Abstract
Background & Aims
Little is known about long-term health outcomes of children with dyspeptic symptoms. We studied the natural history of pediatric patients with dyspeptic symptoms, with and without histologic reflux, compared to healthy controls.
Methods
We performed a prospective study of consecutive new patients, ages 8–16 years, who underwent evaluation for dyspepsia, including upper endoscopy. Patients were assigned to groups with histologic evidence of reflux esophagitis (n=50), or normal histology results (n=53). Healthy children were followed as controls (n=143). Patients and controls were evaluated 5–15 years later. They provided self reports on severity of dyspeptic symptoms, use of acid suppression, quality of life, anxiety, and depression.
Results
When the study began, the groups with histologic evidence for esophagitis and normal histologies did not differ in severity of dyspeptic symptoms, functional disability, or depression. After a mean 7.6-year follow-up period, each group had significantly lower quality of life scores and more severe dyspeptic symptoms and functional disability than controls, but did not differ significantly from each other; both groups were significantly more likely than controls to meet criteria for an anxiety disorder. At time of follow-up, use of acid suppression medication was significantly greater in the group with histologic evidence for esophagitis, compared with patients that had normal histology findings when the study began.
Conclusion
Among pediatric patients with dyspepsia evaluated by endoscopy and biopsy, those with histologic evidence for esophagitis or normal histology findings are at increased risk for chronic dyspeptic symptoms, anxiety disorder, and reduced quality of life in adolescence and young adulthood.
Keywords: gastroesophageal reflux, functional dyspepsia
Background
Gastroesophageal reflux disease (GERD) affects children as well as adults and carries the highest economic cost of any gastrointestinal illness in the United States 1. In adults, GERD is appreciated as a chronic disease that may be progressive over time 2–3. Several retrospective studies of adult patients with GERD have identified childhood GERD to be a risk factor for GERD in adulthood 4–6. However, most pediatricians believe that the majority of infants with GERD “outgrow” their symptoms by one year of age, even though mucosal histology may remain abnormal 7–11. The few pediatric studies that have followed pediatric GERD patients beyond infancy have yielded conflicting results regarding outcomes 12–16. To date, no studies have followed children with GERD prospectively into adolescence and adulthood to assess their health outcomes.
Treatment of dyspeptic symptoms with acid suppressant medication may increase the risk for enteric infections, pneumonia, fractures, and micronutrient deficiencies 17–18. Untreated GERD, however, is associated with serious complications such as esophageal ulcerations, peptic stricture, Barrett's esophagus, adenocarcinoma and extraesophageal disease 19. Thus, it is important to know the extent to which dyspeptic symptoms and use of acid suppression persist beyond childhood, with the ultimate goal to develop treatment strategies that can minimize both complications of GERD and long term use of acid suppression 20.
Consensus statements emphasize the importance of symptom report and quality of life as indicators of outcome for GERD and functional dyspepsia (FD) 21–23. Both GERD and FD have been linked to poor quality of life among adults in the general population 24–26 and one study has linked pediatric GERD to poor quality of life 27. In addition to an association with general measures of quality of life, dyspeptic symptoms and GERD have been associated with anxiety and depression in adults 28–30. No studies have examined the association of dyspepsia or GERD to anxiety and depression in children.
This study aimed to describe the natural history of a prospective cohort of pediatric patients with dyspeptic symptoms who underwent upper endoscopy with biopsy as part of their pediatric gastroenterology subspecialty evaluation. Based on endscopy results, patients were classified into two groups – those with and those without histologic esophagitis‥ Although dyspepsia with histologic esophagitis is associated with a diagnosis of GERD and dyspepsia with normal esophageal histology is associated with a diagnosis of FD31, we described the groups more descriptively as dyspepsia with and without histologic esophagitis. Patients with and without histologic esophagitis were compared to healthy controls that also were followed prospectively.
Outcomes evaluated at follow-up in adolescence and young adulthood included severity of dyspeptic symptoms, use of acid suppression medication, quality of life, anxiety, and depression. The null hypothesis was that the reflux esophagitis, normal esophageal histology and control groups would not differ significantly on these variables at long-term follow-up.
Materials and Methods
Sample
The study entailed a secondary analysis of an existing database of pediatric patients and community controls. The patient sample was drawn from several studies conducted by Walker and colleagues between 1993 and 2004 e.g. 32–33. Consecutive new patients referred to Vanderbilt Pediatric Gastroenterology Clinic for evaluation of abdominal pain were eligible for those studies if they were between the ages of 8 and 16 years, had abdominal pain of at least 3 months’ duration, no chronic illness or disability, living with at least one parent, and English speaking. Study participants were interviewed and completed questionnaires in the waiting room prior to the child’s medical evaluation. Participants consented to be contacted regarding participation in future studies.
The subgroup of patients in the original studies whose medical evaluation included upper endoscopy with biopsies of the esophagus performed within six months of the initial clinic visit were eligible for the present study if their biopsies were normal or were consistent with reflux esophagitis. Histologic reflux esophagitis was defined by basal cell hyperplasia, spongiosis, and presence of intraepithelial eosinophils 34. Patients with evidence of infectious esophagitis, duodenitis, peptic ulcer disease, chronic active gastritis, or villous blunting were excluded. Patients with more than twenty eosinophils per high power field were excluded due to possible overlap with eosinophilic esophagitis. Patients also were excluded if the medical evaluation resulted in a diagnosis of Crohn’s disease, celiac disease, or other significant organic disease. Helicobacter pylori infection was not a reason for excluding patients with reflux esophagitis.
For the purposes of this study, dyspeptic symptoms included patient report of the following items on a symptom questionnaire: chest pain, abdominal pain, lump in throat, nausea, difficulty swallowing, vomiting, bloating, and food making you sick. Patients with fewer than two dyspeptic symptoms at initial evaluation were excluded from the follow-up study.
