Abstract
BACKGROUND & AIMS
The risk of pancreatic cancer is increased in patients with a strong family history of pancreatic cancer or a predisposing germline mutation. Screening can detect curable, non-invasive pancreatic neoplasms, but the optimal imaging approach is not known. We determined the baseline prevalence and characteristics of pancreatic abnormalities using 3 imaging tests to screen asymptomatic, high-risk individuals (HRI).
METHODS
We screened 225 asymptomatic adult HRI at 5 academic US medical centers once, using computed tomography (CT), magnetic resonance imaging (MRI), and endoscopic ultrasonography (EUS). We compared results in a blinded, independent fashion.
RESULTS
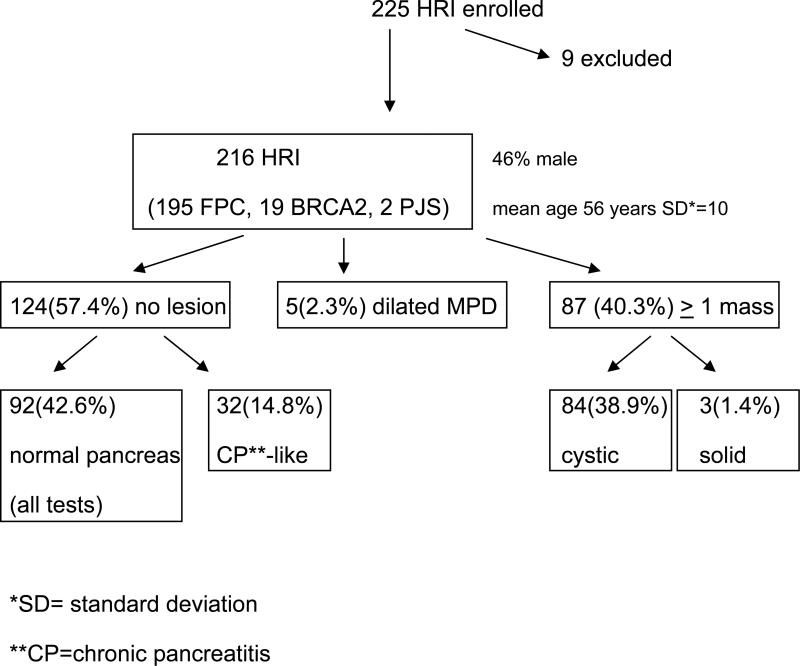
Ninety-two of 216 HRI (42%) were found to have at least 1 pancreatic mass (84 cystic, 3 solid) or a dilated pancreatic duct (n=5) by any of the imaging modalities. Fifty-one of the 84 HRI with a cyst (60.7%) had multiple lesions, typically small (mean 0.55 cm, range 2–39 mm), in multiple locations. The prevalence of pancreatic lesions increased with age; they were detected in 14% of subjects <50 years old, 34% of subjects 50–59 years old, and 53% of subjects 60–69 years old (P<.0001). CT, MRI, and EUS detected a pancreatic abnormality in 11%, 33.3%, and 42.6% of the HRI, respectively. Among these abnormalities, proven or suspected neoplasms were identified in 85 HRI (82 intraductal papillary mucinous neoplasms [IPMN] and 3 pancreatic endocrine tumors). Three of 5 HRI who underwent pancreatic resection had high-grade dysplasia in <3 cm IPMNs and in multiple intraepithelial neoplasias.
CONCLUSIONS
Screening of asymptomatic HRI frequently detects small pancreatic cysts, including curable, non-invasive high-grade neoplasms. EUS and MRI detect pancreatic lesions better than CT.
Keywords: IPMN, PanIN; surveillance; familial pancreatic cancer
INTRODUCTION
Pancreatic ductal adenocarcinoma (PC) is one of the most lethal malignancies. Mortality rates have not significantly changed for decades. Interest in improving the outcomes of PC has led to the use of imaging tests to detect asymptomatic small cancers and pre-invasive lesions in high risk individuals (HRI) with an inherited predisposition, such as Peutz-Jeghers syndrome or PJS (132-fold increased risk1), hereditary pancreatitis (26.3-fold increased risk2), familial atypical mole multiple melanoma or FAMMM (p16 mutation,13-fold increased risk3), familial breast-ovarian cancer (FBOC) with BRCA1 (breast related cancer 1, 2.3-fold increased risk4), BRCA2 (breast related cancer 2, 3.5 to 5.9-fold increased risk5-9) or PALB2 (partner and localizer of BRCA210-12) mutation, and hereditary nonpolyposis colorectal cancer or HNPCC13. Individuals from a family with a pair of affected first degree relatives (familial pancreatic cancer or FPC) also have a higher risk (6.4 to 32-fold) of PC 12, 14,12, 15, 16.
The best approach for PC screening is not yet known. To date, multidisciplinary PC screening programs have screened HRI with one or more imaging tests using different equipment and imaging protocols, with variable diagnostic yield17-20. Five U.S. academic medical centers with pancreatic tumor registries and multidisciplinary PC screening programs formed the American Cancer of the Pancreas Screening (CAPS) Consortium and developed strict criteria for identifying and enrolling HRI, consensus terminology for radiologic and EUS abnormalities, and standardized methods of performing screening tests. We determined the diagnostic yield and concordance of initial screening by MRI, CT, and EUS for pancreatic lesions in screening-naive high risk individuals and calculated the prevalence of pancreatic and extra-pancreatic neoplasms detected by one-time screening. We also describe the factors associated with prevalent pancreatic neoplasia and the radiologic and pathologic correlates of pancreatic neoplasms in asymptomatic HRI who had operative treatment.
METHODS
Study Design and Patients
This was a multicenter prospective cohort study (CAPS 3 study) performed at tertiary care medical centers on an outpatient basis. HRI at the Johns Hopkins Hospital (Baltimore, Maryland), Mayo Clinic (Rochester, Minnesota), University of California (Los Angeles, California), Dana Farber Cancer Institute (Boston, Massachusetts), and M.D. Anderson Cancer Center (Houston, Texas) were identified by each site or through various websites (CAPS 3 study, Lustgarten Foundation, and the NIH clinicaltrials.gov). This study was approved by the institutional review boards of all participating sites. All subjects provided informed consent. The study was registered on the NIH clinicaltrials.gov website (NCT00438906).
Three groups of asymptomatic HRI were studied: PJS patients, FBOC patients with at least 1 affected first- or second-degree relative with PC, and relatives of patients with FPC with at least 1 affected first-degree relative. All participants were 40-80 years old (except for PJS subjects who could be as young as 30 years old) or 10 years younger than their youngest relative with PC. The selection of age 40 for initiation of screening FPC relatives was based upon the youngest age of FPC (age 45) and the age group with the highest risk for FPC (45-64.9 years) in a large FPC registry14. The initiation of screening at age of 30 years for PJS patients was based on the average age of onset of FPC 40.8 +/- 16.2)1. The selection of the age for initiation of screening was also influenced by our goal to potentially detect noninvasive precursor lesions 21,22,23. Patients were not eligible for screening if they were unable to provide informed consent, had prior pancreas screening, Karnofsky performance status of < 60, any suspicion of pancreatic disease, prior pancreas surgery, severe medical illness, bleeding diathesis or thrombocytopenia, renal insufficiency, allergic reaction to radiographic contrast material, morbid obesity, and severe claustrophobia, and upper gastrointestinal tract obstruction.
Study Procedures
Highly experienced radiologists and gastroenterologists at the 5 tertiary academic medical centers participated in multiple conference calls and annual investigator's meetings to discuss the study protocol and promote adherence to study procedures.
