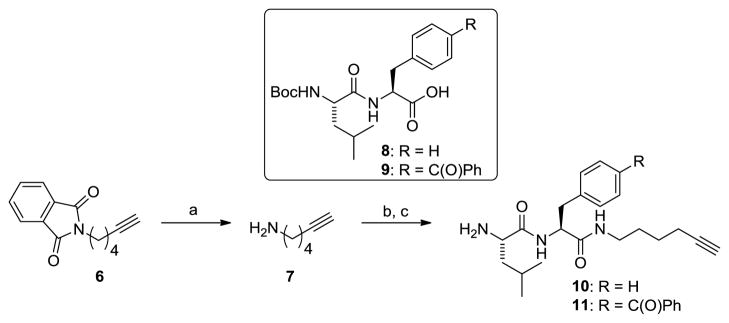
The synthesis of clickable photoprobes 4 and 5 began with preparation of the right-hand side dipeptide incorporating the clickable alkyne handle (Scheme 1). Treatment of commercially available phthalimide 6 with hydrazine in methanol provided aminohexyne 7 in excellent yield.12 Amide coupling of 7 to either Leu-Phe (8) or Leu-Bpa (9) dipeptides followed by removal of the Boc protecting group furnished dipeptides 10 and 11 that incorporated the clickable handle.
Scheme 1.
Reagents and conditions. a) NH2NH2, MeOH, 96%; b) 8 or 9, HATU, DIPEA, DMF, 77–92%; c) TFA, DCM, 92–95%.
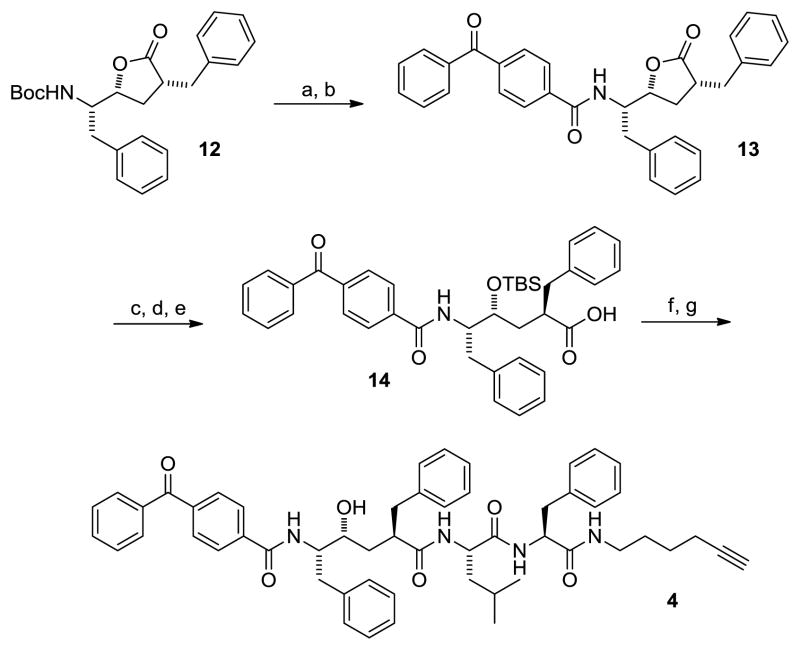
Next, we focused on the hydroxyethylene segment of the molecule. Lactone 12 was synthesized from L-phenylalanine via a sequence similar to that previously reported.13 Subsequent Boc deprotection followed by amidation with benzophenone-4-carboxylic acid provided lactone 13 in 52% yield over 2 steps (Scheme 2). While initial attempts to access 14 proved challenging due to relactonization of the unprotected alcohol intermediate, careful control of pH and temperature during manipulations allowed access to 14 in good overall yield. Furthermore, we found that the reaction worked better on larger scales (> 1 g).14 Treatment of 14 with EDCI, HOBt and the alkyne-containing dipeptide 10 provided the TBS protected probe in 91% yield. Deprotection under standard TBAF conditions afforded the desired clickable photoprobe 4.
Scheme 2.
Reagents and conditions. a) TFA, DCM, rt; b) benzophenone-4-carboxylic acid, EDCI, HOBt, DIPEA, DMF, 52% over 2 steps; c) LiOH, H2O, DME; d) TBSCl, Imidazole, DMF; e) MeOH, 60% over 3 steps; f) 10, EDCI, HOBt, DIPEA, DMF, 91%; g) TBAF, THF, 60%.
