Abstract
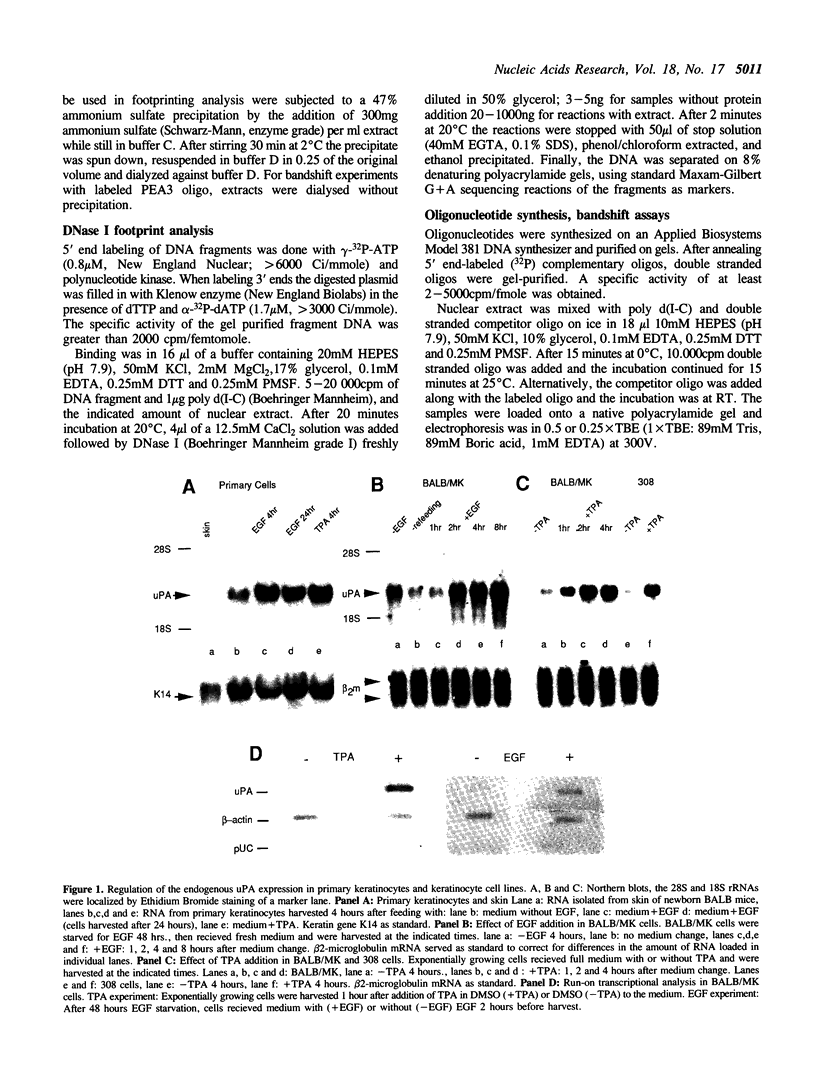
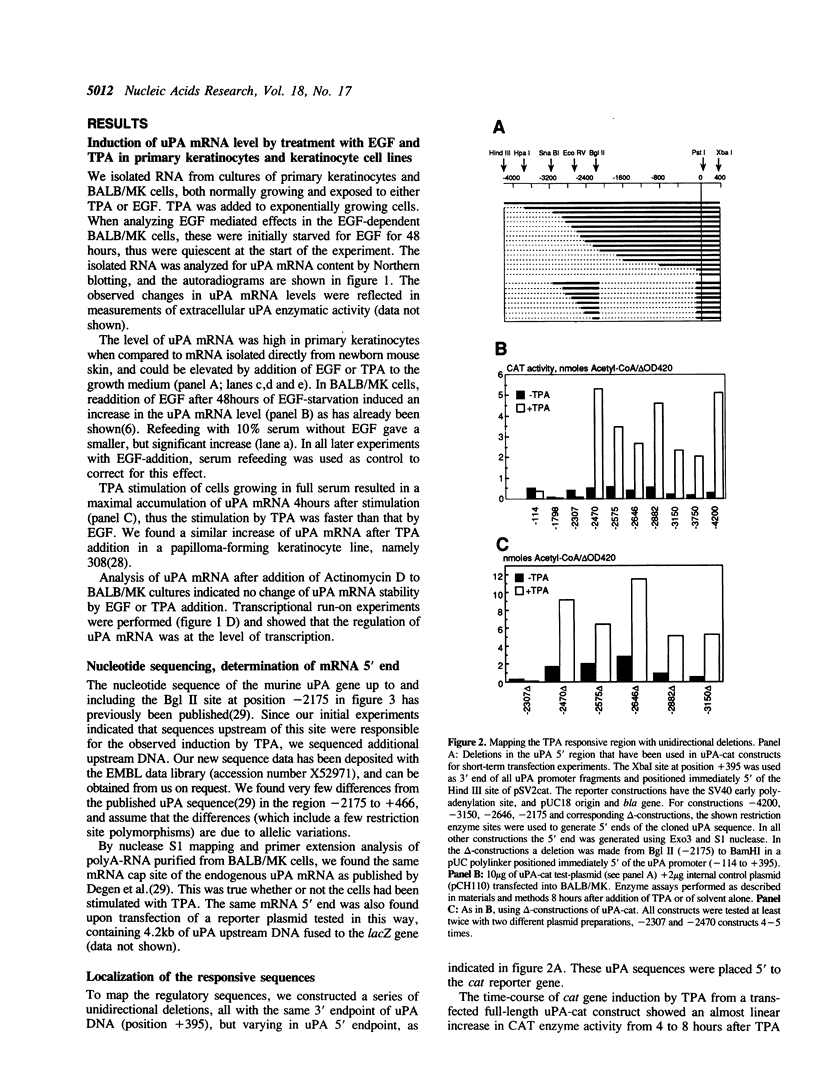
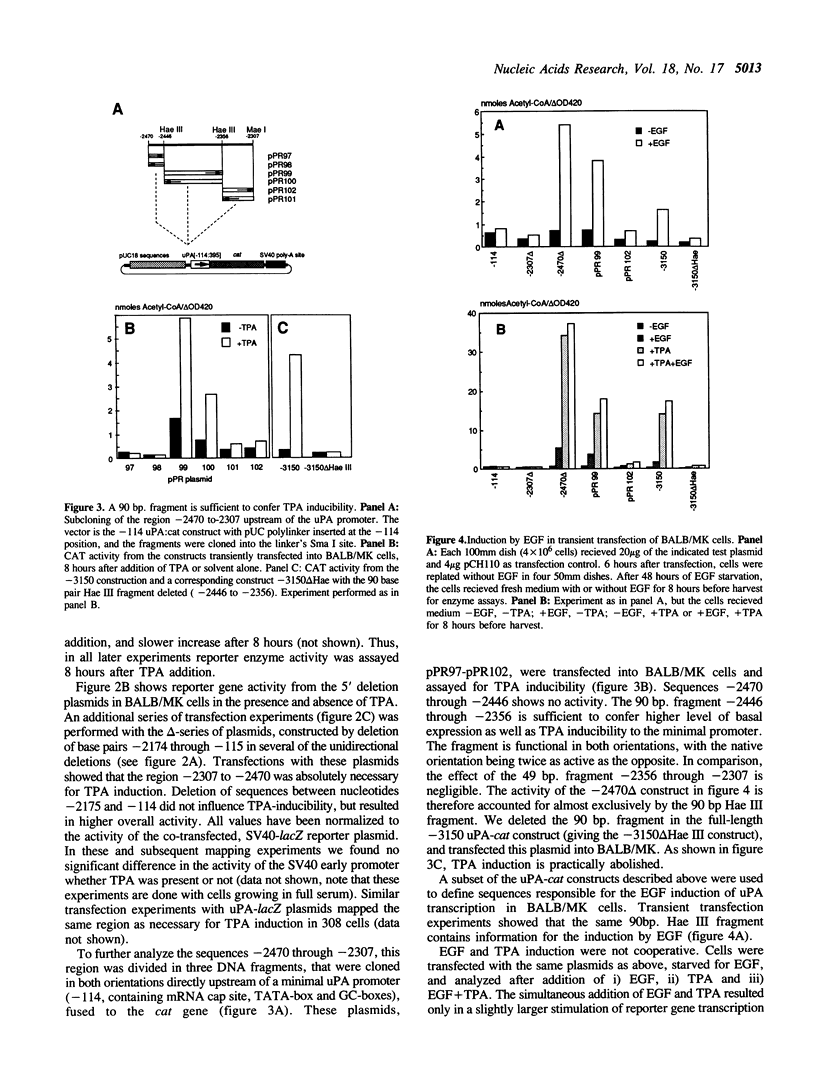
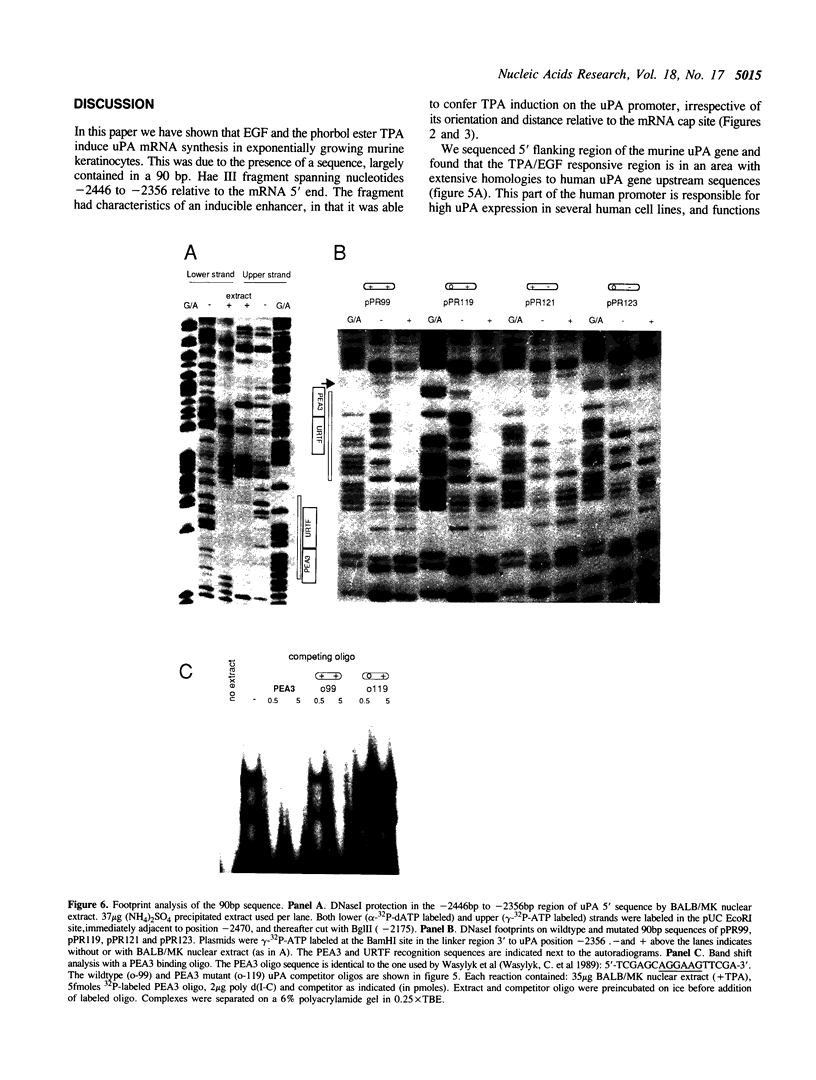
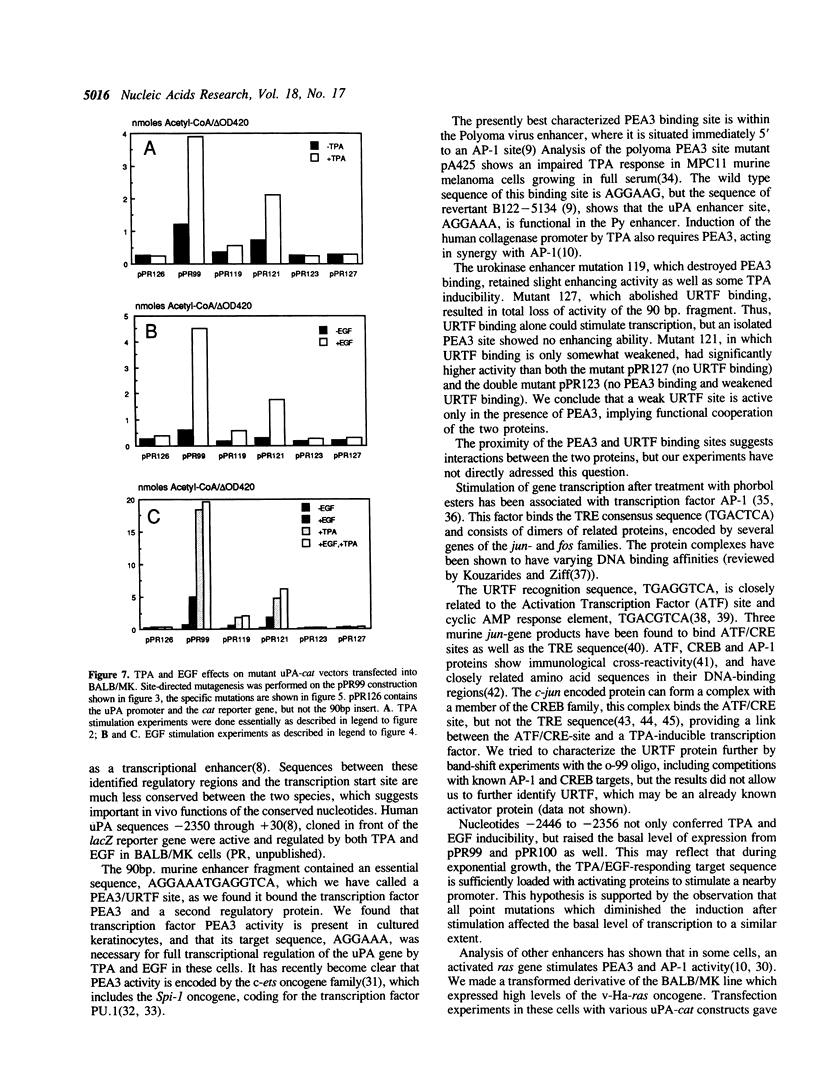
Keratinocytes in culture represent cells which exhibit continued and controlled growth in the organism. We have investigated the synthesis of urokinase plasminogen activator mRNA in exponentially growing cultures of primary murine keratinocytes and the keratinocyte cell line BALB/MK. The tumor promotor 12-O-tetradecanoyl phorbol-13-acetate (TPA) and epidermal growth factor (EGF) induced urokinase mRNA synthesis. We made a series of progressive 5' deletions as well as internal deletions in the region upstream of the murine uPA gene. These were joined to the cat reporter gene, and used to map the TPA and EGF responsive regions of the promoter. We found both responsive sequences within a 90 base pair Hae III fragment, located 2.4 kb. upstream of the mRNA cap site. This DNA fragment conferred TPA inducibility on reporter gene expression independent of its distance and orientation to the transcription initiation site. Footprinting and gel retardation studies identified the responsible sequence to be a binding site for PEA3 juxtaposed to an octameric TRE-element. Transfections with point mutants showed that these target sequences were necessary for TPA and EGF induction of transcription.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Barrandon Y., Green H. Cell migration is essential for sustained growth of keratinocyte colonies: the roles of transforming growth factor-alpha and epidermal growth factor. Cell. 1987 Sep 25;50(7):1131–1137. doi: 10.1016/0092-8674(87)90179-6. [DOI] [PubMed] [Google Scholar]