The healthy control sample for the current study was obtained from control samples in Walker and colleagues’ prior studies during the same time period e.g. 32–33. Participants for those samples were recruited from community schools and were eligible for the present follow up study if they had no chronic illness and no abdominal pain in the month preceding initial study participation.
Procedure
Following approval of the Vanderbilt Institutional Review Board, participants were contacted by telephone or mail by the research coordinator. The coordinator described the study and scheduled an appointment to administer the study protocol by telephone. Parents of adolescent participants were given information about the study and gave consent for the adolescent to be contacted for assent. The follow up interval ranged from 5 to 15 years after the baseline assessment for the original study and was conducted during the years 2008–2011 when participants ranged in age from 12 to 32 years.
At the beginning of the phone interview, the experimenter confirmed consent and assent‥ The experimenter administered self-report questionnaires orally and provided participants with response options for ratings as appropriate. The health services questionnaire was completed by parents for participants less than 18 years of age. All interviews were audio recorded to allow review for accuracy. After completing the telephone interview, participants were sent the STAI-T and CES-D questionnaires to complete on-line or to return in written format.
Measures
The measures examined at baseline and follow-up are listed in Figure 1 and further described below:
Figure 1.
Measures Administered at Baseline and Follow-Up
Abdominal pain severity
The severity of abdominal pain was assessed with the patient-report Abdominal Pain Index 35. This measure comprises five items assessing the frequency, duration, and intensity of abdominal pain episodes experienced during the previous 2 weeks. A total severity score, ranging from 0 to 4, is a composite of these ratings.
Dyspeptic symptom severity
Severity of dyspeptic symptoms was evaluated with 8 items from the Children's Somatization Inventory (CSI) that assess dyspeptic symptoms including chest pain, abdominal pain, nausea, vomiting, difficulty swallowing, lump in the throat, bloating, and food making you sick 36. The stem for symptom report on the CSI is, "In the past two weeks, how much were you bothered by (symptom)?” The response format for each question is a 5-point scale ranging from "not at all" (0) to "a whole lot" (4). Responses to the eight dyspeptic scores were summed to calculate a total score for severity of dyspeptic symptoms.
Health services utilization questionnaire
A questionnaire regarding recent health service utilization was administered at follow-up as a self-report questionnaire for adult participants and as a parent-report questionnaire for participants under the age of 18 years. Participants were asked to report the use of prescription or over- the- counter acid suppression medication during the previous 3 months as well as any history of Nissen fundoplication. Participants also were asked if they had ever been diagnosed with inflammatory bowel or other GI disease by a physician or medical professional.
Psychological functioning
Depressive symptoms were assessed at baseline in the original studies with the Children’s Depression Inventory (CDI), a validated self-report measure for children between 7– 17 years of age 37–38. A total CDI score is computed with a higher score indicating greater severity of depressive symptoms. At follow-up, depressive symptoms were assessed with a validated self-report measure for adolescents and adults, the Center for Epidemiological Studies-Depression Scale (CES-D) 39. The CES-D assesses the frequency of 20 depressive symptoms during the past week. Scoring is a simple sum of item responses with a higher score indicating greater severity of depressive symptoms. Also at follow up, the State-Trait Anxiety Inventory, Trait Form (STAI-T) was administered 40. This validated 20-item self-report measure assesses the tendency to experience anxiety symptoms. Responses are summed to obtain a total score with a higher score indicating greater frequency and number of symptoms of anxiety.
In addition to self-report measures of anxiety and depression, a semi-structured psychiatric diagnostic interview -- the Anxiety Disorders Interview Schedule IV (ADIS) – was administered at follow up by trained mental health professionals to assess DSM-IV criteria for anxiety and mood disorders 41–42. Anxiety disorders included separation anxiety, panic, agoraphobia, social anxiety, generalized anxiety disorder, obsessive compulsive disorder, specific phobia, post-traumatic stress disorder, or anxiety disorder not otherwise specified. Mood disorders included major depressive disorder, dysthmia, or depressive disorder not otherwise specified. Participants were evaluated for current presence of each disorder as well as lifetime history of the disorder. For participants under 18 years, the participant and a parent were interviewed separately and results were collated to determine whether the participant met diagnostic criteria for any psychiatric disorder. The interviewer was blind to the health status of participants.
Quality of life
Health-related impairment in activities, an aspect of quality of life, was assessed both at baseline and at follow-up with the Functional Disability Inventory (FDI) 43–44. This validated self-report measure assesses difficulty in physical and psychosocial functioning due to physical health during the previous two weeks. Respondents rate 15 items on a 5-point scale, ranging from (0) no trouble to (4) impossible, and these ratings are summed to yield a total score that can range from 0 to 60. Higher scores indicate greater disability.
At follow-up, an additional measure of health-related quality of life also was administered. The Short Form Health Survey (SF-36) is a 36-item self-report questionnaire that assesses eight dimensions of health: 1) limitations in physical activities because of health problems; 2) limitations in social activities due to physical or emotional problems; 3) limitations in usual role activities because of physical health problems; 4) bodily pain; 5) general mental health including psychological distress and well-being; 6) limitations in usual role activities because of emotional problems; 7) vitality including energy and fatigue; and 8) general health perceptions 45. The eight scales are aggregated to create two component scores, the physical component score (PCS) and mental component score (MCS). A total summary score is also calculated.
Demographic variables
Demographic information collected at baseline and follow-up included gender, date of birth, racial and ethnic group identification, and living circumstances.
Medical Records including clinic notes, gross endoscopic findings, pathology report, laboratory data and imaging were retrospectively reviewed.