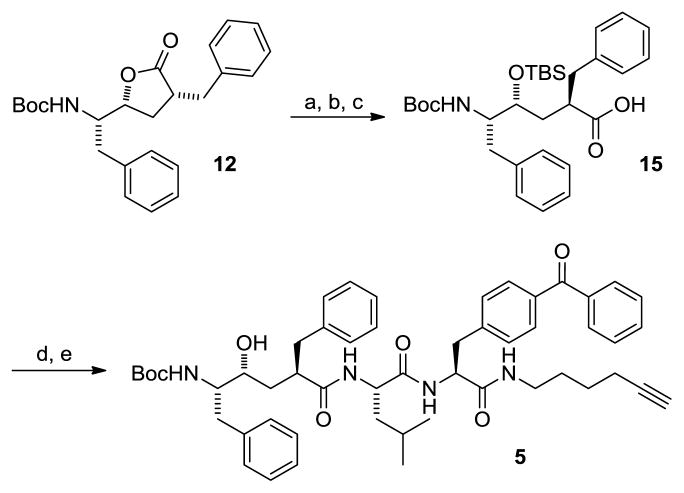
Photoprobe 5 was constructed in a similar fashion to that shown for 4; however, in this instance, lactone 12 was hydrolyzed and silyl protected to supply 15 in good overall yield (Scheme 3). Again, the unprotected alcohol was susceptible to relactonization and therefore, care was taken to minimize exposure to acidic conditions. An amide coupling with the Leu-Bpa dipeptide 11 joined the requisite clickable alkyne and photoprobe portions of the GSI. Removal of the TBS group was accomplished in 95% yield to provide 5.
Scheme 3.
Reagents and conditions. a) LiOH, H2O, DME; b) TBSCl, Imidazole, DMF; c) MeOH, 75% over 3 steps; d) 11, EDCI, HOBt, DIPEA, DMF, 88%; g) TBAF, THF, 95%.
With the clickable photoaffinity probes 4 and 5 in hand, we determined their in vitro potencies in CHO-APP cells and in a cell-free HeLa membrane assay using a recombinant amyloid precursor protein (APP) substrate (Table 1).15,16 Both 4 and 5 are potent γ-secretase inhibitors with IC50 values of 2.3 and 0.2 nM, respectively, in HeLa membranes. However, they exhibit a significant difference in a cell-based setting. Although probe 5 maintains good cellular potency (4 nM) and is improved in comparison to the biotinylated compound 3, the cellular potency of 4 is quite low, which is likely due to poor cell permeability. This is consistent with previous studies which indicate that 3 is able to penetrate the cell plasma membrane and access intracellular γ-secretase to inhibit APP processing, while 2 cannot.7
Table 1.
Potencies of 1–5 for inhibiting production of Aβ42.
| compound | Aβ42 IC50 (nM) (HeLa membranes) | Aβ42 IC50 (nM) (CHO-APP) |
|---|---|---|
| 1 | 0.3 | 36 |
| 2 | 1.0 | 817 |
| 3 | 0.3 | 30 |
| 4 | 2.3 | 1000 |
| 5 | 0.2 | 4.0 |
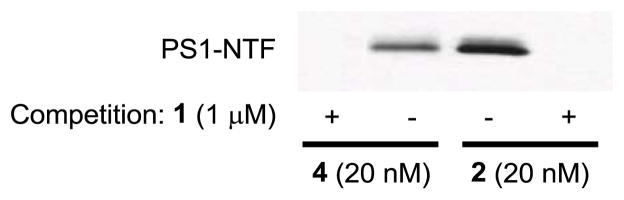
Next, we developed and optimized procedures for photoaffinity labeling of 4 and 5 and tested the efficiency of the click reaction. To this end, we incubated HeLa cell membranes (800 μg/1.2 mL PBS) at 37 °C for one hour with either 2 or 4 in the presence or absence of competitor compound 1 (1 μM). After incubation, membrane fractions were photoirradiated at ~350 nm for 30 minutes at 4 °C followed by centrifugation to remove excess reagents. Cell pellets were resuspended in PBS (500 μL) and subjected to click chemistry conditions employing TCEP (1.0 mM), CuSO4 (1.0 mM), TBTA (0.1 mM) and biotin-azide (0.1 mM Invitrogen) for 1 hour at room temperature with slight agitation. HeLa membranes were centrifuged to remove excess reagents and the resulting cell pellets were resuspended into 1X RIPA buffer. The resulting solubilized labeled membranes were affinity enriched with streptavidin beads, eluted with loading buffer and separated using SDS-PAGE followed by Western-blot analysis with anti-PS1-NTF antibody (Figure 1). Clickable L646 analog 4 displayed efficient PS1-NTF photolabeling and the click reaction effectively appended biotin post-labeling for affinity enrichment. Importantly, PS1-NTF labeling was blocked when the parent non-photoreactive GSI L458 (1) compound was included in the reaction demonstrating specific labeling. The labeling efficiency was approximately 2–3-fold less for 4 which probably reflects the efficiency of the click reaction with biotin-azide and/or loss of protein during the click chemistry/precipitation step. Assuming comparable crosslinking efficiency for 2 and 4, it would appear that the click chemistry conjugation protocol with biotin-azide proceeds in approximately 30–50% yield.
