Abstract
Ornithine transcarbamylase deficiency (OTCD) is the most common inborn error of urea synthesis. Complete OTCD can result in hyperammonemic coma in the neonatal period which can rapidly become fatal. Current acute therapy involves dialysis; chronic therapy involves the stimulation of alternate nitrogen clearance pathways; and the only curative approach is liver transplantation. AAV vector based gene therapy would add to current treatment options provided the vector delivers high level and stable transgene expression in liver without dose limiting toxicity. In this study, we employed an AAV2/8-based self-complementary (sc) vector expressing the murine OTC gene under a liver-specific thyroxine-binding globulin (TBG) promoter and examined the therapeutic effects in a mouse model of OTCD, the spf ash mouse. Seven days after a single intravenous injection of vector, treated mice showed complete normalization of urinary orotic acid, a measure of OTC activity. We further improved vector efficacy by incorporating a Kozak or Kozak-like sequence into mOTC cDNA which increased the OTC activity by 5- or 2-fold and achieved sustained correction of orotic aciduria for up to 7 months. Our results demonstrate that vector optimizations can significantly improve the efficacy of gene therapy.
Keywords: Adeno-associated viruses (AAV), liver gene therapy, OTC deficiency, self-complementary
Introduction
Ornithine transcarbamylase deficiency (OTCD) is an X-linked recessive disorder caused by mutations in ornithine transcarbamylase, a key enzyme involved in the detoxification of ammonia into urea. A complete deficiency of OTC manifests with hyperammonemic coma in the neonatal period associated with a high mortality. If diagnosed early, neonates can be rescued with dialysis and stabilized on alternate pathway therapy,1 although survivors often suffer from cognitive deficits and experience repeated episodes of hyperammonemic crises.2 Curative treatment of neonatal onset OTCD involves liver transplantation, usually performed in the first year of life, which carries not insignificant morbidity and mortality.3
Liver-directed gene therapy could be an attractive addition to therapy for OTCD because OTC is primarily expressed in liver. Recombinant adenoviruses were initially explored as vectors for OTC gene therapy because of their high gene transfer efficiency and rapid transgene expression in liver.4-6 However, the high immunogenicity associated with the adenoviral vector limited its use. Only transient expression of the transgene was observed.7,8 The most significant concerns related to systemic inflammation following liver-directed gene transfer as observed in a pilot human gene therapy study involving adult subjects with partial OTC deficiency.9
In contrast to adenovirus, vectors based on adeno-associated virus (AAV) hold promise as both efficient and safe vectors for liver-directed gene therapy.10 The discovery of novel AAV serotypes and the development of self-complementary (sc) AAV vectors has further increased the potentials of AAV vectors.11,12 We previously pursued OTC correction using AAV vectors constructed from several novel serotypes and showed markedly enhanced efficiency of transduction and expression of the OTC transgene from AAV7, 8, or 9 compared to AAV2.13 However, even with the most efficient serotypes, high vector dose (1×1012 GC per mouse) is required to achieve correction in the spf ash mouse, an OTCD mouse model. Such a high dose is not suitable for treatment in humans. More recently, Cunningham et al reported the long-term correction and supra-physiological levels of OTC expression in spf ash using an AAV8 vector.14 In this study, we have further advanced this work by developing and optimizing a self-complementary AAV2/8 vector expressing the murine OTC gene under the control of the liver-specific TBG promoter, and examined the therapeutic effects of this improved vector in spf ash mice.
Results
To improve the efficacy of OTC gene therapy, we first generated a self-complementary AAV2/8 vector carrying the murine OTC cDNA driven by the TBG promoter (AAV2/8sc.TBG.mOTC1.1, Figure 1a). In vivo evaluation was performed in adult spf ash mice by a single intravenous injection at the dose of 3×1011 or 1×1011 GC (genome copy). In the 3×1011 GC dose group, significant reduction of urine orotate was achieved at 3 days after vector injection (p<0.05), and complete normalization of urine orotate was achieved 7 days after injection and sustained through the course of the experiment (56 days; Figure 2a). In the 1×1011 GC dose group, partial reduction of urine orotate was observed at 3 days after vector injection and complete normalization of urine orotate was achieved at 7 days after vector injection and sustained throughout the course of experiment (Figure 2a). Liver OTC activity measured at 3, 7, 14, 28, and 56 days post vector administration showed continuous increase of OTC activity in liver. At day 56, the liver OTC activity in the high dose group reached 68% of the levels in wild-type (WT) mice (Figure 2b).
Figure 1.
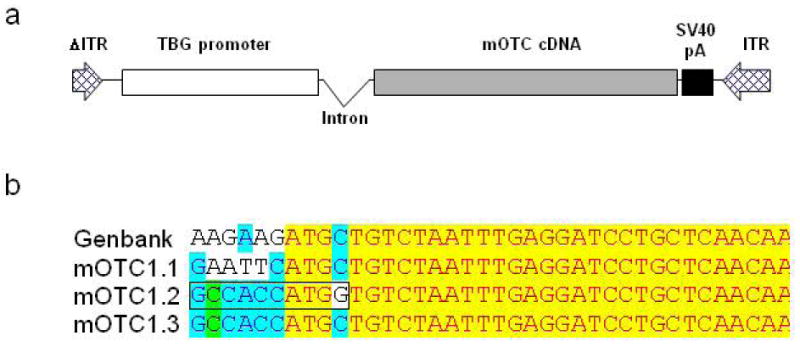
Illustration of self-complementary AAV-mOTC vectors used in this study. (a) Diagram of AAVsc.TBG.mOTC vector. (b) DNA sequences surrounding the ATG start codon of mOTC gene in different variants of AAV.mOTC vectors. The perfect Kozak sequence is indicated by a box in the mOTC1.2 vector. ITR, inverted terminal repeat; TBG, thyroxine-binding globulin promoter; SV40 pA, polyA signal derived from SV40.