Statistical Methods
Fischer’s exact test and chi-square analyses were used to detect significant group differences on the nominal outcome variables. Analysis of variance between groups (ANOVA) and t-tests were used to compare the continuous outcome measures between groups. The Scheffe method was used for multiple comparisons between the three groups within the ANOVA. Subgroup analyses adjusted for gender. Logistic regression was used to evaluate whether baseline characteristics predicted the use of acid suppression at follow-up. A p-value less than 0.05 was considered significant. Data were analyzed using SPSS for Windows Version 19 statistical package (SPSS, Inc., Chicago, IL).
Results
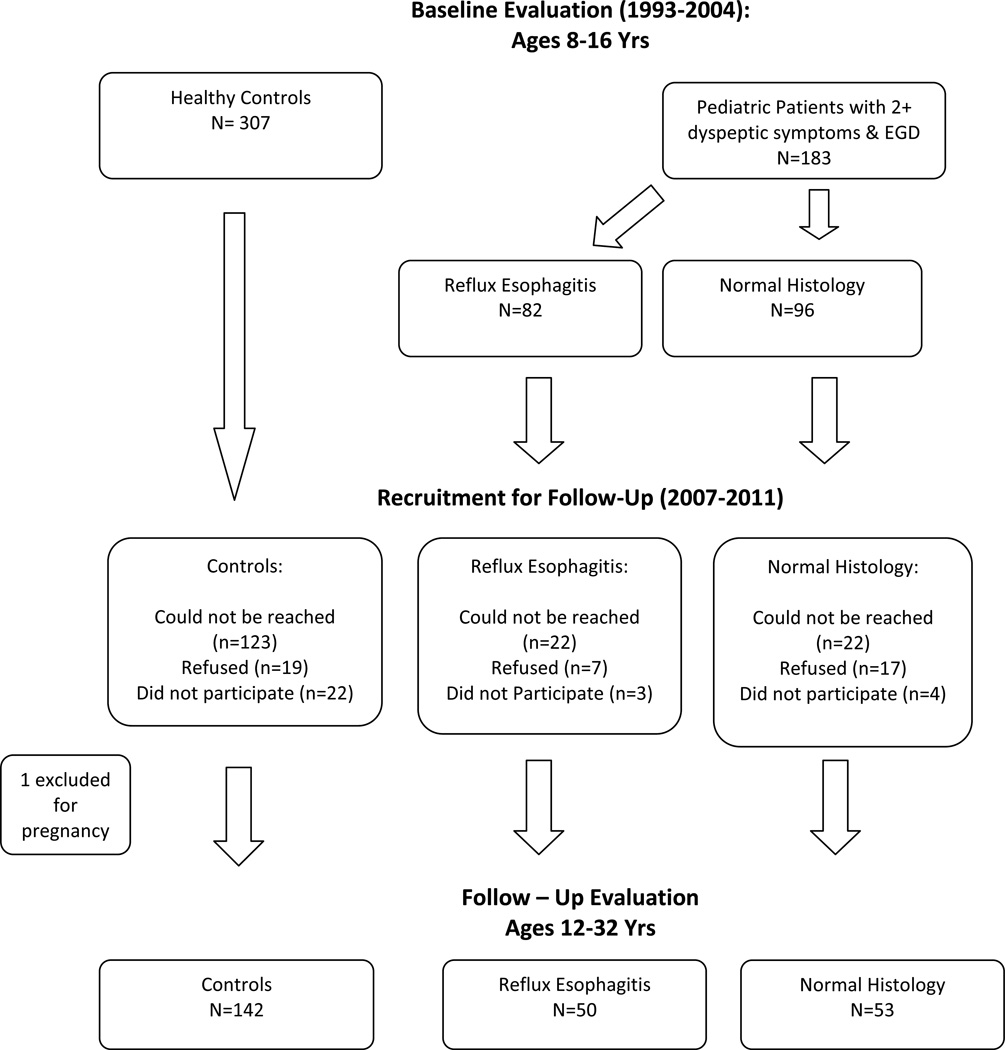
Figure 2 describes patient recruitment and the study timeline. Seven patients who underwent upper endoscopy but endorsed fewer than two dyspeptic symptoms were excluded. Based on review of medical records, four patients with Crohn’s disease and one with cholelithiasis also were excluded. Thus, the final sample consisted of 183 pediatric patients with two or more dyspeptic symptoms who underwent endoscopy with biopsies. Of those whose upper endoscopy exhibited histologic reflux esophagitis, 50 participated in follow-up. Of those with normal biopsies at baseline, 53 participated in follow-up. In the control group, one participant was excluded from due to pregnancy at the time of follow-up, leaving a final sample of 142 participants in the control group.
Figure 2.
Study Timeline & Participant Recruitment
Baseline variables including age, gender, race, dyspeptic symptoms, depression, and disability are presented in Table 1. At baseline, both the esophagitis and normal histology groups had more severe dyspeptic symptoms, abdominal pain, and functional disability compared to the controls but did not differ from each other on any variable. There was a statistically significant difference in age between the normal histology and controls at baseline but no significant age difference between any other groups.
Table 1.