MRI
Gadolinium and secretin-enhanced magnetic resonance cholangiopancreatography (MRCP) was performed according to a standardized protocol. All MRI sequences were obtained at minimum 1.5 Tesla (GE or Siemens) using a phased array torso coil. Human synthetic secretin (0.2 ug/kg, ChiRhoClin Inc, Burtonsville, Maryland) was given intravenously. T2 weighted acquisitions for the MRCP were acquired in the coronal plane. Five post secretin MRCP images were acquired every minute starting 1 minute after secretin administration. The individual slices and reconstructed maximum-intensity projections before and after secretin were used for viewing the 3D MRCP. In addition, axial breath-hold unenhanced and dynamic contrast-enhanced (0.1 mmol per kilogram of body weight) T1-weighted three-dimensional fat-suppressed spoiled gradient-echo imaging were acquired in the arterial, portal venous and delayed phase (20 seconds, 70 seconds, and 3 minutes, respectively).
CT
Standardized pancreatic protocol CT scans were done on a Siemens Somatom Flash Dual Source scanner. Patients drank 1000 cc of water over a 30 minute period prior to the scan with the last 250 cc immediately prior to the study. Intravenous contrast was injected at 4-5 cc/sec using a volume of 100-120 cc of Omnipaque-350 or Visipaque-350 depending on patient renal function and/or age. Dual phase imaging at 30 sec and 60 seconds was routinely obtained. Scan parameters were 120 kVp, body quality reference mass of 320 mAs using Care Dose and .6 mm detectors. Images were reconstructed with thin and thick sections at .75 mm every .5 mm, and 3 mm every 3 mm respectively. Images were analyzed with post processing software including axial imaging, multi-planar reconstruction and 3D rendering. The 3D reconstruction was accomplished with both volume rendering and maximum intensity projection techniques.
Endoscopy
Gastroenterologists with expertise in EUS performed sedated upper endoscopy followed by EUS imaging using both a mechanical or electronic radial (GFUM20, GFUE160-AL5, Olympus Corporation) and linear echoendoscope (CFUC140P, SSD-Alpha5 or Alpha10, Olympus Corporation). EUS was scheduled as the last screening test after CT and MRI to potentially enable fine needle aspiration for any lesions detected by any of the 3 tests. The gastroenterologists performed EUS without prior knowledge of the results of CT and MRI. After EUS imaging was completed and results recorded, the findings of the prior MRI and CT were then disclosed to the endoscopist to allow correlation of radiologic findings, and tissue sampling of any clinically relevant lesions. Fine needle aspiration was performed during the same sedation according to standard medical care guidelines at each institution, with on-site cytopathology review of specimens for adequacy. When pancreatic cysts were aspirated, specimens were also submitted for carcinoembryonic antigen (CEA) testing, if aspirated cyst fluid volume was adequate for laboratory assay (1 ml or greater). Endoscopic retrograde cholangiopancreatography (ERCP) was performed at the discretion of the clinical team to investigate ductal abnormalities.
Image Interpretation and Reporting
Participating gastroenterologists and radiologists were blinded to the results of the other imaging tests. Reporting of imaging findings was standardized across MRI, CT, and EUS using nomenclature developed at a prior 2004 CAPS consensus National Cancer Institute/SPORE-sponsored workshop (Bethesda, MD). Main pancreatic duct dilation (duct diameter > 3 mm in the head, 2 mm in the body, and 1 mm in the tail) was considered abnormal. Pancreatic ductal and parenchymal abnormalities associated with chronic pancreatitis24, 25 were noted. The final imaging diagnosis for the pancreas was given by the radiologist or gastroenterologist interpreting or performing each imaging test. The results of all imaging tests (including discrepancies) were communicated in writing to the patient by the site principal investigator. The determination of a pancreatic abnormality was made based on a positive result on any one of the 3 screening tests.
Final Diagnoses, Follow-up, Clinical Management
Patients were called by the study coordinator within 1 week from screening and seen by their primary physician/gastroenterologist as part of routine care. A follow-up clinical visit and abdominal imaging test one to three years from screening were recommended if a normal pancreas or nonspecific chronic pancreatitis-like abnormalities were seen at baseline, depending upon the patient's age and medical status according to local standard of care. Patients with lesions were managed according to the consensus of the clinical team. The final diagnosis for each screened patient was made at each site at study closure by the principal investigator based upon clinical judgement, repeat imaging, and cytology or surgical pathology.
Recommendations for treatment versus surveillance were individualized. Surgical treatment was offered for suspected prevalent pancreatic neoplasms (solid masses, suspected main duct or mixed intraductal papillary mucinous neoplasm (IPMN), branch duct IPMNs ≥ 2cm or with worrisome features for malignancy such as mural nodules), and/or abnormal cytology. If surgical resection was not imminently scheduled for these lesions, repeat imaging within 3 months was performed. Surveillance with MRI/EUS at 6-12 months was recommended for small cysts (suspected branch-duct IPMN) and other pancreatic lesions without worrisome features. When clinically appropriate, surgery was also offered for suspected extra-pancreatic neoplasms detected during screening.
Pancreatic surgery was performed by one expert pancreatico-biliary surgeon (RS) who had performed ≥ 500 pancreatic operations. One expert pathologist (RHH) provided a pathologic diagnosis using standard26 and consensus international classification systems27. Final diagnoses were made for all patients at study closure by each site's gastroenterologist based upon consensus agreement between baseline imaging tests, repeat imaging, ERCP, cytology, and pathology (when applicable) and clinical follow-up for a minimum of 1 year from baseline.
Statistical Analysis and Sample Size Estimates
The chi-square, Fisher's exact test, t test, and Wilcoxon rank sum test were performed for categorical and numerical variables, where appropriate, to compare patient characteristics. Two-tailed p values < 0.05 were considered statistically significant. The kappa coefficient, Spearman's correlation coefficient, and Lin's concordance correlation coefficient were computed to analyze the agreement in imaging test results for categorical and continuous variables (presence or absence, size and total number of detected pancreatic lesions). Univariate and multivariate logistic regression analyses were performed to evaluate possible and independent factors associated with pancreatic lesions. Analyses were performed using SAS (v. 9.2, SAS Institute, Cary, NC), R(v. 2.11.0), and Stata SE (Stata Corporation, Dallas, TX) software packages.
The study sample size was based upon the estimated population prevalence (reported prevalence 5-23%18, 28, 29) for a detectable pancreatic neoplasm in 15% of high risk individuals. A sample size of 200 would provide 30 detectable neoplasms with 95% confidence interval and precision of +/-5%.
RESULTS
Patients
Table 1 and Figure 1 summarize the characteristics of the high-risk individuals. Two hundred twenty-five asymptomatic HRI were enrolled. Nine of these were excluded from the analysis (due to prior recent screening imaging test, health reasons, failure to confirm the diagnosis of familial PC, and patient-initiated withdrawal) resulting in 216 evaluable patients (Johns Hopkins Hospital=112, Mayo Clinic=51, UCLA=16, MD Anderson Cancer Center = 20, Dana Farber Cancer Institute=17). Ninety percent of the participants were first degree relatives of patients with familial PC and the remaining were mutation carriers. The mean age of our patients was 56.1 years (range 28 – 79).
Table 1.