Figure 2.
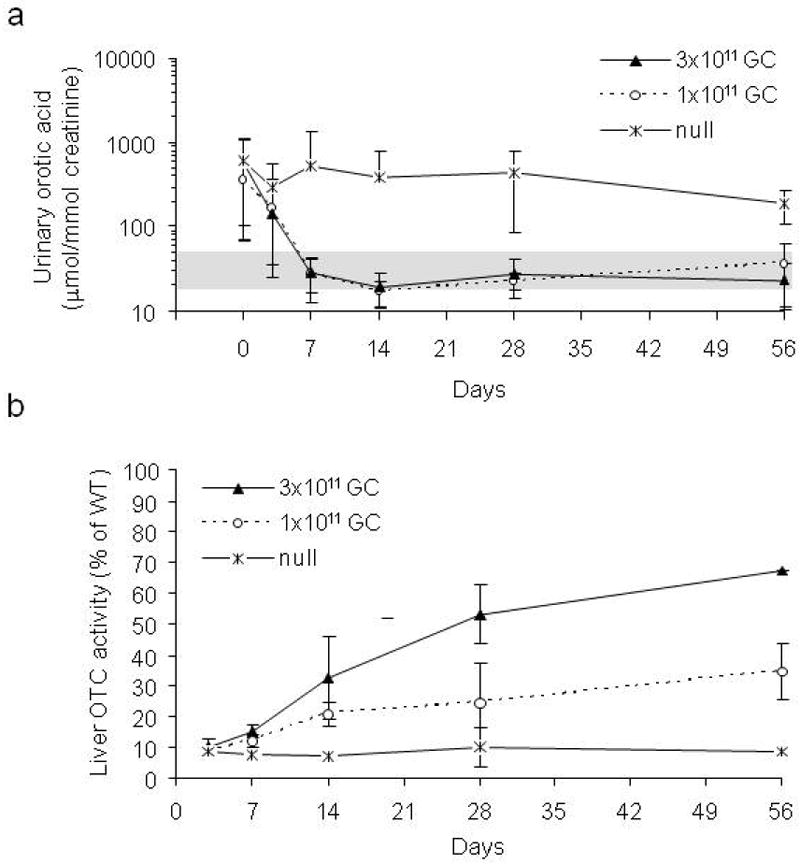
Fast kinetics of correction of orotic aciduria by AAV2/8sc.TBG.mOTC1.1 vector in spf ash mice. Adult male spf ash mice were injected intravenously with 3×1011 or 1×1011 GC of AAV2/8sc.TBG.mOTC1.1 vector, or 3×1011 GC of AAV2/8.TBG.null (no transgene). (a) Rapid decrease and sustained correction of urinary orotic acid levels in the treated spf ash mice (n=4-5 for each time point). Urinary orotic levels were significantly reduced starting at day 3 (3×1011 GC) or day 7 (1×1011 GC) after vector treatment (p<0.05, ANOVA). Shaded area indicates orotatic acid levels in normal mice. (b) Liver OTC activity following vector treatment (n=2-4 for each time point). OTC activity levels were presented as percentages of the mean level in WT mice (n=7).
Improved OTC gene expression and activity by incorporation of an engineered Kozak or Kozak-like sequence in mOTC gene
Although gene transfer was very efficient using the self-complementary AAV8 vector in murine liver, OTC activity in the high dose group was still below the levels in WT mice. We hypothesized that the lack of the Kozak sequence in the mOTC gene might contribute to the apparent inefficiency in translation. To test this, we engineered two constructs: mOTC1.2, that contains a perfect Kozak sequence (GCCACCATGG) which also causes a single amino acid change (Leu to Val), and mOTC1.3, which contains a Kozak-like sequence without an amino acid change in the coding sequence (Figure 1b). The amino acid substitution in mOTC1.2 occurs in the OTC mitochondrial targeting peptide that is normally removed upon mitochondrial import. The efficiency of mOTC protein expression levels were evaluated in adult spf ash mice following a single intravenous injection of 3×1011, 1×1011 or 3×1010 GC of AAV2/8sc.TBG.mOTC variants. Two weeks after injection, liver was harvested for Western blot analysis, liver OTC activity assay, and OTC histochemistry staining. As demonstrated by the Western analysis, the mOTC1.2 vector gave rise to the highest OTC protein levels in spf ash mice, followed by the mOTC1.3 vector (Figure 3a). Spf ash mice treated with 3×1011 GC of the mOTC1.2 vector had liver OTC activity levels that were 140% of normal; and mice treated with 3×1011 GC of the mOTC1.3 vector or 1×1011 GC of the mOTC1.2 vector had OTC activity levels equivalent to 71% and 61% of levels in WT mice respectively (Figure 3b). Overall, at two weeks after vector treatment, mOTC1.2 vector treated mice had statistically higher OTC liver enzyme activities than those treated with high (3×1011) and medium dose (1×1011) of mOTC1.1 or 1.3 vectors (p<0.05, ANOVA); and mice treated with high dose of the mOTC1.3 vector had statistically higher OTC activity levels then those treated with mOTC1.1 vector. Histochemical staining for OTC activity in liver sections of mice treated with the high dose of the mOTC1.2 and 1.3 vectors demonstrated substantial levels of heavily stained hepatocytes, especially in areas surrounding the central veins (Figure 3c), similar to a previous report.14 Although mOTC1.3 had lower expression levels than mOTC1.2, we decided to proceed with further studies using mOTC1.3 because it does not contain an amino acid substitution which could lead to problems of immunogenicity.
