Abstract
Background & Aims
Myofibroblasts are the primary cell type involved in physiologic wound healing and its pathologic counterpart, fibrosis. Cellular fibronectin that contains the alternatively spliced extra domain A (EIIIA) is upregulated during these processes, and is believed to promote myofibroblast differentiation. We sought to determine the requirement for EIIIA in fibrosis and differentiation of myofibroblasts in rodent livers.
Methods
We used a mechanically tunable hydrogel cell culture system to study differentiation of primary hepatic stellate cells and portal fibroblasts from rats into myofibroblasts. Liver fibrosis was induced in mice by bile duct ligation or administration of thioacetamide.
Results
EIIIA was not required for differentiation of rat hepatic stellate cells or portal fibroblasts into fibrogenic myofibroblasts. Instead, hepatic stellate cells cultured on EIIIA-containing cellular fibronectin formed increased numbers of lamellipodia; their random motility and chemotaxis also increased. These increases required the receptor for EIIIA, the integrin α9β1. In contrast, the motility of portal fibroblasts did not increase on EIIIA and these cells expressed little α9β1. Male EIIIA−/− mice were modestly protected from thioacetamide-induced fibrosis, which requires motile hepatic stellate cells, but not from bile duct ligation-induced fibrosis, in which portal fibroblasts are more important. Notably, myofibroblasts developed during induction of fibrosis with either thioacetamide or bile duct ligation in EIIIA−/− mice.
Conclusions
EIIIA is dispensable for differentiation of hepatic stellate cells and portal fibroblasts to myofibroblasts, both in culture and in mouse models of fibrosis. These findings indicate a role for EIIIA in promoting stellate cell motility and parenchymal liver fibrosis.
Keywords: extracellular matrix, animal model, liver disease, cirrhosis
Introduction
Extracellular matrix (ECM) deposition during wound healing and fibrosis requires the differentiation of precursor cells to fibrogenic, α-smooth muscle actin (αSMA)-expressing myofibroblasts. Cells of diverse lineages have been identified as myofibroblast precursors, but pericytes and fibroblasts are the most common. In the liver, hepatic stellate cells and portal fibroblasts are the pericyte and fibroblast precursors of myofibroblasts, respectively. Defining the factors that drive the differentiation and function of these myofibroblast populations is essential for understanding the mechanism of wound healing and identifying therapeutic targets for fibrosis.
The fibronectin splice variant EIIIA is one factor hypothesized to mediate differentiation of myofibroblasts. Fibronectins are among the first ECM proteins upregulated after injury. There are two forms of fibronectin, plasma fibronectin (pFN) and cellular fibronectin (cFN), which are encoded by a single gene. pFN is secreted exclusively by hepatocytes as a soluble dimer, whereas cFN is secreted by many cells, which assemble it into insoluble fibrils1. cFN, but not pFN, can contain either of two alternatively spliced type III repeats, termed extra domain A (EDA) and extra domain B (EDB) in humans, and EIIIA and EIIIB in rodents2,3. For clarity, we use the rodent nomenclature throughout this work.
EIIIA+ cFN and EIIIB+ cFN are expressed nearly ubiquitously in embryonic tissues4 and appear to have distinct but overlapping roles during development5. Their expression is highly restricted in healthy adult tissue6, but is increased in a wide range of disease processes, including wound healing and fibrosis7. EIIIA+ cFN promotes myofibroblast differentiation of fibroblasts in the skin8 and lungs9. EIIIA−/− mice10,11, although viable and fertile, exhibit defective wound healing11 and are less susceptible to pulmonary fibrosis9; myofibroblasts are present, although in decreased numbers.
A requirement for EIIIA+ cFN in liver fibrosis has not been determined. Expression of EIIIA+ cFN is minimal in the healthy liver, but TGFβ induces its upregulation within 12 hours of liver injury12, 13. A 1994 study showed that primary hepatic stellate cells cultured on EIIIA+ cFN express higher levels of αSMA than do those cultured on pFN14, suggesting that EIIIA promotes fibrosis. More recent in vivo work, however, showed that total fibronectin is dispensable for fibrosis after CCl4-intoxication15. Our goal was to determine the requirement for EIIIA in myofibroblast differentiation using both in vivo and in vitro systems.
Materials and Methods
Primary cell isolation and culture
Hepatic stellate cells were isolated from male retired breeder Sprague-Dawley rats. Primary cells were cultured on tissue culture plastic (for transwell chemotaxis assays) or on polyacrylamide hydrogels16–20 (for all other experiments) coated with a saturating concentration of 0.1 mg/ml pFN or cFN (Sigma) in M199 media (Invitrogen) supplemented with 10% FBS (Gemini) and antibiotics. Portal fibroblasts were isolated as described16.
Animal studies
EIIIA−/− mice (on a pure C57Bl/6 background) were established from a litter resuscitated from cryopreserved embryos from the Mutant Mouse Regional Resource Center (B6.129S4-Fn1tm1Bwg/Mmnc, deposited by Dr. Elizabeth George)10. Animals were cared for in compliance with the US Department of Health and Human Services Guide for the Care and Use of Laboratory Animals and with the approval of the University of Pennsylvania Institutional Animal Care and Use Committee. A heterozygous breeding strategy was used so that EIIIA−/− mice could be compared to wild type littermates in all experiments.
Thioacetamide
Thioacetamide (200 mg/L) was prepared fresh every 3 days and administered in the drinking water.
Bile duct ligation
Mice underwent ligation of the common bile duct by standard techniques. Sham operated animals served as negative controls. Animals received enrofloxacin, administered via drinking water, prophylactically for 2 days prior to surgery and for an additional 7 days following surgery.
Statistical Analysis
Statistical significance was determined using Student’s t-test when only 2 groups were compared. One-way ANOVA with Bonferroni’s post-test for multiple comparisons was used when 3 groups were compared. p-values were considered significant when less than or equal to 0.05. * = p ≤ 0.05, ** = p ≤ 0.01, *** = p ≤ 0.005.
Additional methods are available as supplemental material.
