Abstract
Background
Many epidemiologic studies have evaluated the association between caffeine and fertility, with inconsistent results. Some studies suggest that various caffeine-containing beverages may affect fertility differently.
Methods
We evaluated the relation of caffeine, coffee, tea, and sodas with time to pregnancy in a prospective cohort study of 3628 women planning a pregnancy in Denmark (2007–2010). Women reported beverage intake at baseline and every eight weeks during follow-up until they became pregnant or for up to 12 cycles. We used discrete-time Cox proportional hazards regression to estimate fecundability ratios (FRs) and 95% confidence intervals (CI), controlling for potential confounders.
Results
There was little relation between fecundability and caffeine intake of 300+mg/day compared with <100 mg/day (FR=1.04 [95% CI= 0.90–1.21]) or coffee intake of 3+ servings/day compared with none (1.05 [0.85–1.33]). Soda consumption was associated with reduced fecundability: for all types of sodas combined, the adjusted FRs were 0.89 (0.80–0.98), 0.85 (0.71–1.02), 0.84 (0.57–1.25), and 0.48 (0.21–1.13) for <1, 1, 2 and 3+ servings per day, respectively, compared with none. Tea drinking was associated with a slight increase in fecundability, with FR=1.27 (0.98–1.64) for 2+ servings/day vs. none.
Conclusion
In this prospective study of time to pregnancy, the association between caffeine intake and fertility differed by beverage type. Although we controlled for many confounders, our findings of reduced fecundability among soda drinkers and increased fecundability among tea drinkers could have resulted from confounding by unmeasured lifestyle characteristics.
The association between caffeine intake and female fertility has been studied extensively, with inconsistent findings. Most studies have been retrospective, assessing caffeine consumption in relation to time to pregnancy among women who are already pregnant. They have shown either reduced fecundability1–5 or little association6–8 with fecundability. Studies with prospective ascertainment of caffeine consumption also have had discrepant findings, including inverse,9, 10 positive,11–13 and no association11, 14, 15 between caffeine and fecundability or ovulatory infertility.15 Studies that evaluated individual caffeinated beverages suggest different effects by type of beverage. One study found increased fertility among coffee drinkers but not among tea drinkers13; two studies reported increased fertility among tea drinkers11, 12; and four studies reported reduced fertility among women consuming soda.3, 11, 15, 16
Caffeine is an adenosine receptor antagonist with short-term physiologic effects, including release of catecholamines,17 transient increases in blood pressure,18, 19 improvements in exercise performance,20 and (among habitual users) a well-known withdrawal syndrome.21 The mechanisms through which caffeine or other constituents of particular caffeinated beverages or sodas could affect fertility are uncertain. Caffeine has been associated with alterations in estradiol and other hormones,22–25 which in turn may affect ovulation, the length of the follicular or luteal phase, or other menstrual characteristics. Caffeine has also been related to shorter menstrual-cycle length and a lower risk of very long menstrual cycles.26 Caffeine has been reported to have no effect27 or possibly beneficial effects on markers of oocyte aging.28 Soda, both with and without caffeine, has been associated with increased insulin resistance, metabolic syndrome,29 and weight gain,30 which in turn are related to polycystic ovary syndrome, a leading cause of ovulatory infertility.31
We evaluated the relation between time to pregnancy and consumption of caffeinated beverages and soda in a large prospective cohort study of Danish women who were trying to become pregnant.
Methods
Study population
The Snart Gravid study is an Internet-based prospective cohort study of Danish women who have recently stopped using birth control to become pregnant. Study methods have been described in detail previously.32, 33 Briefly, study enrollment began in June 2007 with the launch of the study Web site (www.Snart-Gravid.dk). Potential participants learned about the study via an advertisement on a popular Danish health-related Web site (www.netdoktor.dk), or through publicity in other media. The study Web site contains an on-line consent form and a brief screening questionnaire. Eligible women were Danish residents, aged 18–40 years, in a stable relationship with a male partner, not currently using any form of birth control or fertility drugs, who had been trying to conceive for no more than 12 months. Women also had to agree to provide their Civil Registration Number and e-mail address. After completing an extensive baseline questionnaire, women were e-mailed short follow-up questionnaires every eight weeks to ascertain pregnancies and to update exposures. Women were followed until they reported a pregnancy, stopped trying to become pregnant, or began fertility treatment. If they did not become pregnant, they were followed for up to 12 cycles (a maximum of 6 follow-up questionnaires). Cohort retention (defined as follow-up until a study event or for 12 cycles) was 82%.34
After 30 months of recruitment, 5460 women were enrolled in the study. Of these, we excluded 1063 women who had been trying to conceive for >6 cycles at study entry, 263 women with insufficient or implausible information about their last menstrual period date or length of time trying to conceive, and 495 women who completed only the baseline questionnaire. After these exclusions, 3628 women remained in the cohort. The study was approved by the Danish Data Protection Board and the Institutional Review Board at Boston University. Participant consent was obtained via an on-line consent form.
Ascertainment of caffeinated beverage and soda consumption
The baseline questionnaire asked women to report beverage intake over the past month, specifically the number of mugs (250 milliliters (ml)) of regular coffee, decaffeinated coffee, regular tea, herbal or green tea, and bottles (500 ml) of colas (sweetened and diet separately) and sodas without caffeine (sweetened and diet separately) consumed each week. Each bimonthly follow-up questionnaire asked identical questions about beverage consumption during the month preceding the questionnaire.
Assessment of time-to-pregnancy
We estimated time to pregnancy using data from the screening questionnaire, the baseline questionnaire, and each follow-up questionnaire. In the screening questionnaire, we asked women how many months they had been trying to conceive. In the baseline questionnaire (completed immediately after the screening questionnaire), we asked for date of last menstrual period (LMP), and when they expected to get their next period if they did not become pregnant. We also asked whether they had regular menstrual cycles (defined in a help button as: “usually being able to predict from one menstrual period to the next about when the next menstrual period would start”). Women with regular cycles were asked to report their usual cycle length. For women with irregular cycles or missing data on menstrual-cycle length, we estimated their usual cycle length with data from the baseline questionnaire (LMP, date of questionnaire completion, the question on when they expected to get their next period), and actual LMP dates recorded during each follow-up.
We estimated time to pregnancy in cycles based on the following formula:
(months of trying at study entry/cycle length) + ((LMP date from most recent follow-up questionnaire - date of baseline questionnaire completion)/cycle length) + 1).
We added one cycle to account for the fact that the average woman would have been at mid-cycle when she filled out the baseline questionnaire. Observed cycles at risk were defined as those occurring after study entry.
During each follow-up questionnaire, women were asked whether they were currently pregnant or had had a miscarriage or other pregnancy outcome since the last questionnaire. The study event of interest was the occurrence of any pregnancy, regardless of pregnancy outcome. In secondary analyses, we evaluated whether the results were similar when women with reported miscarriages were excluded from the outcome definition.