- Belin D., Vassalli J. D., Combépine C., Godeau F., Nagamine Y., Reich E., Kocher H. P., Duvoisin R. M. Cloning, nucleotide sequencing and expression of cDNAs encoding mouse urokinase-type plasminogen activator. Eur J Biochem. 1985 Apr 15;148(2):225–232. doi: 10.1111/j.1432-1033.1985.tb08829.x. [DOI] [PubMed] [Google Scholar]
- Benbrook D. M., Jones N. C. Heterodimer formation between CREB and JUN proteins. Oncogene. 1990 Mar;5(3):295–302. [PubMed] [Google Scholar]
- Blasi F., Verde P. Urokinase-dependent cell surface proteolysis and cancer. Semin Cancer Biol. 1990 Apr;1(2):117–126. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chu G., Hayakawa H., Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987 Feb 11;15(3):1311–1326. doi: 10.1093/nar/15.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Danø K., Andreasen P. A., Grøndahl-Hansen J., Kristensen P., Nielsen L. S., Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- Degen S. J., Heckel J. L., Reich E., Degen J. L. The murine urokinase-type plasminogen activator gene. Biochemistry. 1987 Dec 15;26(25):8270–8279. doi: 10.1021/bi00399a038. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebl M. K. The PU.1 transcription factor is the product of the putative oncogene Spi-1. Cell. 1990 Jun 29;61(7):1165–1166. doi: 10.1016/0092-8674(90)90676-6. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Grimaldi G., Di Fiore P., Locatelli E. K., Falco J., Blasi F. Modulation of urokinase plasminogen activator gene expression during the transition from quiescent to proliferative state in normal mouse cells. EMBO J. 1986 May;5(5):855–861. doi: 10.1002/j.1460-2075.1986.tb04295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grøndahl-Hansen J., Lund L. R., Ralfkiaer E., Ottevanger V., Danø K. Urokinase- and tissue-type plasminogen activators in keratinocytes during wound reepithelialization in vivo. J Invest Dermatol. 1988 Jun;90(6):790–795. doi: 10.1111/1523-1747.ep12461511. [DOI] [PubMed] [Google Scholar]