Results
EIIIA is not required for myofibroblast differentiation
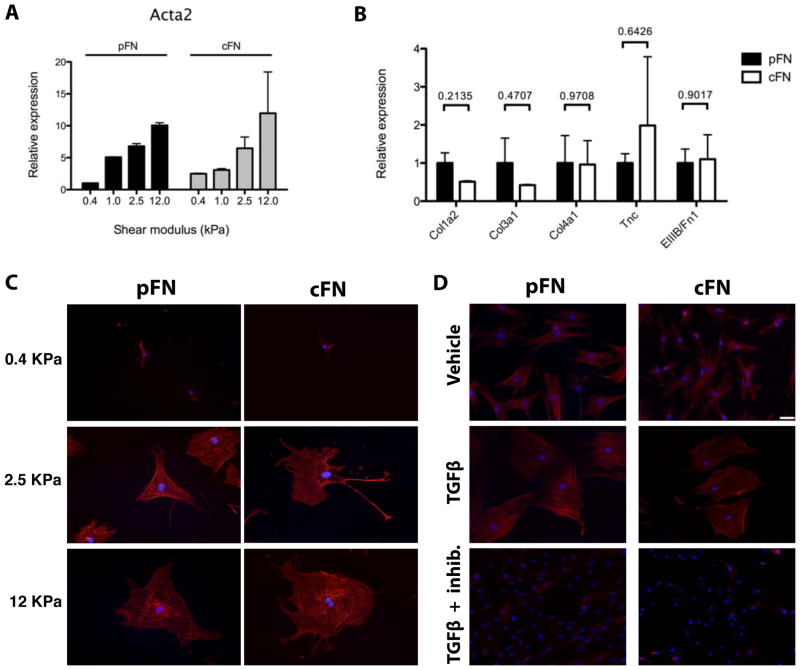
To determine whether EIIIA+ cFN is required for myofibroblast differentiation in the liver, we compared the response of hepatic stellate cells and portal fibroblasts to culture on pFN or cFN. Because the differentiation of both cell types is highly mechanosensitive16, we cultured cells in vitro on pFN- and cFN-coated polyacrylamide hydrogels with defined shear moduli (G′; stiffness) that mimic the mechanical environment of the normal (soft, average 0.5 kPa) or injured (stiff, 1–22 kPa) liver21. Surprisingly, hepatic stellate cells expressed equivalent levels of mRNA for αSMA and ECM proteins, and they had similar expression of αSMA by immunostaining, regardless of the presence or absence of EIIIA (Figure 1A,B,C). Culture on pFN versus cFN did not differentially affect the response of either portal fibroblasts or stellate cells to addition of exogenous TGF-β1 or inhibition of TGF-β signaling, though portal fibroblasts were more sensitive to TGF-β inhibition (Figure 1D) than were hepatic stellate cells (Supplemental Figure 1A,B,C), as previously reported22.
Figure 1. EIIIA is not required for myofibroblast differentiation of hepatic stellate cells or portal fibroblasts.
A. Stellate cells were cultured on pFN-coated or cFN-coated gels for 7 days. qRT-PCR for αSMA (Acta2) on 0.4–12 kPa gels. Error bars represent SD from 2 experiments. B. qRT-PCR on 12 kPa gels. Col1a2 = collagen I, Col1a3 = collagen III, Col4a1 = collagen IV, Tnc = tenascin C, and EIIIB/FN1 = ratio of EIIIB+ cFN to total fibronectin. Error bars represent SEM from 4 experiments. p values from a paired t-test are indicated. C. Stellate cells were cultured on pFN-coated or cFN-coated gels for 7 days then stained for αSMA (red) and DAPI (blue). Scale is the same as in (D). D. Portal fibroblasts were cultured on 12 kPa hydrogels coated with pFN or cFN in the presence or absence of TGF-β1 and a TβR1 inhibitor. Cells were stained for αSMA and DAPI (blue). Scale bar = 50 μm.
EIIIA+ cFN increases hepatic stellate cell but not portal fibroblast motility
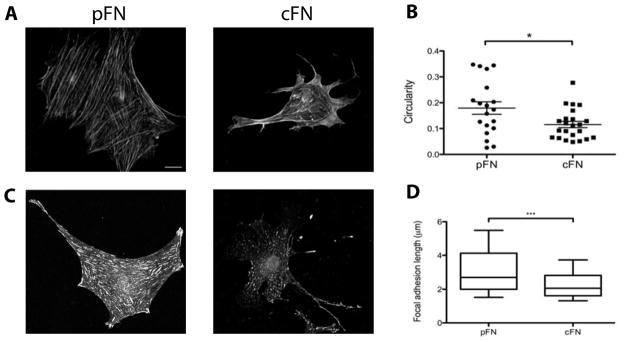
Whereas portal fibroblasts displayed a typical myofibroblast appearance on both pFN and cFN, with an angular shape and prominent stress fibers (Figure 1D), stellate cells on cFN had more diffuse organization of αSMA and a more elongated cell shape when cultured in the presence of 10% FBS (Figure 1C, 2A, 2B), TGF-β1 (Supplemental Figure 1D), or platelet derived growth factor (PDGF-BB) (Supplemental Figure 2A,B). Staining for the focal adhesion marker vinculin showed long, mature-appearing focal adhesions in stellate cells cultured on pFN, whereas stellate cells on cFN had punctate focal contacts (Figure 2C,D). These morphologic attributes are characteristic of motile cells, suggesting that cFN promotes motility rather than fibrogenesis.
Figure 2. Hepatic stellate cells cultured on cFN have a motile morphology.
Stellate cells were cultured on pFN-coated or cFN-coated gels for 7 days. A. Cells on 12 kPa gels stained for αSMA. Scale bar = 20 μm. B. Cell circularity was quantified (circularity = 4πA/P2, where A = cell area and P = perimeter). n = 18–23 cells per condition from 2 experiments. C. Stellate cells on 12 kPa gels stained for vinculin. Scale is the same as in (A). D. Focal adhesion length was calculated using NIH ImageJ. N = 125–150 focal adhesions per cell from 3 representative cells per matrix.
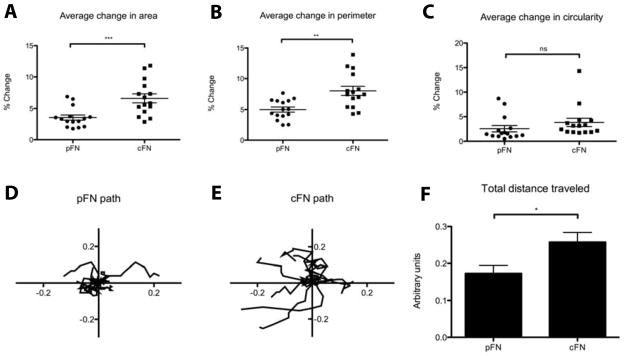
To determine whether the difference in morphology on pFN versus cFN translated to functional differences in motility, we performed time-lapse microscopy. Stellate cells cultured on cFN-coated hydrogels were more dynamic than those cultured on pFN-coated gels, with increased extension and retraction of protrusions as reflected in a greater average change in cell area and perimeter over time (Figure 3A,B, Supplemental Movies 1–4). Cell circularity was different on the two matrices at baseline but remained constant (Figure 3C). Furthermore, stellate cells cultured on cFN were more motile than those on pFN, as measured by nuclear travel (Figure 3D,E,F, Supplemental Movies 1–4).
Figure 3. Hepatic stellate cells cultured on cFN are more motile.
Cells were cultured on pFN-coated or cFN-coated 12 kPA gels for 7 days, followed by time-lapse microscopy performed over 6 hours. A–C. Cells were outlined on still frames taken at 40 minute intervals. Area, perimeter, and circularity were calculated, and the average percent change in each parameter from one frame to the next is graphed. N = 15 cells per matrix. D–E. Nuclear coordinates were traced. Each line represents the path of an individual cell. N = 15 cells per matrix. F. The average total path length per cell was calculated.