Assessment of covariates
We collected data on potential covariates in the baseline questionnaire, including age, partner’s age, education and income, frequency of intercourse, menstrual characteristics, reproductive history, height, weight, medical history, physical activity, smoking history, and alcohol intake. Data on lifestyle factors were updated in each follow-up questionnaire. We calculated body mass index (BMI) as weight (kilograms) / height (meters2). Self-reported data on height and weight were validated in a subset of women using data from the Danish Birth Registry, with high reproducibility (Pearson’s r=0.96).35 We asked about time spent per week doing vigorous and moderate physical activity and estimated total metabolic equivalents (METs) of physical activity per week by summing the METs from moderate exercise (hours per week multiplied by 3.5) and vigorous exercise (hours per week multiplied by 7.0).36
Data analysis
We estimated total caffeine content by assuming that one serving of coffee contained 141 milligrams (mg) of caffeine; one serving of decaffeinated coffee, 5 mg; one serving of regular tea, 56 mg; one serving of regular cola, 51 mg; and one serving of diet cola, 66 mg of caffeine.37 These are similar to estimates used in other studies,9, 38–40) and similar to those provided by a widely-used European Web site with caffeine information.41 Nevertheless, total caffeine amounts in beverages are quite variable, especially for coffee, because the caffeine content is dependent upon both the type of beans used and the brewing method.42 We categorized total daily caffeine intake into 4 levels (<100, 100–199, 200–299, and 300+ mg/day). As our questionnaire did not distinguish between green and herbal tea, we were not able to assess green tea separately. Cola is the only caffeinated soda commonly consumed in Denmark; other sodas were not included in the total caffeine calculation.
To evaluate whether soda per se, rather than its caffeine content, affected fertility, we created a variable for total soda consumption by summing all cola drinks (regular and diet) and all other sodas (regular and diet). We also created variables for all diet sodas combined, and all sugar-sweetened sodas combined. Because of evidence that effects may vary by beverage type, we also evaluated the effect of individual beverages (regular coffee, regular tea, herbal and green tea, cola, other soda, and total soda) according to number of servings per day (for tea and soda; none, <1, 1, 2 or more; for coffee, 3 or more). One serving was defined as a 250 ml mug for coffee and tea, and a 500 ml bottle for sodas. For our main analysis, we evaluated time-varying caffeine and individual beverage intake, updating beverage consumption based on the data in the follow-up questionnaires. If a woman missed a follow-up questionnaire, we carried forward her beverage information from the most recent questionnaire. We used restricted cubic splines to depict the trend by intake level between each beverage type and fecundabilty.43, 44 In secondary analyses, we evaluated caffeine and individual beverage consumption at baseline in relation to time to pregnancy.
We used Cox discrete-time proportional hazards regression models to compute fecundability ratios (FR) and 95% confidence intervals (CI) by level of caffeine and individual beverage intake, adjusting for potential confounders. The FR represents the per-cycle probability of conception in the exposed versus unexposed women. In our primary analysis, we evaluated time to pregnancy, using cycles as the underlying metric. In secondary analyses, we compared our results using time to pregnancy in months instead of cycles. We used any pregnancy as our outcome of interest in main analyses, but also examined the results when the outcome was defined as a successful pregnancy (conception with no report of early miscarriage). Women were censored if and when they (1) reported use of fertility treatments or change in intention to become pregnant, (2) became lost to follow-up or actively resigned from the study, or (3) reached the end of the observation period (no conception after 12 cycles). Women contributed cycles at risk until they became pregnant or were censored. The Cox model allowed for “delayed entry” into the risk set, such that analyses were based only on cycles at risk observed after study entry.45
Potential confounders were selected based on the literature, their association with other variables at baseline, and assessment of a causal graph. Potential confounders were included in final models if they changed the FR by an appreciable amount compared with the unadjusted FR.46 In general, the unadjusted results were similar to the adjusted FRs except in the highest beverage-consumption categories. The final model adjusted for age, partner’s age, BMI, pack-years of smoking, number of alcoholic beverages consumed per week, physical activity (METs/week), and frequency of intercourse. Caffeinated beverages, sodas, alcohol, and intercourse frequency were included as time-varying covariates in our main analyses, and values from the baseline questionnaire were used for all other covariates. We also examined results using the baseline data for caffeinated beverages and sodas. We used multiple imputation techniques to impute missing data.47 We also evaluated whether the effects of caffeine and individual beverages varied according to levels of other covariates, including age at baseline, parity, smoking, alcohol intake, menstrual cycle regularity, and number of cycle attempts before study entry.
Results
The average age of women in our cohort was 28.4 years (range 18–40 years), 67% were nulliparous, and 69% became pregnant within 12 cycles. Mean caffeine consumption reported in the baseline questionnaire was 137 mg/day (median= 86; range= 0 to 1425); over the follow-up period, average caffeine consumption declined to 130 mg/day (median= 80.6; range= 0 to 1425). In total, 59% of the women reported drinking regular coffee in the previous month, 51% reported drinking regular tea, and 75% reported drinking soda on the baseline questionnaire. Herbal or green tea consumption was reported by 39%, regular cola by 31%, diet cola by 45%, non-cola sodas (regular and diet) by <20% of the women, and decaffeinated coffee by 5%. Few women (7%) reported no consumption of caffeinated beverages, and there was little missing data (for example, 1%, 2%, and 3% women were missing baseline data on the number of coffee, tea, and colas, respectively). In general, consumption of all caffeinated beverages and sodas tended to decline progressively over the follow-up period, with the largest reductions occurring between baseline and the first follow-up.
Baseline characteristics of the study population varied by beverage type (Table 1). Women who consumed the most coffee tended to be older, have higher parity, and were more likely to smoke and drink alcohol than women who did not consume coffee. Women who drank large amounts of regular tea were somewhat older, drank more alcohol, and were slightly less likely to be current smokers than women who did not drink regular tea. BMI and physical activity were not strongly associated with coffee or tea consumption. In contrast, women who drank soda had higher BMIs and were less physically active than other women. Soda drinkers were also slightly younger and tended to have fewer years of education. Frequency and timing of intercourse were not strongly related to beverage consumption except among the small number of women (n=17) who drank 3 or more sodas per day. These women were less likely to have frequent intercourse, but more likely to time intercourse.
Table 1.