Patient Characteristics
| Patients without a Pancreatic Lesion N=124 | Patients with a Pancreatic Lesion N=92 | Total N=216 | |
|---|---|---|---|
| Familial PC | 110 (89%) | 85 (92%) | 195(90%) |
| 3 or more affected | 70(56%) | 50(54%) | 120 (56%) |
| 2 FDR affected | 40 (32%) | 35 (39%) | 75 (35%) |
| BRCA2 + PC relative | 13 (10%) | 6 (7%) | 19 (9%) |
| Peutz-Jeghers syndrome | 1(0.8%) | 1(1%) | 2(0.9%) |
| Age* | |||
| < 50 years | 50 (40%) | 12 (14%) | 62(29%) |
| 50-59 years | 49 (39%) | 31 (34%) | 80 (37%) |
| 60-69 years | 18 (15%) | 37 (53%) | 55 (25%) |
 70 years 70 years |
7 (6%) | 12 (13%) | 19 (8.8%) |
| Old (age > 50)* | 74(48%) | 80 (52%) | 154 (71.3%) |
| Male gender | 60 (48%) | 40 (40%) | 100 (46.4%) |
| Race and ethnicity | |||
| Non-Hispanic white | 124(98%) | 88(98%) | 212 (98%) |
| Hispanic white | 1 | 0 | 1 (0.5%) |
| Black | 0 | 1 | 2 (0.9%) |
| Native American | 0 | 1 | 1(0.5%) |
| Jewish ancestry | 16(13%) | 12 (13%) | 28 (13%) |
| Ever smoked (%) | 11(9%) | 7 (8%) | 18 (8%) |
| Type II diabetes (%) | 3(3) | 6(6%) | 9(4%) |
| Regular or heavy alcohol use | 73(58%) | 47 (52%) | 120 (55%) |
chi square, p < 0.0001
Figure 1.
Study Schema
Diagnostic Yield
The overall prevalence of a focal pancreatic abnormality (lesion) in any of the 3 screening tests was 42.6% (92/216) (Figure 1). Five of the 92 (2.3%) patients with a pancreatic lesion had an isolated dilated main pancreatic duct (mean 3.3 mm, range 2.8-3.8 mm) without an associated mass. The remaining 87 patients (40.3% of all 216 subjects) had at least one cystic (84/216 or 38.8%) or solid lesion (3/216 or 1.4%) with or without associated pancreatic duct dilation.
The majority of the pancreatic mass lesions detected were small (mean size 0.55, range 0.2-3.9 cm), and cystic (84/87 or 96%). The majority of the patients with cysts had multiple lesions (51/84 or 60.7% of those with a cyst). The median number of cysts per patient with a cyst was 2 (range 1-20). Pancreatic cysts were multifocal (found in more than one region of the pancreas) in 46 of 84 (54.7%) patients with cysts. Three patients had 4 small solid pancreatic lesions, which were not concerning for malignancy by all imaging tests and EUS-guided fine needle aspiration (FNA).
EUS-FNA was performed in 12 patients with 16 lesions (4 solid lesions, 9 cysts, 4 peripancreatic lymph nodes) with size range 0.4 to 2.1 cm. Aspirates from the peripancreatic lymph nodes uniformly showed benign lymphocytes. Aspirates from solid lesions showed 3 pancreatic neuroendocrine tumors and one benign intra-pancreatic lymph node. Aspiration of pancreatic cysts was not routinely performed due to small size and absence of mural nodules. Only 4 of 9 cyst aspirates had diagnostic cytology for a mucinous cyst. Cyst fluid CEA was not analyzed when aspirated cyst fluid volume was < 1 cc, in which case only cytologic examination was performed. Cyst fluid aspirates from 3 patients (cyst size 15-18 mm) had elevated CEA levels diagnostic for mucinous cystic neoplasm (patient 1, Table 1)
Sixty two (28.7%) of HRI had one or more extra-pancreatic lesions detected by CT or MRI. The most common incidental lesions were kidney cysts (9.4%), liver cysts (7.5%), kidney stones (2.8%), and adrenal masses (1.9%).
Factors Associated with Prevalent Pancreatic Lesions
With univariate analyses, the prevalence of a pancreatic lesion (cyst, solid mass, or dilated pancreatic duct) was significantly associated with greater age (Table 1, Fishers exact, p < 0.0001). Pancreatic lesions were more common in patients aged 50-59 years and 60-69 years, than other age groups (Fishers exact, p < 0.0001). There were no differences in the prevalence of a pancreatic lesion in baseline screening tests according to risk group (familial PC versus mutation carrier), gender, race, ethnicity, Jewish ancestry, smoking, regular alcohol consumption, and personal history of type 2 diabetes (Table 1). With multivariate logistic regression analysis, patients older than 50 years were more likely to have a pancreatic lesion (odds ratio 4.4, 95% CI 2.2-9.1, p < 0.0001) after adjusting for male gender and cigarette smoking.
Concordance of CT, MRI, and EUS Pancreatic Abnormalities
The prevalence of pancreatic abnormalities detected in HRI varied by screening modality. A comparison of the detection rate of the CT, MRI, and EUS for any pancreatic lesion according to type of lesion is provided in Table 2.
Table 2.
Comparison of CT, MRI, and EUS for Detection of Prevalent Pancreatic Lesions (Per Patient Analysis)
| Lesion Detected | CT | MRI | EUS |
|---|---|---|---|
| Any pancreatic lesion* detected (in all 216 screened subjects) | 24/216 (11%) | 72/216 (33.3%) | 92//216 (42.5%) |
| Pancreatic lesion type | |||
| Solid mass (any size) | 3/216(1.4%) | 1/216 (0.4%) | 3/216(1.4%) |
| Cystic mass (any size) | 24/216(11%) | 72/216(33%) | 79/216(36%) |
| Cyst communication with MPD | 8/24(36%) | 38/72(53%) | 21/79(27%) |
| Mural nodule | 1/24(4.2%) | 1/72(1.4%) | 3/79 (3.8%) |
| Main pancreatic duct dilation | 5/216(2.4%) | 5/216(2.4%) | 21/216(9.5%) |
| Branch duct dilation | 10/216(4.6%) | 29/216(14%) | 37/216(17.1%) |
A total of 283 cystic or solid lesions were detected in the 216 HRI by any screening modality. CT, MRI/MRCP, and EUS visualized a total of 39 (13.8%), 218 (77%), and 229 (79%) of all detected lesions, respectively. The concordance (percent agreement) for detection of any pancreatic lesion was higher between EUS and MRI (91%) than for EUS and CT (73%) (per patient analysis). There was also strong positive correlation between MRCP and EUS for the size (Spearman correlation coefficient=0.82) and moderate agreement for the number of pancreatic cystic or solid mass lesions (Lin's CCC=0.67). There was substantial agreement between EUS and MRI for the specific location (head or uncinate, body, or tail of the pancreas) of pancreatic mass lesions 1 cm and smaller (agreement 85.4%, kappa=0.79, p< 0.0001) and lesions more than 1 cm in size (agreement = 100%, kappa = 1.0).
CT, MRI, and EUS detected subcentimeter cysts in 11%, 33%, and 36% of 92 screened high risk individuals with a pancreatic lesion, respectively. Communication of cysts with the main pancreatic duct was seen by MRI in 53% of the 72 subjects with a cyst detected by MRI, which was superior to CT and EUS (Table 2). CT detected fewer suspected pancreatic cysts with size > 1 cm (n=12) (62.5%) than either MRI (90%) or EUS (100%) (p=0.007). EUS visualized subtle parenchymal and ductal abnormalities associated with chronic pancreatitis in 25% (5 or more out of 9 criteria present25) of HRI.
Both CT and EUS did not detect 5/72 (6.9%) patients with a cystic lesion seen by MRI. One of these 5 missed cysts was absent at 2 follow-up MRI examinations. The other 4 (5-8 mm) cysts located in head or tail were stable on follow-up imaging (final diagnoses 2 BD-IPMNs, 2 indeterminate cysts). In contrast, MRI and CT missed 19/92 lesions (22.3%) detected by EUS. Three of these 19 lesions were solid nodules (pancreatic neuroendocrine tumors size 1.3, 1,0, and 0.8 cm), 4 had dilated main pancreatic ducts without stricture or mass (stable on follow-up). The remaining 12 patients had cysts 2-11 mm in size (final diagnoses 3 BD-IPMNs, 9 indeterminate cysts).