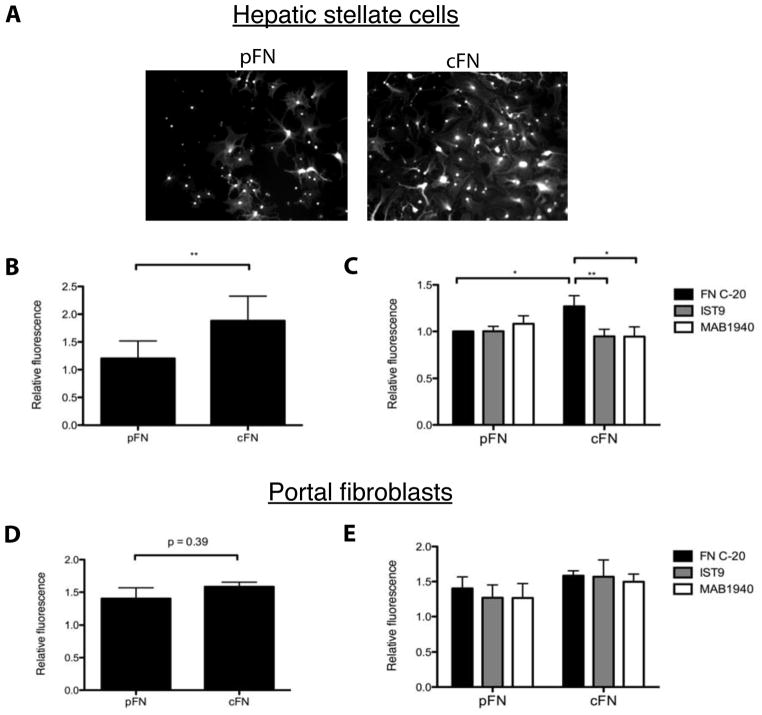
In vivo, stellate cells are likely to move in response to chemokine gradients. To model this, we used transwell chemotaxis assays and found that cells plated on cFN-coated transwell inserts exhibited greater chemotaxis towards serum (Figure 4A,B), TGF-β1 (Supplemental Figure 1E), or PDGF (Supplemental Figure 2C) than those plated on pFN-coated inserts. Increased migration on cFN was attenuated by treatment with two EIIIA blocking antibodies, IST9 and MAB1940, but not by a fibronectin antibody that binds to an unrelated site (FN C-20) (Figure 4C). Thus, increased hepatic stellate cell migration on cFN specifically requires EIIIA. In contrast, portal fibroblasts demonstrate equivalent motility on cFN and pFN (Figure 4D), and treatment with EIIIA blocking antibodies had no effect (Figure 4E).
Figure 4. Hepatic stellate cells but not portal fibroblasts cultured on cFN have increased chemotaxis.
A. Stellate cells were plated on transwell inserts coated with pFN or cFN. After 19 hours, cells were stained with calcein a.m. and visualized (10× magnification). B. Mean fluorescence intensity was measured. p-values were calculated with a paired t-test from 8 experiments. C. Inserts were coated with pFN or cFN, then incubated 1 hour with antibodies before plating cells. Results represent 5 experiments. D. Portal fibroblasts were plated on pFN or cFN-coated inserts. After 19 hours, cells were stained, and mean fluorescence intensity was measured. Results represent 3 experiments. E. Inserts were coated with pFN or cFN, then incubated 1 hour with antibodies before plating cells. Results represent 3 experiments.
Hepatic stellate cells require the fibronectin binding integrin α9β1 for cFN-enhanced motility
To investigate the mechanism of enhanced stellate cell motility on cFN, we first characterized the integrin expression profile for these cells. The classical fibronectin receptors are α5β1 and αvβ3. Inclusion of EIIIA increases α5β1-mediated adhesion to FN23, while the more recently described integrins α4β1 and α9β1 bind specifically to EIIIA itself24,25. We confirmed that hepatic stellate cells express α5β126 and αvβ327 (Supplemental Figure 3) and found that they also express the α9 integrin subunit. We were unable to detect either protein or mRNA for α4 (Supplemental Figure 3B,C and data not shown). Culture on cFN versus pFN did not alter integrin expression (Supplemental Figure 3A).
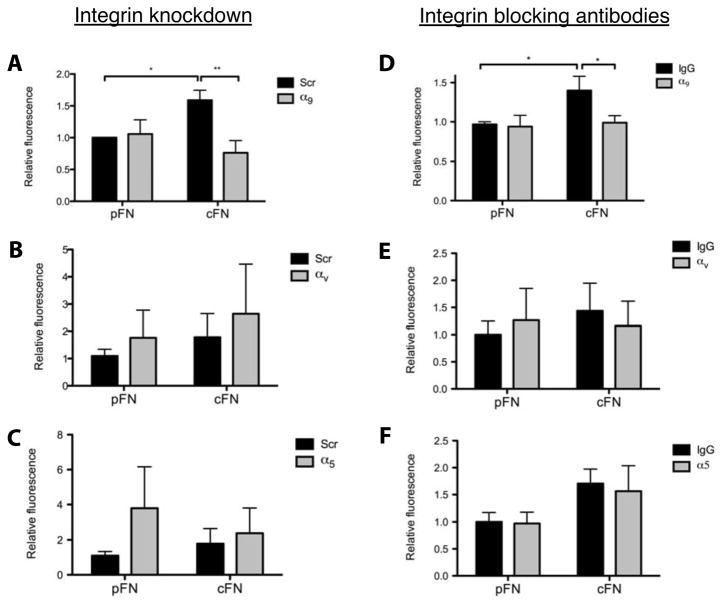
We used shRNAs to inhibit the expression of the α subunits in hepatic stellate cells. Successful knockdown was verified by qRT-PCR (Supplemental Figure 4A) and immunoblotting (Supplemental Figure 4B); knockdown of one subunit did not lead to compensatory upregulation of others. In transwell motility assays, knockdown of α9 attenuated chemotaxis on cFN (Figure 5A), whereas knockdown of αv or α5 had no effect or resulted in increased migration on both matrices (Figure 5B,C). Function blocking antibodies against the integrins yielded similar results (compare Figure 5D with 5E,F). Thus, integrin α9β1 is required for enhanced stellate cell chemotaxis on cFN. Not surprisingly, portal fibroblasts, which failed to show enhanced migration in the presence of EIIIA+ cFN (Figure 4D,E), do not express the integrin α9 or α4 subunits (Supplemental Figure 5 and data not shown).
Figure 5. α9β1 is required for increased stellate cell chemotaxis on cFN.
A–C. Stellate cells were cultured for 4 days then transduced with an adenovirus expressing either an integrin-targeting shRNA or a scrambled (scr) control. Transwell chemotaxis assay was performed 72 hours post-transduction. D–F. Cells were pre-incubated with blocking antibodies against α9β1, α5β1, or αvβ3 for 1 hour prior to beginning the assay. Error bars represent SEM from 3–4 experiments.