Selected characteristicsa of 3628 women according to consumption of caffeinated beverages at baseline: the Snart Gravid Study, 2007–2009.
| Coffee No. servingsb/day |
Tea No. servingsb/day |
All sodas No. servingsc/day |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 (n=1486) |
<1 (n=1037) |
1 (n=610) |
2 (n=332) |
3+ (n=163) |
0 (n=1841) |
<1 (n=1404) |
1 (n=251) |
2 (n=99) |
3+ (n=33) |
0 (n=921) |
<1 (n=2358) |
1 (n=279) |
2 (n=53) |
3+ (n=17) |
|
| Age (years); mean | 27.3 | 28.4 | 29.6 | 30.5 | 30.5 | 28.1 | 28.5 | 29.3 | 30.1 | 32.1 | 29.3 | 28.1 | 28.3 | 27.9 | 27.1 |
| Partner’s age (years); mean | 30.4 | 30.9 | 31.0 | 32.0 | 31.5 | 30.8 | 30.7 | 31.0 | 32.3 | 31.4 | 31.2 | 30.7 | 31.0 | 29.8 | 32.0 |
| Age at menarche (years); mean | 13.0 | 13.0 | 13.0 | 12.9 | 12.8 | 12.9 | 13.0 | 13.1 | 12.8 | 12.9 | 13.0 | 13.0 | 12.8 | 13.0 | 12.7 |
| Regular cycles (yes); % | 75 | 77 | 77 | 75 | 82 | 77 | 75 | 75 | 71 | 82 | 74 | 77 | 77 | 72 | 77 |
| Cycle length (days); mean | 30.6 | 30.4 | 30.8 | 30.3 | 31.4 | 30.4 | 30.6 | 31.2 | 31.8 | 29.2 | 31.0 | 30.5 | 30.2 | 30.4 | 29.8 |
| Cycle length <27 days; % | 11 | 12 | 11 | 19 | 11 | 13 | 11 | 14 | 12 | 9 | 13 | 12 | 11 | 19 | 25 |
| Cycle length >31 days; % | 24 | 25 | 22 | 19 | 23 | 24 | 25 | 24 | 24 | 3 | 24 | 23 | 28 | 32 | 27 |
| Body mass index (kg/m2); mean | 24.6 | 23.7 | 23.5 | 23.7 | 24.8 | 24.4 | 23.9 | 23.3 | 24.2 | 22.8 | 22.6 | 24.4 | 25.9 | 26.2 | 28.9 |
| Body mass index of partner(kg/m2); mean | 25.6 | 25.2 | 25.2 | 25.5 | 25.3 | 25.5 | 25.2 | 24.9 | 25.1 | 26.0 | 24.7 | 25.4 | 26.3 | 27.2 | 26.0 |
| Physical activity (MET hrs/wk); mean | 24.0 | 25.4 | 26.7 | 23.6 | 22.4 | 24.5 | 24.9 | 25.2 | 23.5 | 28.8 | 27.1 | 24.2 | 23.3 | 18.0 | 16.8 |
| Vocational training; % | |||||||||||||||
| None | 12 | 11 | 12 | 14 | 10 | 12.8 | 11.4 | 10.8 | 8.6 | 18.6 | 12.6 | 11.4 | 14.2 | 13.6 | 19.6 |
| Semi-skilled/basic training | 17 | 15 | 11 | 14 | 14 | 16 | 13 | 15 | 12 | 14 | 11 | 16 | 18 | 18 | 18 |
| Higher education <3 yrs | 17 | 14 | 12 | 15 | 15 | 16 | 14 | 11 | 18 | 9 | 12 | 15 | 22 | 28 | 2 |
| Higher education 3–4 yrs | 35 | 34 | 34 | 32 | 41 | 35 | 35 | 34 | 32 | 31 | 33 | 36 | 31 | 36 | 40 |
| Higher education ≥5 yrs | 20 | 26 | 31 | 24 | 21 | 20 | 27 | 30 | 29 | 29 | 32 | 22 | 16 | 6 | 19 |
| Gravid (ever pregnant); % | 46 | 42 | 45 | 48 | 60 | 49 | 41 | 42 | 49 | 44 | 44 | 45 | 49 | 47 | 74 |
| Parous (ever had live birth); % | 35 | 29 | 31 | 40 | 47 | 36 | 29 | 34 | 36 | 36 | 33 | 33 | 38 | 33 | 66 |
| Parity (no. live births); mean | 0.5 | 0.4 | 0.4 | 0.5 | 0.7 | 0.5 | 0.4 | 0.4 | 0.4 | 0.5 | 0.4 | 0.4 | 0.5 | 0.5 | 1.2 |
| Cigarette smoking | |||||||||||||||
| Pack-years for women | 1.4 | 1.9 | 2.4 | 3.7 | 4.8 | 2.4 | 1.7 | 1.9 | 2.5 | 3.4 | 1.8 | 2.1 | 3.2 | 4.3 | 3.6 |
| Pack-years for partners | 1.6 | 1.7 | 1.8 | 2.6 | 3.0 | 2.1 | 1.5 | 1.4 | 1.9 | 1.9 | 1.6 | 1.8 | 2.6 | 2.1 | 0.2 |
| Current daily smoking; % | 8.4 | 11.8 | 14.3 | 22.3 | 29.6 | 14.1 | 10.1 | 10.1 | 11.7 | 10.2 | 7.8 | 12.2 | 22.5 | 16.0 | 9.5 |
| Current daily smoking by partner; % | 16.5 | 17.3 | 21.2 | 27.3 | 31.6 | 20.3 | 18.0 | 15.5 | 21.8 | 8.5 | 14.2 | 19.1 | 28.2 | 25.4 | 29.5 |
| Mother smoked during pregnancy; % | 32.9 | 33.1 | 36.3 | 38.7 | 42.1 | 36.0 | 32.9 | 31.8 | 28.6 | 47.4 | 29.6 | 34.6 | 42.0 | 57.9 | 52.1 |
| Current alcohol consumption | |||||||||||||||
| No. drinks/wk; mean | 2.1 | 3.0 | 3.8 | 3.7 | 4.4 | 2.8 | 3.0 | 3.2 | 3.5 | 6.7 | 2.5 | 3.0 | 3.0 | 3.9 | 1.9 |
| ≥14 drinks/wk; % | 1.0 | 1.7 | 3.9 | 2.1 | 5.4 | 2.0 | 1.6 | 2.6 | 4.6 | 11.7 | 1.1 | 1.9 | 2.9 | 13.3 | 2.4 |
| Intercourse ≥4 times/wk; % | 18.5 | 20.8 | 17.6 | 19.7 | 17.5 | 20.5 | 18.0 | 17.1 | 15.5 | 18.7 | 19.3 | 19.0 | 20.0 | 22.1 | 12.5 |
| Doing something to time intercourse; % | 46.8 | 46.7 | 42.9 | 43.0 | 50.1 | 46.3 | 46.1 | 40.6 | 47.2 | 55.9 | 46.0 | 45.6 | 45.5 | 58.7 | 60.4 |
| Last method of birth control used | |||||||||||||||
| Barrier | 27.2 | 26.7 | 27.6 | 29.3 | 34. | 28.2 | 27.1 | 29.2 | 27.7 | 32.0 | 32.5 | 26.1 | 26.2 | 26.4 | 52.8 |
| Oral contraceptives | 63.5 | 62.4 | 60.3 | 59.2 | 55.5 | 61.5 | 61.9 | 61.2 | 60.7 | 54.3 | 56.7 | 63.0 | 64.8 | 65.2 | 39.5 |
| High blood pressure, yes; % | 6.7 | 8.0 | 8.4 | 8.0 | 10.9 | 7.5 | 7.9 | 6.5 | 10.8 | 3.2 | 6.1 | 8.1 | 9.5 | 3.6 | 16.1 |
Characteristics are presented as means or percents within caffeine categories and are age-standardized to distribution of cohort at baseline.
Serving size for coffee and tea is 250ml.
Serving size for sodas is 500ml.