Figure 3.
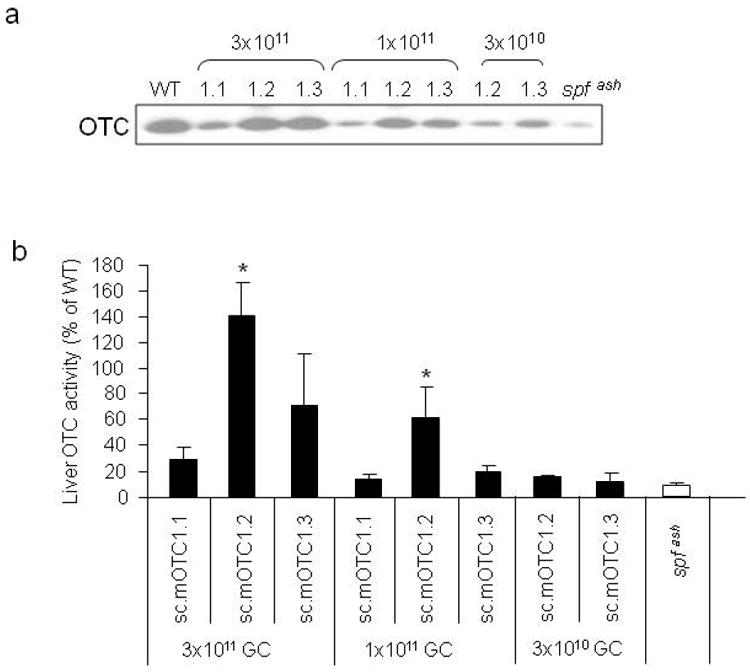
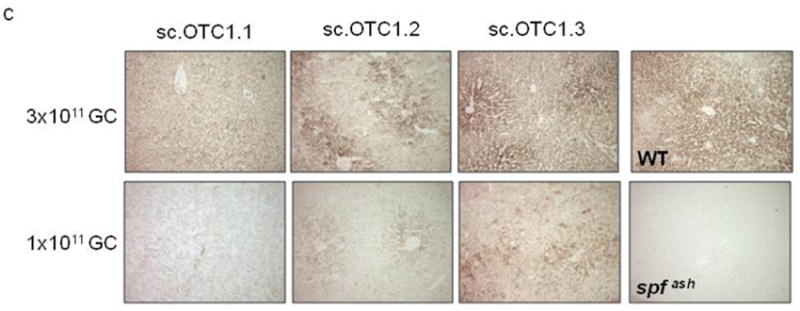
Improved OTC gene expression and activity by incorporation of an engineered Kozak or Kozak-like sequence in mOTC gene. Adult male spf ash mice were injected intravenously with 3 variants (1.1, 1.2, and 1.3) of AAV2/8sc.TBG.mOTC vectors at the dose of 3×1011, 1×1011, or 3×1010 GC. Livers were harvested 2 weeks after injection for analysis. (a) Western analysis of liver lysates (1μg protein per lane) from control and vector-treated spf ash mice. (b) Liver OTC activity in spf ash mice injected with AAV2/8sc.TBG.mOTC vectors. Error bars represent mean ± s.d. (n=3-10). * OTC activity of mOTC1.2 was statistically higher than that of mOTC1.1 and 1.3 at the respective dose of 3×1011 and 1×1011 GC (p<0.05, ANOVA). (c) OTC histochemical enzyme activity in liver of control and vector-treated spf ash mice. Representative pictures from each group are shown.
Comparison of liver-specific promoters
Besides vector genome composition (ss vs. sc) and Kozak sequence, the promoter plays an important role in determining transgene expression levels. We have chosen the thyroxine-binding globulin (TBG) promoter because of its liver specificity and small size (680 bp) which is critical because the size of the transgene cassette is limited in sc vectors. A recent report by Cunningham et al showed long-term correction and supra-physiological levels of OTC expression in spf ash mice using a ss AAV8 vector expressing mOTC under the control of a liver-specific LSP1 promoter (apolipoprotein E/human α1-antitrypsin enhancer/promoter elements).14 Additionally, this single stranded AAV vector also contained the woodchuck hepatitis virus post transcriptional regulatory element (WPRE). In some settings WPRE is capable of enhancing transgene expression levels15-17 but it also contains potential oncogene activity.18 In order to directly compare the promoter strength of LSP1 and TBG, we removed the WPRE element from the AAV.LSP1.mOTC.WPRE and cloned TBG.mOTC1.3 to a single-stranded AAV vector backbone (AAVss.TBG.mOTC1.3). When comparing the three vectors in spf ash mice at 3 different vector doses, mice treated with AAV2/8.LSP1.mOTC.WPRE had statistically higher OTC liver activity than the mice treated with AAV2/8.LSP1.mOTC or AAV2/8.TBG.mOTC1.3, but there was no statistical difference between AAV2/8.LSP1.mOTC and AAV2/8.TBG.mOTC1.3 treated mice (Figure 4). These data suggest LSP1 and TBG have similar promoter strength and WPRE can increase transgene expression levels by 3-fold. Because WPRE has oncogenic potential, we did not pursue it further for consideration of human trials.