Male EIIIA−/− mice are protected from thioacetamide-induced liver fibrosis
To determine whether EIIIA is required for liver fibrosis, we treated EIIIA−/− mice, along with wild type littermates, with thioacetamide, which causes parenchymal injury, for 12 weeks. EIIIA did not significantly affect the degree of liver injury due to thioacetamide (Supplemental Figure 6A–D).
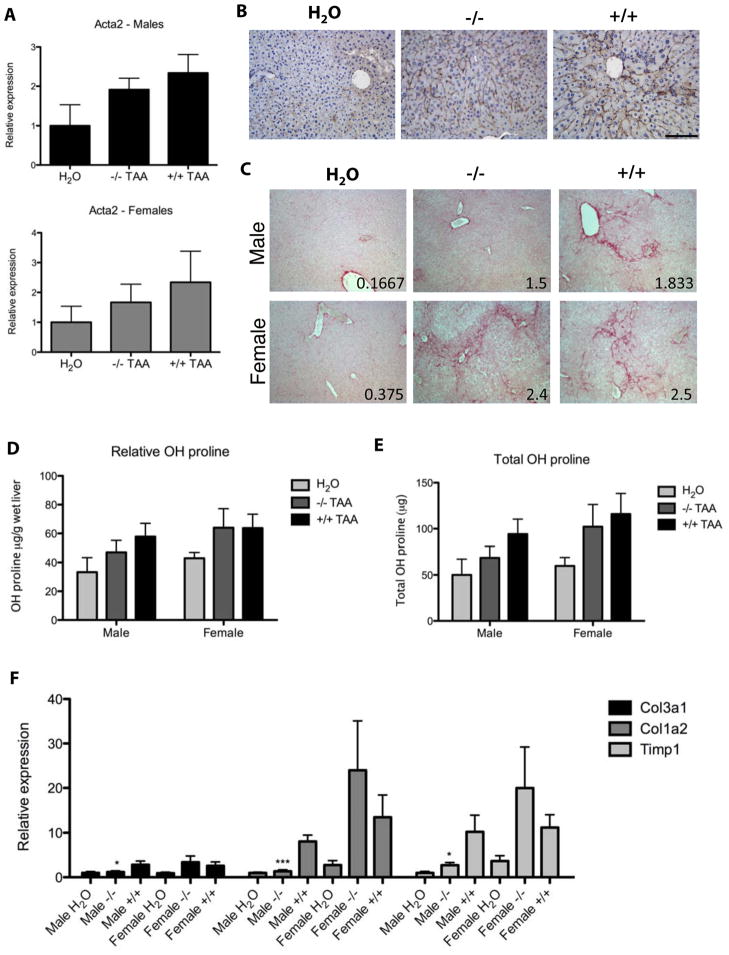
After 12 weeks of thioacetamide treatment, EIIIA−/− mice and wild type mice developed comparable numbers of myofibroblasts (Figure 6A,B). Surprisingly, we found that male, but not female EIIIA−/− mice were modestly protected from thioacetamide-induced fibrosis. Compared to male wild type littermates, male EIIIA−/− mice had less sirius red staining and a lower average fibrosis score, reduced hydroxyproline deposition, and lower expression of fibrillar collagens and tissue inhibitor of metalloproteinases I (TIMP1) (Figure 6C–F). In sharp contrast, female EIIIA−/− mice had similar degrees fibrosis as their female wild type littermates (Figure 6).
Figure 6. Male EIIIA−/− mice are protected from thioacetamide-induced fibrosis.
Mice were administered 200 mg/L thioacetamide for 12 weeks. n = 13 H2O control mice (7 male, 6 female), 11 EIIIA−/− mice (6 male, 5 female), and 12 wild type mice (6 male, 6 female). A. αSMA (Act2a) was measured by qRT-PCR and normalized to glyceraldehyde-3-phosphate dehydrogenase (Gapdh) and beta 2 microglobulin (B2M). B. Liver sections were stained for αSMA (brown) and with hematoxylin (blue). Scale bar = 100 μm. C. Sections were stained with sirius red. Fibrosis was scored by a blinded observer on a scale of 0 (normal) to 4 (cirrhosis). Mean fibrosis score is indicated in the bottom right corner of each photo. D–E. Hydroxyproline content was assayed and reported as relative (D) or total hydroxyproline (E). F. ECM transcripts were measured by qRT-PCR. Col1a2 = collagen I, Col3a1 = collagen III.
Wild type males and females developed a similar degree of fibrosis (Figure 6), and similar results were observed after 16 weeks of thioacetamide treatment (data not shown), suggesting that slower rates of fibrosis progression do not account for the phenotype in males. The different phenotypes are also unlikely to be explained by differences in fibronectin or integrin expression, though females had slightly higher baseline α5 expression (Supplemental Figure 7). Instead, this phenotype may be accounted for by differences in TGFβ. Female mice, regardless of EIIIA status, upregulated TGFβ after thioacetamide treatment, whereas male mice did not (Supplemental Figure 8A), and only in female mice was TGFβ expression correlated with collagen I expression (Supplemental Figure 8D,E), suggesting that regulation of EIIIA and TGFβ expression is uncoupled in this model and that either EIIIA or TGFβ upregulation is sufficient for fibrosis (Supplemental Figure 8F).
EIIIA−/− mice are not protected from bile duct ligation induced fibrosis
To determine whether EIIIA is required for biliary as opposed to parenchymal fibrosis, we subjected EIIIA−/− and wild type mice to ligation of the common bile duct for 14 days. Levels of injury were similar (Supplemental Figure 9A,B). EIIIA was markedly upregulated in wild type mice (Supplemental Figure 9C), and there was a modest increase in EIIIB+ cFN (but not total fibronectin) in both EIIIA−/− and wild type mice (Supplemental Figure 9C).
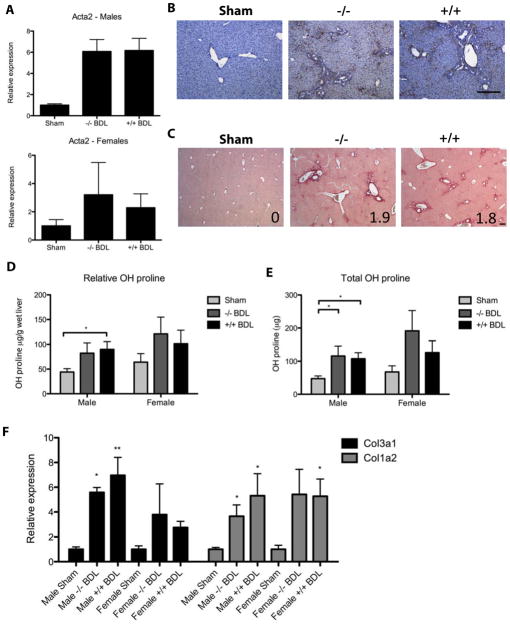
As seen in the thioacetamide model, EIIIA−/− and wild type mice demonstrated equivalent expression of αSMA (Figure 7A,B). They also developed similar degrees of fibrosis, as measured by liver hydroxyproline, sirius red staining, fibrosis score, and expression of fibrillar collagens (Figure 7C–F). Importantly, there were no gender-related differences in the susceptibility of EIIIA−/− mice or wild type mice to bile duct ligation induced fibrosis (Figure 7). Similar findings were seen in mice 6 days after bile duct ligation (data not shown).