Overall, we found little association between total caffeine intake (mg/day) and fecundability, using either caffeine exposure at baseline or updating exposure over follow-up; adjusted FRs for time-varying data ranged from 0.98 to 1.07 for categories of consumption above 100 mg/day compared with <100 mg/day (Table 2). We did not find a monotonic trend of coffee consumption on fecundability, and all FRs for coffee consumption were close to 1.0, whether using baseline or time-varying data.
Table 2.
Association of Baseline and Time-varying caffeine and beverage consumption with time to pregnancy (censored at 12 cycles)
| Results using Beverage Data at Baseline | Results using Time-varying Data on Beverages | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. pregnancies |
Cycles at Risk |
Unadjusted | Adjusteda | No. pregnancies |
Cycles at risk |
Unadjusted | Adjusteda | ||||||
| FR | (95% CI) | FR | (95% CI) | FR | (95% CI) | FR | (95% CI) | ||||||
| Caffeine (mg/day) | |||||||||||||
| <100b | 1318 | 8233 | 1.00 | 1.00 | 1360 | 8396 | 1.00 | 1.00 | |||||
| 100–199 | 553 | 3300 | 1.06 | (0.95–1.19) | 1.05 | (0.93–1.17) | 544 | 3389 | 1.00 | (0.90–1.12) | 0.98 | (0.88–1.10) | |
| 200–299 | 299 | 1763 | 1.07 | (0.93–1.23) | 1.08 | (0.93–1.25) | 280 | 1613 | 1.06 | (0.91–1.22) | 1.07 | (0.92–1.24) | |
| 300+ | 314 | 1929 | 1.03 | (0.90–1.19) | 1.06 | (0.92–1.23) | 300 | 1827 | 1.01 | (0.88–1.16) | 1.04 | (0.90–1.21) | |
| Coffee (servings/day) | |||||||||||||
| Noneb | |||||||||||||
| <1 | 1001 | 6403 | 1.00 | 1.00 | 1018 | 6445 | 1.00 | 1.00 | |||||
| 1 | 710 | 4307 | 1.06 | (0.95–1.18) | 1.04 | (0.93–1.16) | 709 | 4307 | 1.05 | (0.95–1.17) | 1.03 | (0.92–1.15) | |
| 2 | 431 | 2452 | 1.14 | (1.00–1.29) | 1.13 | (0.99–1.29) | 420 | 2440 | 1.10 | (0.97–1.25) | 1.08 | (0.95–1.24) | |
| 3+ | 235 | 1361 | 1.14 | (0.97–1.33) | 1.18 | (1.00–1.40) | 232 | 1383 | 1.09 | (0.93–1.27) | 1.11 | (0.94–1.31) | |
| 107 | 702 | 0.97 | (0.78–1.21) | 1.01 | (0.80–1.27) | 105 | 650 | 1.01 | (0.81–1.26) | 1.05 | (0.83–1.33) | ||
| Tea (servings/day) | |||||||||||||
| Noneb | 1131 | 7915 | 1.00 | 1.00 | 1272 | 8238 | 1.00 | 1.00 | |||||
| <1 | 984 | 5757 | 1.11 | (1.01–1.22) | 1.10 | (1.00–1.22) | 951 | 5463 | 1.12 | (1.02–1.23) | 1.11 | (1.01–1.22) | |
| 1 | 179 | 1060 | 1.08 | (0.91–1.29) | 1.08 | (0.91–1.29) | 177 | 1065 | 1.06 | (0.89–1.26) | 1.04 | (0.87–1.24) | |
| 2+ | 90 | 493 | 1.19 | (0.93–1.53) | 1.27 | (0.99–1.64) | 84 | 459 | 1.20 | (0.93–1.54) | 1.27 | (0.98–1.64) | |
| Herbal/green tea (servings/day) | |||||||||||||
| Noneb | |||||||||||||
| <1 | 1498 | 9208 | 1.00 | 1.00 | 1500 | 9376 | 1.00 | 1.00 | |||||
| 1 | 718 | 4505 | 0.97 | (0.88–1.08) | 0.93 | (0.84–1.03) | 712 | 4256 | 1.02 | (0.93–1.13) | 0.98 | (0.89–1.09) | |
| 2+ | 174 | 982 | 1.07 | (0.89–1.27) | 1.03 | (0.86–1.23) | 173 | 1035 | 1.02 | (0.85–1.21) | 0.97 | (0.81–1.16) | |
| 94 | 530 | 1.06 | (0.84–1.34) | 1.07 | (0.84–1.35) | 99 | 558 | 1.09 | (0.86–1.36) | 1.07 | (0.85–1.35) | ||
| Cola (servings/day) | |||||||||||||
| Noneb | |||||||||||||
| <1 | 846 | 4886 | 1.00 | 1.00 | 880 | 5131 | 1.00 | 1.00 | |||||
| 1 | 1449 | 9124 | 0.90 | (0.82–0.99) | 0.92 | (0.83–1.01) | 1420 | 8896 | 0.90 | (0.82–0.99) | 0.92 | (0.84–1.02) | |
| 2+ | 153 | 950 | 0.91 | (0.75–1.10) | 0.97 | (0.80–1.18) | 157 | 964 | 0.94 | (0.78–1.14) | 1.01 | (0.83–1.23) | |
| 36 | 265 | 0.78 | (0.54–1.13) | 0.92 | (0.64–1.34) | 27 | 234 | 0.63 | (0.42–0.95) | 0.75 | (0.49–1.13) | ||
| Other soda (servings /day) | |||||||||||||
| Noneb | |||||||||||||
| <1 | 1834 | 10945 | 1.00 | 1.00 | 1841 | 11061 | 1.00 | 1.00 | |||||
| 1 | 636 | 4155 | 0.92 | (0.83–1.01) | 0.94 | (0.85–1.04) | 624 | 4000 | 0.93 | (0.84–1.03) | 0.95 | (0.86–1.05) | |
| 2+ | 11 | 106 | 0.58 | (0.31–1.08) | 0.59 | (0.32–1.11) | 17 | 132 | 0.79 | (0.47–1.32) | 0.81 | (0.48–1.36) | |
| 3 | 19 | 0.96 | (0.28–3.32) | 1.22 | (0.35–4.26) | 2 | 32 | 0.38 | (0.09–1.59) | 0.48 | (0.11–2.01) | ||