Figure 4.
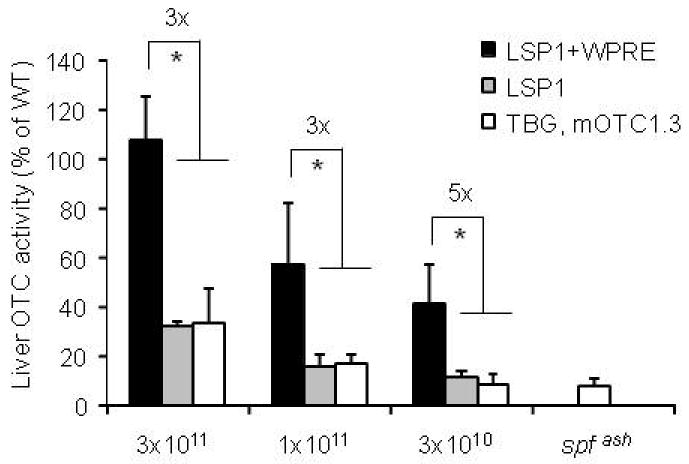
Comparison of promoter strength and contribution of post transcriptional regulatory element on OTC activity. Adult male spf ash mice were injected intravenously with single stranded (ss) AAV2/8.LSP1.mOTC.WPRE, AAV2/8.LSP1.mOTC, or AAV2/8.TBG.mOTC1.3 vectors at the dose of 3×1011, 1×1011, or 3×1010 GC. Livers were harvested 2 weeks after injection for OTC activity analysis. Error bars represent mean ± s.d. (n=3-5). * OTC activity of LSP1-WPRE vector was statistically higher than that of LSP1 and TBG.mOTC1.3 vectors at each of the tested doses (p<0.05, ANOVA). There were no statistical differences between LSP1 and TBG.mOTC1.3 groups at each of the tested doses.
Comparison of ss and sc AAV vectors
The advantages of the scAAV vector were confirmed by comparing OTC activity in mice treated with sc and ss AAV2/8.TBG.mOTC1.3 vectors. As shown in Figure 3b and Figure 4, mice injected with 3×1011 GC of the sc.mOTC1.3 vector had 2-fold higher liver OTC activity than mice injected with the ss.mOTC1.3 vector at the equivalent dose, although the lower dose groups did not show obvious differences at the 2 week time point. Vector genome copies were significantly higher in the liver of sc vector-injected mice as compared to the corresponding ss vector: 5-fold at 3×1011 GC dose (p<0.05, student t-test), and 3-fold at 1×1011 GC dose (p<0.001, student t-test) (Figure 5).
Figure 5.
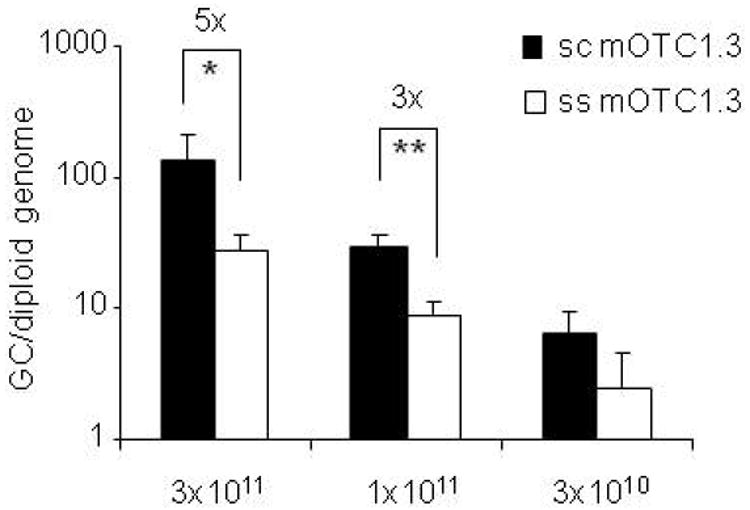
Comparison of vector genome copies in the liver of mice treated with sc or ss AAV2/8.TBG.mOTC1.3 vectors. Total liver DNA were isolated from spf ash mice two weeks after i.v. injection of AAV2/8sc.TBG.mOTC1.3 or AAV2/8ss.TBG.mOTC1.3 vectors at doses of 3×1011, 1×1011, or 3×1010 GC. Vector genome copies were measured by real-time PCR. Error bars represent mean ± s.d. (n=3-5). p<0.05 (*) or p<0.001 (**) by non-paired student t-test (two-tailed).
Robust and sustained correction of OTC deficiency in spf ash mice by the AAV2/8sc.TBG.mOTC1.3 vector
The kinetics and efficacy of AAV2/8sc.TBG.mOTC1.3 were studied at 3 doses (3×1011, 1×1011, and 3×1010 GC) in adult spf ash mice. Three days after a single intravenous injection, urinary orotic acid levels in both the high (3×1011 GC) and medium (1×1011 GC) dose groups were significantly reduced from levels before treatment to levels close to normal range (Figure 6a). The low dose (3×1010 GC) group did not show any reduction at day 3, but at day 7 post vector injection, the urinary orotic acid levels in all groups were in the normal range and sustained through the experiment (Figure 6a). OTC activity was measured in the liver lysates harvested at 4, 12, and 32 weeks post vector treatment. The liver OTC activity in the high dose group reached 150% of WT levels, and the activity levels in the medium dose group were equivalent to those in the WT mice (Figure 6b). The low dose group had a marginal increase in activity as compared to the untreated spf ash mice. Overall, OTC activity levels were maintained through the course of the experiment. Efficient and stable gene transfer by the sc vector was demonstrated by high vector genome copies detected in the livers harvested at 4, 12 and 32 weeks post gene transfer, and the vector genome copy numbers correlated well with the vector dose (Figure 6c). Expression of OTC protein in the treated spf ash mice was further confirmed by Western blot analysis on liver lysates and immunostaining of liver sections harvested 4 weeks after vector infusion. (Figure 6d and 6f). Immunostaining showed that there were some hepatocytes expressing very high levels of OTC protein; those high expressing-cells were scattered around the liver, with slightly higher frequency around the central vein. At 4 weeks post infusion, vector-treated spf ash mice along with untreated spf ash mice and normal littermates (WT) were subjected to a NH4Cl challenge. As shown in Figure 6e, the majority of treated mice (73%) showed improved behavior scores as compared to the untreated spf ash mice, suggesting clinical improvement resulting from gene therapy.
