Abstract
Emerging evidence suggests that the TH17 subset of αβ T cells contributes to the development of allergic asthma. In this study we found that mice lacking αvβ8 on dendritic cells failed to generate TH17 cells in the lung and were protected from AHR in response to house dust mite and ovalbumin sensitization and challenge. Because loss of TH17 cells inhibited airway narrowing without obvious effects on airway inflammation or epithelial morphology, we examined the direct effects of TH17 cytokines on mouse and human airway smooth muscle function. IL-17A enhanced contractile force generation through a NF-κB/RhoA/ROCK2 signaling cascade. Mice lacking integrin αvβ8 on dendritic cells showed impaired activation of this pathway after OVA sensitization and challenge, and the diminished contraction of tracheal rings from these mice was reversed by IL-17A. These data indicate that IL-17A produced by TH17 cells contributes to allergen-induced AHR through direct effects on airway smooth muscle.
T helper 2 (TH2) cells are classically thought to mediate the development of asthma through the production of interleukin-4 (IL-4), IL-5, IL-9 and IL-131,2, but recent evidence suggests that the TH17-derived cytokine, IL-17A, may also play an important role3–5. T helper 17 (TH17) cells are a pro-inflammatory T helper subset defined by production of IL-17A, IL-17F, and IL-22, and are thought to have evolved to aid in host defense against bacteria and fungi6. Elevated levels of IL-17A have been found in the serum, sputum and bronchoalveolar lavage fluids of patients with asthma and the IL-17A concentration found at these sites positively correlates with asthma severity7,8. Furthermore, genetic association studies with asthmatic patients have identified variations in several genes important for TH17 cell differentiation, including TGF-β19–11, IL-1β12, and IL-613. While TH17 cells appear to play a role in the development of allergic asthma, the mechanisms controlling their development in the lung are less clear.
Initial studies on TH17 differentiation showed that IL-6 and TGF-β are sufficient for the conversion of naïve T cells to TH17 cells14,15. The combination of TCR stimulation and TGF-β induces the synthesis of the retinoic acid-related orphan receptor gamma (RORγt) transcription factor, which is specific for the TH17 lineage among T cell subsets16. IL-6 signaling triggers binding of STAT3 to the RORγt locus, resulting in a positive feedback loop that drives T cells toward the TH17 lineage17. More recent studies have shown that other stimuli, such as IL-1β, IL- 21, IL-23, commensal bacteria-derived ATP, segmented filamentous bacterium, and activators of the aryl hydrocarbon receptor, also promote TH17 conversion or expand TH17 populations18–22. Still, TGF-β is a required factor for TH17 cell development in both humans and mice, and its regulation is likely a critical mechanism controlling the differentiation of TH17 cells22,23. TGF-β is produced as part of a latent complex and needs to be activated to bind TGF-β receptors24. We recently reported that integrin αvβ8 expressed on dendritic cells activates latent TGF-β and mediates the differentiation of both adaptive regulatory T cells and TH17 cells in the colon25,26. However, the relevance of this pathway for T cell differentiation in the lung and in the development of allergic airway disease has not been previously investigated.
Several activities of TH17 cells have been proposed as mechanisms for their role in promoting inflammation. IL-17A enhances neutrophil recruitment by stimulating bone marrow stromal cells to produce G-CSF, which augments granulopoiesis and the conversion of neutrophil progenitors from CD34+ cells27. IL-17A also induces neutrophil recruitment by stimulating the expression of CXCL1, CXCL2, CXCL5, and CXCL8 in epithelial cells28. Additional roles for IL-17A have been described. It promotes antibody isotype class switching in B cells and stimulates TH1-type immune responses by inducing macrophages to secrete IL- 12 29,30. While IL-17A is generally thought to serve a pro-inflammatory role in asthma through enhancement of neutrophil recruitment, additional roles for this and other TH17-derived cytokines in this disease remain largely unexplored.
In this study we began by determining the importance of integrin αvβ8 expressed on dendritic cells in a mouse model of allergic airway disease. Interestingly, we observed that these mice were protected from AHR without changes in lung inflammation and found that this protection correlated with a near-complete absence of lung TH17 cells. We therefore tested cytokines produced by TH17 cells for their ability to directly stimulate airway smooth muscle hyper-contractility. Treatment of airway smooth muscle with IL-17A, but not IL-17F or IL-22, triggered enhanced tracheal ring contraction upon stimulation with MCh or KCl. Investigation into how IL-17A exerts this effect on airway smooth muscle revealed that IL-17A induces a NF-κB/RhoA/ROCK2 signaling cascade that results in increased myosin light chain phosphorylation after treatment with MCh. Tracheal rings isolated from OVA-sensitized and challenged mice lacking integrin αvβ8 expressed on dendritic cells showed impaired activity of this signaling pathway, and treatment of tracheal rings with IL-17A completely restored OVA-induced hyper- contractility. These findings identify a critical role for the αvβ8 integrin on dendritic cells for generating pathogenic TH17 cells in allergic asthma and demonstrate, for the first time, that IL- 17A released from these cells contributes to airway hyperresponsiveness by directly increasing the contractility of airway smooth muscle.