| Sweetened sodas (servings/day) | |||||||||||||
| Noneb | |||||||||||||
| <1 | 1506 | 8810 | 1.00 | 1.00 | 1508 | 8813 | 1.00 | 1.00 | |||||
| 1 | 929 | 6035 | 0.90 | (0.82–0.99) | 0.91 | (0.83–1.00) | 929 | 5992 | 0.89 | (0.82–0.98) | 0.91 | (0.83–1.00) | |
| 2+ | 40 | 316 | 0.73 | (0.52–1.03) | 0.77 | (0.55–1.09) | 41 | 352 | 0.68 | (0.49–0.95) | 0.72 | (0.52–1.01) | |
| 9 | 64 | 0.84 | (0.41–1.70) | 0.97 | (0.47–2.00) | 6 | 68 | 0.51 | (0.22–1.18) | 0.58 | (0.25–1.36) | ||
| Diet soda (servings/day) | |||||||||||||
| Noneb | 1306 | 7895 | 1.00 | 1.00 | 1347 | 8147 | 1.00 | 1.00 | |||||
| <1 | 1018 | 6311 | 0.97 | (0.89–1.06) | 0.99 | (0.90–1.09) | 980 | 6100 | 0.96 | (0.87–1.05) | 0.97 | (0.88–1.07) | |
| 1 | 131 | 794 | 0.99 | (0.81–1.21) | 1.02 | (0.83–1.25) | 133 | 782 | 1.03 | (0.85–1.26) | 1.07 | (0.88–1.32) | |
| 2+ | 29 | 225 | 0.77 | (0.52–1.15) | 0.87 | (0.58–1.30) | 24 | 196 | 0.70 | (0.46–1.09) | 0.79 | (0.51–1.23) | |
| All sodas (servings/day) | |||||||||||||
| Noneb | 654 | 3647 | 1.00 | 1.00 | 689 | 3861 | 1.00 | 1.00 | |||||
| <1 | 1600 | 9991 | 0.88 | (0.80–0.98) | 0.90 | (0.81–1.00) | 1570 | 9799 | 0.87 | (0.79–0.96) | 0.89 | (0.80–0.98) | |
| 1 | 184 | 1266 | 0.79 | (0.66–0.95) | 0.83 | (0.69–1.00) | 187 | 1252 | 0.81 | (0.68–0.97) | 0.85 | (0.71–1.02) | |
| 2 | 35 | 243 | 0.80 | (0.55–1.18) | 0.88 | (0.60–1.29) | 32 | 231 | 0.75 | (0.51–1.11) | 0.84 | (0.57–1.25) | |
| 3+ | 11 | 78 | 0.81 | (0.41–1.61) | 1.10 | (0.55–2.21) | 6 | 82 | 0.39 | (0.17–0.91) | 0.48 | (0.21–1.13) | |
Adjusted for age at baseline, partner’s age at baseline, parity, BMI, METs/week, pack-years of smoking, alcoholic beverages consumed/week, and intercourse frequency. Estimates for coffee, tea, and colas are mutually adjusted for each other in addition to the other covariates.
Reference category.
FR indicates fecundability ratio (cycle-specific probability of conception comparing exposed to unexposed); CI, confidence interval
Only 11% of women reported drinking one or more servings of regular tea per day at baseline. Women who drank regular tea had moderate increases in fecundability for 2+ servings per day (FR= 1.27 [95% CI= 0.98–1.64]), with no monotonic trend according to servings per day. There was little association between herbal or green tea consumption and fecundability (Table 2). The associations were similar using baseline data.
Women who drank 2 or more servings per day of any type of soda appeared to have lower fecundability (FRs ranging from from 0.48 to 0.79), but these estimates were imprecise. For all sodas combined, the adjusted FRs were 0.89 (95% CI= 0.80–0.98), 0.85 (0.71–1.02), 0.84 (0.57–1.25), and 0.48 (0.21–1.13) for <1, 1, 2 and 3+ servings per day respectively, compared with none. Sugar-sweetened sodas appeared to have a slightly stronger inverse association with fecundability than diet sodas, with FRs of 0.91 (0.83–1.00), 0.72 (0.52–1.01), and 0.58 (0.25–1.36), respectively for <1, 1, and 2+ sugar-sweetened sodas per day versus none and FRs of 0.97 (0.88–1.07), 1.07 (0.88–1.32), and 0.79 (0.51–1.23), respectively for <1, 1, and 2+ unsweetened sodas per day versus none (Table 2). For most soda variables, the FRs for baseline beverage consumption tended to be closer to the null than the time-varying results (Table 2).
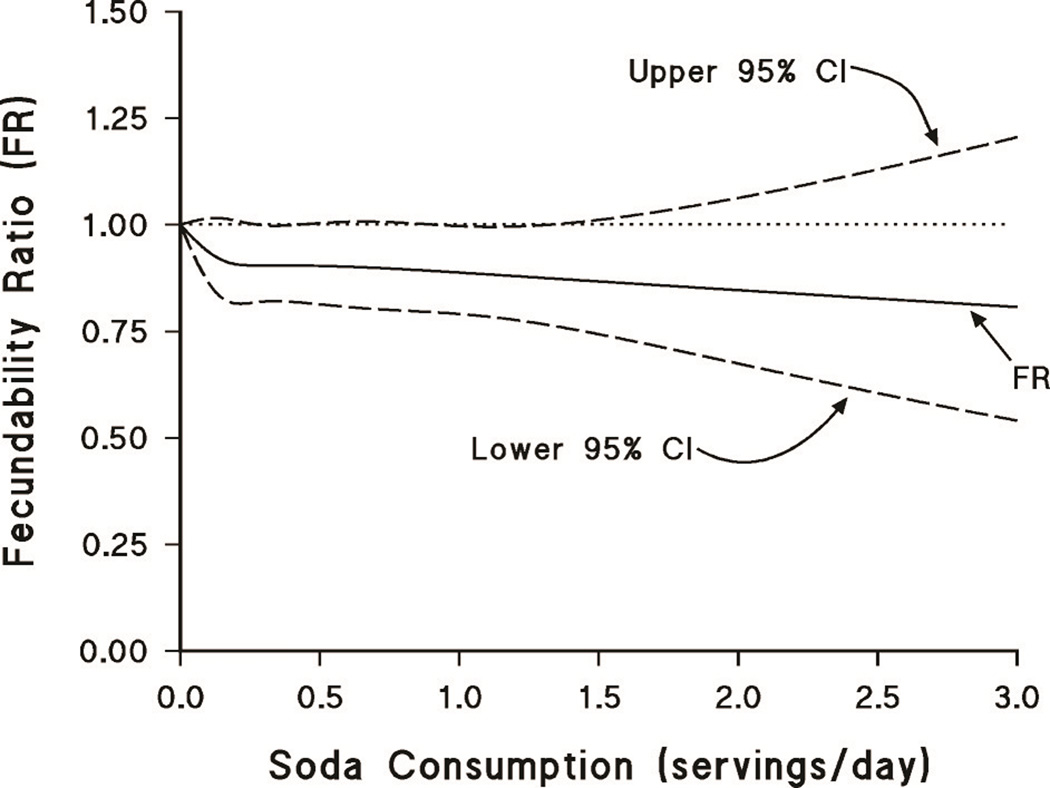
The Figure displays the association between number of servings of all types of soda per day and fecundability, using restricted cubic splines. As in the categorical analysis shown in Table 2, the FR is lower among women who reported drinking sodas compared with those who did not drink any sodas, but the estimates are imprecise.
Figure.
Association between sweetened sodas and fecundability, fitted by restricted cubic splines. The curves are adjusted for age, partner age, pack-years of smoking, alcohol intake, body mass index, level of physical activity, and intercourse frequency.