Measures at Baseline by Reflux Esophagitis, Normal Histology, and Control Group
| Reflux Esophagitis (n=50) |
Normal Histology (n=53) |
Controls (n=142) |
p-value GER vs. FD |
p-value GER vs. Control |
p-value FD vs. Control |
|
|---|---|---|---|---|---|---|
| Mean Age in Years mean ± SD |
11.58 ± 2.43 | 11.98 ± 2.55 | 10.96 ± 2.11 | NS | NS | <0.05 |
| Gender (% female) | 48 % (24/50) |
68% (36/53) |
56% (79/142) |
NA | NA | NA |
| Race (% white) | 94% (47/50) |
89% (47/53) |
96% (135/141) |
NA | NA | NA |
| Intact Family (% live both parents) |
60% (30/50) |
62% (33/53) |
46% (65/142) |
NA | NA | NA |
| Dyspeptic Severity (CSI) mean ± SD |
9.96 ± 4.79 | 9.42 ± 4.58 | 2.67 ± 3.72 | NS | <0.001 | <0.001 |
| Abdominal Pain Severity (API); mean ± SD |
2.16 ± 0.81 | 2.08 ± 0.83 | 0.65 ± 0.79 | NS | <0.001 | <0.001 |
| Depressive Score (CDI) mean ± SD |
8.76 ± 5.89 | 8.77 ± 7.71 | NA | NS | NA | NA |
| Functional Disability (FDI)a; mean ± SD |
11.05 ± 8.80 | 9.15 ± 8.22 | 4.26 ± 5.58 | NS | <0.001 | <0.001 |
Child Report FDI not available for all subjects (Reflux Esophagitis=39, Normal Histology=33, Control=82)
Note: CSI= Children's Somatization Inventory; API= Abdominal Pain Index; CDI= Children's Depression Inventory; FDI= Functional Disability Inventory; NS= not significant; NA= not applicable
The average follow-up interval was 7.6 years. The follow up interval for the control group (6.9 years) was significantly shorter than that for the reflux esophagitis and normal histology groups (8.3 and 9.0 years, respectively) which did not differ significantly from each other. The mean age of the control group at follow-up, (18.3 years) was significantly younger than the reflux esophagitis group (20.3 years) and the normal histology group (21.6 years) which did not differ significantly from each other.
As shown in Table 2, the groups differed significantly in use of acid suppression medication at follow-up. The reflux esophagitis group was more likely to use acid suppressants than the normal histology group which, in turn, was significantly more likely to use acid suppressants than the control group. Two participants in the reflux esophagitis group reported a history of Nissen fundoplication but no participants in the other groups reported having had this procedure. The reflux esophagitis and normal histology groups had similar severity of dyspeptic symptoms and both groups had significantly higher symptom severity compared to controls.
Table 2.
Symptom Related Measures at Follow-Up by Reflux Esophagitis, Normal Histology, and Control Group
| Reflux Esophagitis (n=50) |
Normal Histology (n=53) |
Controls (n=142) |
p-value Reflux vs. Normal |
p-value Reflux vs. Control |
p-value Normal vs. Control |
|
|---|---|---|---|---|---|---|
| Self Reported Outcomes | ||||||
| Acid Suppression Use | 31% (15/49) |
13% (7/53) |
4% (5/142) |
<0.05 | <0.05 | <0.001 |
| Nissen Fundoplication | 4% (2/50) |
0% (0/53) |
0% (0/142) |
NA | NA | NA |
| Dyspeptic Symptom Severity mean ± SD |
4.80 ± 3.99 | 3.96 ± 3.65 | 1.96 ± 2.25 | NS | <0.001 | <0.001 |
| Abdominal Pain Severity mean ± SD |
1.16 ± 1.00 | 1.28 ± 1.02 | 0.45 ± 0.60 | NS | <0.001 | <0.001 |
Note: NS= not significant; NA= not applicable; GI= Gastrointestinal
Table 3 presents measures of quality of life and psychological functioning at follow up. Both the reflux esophagitis and normal histology groups reported poorer overall health related quality of life and higher levels of functional disability at follow up compared to controls. However, the esophagitis and normal endoscopy groups did not differ significantly from each other on quality of life measures at follow up. The normal histology group, but not the esophagitis group, endorsed significantly higher levels of depression and anxiety as well as significantly reduced quality of life on the SF-36 mental health dimension at follow up compared to the control group.
Table 3.
Quality of Life and Psychological Functioning at Follow-Up by Reflux Esophagitis, Normal Histology, and Control Group
| Measure | Reflux Esophagitis (n=50) |
Normal Histology (n=53) |
Controls (n=142) |
p-value Reflux vs. Normal |
p-value Reflux vs. Control |
p-value Normal vs. Control |
|---|---|---|---|---|---|---|
| HRQOL, Total Total SF-36; mean ± SD |
78.82 ± 15.69 | 75.79 ± 14.33 | 84.97 ± 9.37 | NS | <0.05 | <0.001 |
| HRQOL, Physical Component SF-36 PCS; mean ± SD |
76.28 ± 17.50 | 72.25 ± 15.60 | 83.71 ± 9.17 | NS | <0.05 | <0.001 |
| HRQOL, Mental Component SF-36 MCS; mean ± SD |
75.89 ± 15.38 | 72.91 ± 15.60 | 81.22 ± 12.17 | NS | NS | <0.05 |
| Functional Disability (FDI), mean ± SD |
4.49 ± 6.21 | 5.02 ± 6.25 | 1.73 ± 3.08 | NS | <0.05 | <0.001 |
| Depression (CES-D) mean ± SD |
9.00 ± 7.70 | 11.17 ± 9.93 | 7.6 ± 6.78 | NS | NS | <0.05 |
| Anxiety (STAI-T) mean ± SD |
16.98 ± 9.26 | 18.89 ± 10.89 | 13.19 ± 9.12 | NS | NS | <0.05 |
Note: HRQOL= Health Related Quality of Life; SF-36= Short Form Health Survey; PCS: Physical Component Score; MCS: Mental Component Score; FDI= Functional Disability Inventory; CES-D= Center for Epidemiological Studies-Depression Scale; STAI-T= State-Trait Anxiety Inventory, Trait Form; NS= not significant; NA= not applicable
Psychiatric diagnostic interviews (Table 4) indicated that significantly more patients in the esophagitis group (28%) met criteria for a current anxiety disorder at follow up compared to controls (13.6%). Both the esophagitis and normal histology groups were significantly more likely to have met diagnostic criteria for anxiety disorders and mood disorders during their lifetimes compared to controls.
Table 4.