- Gutman A., Wasylyk B. The collagenase gene promoter contains a TPA and oncogene-responsive unit encompassing the PEA3 and AP-1 binding sites. EMBO J. 1990 Jul;9(7):2241–2246. doi: 10.1002/j.1460-2075.1990.tb07394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai T. W., Liu F., Allegretto E. A., Karin M., Green M. R. A family of immunologically related transcription factors that includes multiple forms of ATF and AP-1. Genes Dev. 1988 Oct;2(10):1216–1226. doi: 10.1101/gad.2.10.1216. [DOI] [PubMed] [Google Scholar]
- Hai T. W., Liu F., Coukos W. J., Green M. R. Transcription factor ATF cDNA clones: an extensive family of leucine zipper proteins able to selectively form DNA-binding heterodimers. Genes Dev. 1989 Dec;3(12B):2083–2090. doi: 10.1101/gad.3.12b.2083. [DOI] [PubMed] [Google Scholar]
- Hall C. V., Jacob P. E., Ringold G. M., Lee F. Expression and regulation of Escherichia coli lacZ gene fusions in mammalian cells. J Mol Appl Genet. 1983;2(1):101–109. [PubMed] [Google Scholar]
- Harper J. R., Greenhalgh D. A., Yuspa S. H. Expression of transfected DNA by primary murine keratinocytes. J Invest Dermatol. 1988 Aug;91(2):150–153. doi: 10.1111/1523-1747.ep12464396. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Imler J. L., Schatz C., Wasylyk C., Chatton B., Wasylyk B. A Harvey-ras responsive transcription element is also responsive to a tumour-promoter and to serum. Nature. 1988 Mar 17;332(6161):275–278. doi: 10.1038/332275a0. [DOI] [PubMed] [Google Scholar]
- Isseroff R. R., Rifkin D. B. Plasminogen is present in the basal layer of the epidermis. J Invest Dermatol. 1983 Apr;80(4):297–299. doi: 10.1111/1523-1747.ep12534677. [DOI] [PubMed] [Google Scholar]