Figure 7. EIIIA−/− and wild type mice develop equivalent fibrosis after bile duct ligation.
Mice were euthanized 14 days post-bile duct ligation. Depending on the experiment, n = 6–16 sham-operated animals (up to 10 male, 6 female), 12 EIIIA−/− mice (7 male, 5 female), and 13 wild type mice (7 male, 6 female). A. Liver sections were stained for αSMA (brown) and with hematoxylin (blue). Scale bar = 200 μm. B. αSMA transcript (Act2a) was measured by qRT-PCR and normalized to expression of 18s ribosomal rRNA and glucuronidase B (Gusb). C. Sections were stained with sirius red. Mean fibrosis score is indicated in the bottom right corner of each photo. D–E. Hydroxyproline content was assayed and reported as relative (D) or total hydroxyproline (E). F. Transcripts were measured by qRT-PCR. Col1a2 = collagen I and Col3a1 = collagen III. *, ** = significant over sham control.
Portal fibroblasts are the dominant myofibroblast precursors in biliary fibrosis
Although hepatic stellate cells are well established as the predominant source of ECM in parenchymal fibrosis, portal mesenchymal cells play an important role in biliary fibrosis28–31. In agreement with these reports, we found that, while there were many αSMA-expressing myofibroblasts in the portal region following bile duct ligation (Supplemental Figure 10A), there were few hepatic stellate cells (as determined by staining for desmin, red) (Supplemental Figure 10B). Instead, many cells in the area expressed elastin, a portal fibroblast marker (Supplemental Figure 10C,D)16. Immunostaining of fibrotic livers from mice that underwent bile duct ligation demonstrated many α9-expressing cells in the sinusoidal region, but, not surprisingly given that portal fibroblasts do not express α9, few in the portal scar (Supplemental Figure 10E). As expected, α9-expressing cells were found in the sinusoidal region after thioacetamide treatment (Supplemental Figure 10F).
Discussion
We demonstrate conclusively that EIIIA+ cFN is not required for myofibroblast differentiation in vitro or in vivo. Instead, it enhances motility in hepatic stellate cells but not portal fibroblasts, a phenotype that specifically requires EIIIA and integrin α9β1. Male EIIIA−/− mice are protected from thioacetamide-induced fibrosis, which primarily affects the sinusoids, but not from bile duct ligation-induced fibrosis, which affects the portal area and may depend more on portal fibroblasts28–31. The data provide proof of concept that subtle changes in the ECM can have large effects on myofibroblast behavior and liver fibrosis, and they highlight a previously unappreciated function of EIIIA+ cFN in promoting stellate cell motility rather than myofibroblast differentiation.
Although some investigators have suggested that EIIIA+ cFN drives myofibroblast differentiation8, 9,14, we found no difference in αSMA expression in cells cultured on pFN versus cFN, perhaps because we employed a culture system in which substrate stiffness is within a physiologic range. Fibronectin unfolding and fibrillogenesis are cell-driven mechanosensitive processes32–34 that may be stimulated by splicing in of EIIIA and EIIIB7, 35–37. Plastic and glass have stiffnesses in the GPa range, orders of magnitude stiffer than even a cirrhotic liver, and fibronectin adherent to these surfaces may be non-physiologically rigid. Additionally, fibronectin conformation is regulated by the chemistry of the substrate38, and adhesion is different on native matrix versus glass-adsorbed fibronectin39. Thus, for both chemical and mechanical reasons, it is likely that cells on plastic or glass are prevented from dynamically interacting with fibronectin in a physiologic conformation.
In line with our cell culture results, all mice developed myofibroblasts in vivo, regardless of sex, genotype, or model of fibrosis. Our in vitro data suggest that EIIIA instead acts on motility. An emerging body of literature supports a role for α9β1 and EIIIA+ cFN in cell motility40–45, although few studies have examined the pair together. Endothelial cell specific α9-knockout mice have impaired lymphatic valve formation due to a failure of fibronectin fibril assembly, a phenotype that is partly copied by EIIIA−/− mice37. EIIIA and α9β1 may also promote lymphangiogenesis in colon cancer46, 47, and inhibiting α9β1 has been demonstrated to impair the formation of granulation tissue during wound healing48.
EIIIA may also exert its effects on myofibroblasts by acting synergistically with TGFβ: TGFβ can promote splicing in of EIIIA49–52, EIIIA+ cFN is required for TGFβ-dependent induction of αSMA expression in dermal myofibroblasts53, and EIIIA−/− pulmonary myofibroblasts fail to activate TGFβ from the ECM9. Interestingly, multiple studies examining the relationship between EIIIA+ cFN and TGFβ have demonstrated different results in cell culture systems versus whole animal models. Moriya et al. recently reported that liver-specific total FN−/− mice display normal collagen fibril formation in vivo but not in vitro, because only in vivo do the mice upregulate TGFβ, which compensates for lack of FN15. Here we report a similar phenomenon: in vitro the effects of EIIIA+ cFN on liver myofibroblasts appeared to be independent of TGFβ (Figure 1, Supplemental Figure 1), whereas in vivo we observed a more complicated relationship between EIIIA and TGFβ (Supplemental Figure 8).
In addition to differences between in vitro and in vivo results, the relationship between EIIIA and TGFβ may be further complicated by sex specific differences in TGFβ expression. In males, TGFβ was not upregulated (Supplemental Figure 8A), nor was its expression correlated with that of fibrosis markers (Supplemental Figure 8B). Instead, expression of EIIIA determined the degree of fibrosis (Supplemental Figure 8C). In contrast, in females, TGFβ was upregulated (Supplemental Figure 8A) and its expression correlated with markers of fibrosis (Supplemental Figure 8D), whereas EIIIA status did not (Supplemental Figure 8E).
Others have also reported sex-specific differences in TGFβ signaling. Lane et al. found that while female rats had greater renal TGFβ expression than did males, male rats were more efficient at activating TGFβ54. Furthermore, adult female rats had higher renal TGFβ levels than did ovariectomized female rats in a model of diabetes mellitus, supporting a link between estrogen and TGFβ expression55. It is possible that wild type males express little TGFβ but activate it efficiently, whereas wild type females overcome their inefficient activation by expressing more. EIIIA−/− mice of both sexes may have impaired TGFβ activation, as has been suggested in the literature, but female EIIIA−/− mice are able to overcome this by producing more TGFβ. Future studies will need to be conducted to validate this model (Supplemental Figure 8F).