Psychiatric Disorders at Follow-Up by Reflux Esophagitis, Normal Histology, and Control Group
| Reflux Esophagitis (n=50) |
Normal Histology (n=53) |
Controls (n=142) |
p-value Reflux vs. Normal |
p-value Reflux vs. Control |
p-value Normal vs. Control |
|
|---|---|---|---|---|---|---|
| Current | ||||||
| Anxiety Disorder | 28.3% (13/46) |
24.5% (13/53) |
13.6% (19/140) |
NS | <0.05 | NS |
| Mood Disorder | 6.5% (3/46) |
7.5% (4/53) |
2.9% (4/140) |
NA | NA | NA |
| Lifetime | ||||||
| Anxiety Disorder | 43.5% (20/46) |
54.7% (29/53) |
23.6% (33/140) |
NS | <0.05 | <0.001 |
| Mood Disorder | 34.8% (16/46) |
49.1% (26/53) |
20.0% (28/140) |
NS | <0.05 | <0.001 |
Note: Current and Lifetime Anxiety and Mood Disorder criteria based upon The Anxiety Disorders Interview (ADIS), Schedule-IV; GER= gastroesophageal reflux; FD= functional dyspepsia
Finally, we compared characteristics of the individuals on acid suppression therapy at follow-up (n=80) to see if they differed from those not on acid suppression at follow up (n=22), regardless of previous histologic findings. Controls were excluded from this analysis. Individuals on antacids at follow up had significantly more dyspeptic symptoms compared to those not on antacids at follow up (CSI dyspeptic score, mean 6.0 vs. 3.9, p< 0.05) but did not differ on severity of abdominal pain at follow up (API mean, 1.16 vs. 1.45, p>0.05). SF-36 scores indicated that quality of life was poorer in those on antacids at follow up compared to those not on antacids (SF-36 total 69.2 vs. 79.8, p<0.05). Those using antacids at follow up reported higher anxiety and depression than those not using antacids, but this difference did not reach statistical significance (CESD sum depression 13.4 vs. 9.4, p=0.054; STAI-T 16.8 vs. 21.71, p=0.053). We performed binary logistic regression analysis to assess predictors for acid suppression use at follow-up. The model included baseline gender, age, histology findings, abdominal pain, dyspeptic symptoms, functional disability, and depression score. None of these variables were significant predictors of the use of acid suppression at follow-up.
Conclusion
This prospective cohort study of pediatric patients evaluated with upper endoscopy makes two important contributions to the literature. First, we found that these pediatric patients with dyspeptic symptoms, both with and without abnormal esophageal histology, had more dyspeptic symptoms, greater functional disability, and poorer health related quality of life compared to controls in adolescence and young adulthood. Related studies have reported poorer health related quality of life in clinical and community samples of adults with GERD and with dyspepsia 46, 24–26. Ours is the first study to show that pediatric patients with dyspeptic symptoms evaluated by endoscopy are at increased risk of reduced quality of life later in their development and that this risk applies equally to patients with and without histologic esophagitis. This finding suggests that, for many pediatric patients evaluated with endoscopy in the tertiary care setting, dyspepsia may become a chronic condition that negatively impacts their daily lives as they transition to adulthood.
Our second important finding is a strong association between pediatric dyspepsia and anxiety. Results of a psychiatric diagnostic interview by a trained clinician blind to participants’ health status indicated that, at the time of follow up in adolescent and young adulthood, approximately half of both the esophagitis and normal histology patients had a lifetime history of one or more anxiety disorders and a quarter currently met criteria for an anxiety disorder at follow up. These rates of lifetime and current anxiety disorders were double those observed in the control group. The esophagitis and normal histology groups also had elevated rates of lifetime depressive disorders but both they and controls had very low current levels of depressive disorders at follow up. Whereas other studies have linked self-reported symptoms of anxiety and depression to GER and FD, ours is the first to demonstrate, using a psychiatric diagnostic interview, that these symptoms reached clinical significance 28–30,47. Our findings also suggest that depressive symptoms may resolve or be episodic in youth with dyspepsia, while anxiety appears to be more chronic.
Anxiety and depression could develop as a consequence of living with chronic dyspeptic symptoms that are poorly controlled by treatment. Clinical studies suggest that, in GERD patients, anxiety rather than disease pathology may lead to poorer quality of life and worsened symptom severity 48–50. It also is possible that anxiety and depression affect clinical outcomes directly by impacting adherence to provider recommendations regarding diet, lifestyle, and medication. Anxiety and depression also may reflect central sensitization of pain and dysregulation of reciprocal communication between the brain and the gut, a factor that is increasingly recognized to play an important role in the pathophysiology of functional GI disorders 51–53. For example, a recent study by Sharma found that anxiety induction increased hyperalgesia to acid infusion in healthy volunteers, likely through central sensitization 54.
The only significant difference at follow-up between those with and without baseline histological esophagitis was greater reported use of acid suppression medication by those with esophagitis. These results regarding use of acid suppression medication are consistent with those of Hyam’s study which reported similar percentages of children on acid suppression at a shorter follow up (24% with normal endoscopy and 26% with abnormal endoscopy). This observation suggests that histology alone is not adequate to discriminate between organic and functional dyspepsia.
In this study, we classified patients with dyspeptic symptoms into two groups – those with histologic evidence of reflux esophagitis and those with normal histology -- based on findings of upper endoscopy and biopsy. Hyams and colleagues used a similar procedure for classifying patients in their study 16. As they noted, upper GI inflammation often occurs in asymptomatic adults and, without comparable data for asymptomatic children, we cannot rule out the possibility that mucosal inflammation was unrelated to dyspeptic symptoms in our pediatric sample. It also is possible that alternative criteria for classifying patients, for example, based on results of a pH probe or symptom response to a trial of acid suppression, would have classified our patients differently 55.