- Ivashkiv L. B., Liou H. C., Kara C. J., Lamph W. W., Verma I. M., Glimcher L. H. mXBP/CRE-BP2 and c-Jun form a complex which binds to the cyclic AMP, but not to the 12-O-tetradecanoylphorbol-13-acetate, response element. Mol Cell Biol. 1990 Apr;10(4):1609–1621. doi: 10.1128/mcb.10.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T., Ziff E. Behind the Fos and Jun leucine zipper. Cancer Cells. 1989 Nov;1(3):71–76. [PubMed] [Google Scholar]
- Krieg T. M., Schafer M. P., Cheng C. K., Filpula D., Flaherty P., Steinert P. M., Roop D. R. Organization of a type I keratin gene. Evidence for evolution of intermediate filaments from a common ancestral gene. J Biol Chem. 1985 May 25;260(10):5867–5870. [PubMed] [Google Scholar]
- Larsson L. I., Skriver L., Nielsen L. S., Grøndahl-Hansen J., Kristensen P., Danø K. Distribution of urokinase-type plasminogen activator immunoreactivity in the mouse. J Cell Biol. 1984 Mar;98(3):894–903. doi: 10.1083/jcb.98.3.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Lin Y. S., Green M. R. Interaction of a common cellular transcription factor, ATF, with regulatory elements in both E1a- and cyclic AMP-inducible promoters. Proc Natl Acad Sci U S A. 1988 May;85(10):3396–3400. doi: 10.1073/pnas.85.10.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macgregor P. F., Abate C., Curran T. Direct cloning of leucine zipper proteins: Jun binds cooperatively to the CRE with CRE-BP1. Oncogene. 1990 Apr;5(4):451–458. [PubMed] [Google Scholar]
- Martin M. E., Piette J., Yaniv M., Tang W. J., Folk W. R. Activation of the polyomavirus enhancer by a murine activator protein 1 (AP1) homolog and two contiguous proteins. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5839–5843. doi: 10.1073/pnas.85.16.5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montminy M. R., Sevarino K. A., Wagner J. A., Mandel G., Goodman R. H. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6682–6686. doi: 10.1073/pnas.83.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morioka S., Lazarus G. S., Baird J. L., Jensen P. J. Migrating keratinocytes express urokinase-type plasminogen activator. J Invest Dermatol. 1987 Apr;88(4):418–423. doi: 10.1111/1523-1747.ep12469754. [DOI] [PubMed] [Google Scholar]