Figure 1.
HeLa membranes were photolabeled with 2 or 4 (20 nM) in the presence or absence of 1 (1 μM), followed by click chemistry with biotin-azide (in the case of 4), streptavidin pull down, and Western blot analysis with PS1-NTF antibody.
We next compared the labeling of PS1-NTF, PS1-CTF and signal peptide peptidase (SPP), a related aspartyl intramembrane protease17 that is known to have cross-reactivity with GSIs,18 with both clickable photoprobes 4 and 5. As anticipated, probe 4 (based on L646) strongly labeled PS1-NTF vs. CTF,6 and also displayed minimal labeling of SPP (Figure 2). Probe 5 (based on L505) confirmed the expected preference for PS1-CTF with minimal PS1-NTF labeling,6 but also revealed significant labeling of SPP.
Figure 2.
HeLa membranes were photolabeled with 4 or 5 (20 nM) in the presence or absence of 1 (1 μM), followed by click chemistry with biotin-azide, streptavidin pull down, and Western blot analysis with PS1-NTF, PS1-CTF and SPP antibodies.
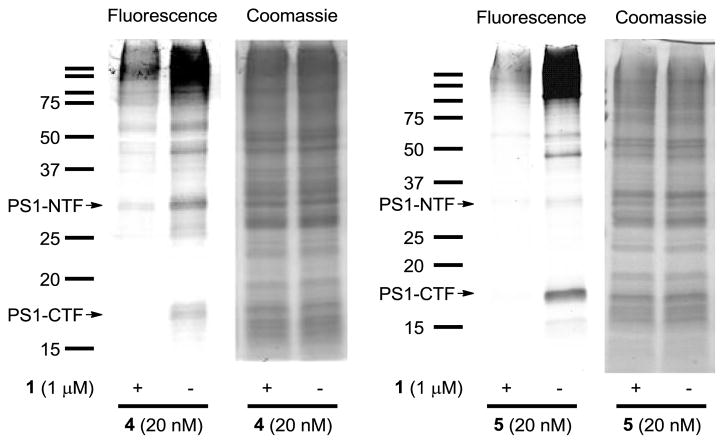
Furthermore, we analyzed the labeled proteins in an unbiased fashion by click chemistry with TAMRA-azide followed by in-gel fluorescence detection (Figure 3). After click chemistry, the fluorescently-tagged probe-labeled HeLa membranes were acetone precipitated to remove excess reagents, re-solubilized in loading buffer, and separated on SDS-PAGE. Gels were then scanned for fluorescent detection of labeled proteins followed by Coomassie blue staining for confirmation of equal protein loading in each of the lanes (Figure 3). Our in-gel fluorescence labeling results support the disparate labeling profiles of 4 and 5, confirming an increase in labeling of PS1-NTF vs CTF for probe 4 and increase in PS1-CTF vs NTF labeling for 5. For example, probe 4 shows a predominate band at ~30 kDa which is consistent with the labeling of PS1-NTF that was observed by Western blot analysis, while probe 5 shows a predominate band at ~18 kDa which is consistent with the preferential labeling of PS1-CTF (Figure 2). Bands that could correspond to fluorescent labeling of SPP (monomer and oligomer forms) were also observed in the gels for 4 and 5. In each case these specific bands were competed by the addition of GSI L458 (1).