Important limitations of the study include the highly selected patient sample, absence of information regarding treatment during the follow up interval, loss of some patients to follow-up, and lack of medical evaluation with upper endoscopy and esophageal biopsy at follow up. Patients evaluated with endoscopy at a tertiary care center may differ substantially from nonreferred youth with dyspepsia; study findings cannot be generalized to those youth. Moreover, without repeat endoscopy, it is not possible to know whether patients’ lack of improvement over time may have been due to worsening of disease or a change in underlying diagnosis. Additional limitations are related to advances in clinical practice that have occurred since these patients were evaluated (1993–2004). For example, recent consensus guidelines have put emphasis on mucosal breaks as a finding suggestive of reflux esophagitis 56. This was not a finding commonly described at the time when the endoscopies were done in our study patients; whether study patients with or without esophagitis had mucosal breaks is unknown to us. The use of multichannel impedence monitoring is another advance not available at the time of evaluation; this technique might have yielded evidence of nonacid reflux in some patients.
This study is the largest prospective cohort of its kind to describe the natural history of pediatric dyspepsia with and without positive histologic findings. The five to fifteen year length of follow-up is considerably longer than that for any previous study and allowed us to examine outcomes for children with dyspepsia in adolescence and young adulthood. Of particular note, the study included a control group that also was followed prospectively. Finally, the use of validated measures strengthens confidence in our findings.
An interesting clinical implication of this study is that, within the pediatric subspecialty setting, pediatric patients with and without positive histology associated with dyspeptic symptoms may be at equally increased risk for long term persistence of their symptoms and reduced quality of life. These findings further blur the distinction between organic and functional gastrointestinal disorders 57. Our evidence linking anxiety disorder to dyspepsia with and without positive histology suggests that pediatric gastroenterologists should consider evaluation of psychological functioning as an integral part of the medical evaluation for dyspeptic symptoms. Research is needed to identify factors in childhood that may be prognostic indicators of long term outcomes of pediatric dyspepsia. Finally, treatment studies are needed to evaluate the extent to which reduction in anxiety may be associated with reductions in dyspeptic symptoms and vice versa.
Acknowledgments
Grant Support: R01 HD23264, T32DK007673, DK058404, UL1 RR024975, P30 HD15052
Abbreviations
- FD
functional dyspepsia
- GERD
gastroesophageal reflux disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: No disclosures for all authors
Writing Assistance-not applicable
Author Involvement: Study Concept and Design (Walker, Acra, Correa, Vaezi); Acquisition of Data (Rippel, Walker); Analysis and Interpretation of Data (Rippel, Acra, Correa, Vaezi, Di Lorenzo, Walker); Drafting of the Manuscript (Rippel, Walker); Critical Revision of the Manuscript for Important Intellectual Content (Acra, Correa, Vaezi, Di Lorenzo); Statistical Analysis (Rippel, Walker); Obtained Funding (Walker, Rippel); Technical or Material Support (Not Applicable); Study Supervision (Not Applicable).
References
- 1.Sandler RS, Everhart JE, Donowitz M, et al. The Burden of Selected Digestive Diseases in the United States. Gastroenterology. 2002;122:1500–1511. doi: 10.1053/gast.2002.32978. [DOI] [PubMed] [Google Scholar]
- 2.Pace F, Pallotta S, Vakil N. Gastroesophageal reflux disease is a progressive disease. Dig Liver Dis. 2007;39:409–414. doi: 10.1016/j.dld.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Agréus L, Svärdsudd K, Talley NJ, et al. Natural history of gastroesophageal reflux disease and functional abdominal disorders: a population-based study. Am J Gastroenterol. 2001;96:2905–2914. doi: 10.1111/j.1572-0241.2001.04680.x. [DOI] [PubMed] [Google Scholar]
- 4.El-Serag HB, Gilger M, Carter J, et al. Childhood GERD is a Risk Factor for GERD in Adolescents and Young Adults. Am J Gastroenterol. 2004;99:806–812. doi: 10.1111/j.1572-0241.2004.30098.x. [DOI] [PubMed] [Google Scholar]
- 5.Waring JP, Feiler MJ, Hunter JG, et al. Childhood Gastroesophageal Reflux Symptoms in Adult Patients. J Pediatr Gastroenterol Nutr. 2002;35:334–338. doi: 10.1097/00005176-200209000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Young RJ, Lyden E, Ward B, et al. A Retrospective, Case-control Pilot Study of the Natural History of Pediatric Gastroesophageal Reflux. Dig Dis Sci. 2007;52:457–462. doi: 10.1007/s10620-006-9516-3. [DOI] [PubMed] [Google Scholar]
- 7.Gold BD. Editorial: Is Gastroesophageal Reflux Disease Really a Life-Long Disease: Do Babies Who Regurgitate Grow up to Be Adults with GERD Complications? Am J Gastroenterol. 2006;101:641–644. doi: 10.1111/j.1572-0241.2006.00436.x. [DOI] [PubMed] [Google Scholar]