Analyses of viable pregnancies, rather than all pregnancies, did not produce any major change in effect estimates for total caffeine or any individual beverage (data not shown). The results using months instead of cycles were very similar to those using estimated number of menstrual cycles as the underlying metric for time to pregnancy (data not shown).
In general, we found little evidence that the effect measures for each beverage varied by age, parity, smoking, alcohol intake, menstrual cycle regularity, and number of cycle attempts before study entry (eTables 1–3, http://links.lww.com). For example, results were similar to our overall results among women with regular cycles (FR= 1.04 [95% CI= 0.80–1.34], 1.18 [0.88–1.57] and 0.71 [0.47–1.08] for coffee [3+ servings/day], tea [2+servings/day], and sodas [2+ servings/day], respectively, compared with none). Similar results were also found in women who had been trying for 2 cycles or fewer at entry (1.12 [0.85–1.46], 1.22 [0.91–1.64] and 0.64 [0.41–1.00] for highest level of coffee, tea, and sodas, respectively, compared with none). Coffee appeared to have slight detrimental effects on fecundability among older women (age 30+ years) and parous women, at high levels of coffee intake (3+ servings per day). In contrast, consuming large amounts of coffee was associated with increased fecundability among younger women (age <30 years) and nulliparous women. Smokers who drank coffee also seemed to have slightly increased fecundability, while fecundability was reduced among non-smokers who consumed 3+ servings per day vs. none (FR=0.83 [95% CI= 0.55–1.23]). Coffee also appeared to be inversely associated with fecundability among the women who had been trying to conceive for 3–6 cycles at entry, while all FRs for coffee were greater than 1.0 among women who had been trying for 2 cycles or less. The associations between either tea or soda consumption and fecundability appeared to vary little according to categories of age, parity, smoking, cycle regularity, or the number of cycle attempts before study entry.
Discussion
We found little evidence that total caffeine or coffee consumption was associated with fecundability in this large prospective study of Danish women planning a pregnancy. However, results differed by type of caffeinated beverage, indicating that a combined caffeine exposure variable may not be appropriate. Most previous studies of caffeinated beverages, sodas, and fertility have focused on total caffeine consumption from beverages. Some studies have also collected information on chocolate and caffeine containing medications.9 The definition of high caffeine consumption in other prospective studies has varied substantially, from above 107 mg/day in a U.S. study11 to over 700 mgs/day in a Danish study,9 although several studies used definitions close to our highest category of greater than 300 mgs/day.10, 14, 15 The majority of studies have assessed caffeine and individual beverages retrospectively—in pregnant women,3, 6 shortly after birth,7 or in some cases, many years after pregnancy attempts.8 Besides the obvious potential for exposure and outcome misclassification, retrospective studies may suffer from selection bias, because they are usually limited to women who have become pregnant, and thus could theoretically miss an association that was present only among less fertile or sterile women. Prospective data collection, especially using frequent diaries, or on a monthly or per cycle basis,9, 10 is more accurate. On the other hand, prospective studies of time to pregnancy are limited to couples with planned pregnancies, and thus may be affected by selection bias caused by exclusion of more fertile couples.
Our finding that neither total caffeine intake nor coffee consumption is associated with fecundability is consistent with most,11–13, 15 but not all,9, 10 prospective studies. Jensen and Colleagues9 enrolled 430 couples planning a pregnancy and began follow-up as soon as they discontinued birth control. Daily diaries were used to record vaginal bleeding and intercourse, and monthly data were collected on exposures. Reductions in fecundability were found for coffee and caffeine in both smoking and non-smoking women. However, findings for total caffeine were stronger among non-smokers, and findings for coffee alone were stronger among smokers. The high level of caffeine consumption and use of a referent group consuming 0–299 mgs/caffeine per day make it difficult to compare these results with our study. In a study of 104 women who had attempted to conceive for 3 cycles, Wilcox et al.10 found an adjusted FR of 0.51 (95% CI=0.35–75) for greater than 3150 mg/month of caffeine consumption versus less than that amount. When all women, including the 117 who became pregnant in the first 3 cycles, were included in the analysis, the result was attenuated (FR=0.80). The authors suggested that the association between caffeine and fecundability may be more apparent among less fertile women. In our study, we also found suggestive differences in the FRs for coffee and total caffeine according to the length of time the women had been trying to conceive at study entry.
Several studies have indicated varying effects of different caffeinated beverages on fecundability.11, 12, 15, 16 If true, then studies focused primarily on total caffeine consumption may misclassify relevant exposure, especially given the opposite effects reported for some beverages. Like our study, three other prospective studies found reduced fertility in women consuming the largest amounts of sodas. Results ranged from a 19% reduction in monthly fertility rates11 for ½ serving per day to 50% reduction for 1 serving per day16 in the two studies investigating time to pregnancy. The Nurses’ Health Study15 found 33% to 54% increased risk of ovulatory infertility among women who consumed 2 or more sodas per day. In contrast, a Dutch study12 reported that there was little association between cola drinks and fertility; however, effect estimates were not reported, and a large proportion of women in the study had been trying to conceive for at least 12 months. In our study, results were slightly stronger for sugar-sweetened beverages but some reduction in fertility was seen in all categories of soft drink consumption. It is possible that chemical additives or contaminants could explain the findings. For example, bisphenol A has been suggested to have an adverse effect on fertility48 and it has been found in canned soft drinks.49 On the other hand, another plausible explanation is residual confounding by unmeasured dietary factors or other lifestyle characteristics. Our finding of slightly increased fecundability among tea drinkers is consistent with two previous prospective studies11, 12 reporting an approximate two-fold increase in fecundability among tea drinkers, but disagrees with two other studies that reported null associations.15, 16
Our study is the largest prospective study thus far to evaluate the association between caffeinated beverages and sodas and time to pregnancy. We updated data on beverage consumption over time, which may provide a more accurate estimate of exposure, if one assumes a relatively short induction time for the relation between caffeine and soda consumption and fecundability. When we modeled beverage consumption at baseline, there were few differences for coffee and tea, whereas baseline data on sodas showed weaker associations with fecundability. This might reflect a greater problem with confounding in the time-varying results, but if short-term effects of beverages are more important, the stronger results could be due to better measurement of exposure (and less non-differential misclassification) using the exposure data that is updated over time.
We collected data on numerous potential confounders. Nevertheless, our findings may be affected by other unmeasured confounding factors, such as dietary patterns or other lifestyle factors. Tea drinkers may have healthier lifestyles that could affect fertility, while women who regularly consume sodas may have more unhealthy lifestyles, as exemplified by the strong relationship between BMI and soda consumption (Table 1). In general, once we controlled for known confounders, our fecundability ratios increased slightly, but, overall, confounding had only very small effects, suggesting that residual confounding is unlikely to completely explain our results.