Migration of myofibroblasts to areas of tissue injury is a critical early event in both wound healing and fibrosis and our data may have implications for understanding the basic nature of these processes. Quiescent hepatic stellate cells have an innate ability to undergo chemotaxis, suggesting that they may be poised to respond to injury, and this capacity increases further as they differentiate to myofibroblasts56. Many signaling pathways have been demonstrated to play a role in stellate cell motility57, 58, and sophisticated culture systems are now allowing mechanistic exploration of the unique cellular structures required59, 60. Furthermore, over-expression of one downstream integrin signaling molecule known to promote stellate cell motility, integrin-linked kinase, is sufficient to exaggerate fibrotic response61, providing strong evidence that motility can drive fibrosis. A better understanding of downstream signaling effectors of α9β1 may suggest other mediators of motility40,62, and EIIIA and α9β1 represent attractive, novel targets for exploring myofibroblast motility during fibrosis.
Supplementary Material
Acknowledgments
Grant support: This work was supported by NIH R01 DK-058123 (to R.G.W.) and NIH F30 DK081265-01 (to A.L.O.).
We thank Hyunsook Ahn, Ariel Lefkovith, and Jia-Ji Hui for technical contributions, as well as the Morphology Core of the NIDDK Center for the Study of Digestive and Liver Diseases (P30 DK50306) and the Biomedical Imaging Core Laboratory of the Department of Pathology and Laboratory Medicine, both at the University of Pennsylvania, and the Wistar Institute Microscopy Core Facility. This work was supported by NIH R01 DK-058123 (to R.G.W.) and NIH F30 DK081265-01 (to A.L.O.).
Abbreviations
- EIIIA
extra domain A
- α-SMA
α-smooth muscle actin
- ECM
extracellular matrix
- PDGF
platelet derived growth factor
- TGFβ
transforming growth factor β
- EIIIB
extra domain B
Footnotes
Disclosures: The authors have nothing to disclose.
Author contributions: Abby Olsen helped design, perform, and interpret experiments, and wrote the manuscript. Bridget Sackey helped design, perform, and interpret experiments. Cezary Marcinkiewiecz contributed intellectually at preliminary stages of the project, provided essential reagents, and aided in data interpretation. David Boettiger provided intellectual input and assisted in interpreting data. Rebecca Wells supervised all aspects of the work, helped design all experiments, provided intellectual input, aided in writing the manuscript, and obtained funding.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hynes RO, Yamada KM. Fibronectins: multifunctional modular glycoproteins. J Cell Biol. 1982;95:369–77. doi: 10.1083/jcb.95.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwarzbauer JE, Tamkun JW, Lemischka IR, Hynes RO. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell. 1983;35:421–31. doi: 10.1016/0092-8674(83)90175-7. [DOI] [PubMed] [Google Scholar]
- 3.Schwarzbauer JE, Patel RS, Fonda D, Hynes RO. Multiple sites of alternative splicing of the rat fibronectin gene transcript. EMBO J. 1987;6:2573–80. doi: 10.1002/j.1460-2075.1987.tb02547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peters JH, Hynes RO. Fibronectin isoform distribution in the mouse. I. The alternatively spliced EIIIB, EIIIA, and V segments show widespread codistribution in the developing mouse embryo. Cell Adhes Commun. 1996;4:103–25. doi: 10.3109/15419069609010766. [DOI] [PubMed] [Google Scholar]
- 5.Astrof S, Crowley D, Hynes RO. Multiple cardiovascular defects caused by the absence of alternatively spliced segments of fibronectin. Dev Biol. 2007;311:11–24. doi: 10.1016/j.ydbio.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peters JH, Chen GE, Hynes RO. Fibronectin isoform distribution in the mouse. II. Differential distribution of the alternatively spliced EIIIB, EIIIA, and V segments in the adult mouse. Cell Adhes Commun. 1996;4:127–48. doi: 10.3109/15419069609010767. [DOI] [PubMed] [Google Scholar]
- 7.White ES, Baralle FE, Muro AF. New insights into form and function of fibronectin splice variants. J Pathol. 2008;216:1–14. doi: 10.1002/path.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L, Gabbiani G. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol. 1998;142:873–81. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muro AF, Moretti FA, Moore BB, Yan M, Atrasz RG, Wilke CA, Flaherty KR, Martinez FJ, Tsui JL, Sheppard D, Baralle FE, Toews GB, White ES. An essential role for fibronectin extra type III domain A in pulmonary fibrosis. Am J Respir CritCare Med. 2008;177:638–45. doi: 10.1164/rccm.200708-1291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan MH, Sun Z, Opitz SL, Schmidt TE, Peters JH, George EL. Deletion of the alternatively spliced fibronectin EIIIA domain in mice reduces atherosclerosis. Blood. 2004;104:11–8. doi: 10.1182/blood-2003-09-3363. [DOI] [PubMed] [Google Scholar]
- 11.Muro AF, Chauhan AK, Gajovic S, Iaconcig A, Porro F, Stanta G, Baralle FE. Regulated splicing of the fibronectin EDA exon is essential for proper skin wound healing and normal lifespan. J Cell Biol. 2003;162:149–60. doi: 10.1083/jcb.200212079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.George J, Wang SS, Sevcsik AM, Sanicola M, Cate RL, Koteliansky VE, Bissell DM. Transforming growth factor-beta initiates wound repair in rat liver through induction of the EIIIA-fibronectin splice isoform. Am J Pathol. 2000;156:115–24. doi: 10.1016/s0002-9440(10)64711-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang ML, Chen JC, Alonso CR, Kornblihtt AR, Bissell DM. Regulation of fibronectin splicing in sinusoidal endothelial cells from normal or injured liver. Proc Natl Acad Sci U S A. 2004;101:18093–8. doi: 10.1073/pnas.0408439102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarnagin WR, Rockey DC, Koteliansky VE, Wang SS, Bissell DM. Expression of variant fibronectins in wound healing: cellular source and biological activity of the EIIIA segment in rat hepatic fibrogenesis. J Cell Biol. 1994;127:2037–48. doi: 10.1083/jcb.127.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moriya K, Bae E, Honda K, Sakai K, Sakaguchi T, Tsujimoto I, Kamisoyama H, Keene DR, Sasaki T, Sakai T. A Fibronectin-Independent Mechanism of Collagen Fibrillogenesis in Adult Liver Remodeling. Gastroenterology. doi: 10.1053/j.gastro.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z, Dranoff JA, Chan EP, Uemura M, Sevigny J, Wells RG. Transforming growth factor-beta and substrate stiffness regulate portal fibroblast activation in culture. Hepatology. 2007;46:1246–56. doi: 10.1002/hep.21792. [DOI] [PubMed] [Google Scholar]