RESULTS
Itgb8f/f x CD11c-cre mice are protected from AHR
To determine the role of integrin αvβ8 on dendritic cells in allergic airway disease, we sensitized and challenged control mice or mice lacking integrin αvβ8 on dendritic cells (Itgb8f/f x CD11c-cre mice) we have previously described25,26. In control mice, ovalbumin sensitization and challenge increased airway hyper-responsiveness (AHR) to the bronchoconstrictor, acetylcholine, but Itgb8f/f x CD11c-cre mice were significantly protected from allergen-induced AHR (Fig. 1a). To determine the mechanism for the diminished AHR seen in Itgb8f/f x CD11c-cre mice, we characterized mucus production and leukocyte accumulation in the lungs of these mice after OVA sensitization and challenge. Both control mice and Itgb8f/f x CD11c-cre mice showed similar numbers of mucus-secreting cells by Periodic Acid-Schiff (PAS) staining, as well as similar numbers of macrophages, eosinophils, lymphocytes and polymorphonuclear cells in the bronchoalveolar lavage fluid (BAL) (Fig. 1b–d). We also examined whether Itgb8f/f x CD11c-cre mice were protected from AHR in the house dust mice (HDM) asthma model. Itgb8f/f x CD11c-cre mice had significantly reduced AHR after house dust mite sensitization and challenge (Supplementary Fig. 1a). Intranasal delivery of IL-13 is known to provoke AHR, mucus production and leukocyte accumulation in the lungs of mice2,31. We tested if Itgb8f/f x CD11c-cre mice were capable of developing AHR by administering IL-13 directly to the lungs. Treatment of Itgb8f/f x CD11c-cre mice with intranasal IL-13 resulted in similar AHR as control mice, indicating that Itgb8f/f x CD11c-cre mice have the capacity to develop AHR (Supplementary Fig. 1b).
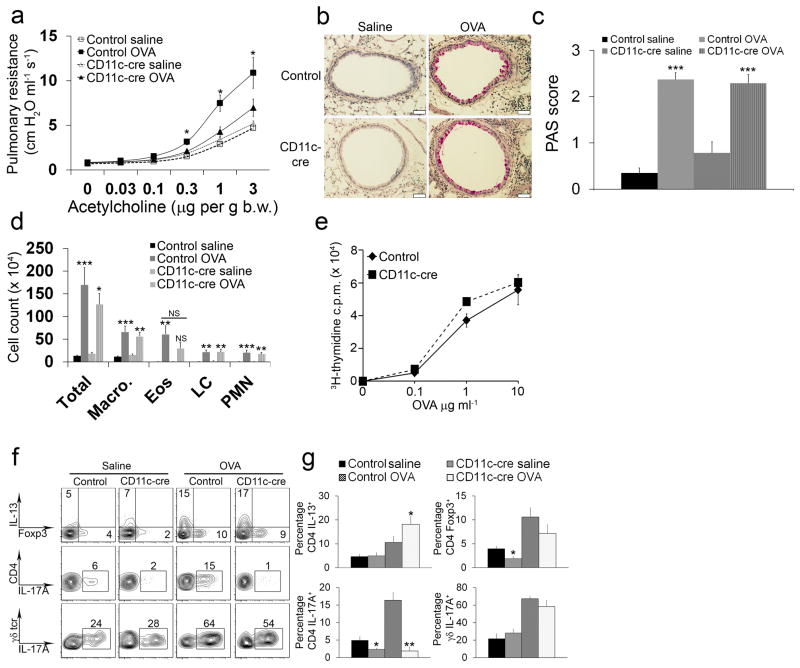
Figure 1. Itgb8f/f x CD11c-cre mice are protected from AHR and fail to develop Th17 cells in the lung.