Another limitation of our study, despite its large size, is that relatively few women were regular consumers (at least 7 servings per week) of caffeinated tea (11%) and sodas (10%), limiting our ability to precisely estimate associations with fecundability. Also, we collected data bimonthly instead of during each menstrual cycle, which may have led to some misclassification, not only of exposure and confounding variables, but also of cycles to pregnancy. We found similar FRs when we used months instead of cycles, suggesting that our results were relatively insensitive to the choice of metric. Cohort retention was 82%,34 which is comparable with that of many other prospective cohort studies,50, 51 and the characteristics of women who dropped out of the study before reaching a study endpoint were not notably different from those with complete follow-up data (data not shown). Our study, like nearly all prospective studies of time to pregnancy, cannot evaluate exposures that affect the most fecund women, who are usually underrepresented in a study of pregnancy planners. We addressed this issue by restricting the study population to women who had been trying to conceive for six cycles or less. We found few differences in results across strata defined by attempt time of less than 3 versus 3–6 cycles, implying that length-biased sampling was not a major problem.
In summary, we found little overall relation between caffeine or coffee consumption and time to pregnancy. We did, however, find some evidence for decreased fecundability among women who consumed sodas and increased fecundability among women who drank tea. We caution that these associations may reflect unmeasured confounding by diet or other lifestyle factors.
Supplementary Material
Acknowledgements
We appreciate the technical assistance of Kristen Hahn and Rose Radin.
Funding: This study was supported by the National Institute for Child Health and Development (R21-050264 and R01-060680) and the Danish Medical Research Council (271-07-0338).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SDC Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com). This content is not peer-reviewed or copy-edited; it is the sole responsibility of the author.
References
- 1.Bolumar F, Olsen J, Rebagliato M, Bisanti L. Caffeine intake and delayed conception: a European multicenter study on infertility and subfecundity. European Study Group on Infertility Subfecundity. Am J Epidemiol. 1997;145(4):324–334. doi: 10.1093/oxfordjournals.aje.a009109. [DOI] [PubMed] [Google Scholar]
- 2.Christianson RE, Oechsli FW, van den Berg BJ. Caffeinated beverages and decreased fertility. Lancet. 1989;1(8634):378. doi: 10.1016/s0140-6736(89)91745-5. [DOI] [PubMed] [Google Scholar]
- 3.Hatch EE, Bracken MB. Association of delayed conception with caffeine consumption. Am J Epidemiol. 1993;138(12):1082–1092. doi: 10.1093/oxfordjournals.aje.a116826. [DOI] [PubMed] [Google Scholar]
- 4.Olsen J. Cigarette smoking, tea and coffee drinking, and subfecundity. Am J Epidemiol. 1991;133(7):734–739. doi: 10.1093/oxfordjournals.aje.a115948. [DOI] [PubMed] [Google Scholar]
- 5.Williams CJ, Fargnoli JL, Hwang JJ, van Dam RM, Blackburn GL, Hu FB, Mantzoros CS. Coffee consumption is associated with higher plasma adiponectin concentrations in women with or without type 2 diabetes: a prospective cohort study. Diabetes Care. 2008;31(3):504–507. doi: 10.2337/dc07-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alderete E, Eskenazi B, Sholtz R. Effect of cigarette smoking and coffee drinking on time to conception. Epidemiology. 1995;6(4):403–408. doi: 10.1097/00001648-199507000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Joesoef MR, Beral V, Rolfs RT, Aral SO, Cramer DW. Are caffeinated beverages risk factors for delayed conception? Lancet. 1990;335(8682):136–137. doi: 10.1016/0140-6736(90)90005-p. [DOI] [PubMed] [Google Scholar]
- 8.Curtis KM, Savitz DA, Arbuckle TE. Effects of cigarette smoking, caffeine consumption, and alcohol intake on fecundability. Am J Epidemiol. 1997;146(1):32–41. doi: 10.1093/oxfordjournals.aje.a009189. [DOI] [PubMed] [Google Scholar]
- 9.Jensen TK, Henriksen TB, Hjollund NH, Scheike T, Kolstad H, Giwercman A, Ernst E, Bonde JP, Skakkebaek NE, Olsen J. Caffeine intake and fecundability: a follow-up study among 430 Danish couples planning their first pregnancy. Reprod Toxicol. 1998;12(3):289–295. doi: 10.1016/s0890-6238(98)00002-1. [DOI] [PubMed] [Google Scholar]
- 10.Wilcox A, Weinberg C, Baird D. Caffeinated beverages and decreased fertility. Lancet. 1988;2(8626–8627):1453–1456. doi: 10.1016/s0140-6736(88)90933-6. [DOI] [PubMed] [Google Scholar]
- 11.Caan B, Quesenberry CP, Jr, Coates AO. Differences in fertility associated with caffeinated beverage consumption. Am J Public Health. 1998;88(2):270–274. doi: 10.2105/ajph.88.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Florack EI, Zielhuis GA, Rolland R. Cigarette smoking, alcohol consumption, and caffeine intake and fecundability. Prev Med. 1994;23(2):175–180. doi: 10.1006/pmed.1994.1024. [DOI] [PubMed] [Google Scholar]
- 13.Spinelli A, Figa-Talamanca I, Osborn J. Time to pregnancy and occupation in a group of Italian women. Int J Epidemiol. 1997;26(3):601–609. doi: 10.1093/ije/26.3.601. [DOI] [PubMed] [Google Scholar]
- 14.Hakim RB, Gray RH, Zacur H. Alcohol and caffeine consumption and decreased fertility. Fertil Steril. 1998;70(4):632–637. doi: 10.1016/s0015-0282(98)00257-x. [DOI] [PubMed] [Google Scholar]
- 15.Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. Caffeinated and alcoholic beverage intake in relation to ovulatory disorder infertility. Epidemiology. 2009;20(3):374–381. doi: 10.1097/EDE.0b013e31819d68cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilcox AJ, Weinberg CR. Tea and fertility. Lancet. 1991;337(8750):1159–1160. doi: 10.1016/0140-6736(91)92825-m. [DOI] [PubMed] [Google Scholar]
- 17.Benowitz NL. Clinical pharmacology of caffeine. Annu Rev Med. 1990;41:277–288. doi: 10.1146/annurev.me.41.020190.001425. [DOI] [PubMed] [Google Scholar]
- 18.Nurminen ML, Niittynen L, Korpela R, Vapaatalo H. Coffee, caffeine and blood pressure: a critical review. Eur J Clin Nutr. 1999;53(11):831–839. doi: 10.1038/sj.ejcn.1600899. [DOI] [PubMed] [Google Scholar]
- 19.Jee SH, He J, Whelton PK, Suh I, Klag MJ. The effect of chronic coffee drinking on blood pressure: a meta-analysis of controlled clinical trials. Hypertension. 1999;33(2):647–652. doi: 10.1161/01.hyp.33.2.647. [DOI] [PubMed] [Google Scholar]