- 17.Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94:13661–5. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byfield FJ, Wen Q, Levental I, Nordstrom K, Arratia PE, Miller RT, Janmey PA. Absence of filamin A prevents cells from responding to stiffness gradients on gels coated with collagen but not fibronectin. Biophys J. 2009;96:5095–102. doi: 10.1016/j.bpj.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flanagan LA, Ju YE, Marg B, Osterfield M, Janmey PA. Neurite branching on deformable substrates. Neuroreport. 2002;13:2411–5. doi: 10.1097/01.wnr.0000048003.96487.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, Ming W, Weaver V, Janmey PA. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 21.Georges PC, Hui JJ, Gombos Z, McCormick ME, Wang AY, Uemura M, Mick R, Janmey PA, Furth EE, Wells RG. Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1147–54. doi: 10.1152/ajpgi.00032.2007. [DOI] [PubMed] [Google Scholar]
- 22.Olsen AL, Bloomer SA, Chan EP, Gaca MD, Georges PC, Sackey B, Uemura M, Janmey PA, Wells RG. Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. Am J Physiol Gastrointest Liver Physiol. 301:G110–8. doi: 10.1152/ajpgi.00412.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manabe R, Ohe N, Maeda T, Fukuda T, Sekiguchi K. Modulation of cell-adhesive activity of fibronectin by the alternatively spliced EDA segment. J Cell Biol. 1997;139:295–307. doi: 10.1083/jcb.139.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao YF, Gotwals PJ, Koteliansky VE, Sheppard D, Van De Water L. The EIIIA segment of fibronectin is a ligand for integrins alpha 9beta 1 and alpha 4beta 1 providing a novel mechanism for regulating cell adhesion by alternative splicing. J Biol Chem. 2002;277:14467–74. doi: 10.1074/jbc.M201100200. [DOI] [PubMed] [Google Scholar]
- 25.Shinde AV, Bystroff C, Wang C, Vogelezang MG, Vincent PA, Hynes RO, Van De Water L. Identification of the peptide sequences within the EIIIA (EDA) segment of fibronectin that mediate integrin alpha9beta1-dependent cellular activities. J Biol Chem. 2008;283:2858–70. doi: 10.1074/jbc.M708306200. [DOI] [PubMed] [Google Scholar]
- 26.Zhou X, Zhang Y, Zhang J, Zhu H, Du W, Zhang X, Chen Q. Expression of fibronectin receptor, integrin alpha 5 beta 1 of hepatic stellate cells in rat liver fibrosis. Chin Med J (Engl) 2000;113:272–6. [PubMed] [Google Scholar]
- 27.Zhou X, Murphy FR, Gehdu N, Zhang J, Iredale JP, Benyon RC. Engagement of alphavbeta3 integrin regulates proliferation and apoptosis of hepatic stellate cells. J Biol Chem. 2004;279:23996–4006. doi: 10.1074/jbc.M311668200. [DOI] [PubMed] [Google Scholar]
- 28.Tang L, Tanaka Y, Marumo F, Sato C. Phenotypic change in portal fibroblasts in biliary fibrosis. Liver. 1994;14:76–82. doi: 10.1111/j.1600-0676.1994.tb00051.x. [DOI] [PubMed] [Google Scholar]
- 29.Tuchweber B, Desmouliere A, Bochaton-Piallat ML, Rubbia-Brandt L, Gabbiani G. Proliferation and phenotypic modulation of portal fibroblasts in the early stages of cholestatic fibrosis in the rat. Lab Invest. 1996;74:265–78. [PubMed] [Google Scholar]
- 30.Kinnman N, Francoz C, Barbu V, Wendum D, Rey C, Hultcrantz R, Poupon R, Housset C. The myofibroblastic conversion of peribiliary fibrogenic cells distinct from hepatic stellate cells is stimulated by plateleterived growth factor during liver fibrogenesis. Lab Invest. 2003;83:163–73. doi: 10.1097/01.lab.0000054178.01162.e4. [DOI] [PubMed] [Google Scholar]
- 31.Beaussier M, Wendum D, Schiffer E, Dumont S, Rey C, Lienhart A, Housset C. Prominent contribution of portal mesenchymal cells to liver fibrosis in ischemic and obstructive cholestatic injuries. Lab Invest. 2007;87:292–303. doi: 10.1038/labinvest.3700513. [DOI] [PubMed] [Google Scholar]
- 32.Mao Y, Schwarzbauer JE. Fibronectin fibrillogenesis, a cellediated matrix assembly process. Matrix Biol. 2005;24:389–99. doi: 10.1016/j.matbio.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 33.Antia M, Baneyx G, Kubow KE, Vogel V. Fibronectin in aging extracellular matrix fibrils is progressively unfolded by cells and elicits an enhanced rigidity response. Faraday Discuss. 2008;139:229–49. doi: 10.1039/b718714a. discussion 309–25, 419–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klotzsch E, Smith ML, Kubow KE, Muntwyler S, Little WC, Beyeler F, Gourdon D, Nelson BJ, Vogel V. Fibronectin forms the most extensible biological fibers displaying switchable force-exposed cryptic binding sites. Proc Natl Acad Sci U S A. 2009;106:18267–72. doi: 10.1073/pnas.0907518106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukuda T, Yoshida N, Kataoka Y, Manabe R, Mizuno-Horikawa Y, Sato M, Kuriyama K, Yasui N, Sekiguchi K. Mice lacking the EDB segment of fibronectin develop normally but exhibit reduced cell growth and fibronectin matrix assembly in vitro. Cancer Res. 2002;62:5603–10. [PubMed] [Google Scholar]
- 36.Abe Y, Bui-Thanh NA, Ballantyne CM, Burns AR. Extra domain A and type III connecting segment of fibronectin in assembly and cleavage. Biochem Biophys Res Commun. 2005;338:1640–7. doi: 10.1016/j.bbrc.2005.10.134. [DOI] [PubMed] [Google Scholar]
- 37.Bazigou E, Xie S, Chen C, Weston A, Miura N, Sorokin L, Adams R, Muro AF, Sheppard D, Makinen T. Integrin-alpha9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev Cell. 2009;17:175–86. doi: 10.1016/j.devcel.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia AJ, Vega MD, Boettiger D. Modulation of cell proliferation and differentiation through substrate-dependent changes in fibronectin conformation. Mol Biol Cell. 1999;10:785–98. doi: 10.1091/mbc.10.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Engler AJ, Chan M, Boettiger D, Schwarzbauer JE. A novel mode of cell detachment from fibrillar fibronectin matrix under shear. J Cell Sci. 2009;122:1647–53. doi: 10.1242/jcs.040824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta SK, Vlahakis NE. Integrin alpha9beta1 mediates enhanced cell migration through nitric oxide synthase activity regulated by Src tyrosine kinase. J Cell Sci. 2009;122:2043–54. doi: 10.1242/jcs.041632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ito K, Kon S, Nakayama Y, Kurotaki D, Saito Y, Kanayama M, Kimura C, Diao H, Morimoto J, Matsui Y, Uede T. The differential amino acid requirement within osteopontin in alpha4 and alpha9 integrin-mediated cell binding and migration. Matrix Biol. 2009;28:11–9. doi: 10.1016/j.matbio.