Figure 6.
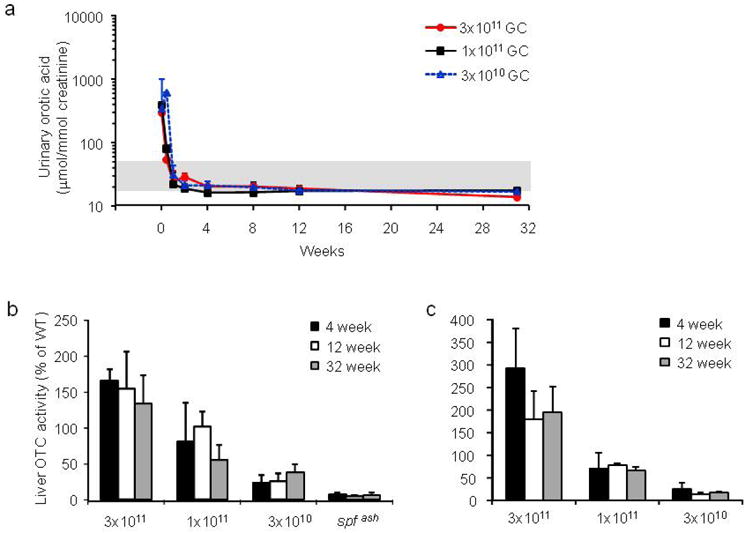
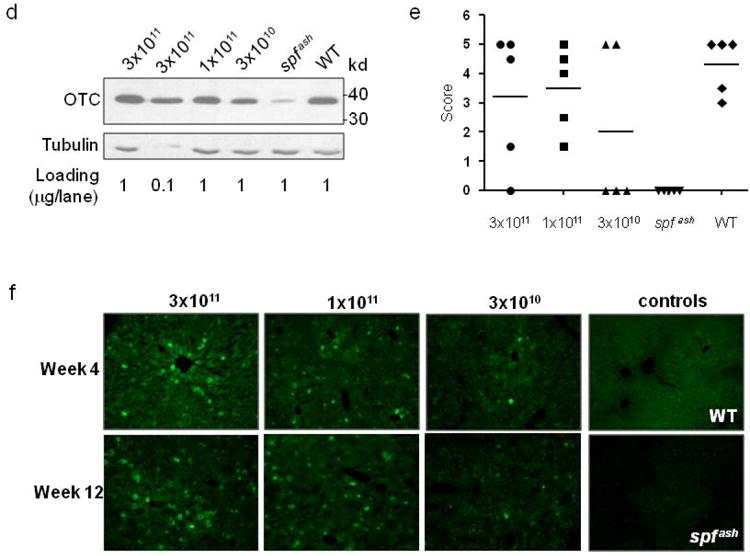
Robust and sustained correction of OTC-deficiency in adult spf ash mice treated with scAAV2/8.TBG.mOTC1.3 vector. Adult male spf ash mice were injected intravenously with AAV2/8sc.TBG.mOTC1.3 vectors at the dose of 3×1011, 1×1011, or 3×1010 GC. (a) Normalization of urinary orotic acid levels following AAV gene therapy. (b) Liver OTC activity at 4, 12, and 32 weeks after vector treatment. (c) Vector genome copies in the liver of treated mice at 4, 12 and 32 weeks. (d) Western blot analysis on liver lysates (1μg or 0.1μg protein per lane) from control and vector-treated spf ash mice at 4 weeks. (e) Outcome of NH4Cl challenge performed at 4 weeks. (f) Immunostaining of OTC in the liver of control or treated spf ash mice. Representative pictures from each group are shown.
Discussion
The requirements for gene therapy in diseases caused by defects in secreted proteins, such as clotting factors and α1-antitrypsin, are relatively simple. A number of cellular targets can be considered, and limitations in transduction efficiency can be overcome by driving high levels of transgene expression in cells that are actually transduced. Gene therapy of a metabolic enzyme such as OTC presents a more challenging model for gene replacement therapy. The gene acts in an autocrine manner (i.e., it can only influence the cell in which it is expressed), and the net therapeutic effect is a function of the total number of target cells tansduced. Previous studies using adenoviral or AAV vectors have illustrated the difficulties of expressing sufficient levels of OTC in vivo to show efficacy. 4,13,16 In our previous study, we demonstrated that in OTC deficient mouse models, AAV7, 8, and 9 vectors expressing mOTC (i.e., a single stranded genome with a TBG promoter) were able to restore urinary orotate levels to normal 15 days after intraportal vector infusion.13 However, these therapeutic effects were achieved only at a high vector dose of 1×1012 GC/mouse, an unrealistic dose (equivalent to 4×10e13 GC/kg) for treatment of human patients. More recently, Cunningham et al reported encouraging results of achieving over 200% of normal levels of OTC activity with a single-stranded AAV2/8 vector at the dose of 1.5×1011 GC/mouse.14 The use of a strong liver-specific promoter (LSP1) was indicated as the main factor for superior efficacy.