- 8.Orenstein SR, Shalaby TM, Kelsey SF, et al. Natural History of Infant Reflux Esophagitis: Symptoms and Morphometric Histology During One Year Without Pharmacotherapy. Am J Gastroenterol. 2006;101:628–640. doi: 10.1111/j.1572-0241.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 9.Hegar B, Dewanti NR, Kadim M, et al. Natural evolution of regurgitation in healthy infants. Acta Paediatr. 2009;98:1189–1193. doi: 10.1111/j.1651-2227.2009.01306.x. [DOI] [PubMed] [Google Scholar]
- 10.Campanozzi A, Boccia G, Pensabene L, et al. Prevalence and natural history of gastroesophageal reflux: pediatric prospective survey. Pediatrics. 2009;123:779–783. doi: 10.1542/peds.2007-3569. [DOI] [PubMed] [Google Scholar]
- 11.Nelson SP, Chen EH, Syniar GM, Christoffel KK. One-year follow-up of symptoms of gastroesophageal reflux during infancy. Pediatric Practice Research Group. Pediatrics. 1998;102:E67. doi: 10.1542/peds.102.6.e67. [DOI] [PubMed] [Google Scholar]
- 12.Ashorn M, Ruuska T, Karikoski R, et al. The natural course of gastroesophageal reflux disease in children. Scand J Gastroenterol. 2002;37:638–641. doi: 10.1080/00365520212507. [DOI] [PubMed] [Google Scholar]
- 13.Treem WR, Davis PM, Hyams JS. Gastroesophageal reflux in the older child: presentation, response to treatment and long-term follow-up. Clin Pediatr. 1991;30:435–440. doi: 10.1177/000992289103000705. [DOI] [PubMed] [Google Scholar]
- 14.Tolaymat N, Chapman DM. Gastroesophageal reflux disease in children older than two years of age. W V Med J. 1998;94:22–25. [PubMed] [Google Scholar]
- 15.Martin AJ, Pratt N, Kennedy JK, et al. Natural History and Familial Relationships of Infant Spilling to 9 Years of Age. Pediatrics. 2002;109:1061–1067. doi: 10.1542/peds.109.6.1061. [DOI] [PubMed] [Google Scholar]
- 16.Hyams JS, Davis P, Sylvester FA, et al. Dyspepsia in Children and Adolescents: A Prospective Study. J Pediatr Gastroenterol Nutr. 2000;4:413–418. doi: 10.1097/00005176-200004000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Yang YX, Metz DC. Safety of proton pump inhibitor exposure. Gastroenterol. 2010;139:1115–1127. doi: 10.1053/j.gastro.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 18.Johnson A, Peura D, Vaezi M. Safety of Proton Pump Inhibitors. J Gastroenterol Hepatol. 2011;7:1–16. (not yet in Pubmed). [Google Scholar]
- 19.Kamal A, Vaezi MF. Diagnosis and initial management of Gastroesophageal complications. Best Pract Res Clin Gastroenterol. 2010;24:799–820. doi: 10.1016/j.bpg.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Colletti RB, Di Lorenzo C. Overview of Pediatric Gastroesophageal Reflux Disease and Proton Pump Inhibitor Therapy. J Pediatr Gasteroenterol Nutr. 2003;37:S7–S11. doi: 10.1097/00005176-200311001-00003. [DOI] [PubMed] [Google Scholar]
- 21.Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–1920. doi: 10.1111/j.1572-0241.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 22.Sherman PM, Hassall E, Fagundes-Neto U, et al. A global, evidence-based consensus on the definition of gastroesophageal reflux disease in the pediatric population. Am J Gastroenterol. 2009;104:1278–1295. doi: 10.1038/ajg.2009.129. [DOI] [PubMed] [Google Scholar]
- 23.Fraser A, Delaney B, Moayyedi P. Symptom-based outcome measures for dyspepsia and GERD trials: a systematic review. Am J Gastroenterol. 2005;100:442–452. doi: 10.1111/j.1572-0241.2005.40122.x. [DOI] [PubMed] [Google Scholar]
- 24.Aro P, Talley NJ, Agréus L, et al. Functional dyspepsia impairs quality of life in the adult population. Aliment Pharmacol Ther. 2011;33:1215–1224. doi: 10.1111/j.1365-2036.2011.04640.x. [DOI] [PubMed] [Google Scholar]
- 25.Wiklund I, Carlsson J, Vakil N. Gastroesophageal reflux symptoms and well-being in a random sample of the general population of a Swedish community. Am J Gastroenterol. 2006;101:18–28. doi: 10.1111/j.1572-0241.2005.00343.x. [DOI] [PubMed] [Google Scholar]
- 26.Wang R, Zou D, Ma X, et al. Impact of gastroesophageal reflux disease on daily life: the Systematic Investigation of Gastrointestinal Diseases in China (SILC) epidemiological study. Health Qual Life Outcomes. 2010;8:128. doi: 10.1186/1477-7525-8-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marlais M, Fishman JR, Köglmeier J, et al. Reduced quality of life in children with gastro-oesophageal reflux disease. Acta Paediatr. 2010;99:418–421. doi: 10.1111/j.1651-2227.2009.01613.x. [DOI] [PubMed] [Google Scholar]
- 28.Van Oudenhove L, Vandenberghe J, Vos R, et al. Risk factors for impaired health-related quality of life in functional dyspepsia. Aliment Pharamacol Ther. 2011;33:261–274. doi: 10.1111/j.1365-2036.2010.04510.x. [DOI] [PubMed] [Google Scholar]
- 29.Kindt S, Van Oudenhove L, Mispelon L, et al. Longitudinal and Cross-Sectional Factors Associated with Long-Term Clinical Course in Functional Dyspepsia: A 5-Year Follow-Up Study. Am J Gastroenterol. 2011;106:340–348. doi: 10.1038/ajg.2010.406. [DOI] [PubMed] [Google Scholar]
- 30.Aro P, Talley N, Ronkainen J, et al. Anxiety is Associated with Uninvestigated and Functional Dyspepsia (Rome III Criteria) in a Swedish Population-Based Study. Gastroenterol. 2009;137:94–100. doi: 10.1053/j.gastro.2009.03.039. [DOI] [PubMed] [Google Scholar]
- 31.Drossman DA, Corazziari E, Delvaux M, Spiller RC, Talley NJ, Thompson WG, Whitehead WE, editors. Rome III: The Functional Gastrointestinal Disorders. 3rd ed. McLean, VA: Degnon Associates; 2006. [Google Scholar]