- 20.Keisler BD, Armsey TD., 2nd Caffeine as an ergogenic aid. Curr Sports Med Rep. 2006;5(4):215–219. doi: 10.1097/01.csmr.0000306510.57644.a7. [DOI] [PubMed] [Google Scholar]
- 21.Silverman K, Evans SM, Strain EC, Griffiths RR. Withdrawal syndrome after the double-blind cessation of caffeine consumption. N Engl J Med. 1992;327(16):1109–1114. doi: 10.1056/NEJM199210153271601. [DOI] [PubMed] [Google Scholar]
- 22.Lucero J, Harlow BL, Barbieri RL, Sluss P, Cramer DW. Early follicular phase hormone levels in relation to patterns of alcohol, tobacco, and coffee use. Fertil Steril. 2001;76(4):723–729. doi: 10.1016/s0015-0282(01)02005-2. [DOI] [PubMed] [Google Scholar]
- 23.Goto A, Song Y, Chen BH, Manson JE, Buring JE, Liu S. Coffee and caffeine consumption in relation to sex hormone-binding globulin and risk of type 2 diabetes in postmenopausal women. Diabetes. 60(1):269–275. doi: 10.2337/db10-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotsopoulos J, Eliassen AH, Missmer SA, Hankinson SE, Tworoger SS. Relationship between caffeine intake and plasma sex hormone concentrations in premenopausal and postmenopausal women. Cancer. 2009;115(12):2765–2774. doi: 10.1002/cncr.24328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sowers MR, Crawford S, McConnell DS, Randolph JF, Jr, Gold EB, Wilkin MK, Lasley B. Selected diet and lifestyle factors are associated with estrogen metabolites in a multiracial/ethnic population of women. J Nutr. 2006;136(6):1588–1595. doi: 10.1093/jn/136.6.1588. [DOI] [PubMed] [Google Scholar]
- 26.Fenster L, Quale C, Waller K, Windham GC, Elkin EP, Benowitz N, Swan SH. Caffeine consumption and menstrual function. Am J Epidemiol. 1999;149(6):550–557. doi: 10.1093/oxfordjournals.aje.a009851. [DOI] [PubMed] [Google Scholar]
- 27.Kinney A, Kline J, Kelly A, Reuss ML, Levin B. Smoking, alcohol and caffeine in relation to ovarian age during the reproductive years. Hum Reprod. 2007;22(4):1175–1185. doi: 10.1093/humrep/del496. [DOI] [PubMed] [Google Scholar]
- 28.Ye XF, Chen SB, Wang LQ, Zhao YC, Lv XF, Liu MJ, Huang JC. Caffeine and dithiothreitol delay ovine oocyte ageing. Reprod Fertil Dev. 22(8):1254–1261. doi: 10.1071/RD10062. [DOI] [PubMed] [Google Scholar]
- 29.Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care. 33(11):2477–2483. doi: 10.2337/dc10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006;84(2):274–288. doi: 10.1093/ajcn/84.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang RJ. The reproductive phenotype in polycystic ovary syndrome. Nat Clin Pract Endocrinol Metab. 2007;3(10):688–695. doi: 10.1038/ncpendmet0637. [DOI] [PubMed] [Google Scholar]
- 32.Mikkelsen EM, Hatch EE, Wise LA, Rothman KJ, Riis A, Sorensen HT. Cohort profile: the Danish Web-based Pregnancy Planning Study--'Snart-Gravid'. Int J Epidemiol. 2009;38(4):938–943. doi: 10.1093/ije/dyn191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rothman KJ, Mikkelsen EM, Riis A, Sorensen HT, Wise LA, Hatch EE. Randomized trial of questionnaire length. Epidemiology. 2009;20(1):154. doi: 10.1097/EDE.0b013e31818f2e96. [DOI] [PubMed] [Google Scholar]
- 34.Huybrechts KF, Mikkelsen EM, Christensen T, Riis AH, Hatch EE, Wise LA, Sorensen HT, Rothman KJ. A successful implementation of e-epidemiology: the Danish pregnancy planning study 'Snart-Gravid'. Eur J Epidemiol. 25(5):297–304. doi: 10.1007/s10654-010-9431-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wise LA, Rothman KJ, Mikkelsen EM, Sorensen HT, Riis A, Hatch EE. An internet-based prospective study of body size and time-to-pregnancy. Hum Reprod. 25(1):253–264. doi: 10.1093/humrep/dep360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacobs DR, Jr, Ainsworth BE, Hartman TJ, Leon AS. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sports Exerc. 1993;25(1):81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 37.Interest CfSitP [Google Scholar]
- 38.Bunker ML, McWilliams M. Caffeine content of common beverages. J Am Diet Assoc. 1979;74(1):28–32. [PubMed] [Google Scholar]
- 39.Barone JJ, Roberts HR. Caffeine consumption. Food Chem Toxicol. 1996;34(1):119–129. doi: 10.1016/0278-6915(95)00093-3. [DOI] [PubMed] [Google Scholar]
- 40.Bech BH, Obel C, Henriksen TB, Olsen J. Effect of reducing caffeine intake on birth weight and length of gestation: randomised controlled trial. BMJ. 2007;334(7590):409. doi: 10.1136/bmj.39062.520648.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foster JT. T.. Caffeine content of UK and European Drinks. Vol. 2011. Exis Holdings, Limited; [Google Scholar]
- 42.Bracken MB, Triche E, Grosso L, Hellenbrand K, Belanger K, Leaderer BP. Heterogeneity in assessing self-reports of caffeine exposure: implications for studies of health effects. Epidemiology. 2002;13(2):165–171. doi: 10.1097/00001648-200203000-00011. [DOI] [PubMed] [Google Scholar]
- 43.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 44.Li RHE, Louie M, Chen L, Spiegelman D. The SAS PSPLINE8 Macro. Boston, MA: Channing Laboratory; 2003. [Google Scholar]
- 45.Wise LA, Rothman KJ, Mikkelsen EM, Sorensen HT, Riis A, Hatch EE. An internet-based prospective study of body size and time-to-pregnancy. Hum Reprod. 2010;25(1):253–264. doi: 10.1093/humrep/dep360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd ed. Philadelphia: Lippincott Williams and Wilkins; 2008. [Google Scholar]
- 47.Zhou XH, Eckert GJ, Tierney WM. Multiple imputation in public health research. Stat Med. 2001;20(9–10):1541–1549. doi: 10.1002/sim.689. [DOI] [PubMed] [Google Scholar]
- 48.Mok-Lin E, Ehrlich S, Williams PL, Petrozza J, Wright DL, Calafat AM, Ye X, Hauser R. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. Int J Androl. 33(2):385–393. doi: 10.1111/j.1365-2605.2009.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao XL, Corriveau J, Popovic S. Levels of bisphenol A in canned soft drink products in Canadian markets. J Agric Food Chem. 2009;57(4):1307–1311. doi: 10.1021/jf803213g. [DOI] [PubMed] [Google Scholar]
- 50.Hunter DJ, Colditz GA, Hankinson SE, Malspeis S, Spiegelman D, Chen W, Stampfer MJ, Willett WC. Oral contraceptive use and breast cancer: a prospective study of young women. Cancer Epidemiol Biomarkers Prev. 19(10):2496–2502. doi: 10.1158/1055-9965.EPI-10-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wise LA, Rosenberg L, Radin RG, Mattox C, Yang EB, Palmer JR, Seddon JM. A prospective study of diabetes, lifestyle factors, and glaucoma among African-american women. Ann Epidemiol. 21(6):430–439. doi: 10.1016/j.annepidem.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.