Our study attempted to determine the precise components of the previously described OTC constructs that impacted on transgene expression, with the goal of developing a vector for human studies. One important difference between our first AAV8-TBG-mOTC vector and the Cunningham construct is that the latter vector contained a WPRE element, which is known to enhance transgene expression levels by increasing mRNA stability.15 We removed the WPRE from the Cunningham vector and compared the LSP1 ss vector with our TBG ss vector. As shown in Figure 4, there was no significant difference between the LSP1 and TBG vectors, however both were substantially reduced in comparison to the related vector that has the WPRE element. This suggests one component of the expression cassette that contributed to high level of expression was the WPRE. Previous work on adenoviral vector-mediated gene therapy for OTCD has also demonstrated the function of the WPRE element.16 Due to the concerns about potential oncogenic activity of WPRE,18 it is probably prudent to eliminate the WPRE in all vectors destined for clinical applications.
To develop a clinical candidate vector for OTC gene therapy, we decided to determine the influence of other components of the vector including capsid, ITR, promoter, and Kozak sequence, on OTC transgene expression. Our previous studies in mouse, dog, and nonhuman models identified that AAV8 was the best candidate for hepatic gene transfer.19-21 Initial studies evaluated the potential benefit of vectors with sc genomes since previous studies showed that they can bypass the rate-limiting second strand DNA synthesis leading to fast kinetics and more efficient transduction.22-24 We first generated AAV2/8sc.TBG.mOTC1.1, a scAAV vector in which expression of the mouse OTC gene was under the control of a liver-specific TBG promoter. Compared to the single-stranded AAV2/8ss.TBG.mOTC used in our previous studies,13 this vector did indeed show improved gene expression kinetics, with partial normalization of the urinary orotate concentration in the spf ash mouse 3 days after a single intravenous injection of 1×1011 and 3× 1011 GC. However, liver OTC activity assays of treated mice at the high dose showed enzyme activity at only one-third of the level in WT control mice. Therefore, the initial AAV2/8sc.TBG.mOTC1.1 vector was redesigned with a perfect Kozak sequence that results in a Leu to Val amino acid substitution at codon 2 (mOTC1.2). The use of this OTC 1.2 vector resulted in dramatically improved OTC enzyme activity in the livers of treated mice. We created another sc vector (mOTC1.3) that preserves the native amino acid sequence with an imperfect but Kozak-like sequence called mOTC1.3. The use of mOTC1.3 at 1×1011 GC restored the OTC enzyme activity in the livers of treated mice to a level similar to WT. Furthermore, comparison with an ssAAV2/8 vector carrying mOTC1.3 shows the self-complementary vector is about 3-fold more efficient than the ss vector, as demonstrated by both OTC enzyme activities and vector genome copies in liver. Our results demonstrate that optimization of the genome of the vector can significantly improve the efficacy of gene therapy. An additional approach that could be explored to further improve OTC expression levels is codon optimization, which have been demonstrated in hemophilia B gene therapy and is currently being tested in humans.22,23,25
Materials and Methods
Vector construction and production
pAAVsc.TBG.mOTC1.1 was constructed containing the D-sequence deleted 5’ AAV ITR and an intact 3’ ITR, the liver-specific TBG promoter, an intron from human immunoglobulin, murine OTC coding sequence, and an SV40 polyA. pAAVsc.TBG.mOTC1.2, carrying a perfect Kozak consensus sequence and a change at the second codon, was generated by site-directed mutagenesis of pAAVsc.TBG.mOTC1.1. Further mutagenesis was performed on pAAVsc.TBG.mOTC1.2 to generate pAAVsc.TBG.mOTC1.3 in which the second codon was restored to the original sequence that results in an imperfect but Kozak-like sequence (Figure 1a). pAAV.LSP1.mOTC.WPRE was a gift from Ian E. Alexander, the Children’s Hospital at Westmead, Australia. pAAV.LSP1.mOTC was generated by removing the WPRE element from pAAV.LSP1.mOTC.WPRE. All vectors used in this study were purified by two rounds of cesium chloride gradient centrifugation, buffered-exchanged with PBS, and concentrated using Amicon Ultra 15 centrifugal filter devices-100K (Millipore, Bedford, MA). Genome titer (GC/ml) of AAV vectors were determined by real-time PCR using a primer/probe set corresponding to the TBG promoter and linearized plasmid standards. Vectors were subjected to additional quality control tests including sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) analysis for vector purity and Limulus amebocyte lysate (LAL) for endotoxin detection (Cambrex Bio Science, Walkersville, MD, USA).
Animals
Spf ash mice were maintained at the Animal Facility of the Translational Research Laboratories at the University of Pennsylvania as described previously.13 Two to five month old spf ash mice and their normal littermates were used in the studies. All animal procedures were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania. Vectors were administered by intravenous (i.v.) injection via the tail vein.