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Staniszewska I, Sariyer IK, Lecht S, Brown MC, Walsh EM, Tuszynski GP, Safak M, Lazarovici P, Marcinkiewicz C. Integrin alpha9 beta1 is a receptor for nerve growth factor and other neurotrophins. J Cell Sci. 2008;121:504–13. doi: 10.1242/jcs.000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taooka Y, Chen J, Yednock T, Sheppard D. The integrin alpha9beta1 mediates adhesion to activated endothelial cells and transendothelial neutrophil migration through interaction with vascular cell adhesion molecule-1. J Cell Biol. 1999;145:413–20. doi: 10.1083/jcb.145.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inoue T, Nabeshima K, Shimao Y, Meng JY, Koono M. Regulation of fibronectin expression and splicing in migrating epithelial cells: migrating MDCK cells produce a lesser amount of, but more active, fibronectin. Biochem Biophys Res Commun. 2001;280:1262–8. doi: 10.1006/bbrc.2001.4264. [DOI] [PubMed] [Google Scholar]
- 45.Shimao Y, Nabeshima K, Inoue T, Koono M. Role of fibroblasts in HGF/SF-induced cohort migration of human colorectal carcinoma cells: fibroblasts stimulate migration associated with increased fibronectin production via upregulated TGF-beta1. Int J Cancer. 1999;82:449–58. doi: 10.1002/(sici)1097-0215(19990730)82:3<449::aid-ijc20>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 46.Ou JJ, Wu F, Liang HJ. Colorectal tumor derived fibronectin alternatively spliced EDA domain exserts lymphangiogenic effect on human lymphatic endothelial cells. Cancer Biol Ther. 9:186–91. doi: 10.4161/cbt.9.3.10651. [DOI] [PubMed] [Google Scholar]
- 47.Ou J, Li J, Pan F, Xie G, Zhou Q, Huang H, Liang H. Endostatin suppresses colorectal tumor-induced lymphangiogenesis by inhibiting expression of fibronectinextra domain A and integrin alpha9. J Cell Biochem. doi: 10.1002/jcb.23130. [DOI] [PubMed] [Google Scholar]
- 48.Nakayama Y, Kon S, Kurotaki D, Morimoto J, Matsui Y, Uede T. Blockade of interaction of alpha9 integrin with its ligands hinders the formation of granulation in cutaneous wound healing. Lab Invest. 90:881–94. doi: 10.1038/labinvest.2010.69. [DOI] [PubMed] [Google Scholar]
- 49.Hackett TL, Warner SM, Stefanowicz D, Shaheen F, Pechkovsky DV, Murray LA, Argentieri R, Kicic A, Stick SM, Bai TR, Knight DA. Induction of epithelial-mesenchymal transition in primary airway epithelial cells from patients with asthma by transforming growth factor-beta1. Am J Respir Crit Care Med. 2009;180:122–33. doi: 10.1164/rccm.200811-1730OC. [DOI] [PubMed] [Google Scholar]
- 50.Baelde HJ, Eikmans M, van Vliet AI, Bergijk EC, de Heer E, Bruijn JA. Alternatively spliced isoforms of fibronectin in immune-mediated glomerulosclerosis: the role of TGFbeta and IL-4. J Pathol. 2004;204:248–57. doi: 10.1002/path.1653. [DOI] [PubMed] [Google Scholar]
- 51.Li J, Tripathi BJ, Tripathi RC. Modulation of pre-mRNA splicing and protein production of fibronectin by TGF-beta2 in porcine trabecular cells. Invest Ophthalmol Vis Sci. 2000;41:3437–43. [PubMed] [Google Scholar]
- 52.Inoue T, Nabeshima K, Shimao Y, Koono M. Hepatocyte growth Factor/Scatter factor (HGF/SF) is a regulator of fibronectin splicing in MDCK cells: comparison between the effects of HGF/SF and TGF-beta1 on fibronectin splicing at the EDA region. Biochem Biophys Res Commun. 1999;260:225–31. doi: 10.1006/bbrc.1999.0881. [DOI] [PubMed] [Google Scholar]
- 53.Dugina V, Fontao L, Chaponnier C, Vasiliev J, Gabbiani G. Focal adhesion features during myofibroblastic differentiation are controlled by intracellular and extracellular factors. J Cell Sci. 2001;114:3285–96. doi: 10.1242/jcs.114.18.3285. [DOI] [PubMed] [Google Scholar]
- 54.Lane PH, Snelling DM, Babushkina-Patz N, Langer WJ. Sex differences in the renal transforming growth factor-beta 1 system after puberty. Pediatr Nephrol. 2001;16:61–8. doi: 10.1007/s004670000502. [DOI] [PubMed] [Google Scholar]
- 55.Lane PH, Sun J, Devish K, Langer WJ. Dissociation of renal TGF-beta and hypertrophy in female rats with diabetes mellitus. Am J Physiol Renal Physiol. 2004;287:F1011–20. doi: 10.1152/ajprenal.00031.2004. [DOI] [PubMed] [Google Scholar]
- 56.Ikeda K, Wakahara T, Wang YQ, Kadoya H, Kawada N, Kaneda K. In vitro migratory potential of rat quiescent hepatic stellate cells and its augmentation by cell activation. Hepatology. 1999;29:1760–7. doi: 10.1002/hep.510290640. [DOI] [PubMed] [Google Scholar]
- 57.Yang C, Zeisberg M, Mosterman B, Sudhakar A, Yerramalla U, Holthaus K, Xu L, Eng F, Afdhal N, Kalluri R. Liver fibrosis: insights into migration of hepatic stellate cells in response to extracellular matrix and growth factors. Gastroenterology. 2003;124:147–59. doi: 10.1053/gast.2003.50012. [DOI] [PubMed] [Google Scholar]
- 58.Marra F, Gentilini A, Pinzani M, Choudhury GG, Parola M, Herbst H, Dianzani MU, Laffi G, Abboud HE, Gentilini P. Phosphatidylinositol 3-kinase is required for platelet-derived growth factor’s actions on hepatic stellate cells. Gastroenterology. 1997;112:1297–306. doi: 10.1016/s0016-5085(97)70144-6. [DOI] [PubMed] [Google Scholar]
- 59.Melton AC, Soon RK, Jr, Park JG, Martinez L, Dehart GW, Yee HF., Jr Focal adhesion disassembly is an essential early event in hepatic stellate cell chemotaxis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1272–80. doi: 10.1152/ajpgi.00134.2007. [DOI] [PubMed] [Google Scholar]
- 60.Melton AC, Yee HF. Hepatic stellate cell protrusions couple platelet-derived growth factor-BB to chemotaxis. Hepatology. 2007;45:1446–53. doi: 10.1002/hep.21606. [DOI] [PubMed] [Google Scholar]
- 61.Shafiei MS, Rockey DC. The role of integrin-linked kinase in liver wound healing. J Biol Chem. 2006;281:24863–72. doi: 10.1074/jbc.M513544200. [DOI] [PubMed] [Google Scholar]
- 62.deHart GW, Jin T, McCloskey DE, Pegg AE, Sheppard D. The alpha9beta1 integrin enhances cell migration by polyamine-mediated modulation of an inward-rectifier potassium channel. Proc Natl Acad Sci U S A. 2008;105:7188–93. doi: 10.1073/pnas.0708044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.