Clinical and Pathologic Diagnoses
The mean follow-up time at study closure was 28.8 months (range 14-47.2). The final diagnoses for all subjects based upon clinical follow-up, repeat abdominal imaging, EUS/FNA, and/or surgery for were: confirmed or suspected branch duct IPMN (n=82), combined IPMN (n=2), pancreatic endocrine tumor (n=3), isolated main pancreatic duct dilation (n=4), indeterminate benign cyst (n=1), nonspecific chronic pancreatitis-like abnormalities (n=32), and normal pancreas (n=92). No HRI was diagnosed with an invasive malignancy within the follow-up period after baseline screening.
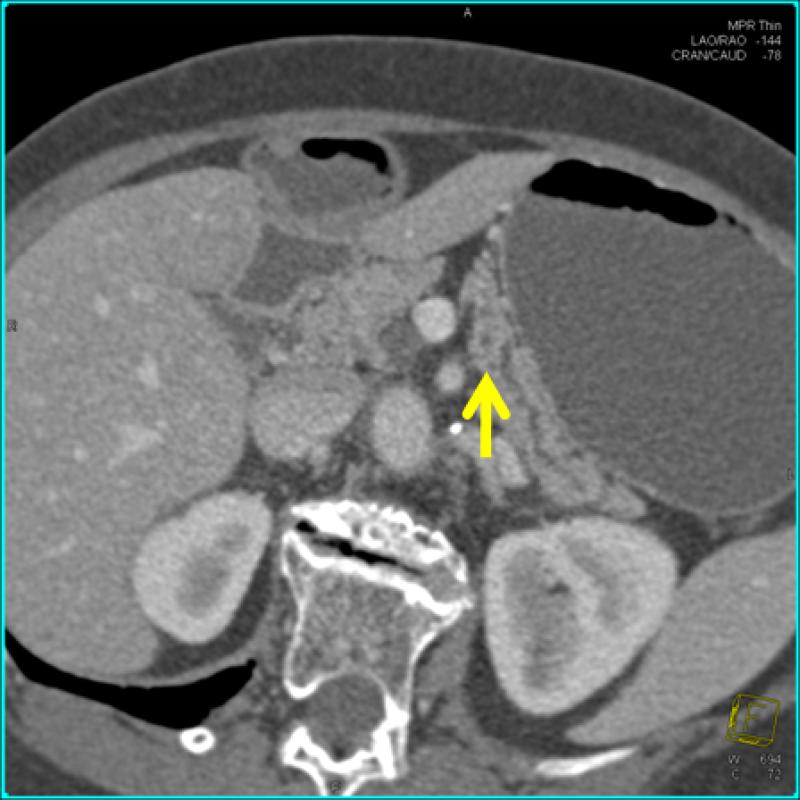
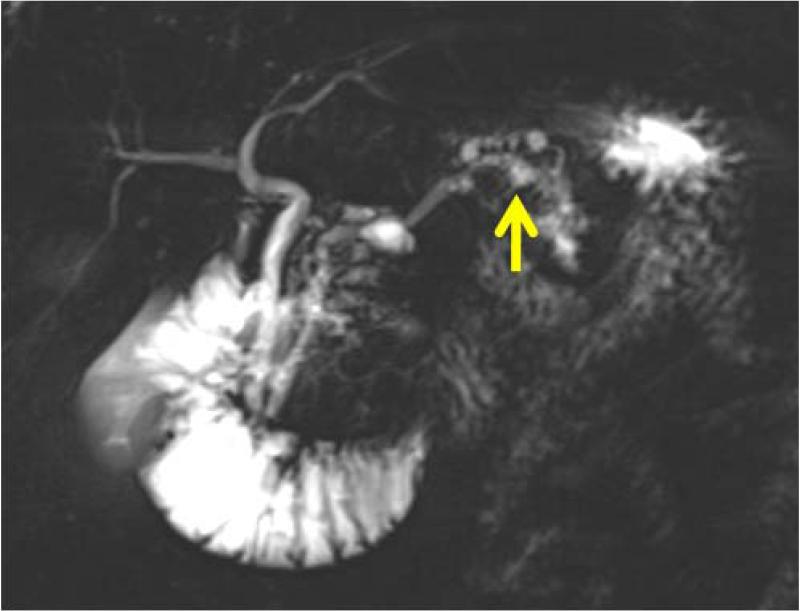
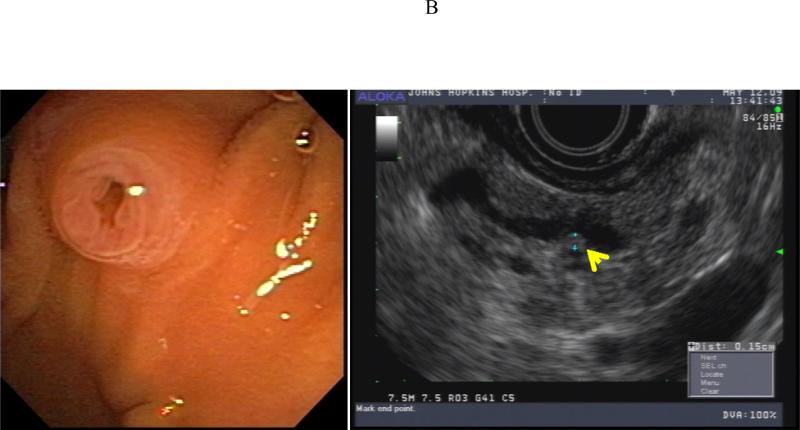
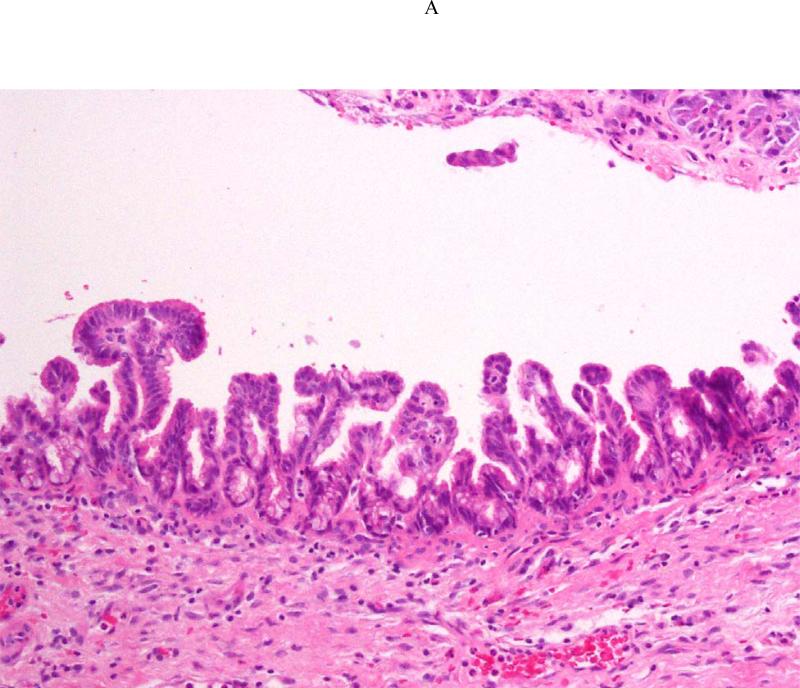
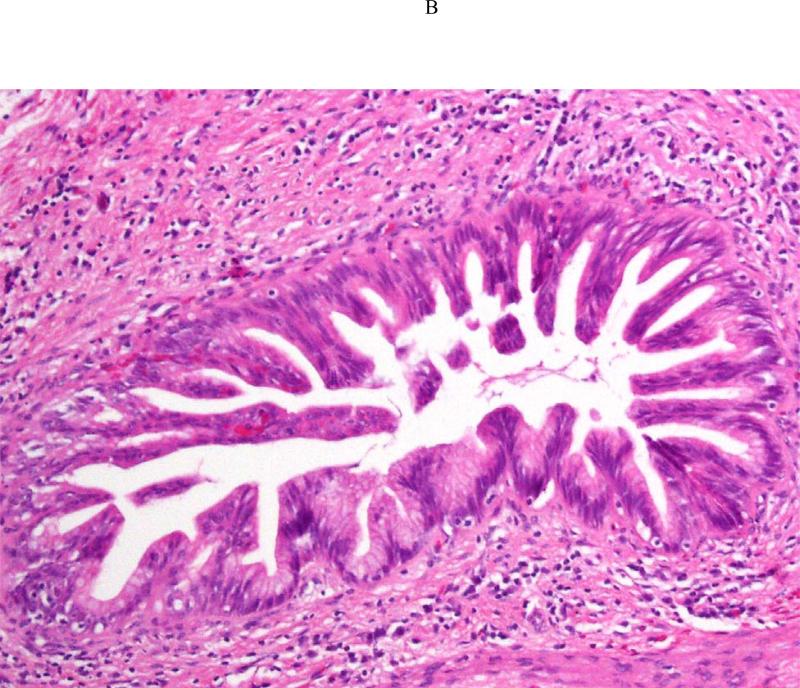
Pancreatectomy was performed on 5 HRI (1 Whipple procedure, 3 distal, 1 total) with no major adverse events. The demographic, radiologic, and pathologic findings are summarized in Table 3. All patients had multiple and multifocal pancreatic neoplasms consisting of IPMN and pancreatic intraepithelial neoplasia (PanIN27). Three of the 5 patients had high-grade dysplasia involving the main duct and/or branch ducts as well as multifocal PanINs but no invasive PC. Patient 1 exemplifies a phenotype seen in high-risk patients characterized by multifocal IPMNs and PanINs involving the main and branch ducts. The CT and MRI detected a prominent main pancreatic duct and branch duct IPMNs but were not conclusive (Figure 2A). Endoscopy + EUS correctly predicted a mixed IPMN (Figure 2B, with video 1). The resected distal pancreas showed a remarkably large 11.5 cm intestinal-type IPMN extending into the branch ducts (Figure 3A) with 95% of the main duct epithelium showing high-grade dysplasia and synchronous multiple, multifocal PanINs (highest grade PanIN-3) (Figure 3B).