(a) Pulmonary resistance measurements after administration of acetylcholine in control and Itgb8f/f x CD11c-cre mice immunized and intranasally challenged with OVA or saline. Data are mean ± SEM values for 10–13 animals in each group. *P < 0.01 in Itgb8f/f x CD11c-cre mice compared to control mice. (b) PAS-stained lung sections from control and Itgb8f/f x CD11c-cre mice immunized and intranasally challenged with OVA or saline (scale bar = 50 μm). (c) Quantitation of PAS-stained goblet cells from lung sections of control and Itgb8f/f x CD11c-cre mice immunized and intranasally challenged with OVA or saline. ***P < 0.001 in OVA-treated control and Itgb8f/f x CD11c-cre mice compared to control and Itgb8f/f x CD11c-cre mice. PAS scores in OVA-treated Itgb8f/f x CD11c-cre mice are not statistically different from OVA-treated control mice (n=6 mice per group). (d) Total number of macrophages, eosinophils (Eos), lymphocytes (LC), and polymorphonuclear cells (PMN) counted in the BAL of control and Itgb8f/f x CD11c-cre mice immunized and intranasally challenged with OVA or saline. *P < 0.05, **P < 0.01 and ***P < 0.001 in OVA-treated control and Itgb8f/f x CD11c-cre samples compared to saline-treated control and Itgb8f/f x CD11c-cre samples. Eosinophil counts from OVA-treated Itgb8f/f x CD11c-cre samples were not statistically significant (N.S) compared to saline-treated Itgb8f/f x CD11c-cre samples. None of the Itgb8f/f x CD11c-cre groups are statistically different from control mice (n=8–13 mice per group). (e) Measurement of incorporated 3H-thymidine upon re-exposure to OVA in lymphocytes isolated from the lungs of control and Itgb8f/f x CD11c-cre mice immunized and intranasally challenged with OVA. (f) Flow cytometry plots of CD4+ and γδ+ T cells isolated from the lungs of control and Itgb8f/f x CD11c-cre mice immunized and intranasally challenged with OVA or saline. Cells were activated with PMA and Ionomycin and then stained for IL-13, Foxp3, and IL-17A. The top and middle panels show CD4+ gated cells, and the bottom panels show γδ+ gated cells. (g) Percentage of IL-13, Foxp3, or IL-17A positive CD4+ T cells and IL-17A positive γδ+ T cells isolated from the lungs of control and Itgb8f/f x CD11c-cre mice immunized and intranasally challenged with OVA or saline. *P < 0.01 and **P < 0.001 in Itgb8f/f x CD11c-cre mice compared to control mice.
Since AHR in response to OVA or HDM requires an adaptive immune response and our previous work implicates integrin αvβ8 on dendritic cells in the regulation of CD4+ T cell differentiation, we tested the recall response of lymphocytes to OVA and characterized the CD4+ T cell subsets in the lungs of these animals. Control and Itgb8f/f x CD11c-cre mice had similar lymphocyte recall responses to OVA (Fig. 1e). TH2 cells, identified by intracellular flow cytometry for IL-13, were increased similarly in control and Itgb8f/f x CD11c-cre mice (Fig. 1f,g). We found a small, but significant, decrease in the percentage of Foxp3+ adaptive regulatory T cells in the lungs of Itgb8f/f x CD11c-cre mice before OVA sensitization and challenge, but there was no significant difference in the generation of regulatory T cells after OVA (Fig. 1f,g). OVA sensitization and challenge increased the percentage of TH17 cells, marked by expression of IL-17A and CD4, in the lungs of control mice, but Itgb8f/f x CD11c-cre mice had a near-absence of TH17 cells before and after OVA sensitization and challenge. Interestingly, the failure to generate IL-17A producing T cells in Itgb8f/f x CD11c-cre mice was restricted to the TH17 lineage, since the number of IL-17A producing γδ T cells in these mice was comparable to control mice (Fig. 1f,g). This finding suggests that Itgb8f/f x CD11c-cre mice may be protected from AHR through a specific defect in the generation of TH17 cells.