Measurement of urinary orotate
Urine samples were collected before and at various time points after vector treatment for orotic acid analysis as described previously.13
OTC enzyme activity assay
OTC enzyme activity was assayed in liver lysates as described previously with modifications.26 Whole liver fragments were frozen in liquid nitrogen, and stored at -80°C until OTC measurements were performed. A homogenate of 50 mg liver tissue/ml was prepared in 50mM Tris acetate buffer pH 7.5, with a Polytron homogenizer. Five hundred microgram of liver tissue was used per assay tube, and assays were performed in triplicate. Protein concentration was determined on the remaining liver homogenate using the BioRad Protein assay kit (Biorad, Hercules, CA, USA) according to the manufacturer’s instructions.
OTC histochemistry
Sliced liver tissue (2 mm) was fixed, embedded, sectioned (9 μm), and mounted onto slides for histochemical staining of OTC enzyme activity as previously described 4.
OTC immunostaining
Immunofluorescence for OTC expression was performed on frozen liver sections. Cryosections (8 μm) were air dried and fixed in 4% paraformaldehyde (all solutions in PBS) for 10 min. Sections were then permeabilized and blocked in 0.2% Triton containing 1% goat serum for 30 min. A rabbit anti-OTC antibody diluted 1:1000 in 1% goat serum was used to incubate the sections for 1 hour. After washing, the sections were stained with FITC-labeled anti-rabbit antibodies (Vector Labs, Burlingame, CA, USA) in 1% goat serum for 30 min, washed, and mounted with Vectashield plus DAPI (Vector Labs).
Western blot analysis
Proteins from liver lysates were separated on 4-12% Bis-Tris NuPAGE gel (Invitrogen, Carlsbad, CA) and transferred to PVDF membrane, blocked, and probed with rabbit anti-OTC antibody (1:5000 dilution) and rabbit anti-tubulin antibody (1:1000 dilution, Abcam, Cambridge, MA). Bound primary antibody was detected with HRP-conjugated goat anti-rabbit IgG antibody (1:10000 dilution, Thermo Fisher, Waltham, MA, USA) and SuperSignal West Pico Chemiluminescence Substrate (Thermo Fisher).
Ammonia challenge
A nitrogen-load challenge test was performed on mice at selective days post-vector treatment as described previously with modifications.5 The negative controls were untreated spf ash mice and the positive controls were normal littermates. Mice were treated with a 0.75 M NH4Cl solution (7.5 mmol/kg, ip) and behavioral measurements were assessed 15-20 min following the injection. The scoring system was based on the gait and response to sound. Seizures were excluded from the scoring system due to the inconsistent incidence of this phenotype in untreated spf ash control mice. Gait analysis was performed as described previously with a blind tunnel.27 The front and back paws of a mouse were dipped in red and blue food dyes, respectively. The foot prints of the mouse going through the tunnel were used for scoring. Hypersensitivity to sound was determined by ringing a 100-dB bell located ~1.5 meter away from the mice 3 times for 5 seconds each time with at least a 20 seconds interval. The total score of a normal mouse would be 5.
Q-PCR
Q-PCR was performed on genomic DNA isolated from mice liver using QIAamp DNA Mini Kit (Qiagen, Valencia, CA, USA). Detection and quantification of vector genomes in extracted DNA were performed by real-time PCR as described previously.28
Statistical analysis
Statistical differences between different treatment groups were determined using ANOVA or Student’s t test.
Acknowledgments
We thank Martin Lock, Julie Johnston, Arbans Sandhu, and Shu-Jen Chen (Penn Vector) for supplying vectors, Guangping Gao and Xun Sun for initial participation of the project. This work was supported in part by the Kettering Family Foundation and the following grants to J.M.W.: P01-HD057247, P01-HL059407, and P30-DK047757. H.W. was supported by a scholarship from China Scholarship Council. J.M.W. is a consultant to ReGenX Holdings, and is a founder of, holds equity in, and receives a grant from affiliates of ReGenX Holdings; in addition, he is an inventor on patents licensed to various biopharmaceutical companies, including affiliates of ReGenX Holdings.
References
- 1.Batshaw ML, MacArthur RB, Tuchman M. Alternative pathway therapy for urea cycle disorders: twenty years later. J Pediatr. 2001;138:S46–54. doi: 10.1067/mpd.2001.111836. discussion S54-45. [DOI] [PubMed] [Google Scholar]
- 2.Krivitzky L, Babikian T, Lee HS, Thomas NH, Burk-Paull KL, Batshaw ML. Intellectual, adaptive, and behavioral functioning in children with urea cycle disorders. Pediatr Res. 2009;66:96–101. doi: 10.1203/PDR.0b013e3181a27a16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leonard JV, McKiernan PJ. The role of liver transplantation in urea cycle disorders. Mol Genet Metab. 2004;81(Suppl 1):S74–78. doi: 10.1016/j.ymgme.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 4.Ye X, Robinson MB, Batshaw ML, Furth EE, Smith I, Wilson JM. Prolonged metabolic correction in adult ornithine transcarbamylase-deficient mice with adenoviral vectors. J Biol Chem. 1996;271:3639–3646. doi: 10.1074/jbc.271.7.3639. [DOI] [PubMed] [Google Scholar]