Table 3.
Pancreatic Radiologic and Pathologic Findings in 5 Surgically-Treated High Risk Patients
| Patient/Age, Risk | CT | MRI/MRCP | EUS | Final Pathologic Diagnosis |
|---|---|---|---|---|
| Patient 1 73 year 2 FDR | 4 cysts (0.6-1.5 cm, non-communicating), 1 cyst with mural nodule), normal MPD Diagnosis BD-IPMN | 6 cysts (0.5-2.1 cm, 2 communicating), normal but prominent MPD, multiple dilated branch ducts Diagnosis: BD-IPMN | 3 cysts (0.5- 1.5 cm, 2 communicating), multiple non-communicating cysts, focally dilated MPD 3.8 mm with mural nodules and echogenic mucin, multiple dilated branch ducts, EUS-FNA cyst fluid CEA >1000 Diagnosis: combined IPMN | Distal pancreatectomy: MD-IPMN (7 cm body and tail, intestinal type) with extensive HGD, involving multiple branch ducts, multiple PanIN (highest grade 3) |
| Patient 2 65 years 2 FDR | 2 cysts (0.9-1.0 cm, non-communicating) Diagnosis: BD-IPMN | 2 cysts (1.7, 1.4 cm, communicating); Diagnosis: BD-IPMN | Dilated MPD 2.8 mm with 2 cysts (0.9, 1.9 cm, communicating, 1 with 5.5 mm mural nodule), multiple dilated branch ducts, Diagnosis: BD-IPMN | Whipple: MD-IPMN (1.3 cm, head and neck, intestinal type) with LGD with BD-IPMN (1.0 with LGD, 1.5 cm with MGD), multifocal PanIN (highest grade 2) |
| Patient 3 67 years 2 FDR | One 1.2 cm non-communicating cyst (body) Diagnosis: BD-IPMN | 2 communicating cysts (body 1.1-1.4 cm) Diagnosis: BD-IPMN | 6 communicating cysts (0.5-1.2 cm head, body, tail), 1 cyst with mural nodule; 1 solid mass 0.7 cm (FNA:PNET) Diagnosis: multiple BD-IPMNs, PNET | Total pancreatectomy: Multiple BD-IPMN with LGD (head, body, tail); multifocal PanIN (highest grade 3); multiple PNET (0.6-1.5-cm) |
| Patient 4 72 years 2 FDR | 1.4 cm non-communicating cyst Diagnosis: BD-IPMN | 15 cysts (0.5-1.6 cm largest communicating), multiple dilated branch ducts Diagnosis: BD-IPMN | 4 cysts (range 0.6 -1.8 cm, largest communicating), multiple dilated branch ducts Diagnosis: BD-IPMN | Distal pancreatectomy: BD-IPMN (1.9 cm, tail) with MGD, incipient IPMN + multifocal PanIN (highest grade 2) |
| Patient 5 61 years 1 FDR, 1SDR, BRCA2 FBOC | No lesion detected Diagnosis: normal pancreas | No lesion detected Diagnosis: normal pancreas | 0.5 cm non-septated communicating cyst (enlarged on follow-up to 0.9 cm) Diagnosis: BD-IPMN | Distal pancreatectomy: Incipient IPMN* (0.9 cm, tail) with LGD + multifocal PanIN (highest grade 2) |
• FDR = first degree relative
• SDR= second degree relative
• MD-IPMN = main duct intraductal papillary mucinous neoplasm
• BD-IPMN = branch duct intraductal papillary mucinous neoplasm
• HGD = high grade dysplasia
• MGD = moderate grade dysplasia
• LGD = low grade dysplasia
• FBOC= familial breast ovarian cancer syndrome
incipient IPMN = pancreatic ductal neoplastic lesions with long finger-like papillae and extensive extracellular mucin
Figure 2.
Asymptomatic prevalent combined (main duct and multiple branch duct) IPMNs in a 74 year old healthy Ashkenazi Jewish woman with 2 affected first-degree and relatives; pancreatic protocol CT (3A, right image), MRI/MRCP (2A left image), and EUS (2B, right image) all showed multiple pancreatic cysts 3-15 mm (arrows indicate suspected BD-IPMNs) and a mildly dilated main pancreatic duct up to 3.8 mm. Endoscopic examination of the ampulla at the time of EUS showed a patulous pancreatic duct orifice with active mucin extrusion (2B, left image). EUS also showed echogenic mucin and polypoid mural nodules in the main duct and multiple cysts typical of mixed IPMN (2B, right image, arrow). See video (for online submission).
Figure 3.
Histologic sections from the distal pancreatectomy performed on Patient 1. The main duct intraductal papillary neoplasm (IPMN) harbored high-grade dysplasia (Figure 3A), and the adjacent pancreatic parenchyma pancreatic intraepithelial with high-grade dysplasia (PanIN-3) (Figure 3B).
DISCUSSION
We report the first multicenter, prospective, blinded comparison of state-of-the-art clinical imaging tests for the first screening of a well-defined asymptomatic population of individuals with an increased risk for developing PC. The diagnostic yield for a pancreatic lesion (masses, cysts, or isolated dilated main pancreatic duct) was 92 of 216 HRI (42.6%). Single center studies using different approaches for imaging have reported variable diagnostic yields for one-time screening of heterogeneous groups of HRI. The prevalence of radiologic lesions in a study of American FPC relatives and mutation carriers was 16.5% (18/109) using an MRI-based screening approach30. In a Dutch study of p16-Leiden gene mutation carriers, MRI detected 3 PCs and 9 benign cysts (17.7%)31. Verna et al reported a baseline diagnostic yield for pancreatic lesions of 33% (11/33) for MRI screening and 45% (14/31) for EUS screening32, similar to our current study.