- Moscat J., Molloy C. J., Fleming T. P., Aaronson S. A. Epidermal growth factor activates phosphoinositide turnover and protein kinase C in BALB/MK keratinocytes. Mol Endocrinol. 1988 Sep;2(9):799–805. doi: 10.1210/mend-2-9-799. [DOI] [PubMed] [Google Scholar]
- Nakabeppu Y., Ryder K., Nathans D. DNA binding activities of three murine Jun proteins: stimulation by Fos. Cell. 1988 Dec 2;55(5):907–915. doi: 10.1016/0092-8674(88)90146-8. [DOI] [PubMed] [Google Scholar]
- Parnes J. R., Seidman J. G. Structure of wild-type and mutant mouse beta 2-microglobulin genes. Cell. 1982 Jun;29(2):661–669. doi: 10.1016/0092-8674(82)90182-9. [DOI] [PubMed] [Google Scholar]
- Sistonen L., Hölttä E., Mäkelä T. P., Keski-Oja J., Alitalo K. The cellular response to induction of the p21 c-Ha-ras oncoprotein includes stimulation of jun gene expression. EMBO J. 1989 Mar;8(3):815–822. doi: 10.1002/j.1460-2075.1989.tb03442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppelli M. P., Verde P., Grimaldi G., Locatelli E. K., Blasi F. Increase in urokinase plasminogen activator mRNA synthesis in human carcinoma cells is a primary effect of the potent tumor promoter, phorbol myristate acetate. J Cell Biol. 1986 Apr;102(4):1235–1241. doi: 10.1083/jcb.102.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde P., Boast S., Franzè A., Robbiati F., Blasi F. An upstream enhancer and a negative element in the 5' flanking region of the human urokinase plasminogen activator gene. Nucleic Acids Res. 1988 Nov 25;16(22):10699–10716. doi: 10.1093/nar/16.22.10699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk B., Wasylyk C., Flores P., Begue A., Leprince D., Stehelin D. The c-ets proto-oncogenes encode transcription factors that cooperate with c-Fos and c-Jun for transcriptional activation. Nature. 1990 Jul 12;346(6280):191–193. doi: 10.1038/346191a0. [DOI] [PubMed] [Google Scholar]