Figure 3.
HeLa membranes were photolabeled with 4 or 5 (20 nM) in the presence or absence of 1 (1 μM), followed by click chemistry with TAMRA-azide, in-gel fluorescence, and Coomassie blue gel staining.
In summary, we have developed two potent clickable active site-directed GSI photoaffinity probes (4 and 5) and demonstrated efficient PS1 labeling within the γ-secretase complex. Utilizing the terminal acetylene, we were able to demonstrate conjugation with both biotin- and TAMRA-azide for affinity enrichment/WB studies and in-gel fluorescence detection. In addition, clickable L505 analog 5 was shown to be cell penetrant and may be a useful tool for imaging γ-secretase. Efforts to this end are under investigation and results will be disclosed in due course.
Acknowledgments
We thank Leslie Pustilnik for measuring Aβ42 in CHO-APP cells. This work is supported by NIH grants 1R01NS076117 and 2R01AG026660 (YML), the American Health Assistance Foundation (YML), Pfizer (YML), Mr. William H. Goodwin and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research, the Experimental Therapeutics Center of MSKCC, and the William Randolph Hearst Fund in Experimental Therapeutics. CJC was supported by Institutional Training Grant T32 GM073546-01A1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Geurink PP, Prely LM, van der Marel GA, Bischoff R, Overkleeft HS. Top Curr Chem. 2011 doi: 10.1007/128_2011_286. [DOI] [PubMed] [Google Scholar]
- 2.Geoghegan KF, Johnson DS. Annu Rep Med Chem. 2010;45:345. [Google Scholar]
- 3.(a) Xu M, Lai MT, Huang Q, DiMuzio-Mower J, Castro JL, Harrison T, Nadin A, Neduvelil JG, Shearman MS, Shafer JA, Gardell SJ, Li YM. Neurobiol Aging. 2002;23:1023. doi: 10.1016/s0197-4580(02)00126-4. [DOI] [PubMed] [Google Scholar]; (b) Crump CJ, Johnson DS, Li YM. EMBO J. 2011;30:4696. doi: 10.1038/emboj.2011.410. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Yokoshima S, Abe Y, Watanabe N, Kita Y, Kan T, Iwatsubo T, Tomita T, Fukuyama T. Bioorg Med Chem Lett. 2009;19:6869. doi: 10.1016/j.bmcl.2009.10.086. [DOI] [PubMed] [Google Scholar]; (d) Fuwa H, Takahashi Y, Konno Y, Watanabe N, Miyashita H, Sasaki M, Natsugari H, Kan T, Fukuyama T, Tomita T, Iwatsubo T. ACS Chem Biol. 2007;2:408. doi: 10.1021/cb700073y. [DOI] [PubMed] [Google Scholar]; (e) Kornilova AY, Das C, Wolfe MS. J Biol Chem. 2003;278:16470. doi: 10.1074/jbc.C300019200. [DOI] [PubMed] [Google Scholar]; (f) Seiffert D, Bradley JD, Rominger CM, Rominger DH, Yang F, Meredith JE, Jr, Wang Q, Roach AH, Thompson LA, Spitz SM, Higaki JN, Prakash SR, Combs AP, Copeland RA, Arneric SP, Hartig PR, Robertson DW, Cordell B, Stern AM, Olson RE, Zaczek R. J Biol Chem. 2000;275:34086. doi: 10.1074/jbc.M005430200. [DOI] [PubMed] [Google Scholar]
- 4.Selkoe DJ, Wolfe MS. Cell. 2007;131:215. doi: 10.1016/j.cell.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 5.For structural studies using cryoelectron microscopy see, Li H, Wolfe MS, Selkoe DJ. Structure. 2009;17:326. doi: 10.1016/j.str.2009.01.007.Osenkowski P, Li H, Ye W, Li D, Aeschbach L, Fraering PC, Wolfe MS, Selkoe DJ. J Mol Biol. 2009;385:642. doi: 10.1016/j.jmb.2008.10.078.Renzi F, Zhang X, Rice WJ, Torres-Arancivia C, Gomez-Llorente Y, Diaz R, Ahn K, Yu C, Li YM, Sisodia SS, Ubarretxena-Belandia I. J Biol Chem. 2011;286:21440. doi: 10.1074/jbc.M110.193326.