- 32.Walker LS, Garber J, Smith CA, et al. The relation of daily stressors to somatic and emotional symptoms in children with recurrent abdominal pain. J Consult Clin Psychol. 2001;69:85–91. [PMC free article] [PubMed] [Google Scholar]
- 33.Walker LS, Smith CA, Garber J, et al. Testing a model of pain appraisal and coping in children with chronic abdominal pain. Health Psychol. 2005;24:364–374. doi: 10.1037/0278-6133.24.4.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allende D, Yerian L. Diagnosing Gastroesophageal Reflux Disease: The Pathologist’s Perspective. Adv Anat Pathol. 2009;16:161–165. doi: 10.1097/PAP.0b013e3181a186a3. [DOI] [PubMed] [Google Scholar]
- 35.Walker LS, Smith CA, Garber J, et al. Development and validation of the pain response inventory for children. Psychol Assess. 1997;9:43–52. [Google Scholar]
- 36.Walker LS, Garber J, Greene JW. Somatization symptoms in pediatric abdominal pain patients: Relation to chronicity of abdominal pain and parent somatization. J Abnorm Child Psych. 1991;19:379–394. doi: 10.1007/BF00919084. [DOI] [PubMed] [Google Scholar]
- 37.Kovacs M. Rating scales to assess depression in school-aged children. Acta Paedopsychiatr. 1981;46:305–315. [PubMed] [Google Scholar]
- 38.Kovacs M, Beck AT. An empirical– clinical approach toward a definition of childhood depression. In: Schulterbrandt J J, Raskin A, editors. Depression in childhood: Diagnosis, treatment, and conceptual models. New York: Raven; 1977. pp. 1–25. [Google Scholar]
- 39.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 40.Spielberger CD, Gorsuch RL, Lushene R, et al. Manual for the State-Trait Anxiety Inventory (Form Y) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 41.Silverman WK, Albano AM. Anxiety Disorders Interview Schedule for DSM-IV: Child and parent version. New York: Oxford University Press Inc.; 1996. [Google Scholar]
- 42.DiNardo PA, Brown TA, Barlow DH. Anxiety Disorders Interview Schedule for DSM-IV: Adult Lifetime Version. New York: Oxford University Press Inc.; 1994. [Google Scholar]
- 43.Claar RL, Walker LS. Functional assessment of pediatric pain patients: psychometric properties of the functional disability inventory. Pain. 2006;121:77–84. doi: 10.1016/j.pain.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walker LS, Greene JW. The functional disability inventory: measuring a neglected dimension of child health status. J Pediatr Psychol. 1991;16:39–58. doi: 10.1093/jpepsy/16.1.39. [DOI] [PubMed] [Google Scholar]
- 45.Ware JE, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36) Medical Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 46.Damiano A, Handley K, Adler E, et al. Measuring Symptom Distress and Health-Related Quality of Life in Clinical Trials of Gastroesophageal Reflux Disease Treatment. Dig Dis Sci. 2002;47:1530–1537. doi: 10.1023/a:1015815102175. [DOI] [PubMed] [Google Scholar]
- 47.Jones MP, Sharp LK, Crowell MD. Psychosocial Correlates of Symptoms in Functional Dyspepsia. Clin Gastroenterol Hepatol. 2005;3:521–528. doi: 10.1016/s1542-3565(05)00245-4. [DOI] [PubMed] [Google Scholar]
- 48.Oh JH, Kim TS, Choi MG, et al. Relationship between Psychological Factors and Quality of Life in Subtypes of Gastroesophageal Reflux Disease. Gut Liver. 2009;4:259–265. doi: 10.5009/gnl.2009.3.4.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamolz T, Valanovich V. Psychological and emotional aspects of gastroesophageal reflux disease. Dis Esophagus. 2002;15:199–203. doi: 10.1046/j.1442-2050.2002.00261.x. [DOI] [PubMed] [Google Scholar]
- 50.Wiklund I, Carlesson R, Carlsson J, et al. Psychological factors as a predictor of treatment response in patients with heartburn: A pooled analysis of clinical trials. Scand J Gastroenterol. 2006;41:288–293. doi: 10.1080/00365520500292970. [DOI] [PubMed] [Google Scholar]
- 51.Barry S, Dinan T. Functional dyspepsia: Are psychosocial factors of relevance? World J Gastroentrol. 2006;12:2701–2707. doi: 10.3748/wjg.v12.i17.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Willert RP, Delaney C, Kelly K, et al. Exploring the neurophysiological basis of chest wall allodynia induced by experimental oeophageal acidification – evidence of central sensitization. Neurogastroenterol Motil. 2007;19:270–278. doi: 10.1111/j.1365-2982.2006.00890.x. [DOI] [PubMed] [Google Scholar]
- 53.Sarkar S, Aziz Q, Woolf C, et al. Contribution of central sensitization to the development of non-cardiac chest pain. Lancet. 356:1154–1159. doi: 10.1016/S0140-6736(00)02758-6. 200. [DOI] [PubMed] [Google Scholar]
- 54.Sharma A, Van Oudenhove L, Paine P, et al. Anxiety increases acid-induced esophageal hyperalgesia. Psychosom Med. 2010;72:802–809. doi: 10.1097/PSY.0b013e3181f5c021. [DOI] [PubMed] [Google Scholar]
- 55.Shaw MJ, Talley NJ, Beebe TJ, et al. Initial validation of a diagnostic questionnaire for gastroesophageal reflux disease. Am J Gastroenterol. 2001;96:52–57. doi: 10.1111/j.1572-0241.2001.03451.x. [DOI] [PubMed] [Google Scholar]
- 56.Vandeplas Y, Rudolph CD, Di Lorenzo C, et al. Pediatric gastroesophagel reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) J Pediatr Gasteroenterol Nutr. 2009;49:498–547. doi: 10.1097/MPG.0b013e3181b7f563. [DOI] [PubMed] [Google Scholar]
- 57.Di Lorenzo C. Abdominal Pain: Is It in the Gut Or in the Head? J Pediatr Gasteroenterol Nutr. 2005;41:S44–S46. doi: 10.1097/01.scs.0000180302.94428.0c. [DOI] [PubMed] [Google Scholar]