- Wasylyk C., Flores P., Gutman A., Wasylyk B. PEA3 is a nuclear target for transcription activation by non-nuclear oncogenes. EMBO J. 1989 Nov;8(11):3371–3378. doi: 10.1002/j.1460-2075.1989.tb08500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. A., Eichner R., Sun T. T. Monoclonal antibody analysis of keratin expression in epidermal diseases: a 48- and 56-kdalton keratin as molecular markers for hyperproliferative keratinocytes. J Cell Biol. 1984 Apr;98(4):1397–1406. doi: 10.1083/jcb.98.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman B. E., Aaronson S. A. BALB and Kirsten murine sarcoma viruses alter growth and differentiation of EGF-dependent balb/c mouse epidermal keratinocyte lines. Cell. 1983 Feb;32(2):599–606. doi: 10.1016/0092-8674(83)90479-8. [DOI] [PubMed] [Google Scholar]
- Yuspa S. H., Harris C. C. Altered differentiation of mouse epidermal cells treated with retinyl acetate in vitro. Exp Cell Res. 1974 May;86(1):95–105. doi: 10.1016/0014-4827(74)90653-3. [DOI] [PubMed] [Google Scholar]
- Yuspa S. H., Koehler B., Kulesz-Martin M., Hennings H. Clonal growth of mouse epidermal cells in medium with reduced calcium concentration. J Invest Dermatol. 1981 Feb;76(2):144–146. doi: 10.1111/1523-1747.ep12525490. [DOI] [PubMed] [Google Scholar]
- Yuspa S. H., Morgan D., Lichti U., Spangler E. F., Michael D., Kilkenny A., Hennings H. Cultivation and characterization of cells derived from mouse skin papillomas induced by an initiation-promotion protocol. Carcinogenesis. 1986 Jun;7(6):949–958. doi: 10.1093/carcin/7.6.949. [DOI] [PubMed] [Google Scholar]