The majority of lesions in the current study were cysts with typical characteristics of branch duct IPMNs, more commonly found in older individuals. MRI and CT may detect small incidental pancreatic cysts in 2.4%33 and 2.8%34 of asymptomatic individuals in the general population, respectively. An autopsy study reported a 24.3% prevalence of pancreatic cysts35. Our study results suggest that, compared to the general population, HRI have significantly more prevalent pancreatic cysts.
If pancreatic lesions are so common among asymptomatic HRI, which ones warrant surgical resection? Most of the lesions identified by screening pancreatic imaging tests in this and other screening studies30-32, 36 were presumed BD-IPMNs based upon typical imaging characteristics. Most sporadic BD-IPMNs have a low risk for malignancy and can be safely observed if they do not meet standard internationally accepted consensus criteria for resection. These criteria include the presence of cyst features worrisome for malignancy (such as mural nodules, cyst size > 3 cm, or positive cyst fluid cytology), or a dilated main pancreatic duct of 1 cm or greater in diameter 37. Because these criteria have been validated only in patients with sporadic pancreatic cysts, it is unclear if they are appropriate for high risk individuals. Individuals with a family history of PC undergoing pancreatic resection are significantly more likely to harbor widespread PC precursor lesions (IPMNs and PanINs)38,39 than those with sporadic PC. Furthermore, these PC precursors are more likely to contain higher grades of dysplasia, than are in pancreata from patients without a family history39. We detected and treated early pancreatic neoplasms associated with PC development. Three of the five asymptomatic patients that we detected and treated had high grade neoplasia in an IPMN and/or multiple PanINs, similar to other screening studies involving HRI18, 28. Large long-term studies are needed to develop better criteria for surveillance and surgical resection of pancreatic lesions detected by screening. Such criteria should balance the morbidity of pancreas surgery with the opportunity to treat patients with a curable precursor lesion before they develop an incurable invasive cancer.
What age should we begin screening for pancreatic neoplasia in HRI? Age of onset is an important predictor of PC risk in familial kindreds15. However, despite their inherited predisposition, patients with familial PC develop their cancers at the same age as those with sporadic forms of the disease40. In the current study, we initiated screening at age 40 or 10 years younger than the youngest PC relative and found the likelihood of a prevalent pancreatic lesion to be much higher in individuals older than 50 years. Similarly, Ludwig and colleagues initiated screening at age 35 but found a higher baseline diagnostic yield in individuals older than 55 years30. Furthermore, all patients with resected high-grade neoplasia in PC precursor lesions in our study were all older than 60 years. Eleven of 14 reported prevalent screening-detected PC have been in patients 50 years or older18-20, 28, 32. Taken together, these results suggest that the age at which baseline pancreatic screening should be raised to at least 50 years, except in individuals with a relative with young-onset PC or known inherited predisposition to young-onset pancreatic cancer.
What might be an optimal method of screening individuals with an inherited increased risk for PC? We found that CT detected much fewer pancreatic lesions (mostly cysts) than MRI or EUS. Other single center studies support our conclusion that MRI and EUS are currently the best initial tests for detecting early pancreatic neoplasia18, 29-32, 36. The relative costs and effectiveness of MRI and EUS imaging for screening need further study. Coupled with the recent heightened concern about the increased risk of radiation-related cancers associated with CT, our study results suggest that routine CT using current technique might not be an optimal approach for screening HRI.
Multidisciplinary screening programs in the United States and Europe have detected 11 prevalent asymptomatic PCs (0.9-6.8%) at baseline evaluation in of 399 HRI 28,18, 30,32,31,29 and 4 incident PCs31 in 79 HRI during surveillance with CT18, MRI30,32,31, or EUS28, 29, 32. However, even with early detection, all but 4 were early stage T1N0 cancers. PC may develop from IPMNs (visualized as cysts and/or dilated ducts) or PanIN. The latter cannot be reliably identified by currently available imaging tests. Resected pancreata in this study and other studies18, 28 had high-grade PanIN that could not be preoperatively diagnosed. This observation underscores a major limitation of using pancreatic imaging as a sole screening test.
Our study has several limitations. First, we used state-of-the-art radiologic and endoscopic imaging tests read/performed by experts in the field. Second, our study focused on one-time screening and prevalent neoplasia. Third, the final diagnoses of all lesions could not be verified by surgical pathology because most were not treated. Fourth, variability in the performance of EUS and interpretation of radiologic and EUS images among multiple physicians involved in this study may have influenced our study results. Finally, each site used standard-of-care for surveillance and treatment, which might result in differential follow-up and treatment. With no evidence-based practice guidelines for recommending surgery for asymptomatic pancreatic lesions detected by screening, each site used a multidisciplinary individualized approach to make decisions about operative treatment. Compared to earlier studies18, 28,41 where high risk individuals with suspected neoplasms (benign BD-IPMNs) were routinely offered surgery, the number of surgeries and pathologically-confirmed pancreatic neoplasms in this study were relatively low.
Since nearly all patients with symptomatic invasive PC and many of those with asymptomatic PC diagnosed in screening programs die of their malignancy, we suggest that the goal of a PC screening and surveillance program should be to detect and selectively treat asymptomatic high-grade precursor neoplasms, rather than focusing screening efforts to detect invasive cancers. Progress in the understanding of the genetic alterations in PC and associated precursors and development of biomarkers might help us improve the early detection and prevention of PC in asymptomatic individuals.
Supplementary Material
Acknowledgements
The authors thank Hilary Cosby, Verna Scheeler, Antonio Almario, Traci Hammer, and Elena Stoffel for their assistance in the conduct and data management of the CAPS 3 study.
Funding and Material Support:
National Cancer Institute Specialized Program in Research Excellence (SPORE) Clinical Intervention Supplement 2 P50 CA62924, The Lustgarten Foundation for Pancreatic Cancer Research, The Michael Rolfe Foundation, Olympus Corporation, Cooke Medical, Karp Family H.H. & M. Metals, Inc. Fund for Cancer Research, ChiRhoClin
Abbreviations
- PJS
Peutz-Jeghers Syndrome
- PC
pancreatic cancer
- EUS
endoscopic ultrasonography
- FNA
fine needle aspiration
- CT
computed tomography
- ERCP
endoscopic retrograde cholangiopancreatography
- IPMN
intraductal papillary mucinous neoplasm
- BD
branch duct
- PanIN
pancreatic intraepithelial neoplasia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: All authors have no relevant potential conflicts.