- 5.Ye X, Robinson MB, Pabin C, Quinn T, Jawad A, Wilson JM, et al. Adenovirus-mediated in vivo gene transfer rapidly protects ornithine transcarbamylase-deficient mice from an ammonium challenge. Pediatr Res. 1997;41:527–534. doi: 10.1203/00006450-199704000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Raper SE, Wilson JM, Yudkoff M, Robinson MB, Ye X, Batshaw ML. Developing adenoviral-mediated in vivo gene therapy for ornithine transcarbamylase deficiency. J Inherit Metab Dis. 1998;21(Suppl 1):119–137. doi: 10.1023/a:1005369926784. [DOI] [PubMed] [Google Scholar]
- 7.Yang Y, Ertl HC, Wilson JM. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity. 1994;1:433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y, Nunes FA, Berencsi K, Furth EE, Gonczol E, Wilson JM. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci U S A. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80:148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 10.Alexander IE, Cunningham SC, Logan GJ, Christodoulou J. Potential of AAV vectors in the treatment of metabolic disease. Gene Ther. 2008;15:831–839. doi: 10.1038/gt.2008.64. [DOI] [PubMed] [Google Scholar]
- 11.Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCarty DM. Self-complementary AAV vectors; advances and applications. Mol Ther. 2008;16:1648–1656. doi: 10.1038/mt.2008.171. [DOI] [PubMed] [Google Scholar]
- 13.Moscioni D, Morizono H, McCarter RJ, Stern A, Cabrera-Luque J, Hoang A, et al. Long-term correction of ammonia metabolism and prolonged survival in ornithine transcarbamylase-deficient mice following liver-directed treatment with adeno-associated viral vectors. Mol Ther. 2006;14:25–33. doi: 10.1016/j.ymthe.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham SC, Spinoulas A, Carpenter KH, Wilcken B, Kuchel PW, Alexander IE. AAV2/8-mediated correction of OTC deficiency is robust in adult but not neonatal Spf(ash) mice. Mol Ther. 2009;17:1340–1346. doi: 10.1038/mt.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loeb JE, Cordier WS, Harris ME, Weitzman MD, Hope TJ. Enhanced expression of transgenes from adeno-associated virus vectors with the woodchuck hepatitis virus posttranscriptional regulatory element: implications for gene therapy. Hum Gene Ther. 1999;10:2295–2305. doi: 10.1089/10430349950016942. [DOI] [PubMed] [Google Scholar]
- 16.Mian A, McCormack WM, Jr, Mane V, Kleppe S, Ng P, Finegold M, et al. Long-term correction of ornithine transcarbamylase deficiency by WPRE-mediated overexpression using a helper-dependent adenovirus. Mol Ther. 2004;10:492–499. doi: 10.1016/j.ymthe.2004.05.036. [DOI] [PubMed] [Google Scholar]
- 17.Paterna JC, Moccetti T, Mura A, Feldon J, Bueler H. Influence of promoter and WHV post-transcriptional regulatory element on AAV-mediated transgene expression in the rat brain. Gene Ther. 2000;7:1304–1311. doi: 10.1038/sj.gt.3301221. [DOI] [PubMed] [Google Scholar]
- 18.Kingsman SM, Mitrophanous K, Olsen JC. Potential oncogene activity of the woodchuck hepatitis post-transcriptional regulatory element (WPRE) Gene Ther. 2005;12:3–4. doi: 10.1038/sj.gt.3302417. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Wang H, Bell P, McCarter RJ, He J, Calcedo R, et al. Systematic evaluation of AAV vectors for liver directed gene transfer in murine models. Mol Ther. 2010;18:118–125. doi: 10.1038/mt.2009.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bell P, Gao G, Haskins ME, Wang L, Sleeper MM, Wang H, et al. Evaluation of AAV Vectors for Liver-directed Gene Transfer in Dogs. Hum Gene Ther. doi: 10.1089/hum.2010.194. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Calcedo R, Wang H, Bell P, Grant R, Vandenberghe LH, et al. The pleiotropic effects of natural AAV infections on liver-directed gene transfer in macaques. Mol Ther. 2010;18:126–134. doi: 10.1038/mt.2009.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nathwani AC, Gray JT, Ng CY, Zhou J, Spence Y, Waddington SN, et al. Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood. 2006;107:2653–2661. doi: 10.1182/blood-2005-10-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Z, Sun J, Zhang T, Yin C, Yin F, Van Dyke T, et al. Optimization of self-complementary AAV vectors for liver-directed expression results in sustained correction of hemophilia B at low vector dose. Mol Ther. 2008;16:280–289. doi: 10.1038/sj.mt.6300355. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Ma HI, Li J, Sun L, Zhang J, Xiao X. Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther. 2003;10:2105–2111. doi: 10.1038/sj.gt.3302133. [DOI] [PubMed] [Google Scholar]
- 25.Nathwani AC, Gray JT, McIntosh J, Ng CY, Zhou J, Spence Y, et al. Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood. 2007;109:1414–1421. doi: 10.1182/blood-2006-03-010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morizono H, Tuchman M, Rajagopal BS, McCann MT, Listrom CD, Yuan X, et al. Expression, purification and kinetic characterization of wild-type human ornithine transcarbamylase and a recurrent mutant that produces ‘late onset’ hyperammonaemia. Biochem J. 1997;322(Pt 2):625–631. doi: 10.1042/bj3220625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sondhi D, Hackett NR, Peterson DA, Stratton J, Baad M, Travis KM, et al. Enhanced survival of the LINCL mouse following CLN2 gene transfer using the rh.10 rhesus macaque-derived adeno-associated virus vector. Mol Ther. 2007;15:481–491. doi: 10.1038/sj.mt.6300049. [DOI] [PubMed] [Google Scholar]
- 28.Bell P, Moscioni AD, McCarter RJ, Wu D, Gao G, Hoang A, et al. Analysis of tumors arising in male B6C3F1 mice with and without AAV vector delivery to liver. Mol Ther. 2006;14:34–44. doi: 10.1016/j.ymthe.2006.03.008. [DOI] [PubMed] [Google Scholar]