- 6.Li YM, Xu M, Lai MT, Huang Q, Castro JL, DiMuzio-Mower J, Harrison T, Lellis C, Nadin A, Neduvelil JG, Register RB, Sardana MK, Shearman MS, Smith AL, Shi XP, Yin KC, Shafer JA, Gardell SJ. Nature. 2000;405:689. doi: 10.1038/35015085. [DOI] [PubMed] [Google Scholar]
- 7.Tarassishin L, Yin YI, Bassit B, Li YM. Proc Natl Acad Sci USA. 2004;101:17050. doi: 10.1073/pnas.0408007101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haapasalo A, Kovacs DM. J Alzheimers Dis. 2011;25:3. doi: 10.3233/JAD-2011-101065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Strooper B, Annaert W. Annu Rev Cell Dev Biol. 2010;26:235. doi: 10.1146/annurev-cellbio-100109-104117. [DOI] [PubMed] [Google Scholar]
- 10.For examples of clickable photoaffinity probes see, Ballell L, Alink KJ, Slijper M, Versluis C, Liskamp RM, Pieters RJ. Chembiochem. 2005;6:291. doi: 10.1002/cbic.200400209.Sieber SA, Niessen S, Hoover HS, Cravatt BF. Nat Chem Biol. 2006;2:274. doi: 10.1038/nchembio781.Salisbury CM, Cravatt BF. Proc Natl Acad Sci USA. 2007;104:1171. doi: 10.1073/pnas.0608659104.MacKinnon AL, Garrison JL, Hegde RS, Taunton J. J Am Chem Soc. 2007;129:14560. doi: 10.1021/ja076250y.Kalesh KA, Sim DS, Wang J, Liu K, Lin Q, Yao SQ. Chem Commun. 2010;46:1118. doi: 10.1039/b919888a.Shi H, Cheng X, Sze SK, Yao SQ. Chem Commun. 2011;47:11306. doi: 10.1039/c1cc14824a.Eirich J, Orth R, Sieber SA. J Am Chem Soc. 2011;133:12144. doi: 10.1021/ja2039979.
- 11.Crump CJ, Fish BA, Castro SV, Chau DM, Gertsik N, Ahn K, Stiff C, Pozdnyakov N, Bales KR, Johnson DS, Li YM. ACS Chem Neurosci. 2011;2:705. doi: 10.1021/cn200098p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rozkiewicz DI, Janczewski D, Verboom W, Ravoo BJ, Reinhoudt DN. Angew Chem Int Ed Engl. 2006;45:5292. doi: 10.1002/anie.200601090. [DOI] [PubMed] [Google Scholar]
- 13.Chun J, Yin YI, Yang G, Tarassishin L, Li YM. J Org Chem. 2004;69:7344. doi: 10.1021/jo0486948. [DOI] [PubMed] [Google Scholar]
- 14.(a) Evans BE, Rittle KE, Homnick CF, Springer JP, Hirshfield J, Veber DF. J Org Chem. 1985;50:4615. [Google Scholar]; (b) Nadin A, Sanchez Lopez JM, Neduvelil JG, Thomas SR. Tetrahedron. 2001;57:1861. [Google Scholar]
- 15.Shelton CC, Tian Y, Frattini MG, Li YM. Mol Neurodegeneration. 2009;4:22. doi: 10.1186/1750-1326-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.(a) Shelton CC, Zhu L, Chau D, Yang L, Wang R, Djaballah H, Zheng H, Li YM. Proc Natl Acad Sci USA. 2009;106:20228. doi: 10.1073/pnas.0910757106. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tian Y, Crump CJ, Li YM. J Biol Chem. 2010;285:32549. doi: 10.1074/jbc.M110.128439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weihofen A, Binns K, Lemberg MK, Ashman K, Martoglio B. Science. 2002;296:2215. doi: 10.1126/science.1070925. [DOI] [PubMed] [Google Scholar]
- 18.Nyborg AC, Kornilova AY, Jansen K, Ladd TB, Wolfe MS, Golde TE. J Biol Chem. 2004;279:15153. doi: 10.1074/jbc.M309305200. [DOI] [PubMed] [Google Scholar]