Author contributions:
Marcia Irene Canto – served as project principal investigator, obtained funding, and supervised the overall conduct of the study; developed the study concept and design, assisted with the acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript
Ralph H. Hruban – assisted with obtaining grant support, assisted with the study concept and design, acquisition of data, drafting of the manuscript, drafting of the manuscript and critical revision of the manuscript
Elliot K. Fishman – assisted with acquisition of data, drafting of the manuscript
Ihab R. Kamel - assisted with acquisition of data, drafting of the manuscript
Richard Schulick - assisted with the study concept and design, assisted with acquisition of data, drafting of the manuscript
Zhe Zhang – assisted with study design and plan for statistical analysis, performed statistical analysis and interpretation of data, and assisted with manuscript preparation
Mark Topazian - assisted with the study concept and design, assisted with acquisition of data, drafting of the manuscript and critical revision of the manuscript
Naoki Takahashi - assisted with acquisition of data, drafting of the manuscript
Fletcher Joel - assisted with acquisition of data, drafting of the manuscript
Gloria Petersen - assisted with the study concept and design and obtaining grant and material support, assisted with drafting of the manuscript
Alison P. Klein - assisted with the study concept and design and obtaining grant and material support, assisted with drafting of the manuscript
Jennifer Axilbund - assisted with the study concept and design and acquisition of data
Constance Griffin - assisted with the study concept and design, acquisition of data, and drafting of the manuscript
Sapna Syngal - assisted with obtaining grant and material support, assisted with study design, acquisition of data, drafting of the manuscript, drafting of the manuscript and critical revision of the manuscript
John R. Saltzman - assisted with acquisition of data, drafting of the manuscript
Koenraad J. Mortele - assisted with acquisition of data, drafting of the manuscript
Jeffrey Lee - assisted with the study concept and design, acquisition of data, and drafting of the manuscript
Eric Tamm - assisted with acquisition of data, drafting of the manuscript
Raghunandan Vikram - assisted with acquisition of data, drafting of the manuscript
Priya Bhosale - assisted with acquisition of data
Daniel Margolis - acquisition of data, drafting of the manuscript, drafting of the manuscript and critical revision of the manuscript
James Farrell - assisted with obtaining grant support, assisted with the study design, acquisition of data, drafting of the manuscript, drafting of the manuscript
Michael Goggins - assisted with obtaining grant and material support, assisted with the study concept and design, acquisition of data, drafting of the manuscript, drafting of the manuscript and critical revision of the manuscript
REFERENCES
- 1.Giardiello FM, Brensinger JD, Tersmette AC, Goodman SN, Petersen GM, Booker SV, Cruz-Correa M, Offerhaus JA. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology. 2000;119:1447–53. doi: 10.1053/gast.2000.20228. [DOI] [PubMed] [Google Scholar]
- 2.Lowenfels AB, Maisonneuve P, Cavallini G, Ammann RW, Lankisch PG, Andersen JR, Dimagno EP, Andren-Sandberg A, Domellof L. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328:1433–7. doi: 10.1056/NEJM199305203282001. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein AM, Fraser MC, Struewing JP, Hussussian CJ, Ranade K, Zametkin DP, Fontaine LS, Organic SM, Dracopoli NC, Clark WH, et al. Increased risk of pancreatic cancer in melanoma-prone kindreds with p16INK4 mutations. N Engl J Med. 1995;333:970–4. doi: 10.1056/NEJM199510123331504. [DOI] [PubMed] [Google Scholar]
- 4.Thompson D, Easton DF. Cancer Incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002;94:1358–65. doi: 10.1093/jnci/94.18.1358. [DOI] [PubMed] [Google Scholar]
- 5.Hahn SA, Greenhalf B, Ellis I, Sina-Frey M, Rieder H, Korte B, Gerdes B, Kress R, Ziegler A, Raeburn JA, Campra D, Grutzmann R, Rehder H, Rothmund M, Schmiegel W, Neoptolemos JP, Bartsch DK. BRCA2 germline mutations in familial pancreatic carcinoma. J Natl Cancer Inst. 2003;95:214–21. doi: 10.1093/jnci/95.3.214. [DOI] [PubMed] [Google Scholar]
- 6.Cancer risks in BRCA2 mutation carriers The Breast Cancer Linkage Consortium. J Natl Cancer Inst. 1999;91:1310–6. doi: 10.1093/jnci/91.15.1310. [DOI] [PubMed] [Google Scholar]
- 7.Couch FJ, Johnson MR, Rabe KG, Brune K, de Andrade M, Goggins M, Rothenmund H, Gallinger S, Klein A, Petersen GM, Hruban RH. The prevalence of BRCA2 mutations in familial pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:342–6. doi: 10.1158/1055-9965.EPI-06-0783. [DOI] [PubMed] [Google Scholar]
- 8.Lal G, Liu G, Schmocker B, Kaurah P, Ozcelik H, Narod SA, Redston M, Gallinger S. Inherited predisposition to pancreatic adenocarcinoma: role of family history and germline p16, BRCA1, and BRCA2 mutations. Cancer Res. 2000;60:409–16. [PubMed] [Google Scholar]
- 9.van Asperen CJ, Brohet RM, Meijers-Heijboer EJ, Hoogerbrugge N, Verhoef S, Vasen HF, Ausems MG, Menko FH, Gomez Garcia EB, Klijn JG, Hogervorst FB, van Houwelingen JC, van't Veer LJ, Rookus MA, van Leeuwen FE. Cancer risks in BRCA2 families: estimates for sites other than breast and ovary. J Med Genet. 2005;42:711–9. doi: 10.1136/jmg.2004.028829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tischkowitz MD, Sabbaghian N, Hamel N, Borgida A, Rosner C, Taherian N, Srivastava A, Holter S, Rothenmund H, Ghadirian P, Foulkes WD, Gallinger S. Analysis of the gene coding for the BRCA2-interacting protein PALB2 in familial and sporadic pancreatic cancer. Gastroenterology. 2009;137:1183–6. doi: 10.1053/j.gastro.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slater EP, Langer P, Niemczyk E, Strauch K, Butler J, Habbe N, Neoptolemos JP, Greenhalf W, Bartsch DK. PALB2 mutations in European familial pancreatic cancer families. Clin Genet. 2010;78:490–4. doi: 10.1111/j.1399-0004.2010.01425.x. [DOI] [PubMed] [Google Scholar]
- 12.Jones S, Hruban RH, Kamiyama M, Borges M, Zhang X, Parsons DW, Lin JC, Palmisano E, Brune K, Jaffee EM, Iacobuzio-Donahue CA, Maitra A, Parmigiani G, Kern SE, Velculescu VE, Kinzler KW, Vogelstein B, Eshleman JR, Goggins M, Klein AP. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science. 2009;324:217. doi: 10.1126/science.1171202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kastrinos F, Mukherjee B, Tayob N, Wang F, Sparr J, Raymond VM, Bandipalliam P, Stoffel EM, Gruber SB, Syngal S. Risk of pancreatic cancer in families with Lynch syndrome. Jama. 2009;302:1790–5. doi: 10.1001/jama.2009.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein AP, Brune KA, Petersen GM, Goggins M, Tersmette AC, Offerhaus GJ, Griffin C, Cameron JL, Yeo CJ, Kern S, Hruban RH. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004;64:2634–8. doi: 10.1158/0008-5472.can-03-3823. [DOI] [PubMed] [Google Scholar]
- 15.Brune KA, Lau B, Palmisano E, Canto M, Goggins MG, Hruban RH, Klein AP. Importance of age of onset in pancreatic cancer kindreds. J Natl Cancer Inst. 2010;102:119–26. doi: 10.1093/jnci/djp466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slater EP, Langer P, Niemczyk E, Strauch K, Butler J, Habbe N, Neoptolemos J, Greenhalf W, Bartsch DK. PALB2 mutations in European familial pancreatic cancer families. Clin Genet. doi: 10.1111/j.1399-0004.2010.01425.x. [DOI] [PubMed] [Google Scholar]
- 17.Canto M, Goggins M, Yeo C, Griffin C, Axilbund JE, Brune K, Ali SZ, Jagannath S, Petersen GM, Fishman EK, M.Giardiello F, Hruban RH. Screening for pancreatic neoplasia in high-risk individuals. Clin Gastro Hepatol. 2004 doi: 10.1016/s1542-3565(04)00244-7. [DOI] [PubMed] [Google Scholar]
- 18.Canto MI, Goggins M, Hruban RH, Petersen GM, Giardiello FM, Yeo C, Fishman EK, Brune K, Axilbund J, Griffin C, Ali S, Richman J, Jagannath S, Kantsevoy SV, Kalloo AN. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol. 2006;4:766–81. doi: 10.1016/j.cgh.2006.02.005. quiz 665. [DOI] [PubMed] [Google Scholar]
- 19.Poley JW, Kluijt I, Gouma DJ, Harinck F, Wagner A, Aalfs C, van Eijck CH, Cats A, Kuipers EJ, Nio Y, Fockens P, Bruno MJ. The Yield of First-Time Endoscopic Ultrasonography in Screening Individuals at a High Risk of Developing Pancreatic Cancer. Am J Gastroenterol. 2009 doi: 10.1038/ajg.2009.276. [DOI] [PubMed] [Google Scholar]
- 20.Vasen HF, Wasser M, van Mil A, Tollenaar RA, Konstantinovski M, Gruis NA, Bergman W, Hes FJ, Hommes DW, Offerhaus GJ, Morreau H, Bonsing BA, de Vos Tot Nederveen Cappel WH. Magnetic Resonance Imaging Surveillance Detects Early-Stage Pancreatic Cancer in Carriers of a p16-Leiden Mutation. Gastroenterology. 2010 doi: 10.1053/j.gastro.2010.11.048. [DOI] [PubMed] [Google Scholar]
- 21.Sohn TA, Yeo CJ, Cameron JL, Hruban RH, Fukushima N, Campbell KA, Lillemoe KD. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239:788–97. doi: 10.1097/01.sla.0000128306.90650.aa. discussion 797-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salvia R, Fernandez-del Castillo C, Bassi C, Thayer SP, Falconi M, Mantovani W, Pederzoli P, Warshaw AL. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239:678–85. doi: 10.1097/01.sla.0000124386.54496.15. discussion 685-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamao K, Ohashi K, Nakamura T, Suzuki T, Shimizu Y, Nakamura Y, Horibe Y, Yanagisawa A, Nakao A, Nimuara Y, Naito Y, Hayakawa T. The prognosis of intraductal papillary mucinous tumors of the pancreas. Hepatogastroenterology. 2000;47:1129–34. [PubMed] [Google Scholar]
- 24.Hollerbach S, Klamann A, Topalidis T, Schmiegel WH. Endoscopic ultrasonography (EUS) and fine-needle aspiration (FNA) cytology for diagnosis of chronic pancreatitis. Endoscopy. 2001;33:824–31. doi: 10.1055/s-2001-17337. [DOI] [PubMed] [Google Scholar]
- 25.Sahai AV, Zimmerman M, Aabakken L, Tarnasky PR, Cunningham JT, van Velse A, Hawes RH, Hoffman BJ. Prospective assessment of the ability of endoscopic ultrasound to diagnose, exclude, or establish the severity of chronic pancreatitis found by endoscopic retrograde cholangiopancreatography. Gastrointest Endosc. 1998;48:18–25. doi: 10.1016/s0016-5107(98)70123-3. [DOI] [PubMed] [Google Scholar]
- 26.Hruban R, Pitman M, Klimstra D. Tumors of the pancreas. Armed Forces Institute of Pathology. 2007 [Google Scholar]
- 27.Hruban RH, Takaori K, Klimstra DS, Adsay NV, Albores-Saavedra J, Biankin AV, Biankin SA, Compton C, Fukushima N, Furukawa T, Goggins M, Kato Y, Kloppel G, Longnecker DS, Luttges J, Maitra A, Offerhaus GJ, Shimizu M, Yonezawa S. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977–87. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- 28.Canto MI, Goggins M, Yeo CJ, Griffin C, Axilbund JE, Brune K, Ali SZ, Jagannath S, Petersen GM, Fishman EK, Piantadosi S, Giardiello FM, Hruban RH. Screening for pancreatic neoplasia in high-risk individuals: an EUS-based approach. Clin Gastroenterol Hepatol. 2004;2:606–21. doi: 10.1016/s1542-3565(04)00244-7. [DOI] [PubMed] [Google Scholar]
- 29.Poley JW, Kluijt I, Gouma DJ, Harinck F, Wagner A, Aalfs C, van Eijck CH, Cats A, Kuipers EJ, Nio Y, Fockens P, Bruno MJ. The yield of first-time endoscopic ultrasonography in screening individuals at a high risk of developing pancreatic cancer. Am J Gastroenterol. 2009;104:2175–81. doi: 10.1038/ajg.2009.276. [DOI] [PubMed] [Google Scholar]
- 30.Ludwig E, Olson SH, Bayuga S, Simon J, Schattner MA, Gerdes H, Allen PJ, Jarnagin WR, Kurtz RC. Feasibility and yield of screening in relatives from familial pancreatic cancer families. Am J Gastroenterol. 2011;106:946–54. doi: 10.1038/ajg.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vasen HF, Wasser M, van Mil A, Tollenaar RA, Konstantinovski M, Gruis NA, Bergman W, Hes FJ, Hommes DW, Offerhaus GJ, Morreau H, Bonsing BA, de Vos tot Nederveen Cappel WH. Magnetic resonance imaging surveillance detects early-stage pancreatic cancer in carriers of a p16-Leiden mutation. Gastroenterology. 2011;140:850–6. doi: 10.1053/j.gastro.2010.11.048. [DOI] [PubMed] [Google Scholar]
- 32.Verna EC, Hwang C, Stevens PD, Rotterdam H, Stavropoulos SN, Sy CD, Prince MA, Chung WK, Fine RL, Chabot JA, Frucht H. Pancreatic Cancer Screening in a Prospective Cohort of High-Risk Patients: A Comprehensive Strategy of Imaging and Genetics. Clin Cancer Res. 2010 doi: 10.1158/1078-0432.CCR-09-3209. [DOI] [PubMed] [Google Scholar]
- 33.de Jong K, Nio CY, Hermans JJ, Dijkgraaf MG, Gouma DJ, van Eijck CH, van Heel E, Klass G, Fockens P, Bruno MJ. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol. 2010;8:806–11. doi: 10.1016/j.cgh.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 34.Laffan TA, Horton KM, Klein AP, Berlanstein B, Siegelman SS, Kawamoto S, Johnson PT, Fishman EK, Hruban RH. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802–7. doi: 10.2214/AJR.07.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimura W, Nagai H, Kuroda A, Muto T, Esaki Y. Analysis of small cystic lesions of the pancreas. Int J Pancreatol. 1995;18:197–206. doi: 10.1007/BF02784942. [DOI] [PubMed] [Google Scholar]
- 36.Langer P, Kann PH, Fendrich V, Habbe N, Schneider M, Sina M, Slater EP, Heverhagen JT, Gress TM, Rothmund M, Bartsch DK. Five years of prospective screening of high-risk individuals from families with familial pancreatic cancer. Gut. 2009;58:1410–8. doi: 10.1136/gut.2008.171611. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 38.Brune K, Abe T, Canto M, O'Malley L, Klein AP, Maitra A, Volkan Adsay N, Fishman EK, Cameron JL, Yeo CJ, Kern SE, Goggins M, Hruban RH. Multifocal neoplastic precursor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. Am J Surg Pathol. 2006;30:1067–76. [PMC free article] [PubMed] [Google Scholar]
- 39.Shi C, Klein AP, Goggins M, Maitra A, Canto M, Ali S, Schulick R, Palmisano E, Hruban RH. Increased Prevalence of Precursor Lesions in Familial Pancreatic Cancer Patients. Clin Cancer Res. 2009;15:7737–7743. doi: 10.1158/1078-0432.CCR-09-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petersen GM, de Andrade M, Goggins M, Hruban RH, Bondy M, Korczak JF, Gallinger S, Lynch HT, Syngal S, Rabe KG, Seminara D, Klein AP. Pancreatic cancer genetic epidemiology consortium. Cancer Epidemiol Biomarkers Prev. 2006;15:704–10. doi: 10.1158/1055-9965.EPI-05-0734. [DOI] [PubMed] [Google Scholar]
- 41.Brentnall TA, Bronner MP, Byrd DR, Haggitt RC, Kimmey MB. Early diagnosis and treatment of pancreatic dysplasia in patients with a family history of pancreatic cancer. Ann Intern Med. 1999;131:247–55. doi: 10.7326/0003-4819-131-4-199908170-00003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.