IL-17A enhances airway smooth muscle contraction
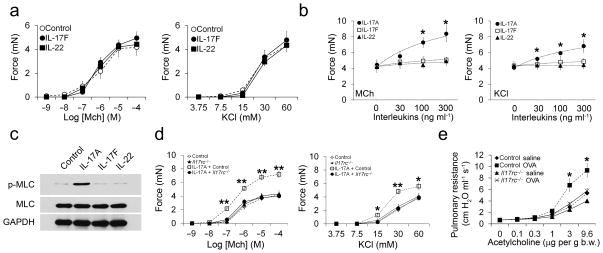
IL-13 and TNF-α are reported to act directly on airway smooth muscle to enhance AHR, and we wondered if IL-17 cytokines might also act in this fashion32,33. We first determined if IL-17 receptors were expressed on mouse airway smooth muscle cells. mRNA for both IL-17 receptor-A and IL-17 receptor-C was detected in cultured primary airway smooth muscle cells (Fig. 2a). We then determined the effects of IL-17A on the contractile force generated by mouse tracheal rings stimulated with methacholine (MCh) or depolarization with potassium chloride (KCl), and on the degree of airway narrowing induced by MCh in agarose-filled lung slices. IL-17A treatment alone had no effects on contraction when applied directly to tracheal rings in the organ bath for 15 minutes (data not shown). However, 12 hour incubation with IL-17A significantly enhanced both MCh and KCl-induced tracheal ring contraction (Fig. 2b) and MCh-induced airway narrowing in lung slices (Fig. 2c,d). To determine if IL-17A was acting on the smooth muscle or epithelium, we denuded the epithelium from tracheal rings and measured the effects of IL-17A on contraction. As others have reported, epithelial removal enhanced the amount of force generation in response to MCh and KCl (Supplementary Fig. 2)34,35. In tracheal rings completely denuded of epithelium, IL-17A increased contraction to a similar degree as intact tracheal rings, indicating that IL-17A acts directly on smooth muscle to enhance contraction. We next determined whether IL-17A also enhanced airway smooth muscle contraction in humans. Human bronchi were isolated and incubated with IL-17A or carrier for 12 hours. As we observed with mouse tracheal rings, pretreatment with IL-17A significantly increased contraction of human bronchi in response to MCh and KCl (Fig. 2e). These data suggest that IL-17A acts on airway smooth muscle in mice and humans to enhance contractile responses.
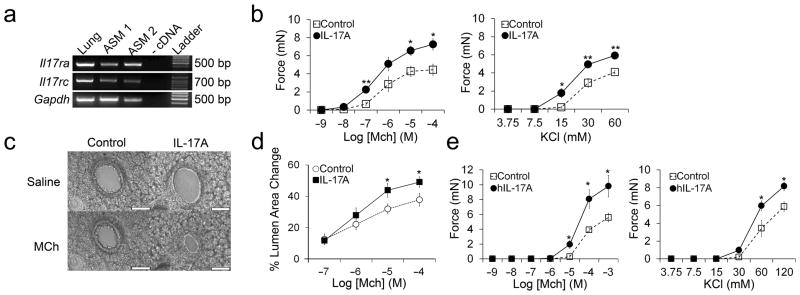
Figure 2. IL-17A enhances mouse and human airway smooth muscle contraction.
(a) RT-PCR products after amplification with primers to Il17ra, Il17rc, and Gapdh and RNA isolated from whole lung or primary airway smooth muscle cells cultured from 2 different mice. Reactions without cDNA were used as a negative control. (b) Contractile force measurements from mouse tracheal rings treated for 12 hours with or without 100 ng ml−1 IL-17A and stimulated with methacholine (MCh) or potassium chloride (KCl). Data are means ± SEM of at least 5 tracheal rings per group. (c) Phase contrast images of lung slices treated for 12 hours with or without 100 ng ml−1 IL-17A and stimulated with saline or MCh for 10 minutes (scale bar = 100 μm). (d) Measurement of the % lumen area change of airways in lung slices treated for 12 hours with or without 100 ng ml−1 IL-17A and stimulated with saline or MCh for 10 minutes. (e) Contractile force measurements from human bronchi treated for 12 hours with or without 100 ng ml−1 human IL-17A (hIL-17A) and stimulated with methacholine (MCh) or potassium chloride (KCl). *P < 0.05 and **P < 0.001 in IL-17A treated samples compared to control samples.
We next performed a series of experiments to test whether IL-17A was interacting directly or indirectly with airway smooth muscle. Atropine, a muscarinic receptor antagonist, was added to tracheal rings to determine if IL-17A augmented contraction through modulation of MCh signaling. Atropine completely prevented contraction triggered by MCh in tracheal rings pretreated with IL-17A, but had no effect on contraction stimulated with KCl (Fig. 3a). We then confirmed that enhanced contraction was due to IL-17A and not other contaminants in our IL-17A preparation. Concurrent incubation with IL-17A and antibodies that block IL-17A activity prevented the enhanced contraction seen with IL-17A alone in response to MCh and KCl (Fig. 3b). TNF-α is known to enhance airway smooth muscle contraction and we wondered whether pretreatment with IL-17A was indirectly stimulating contractile responses through release of TNF-α33. TNF-α blocking antibodies had no effect on enhanced tracheal ring contraction induced by IL-17A (Fig. 3c). To confirm that the TNF-α blocking antibodies were functional, we incubated tracheal rings with TNF-α in the presence or absence of TNF-α blocking antibodies. Pre-incubation with TNF-α increased tracheal ring contraction to both MCh and KCl and this enhancement was prevented with TNF-α blocking antibodies (Fig. 3d). These data indicate that IL-17A augments tracheal ring contraction through direct effects on airway smooth muscle.
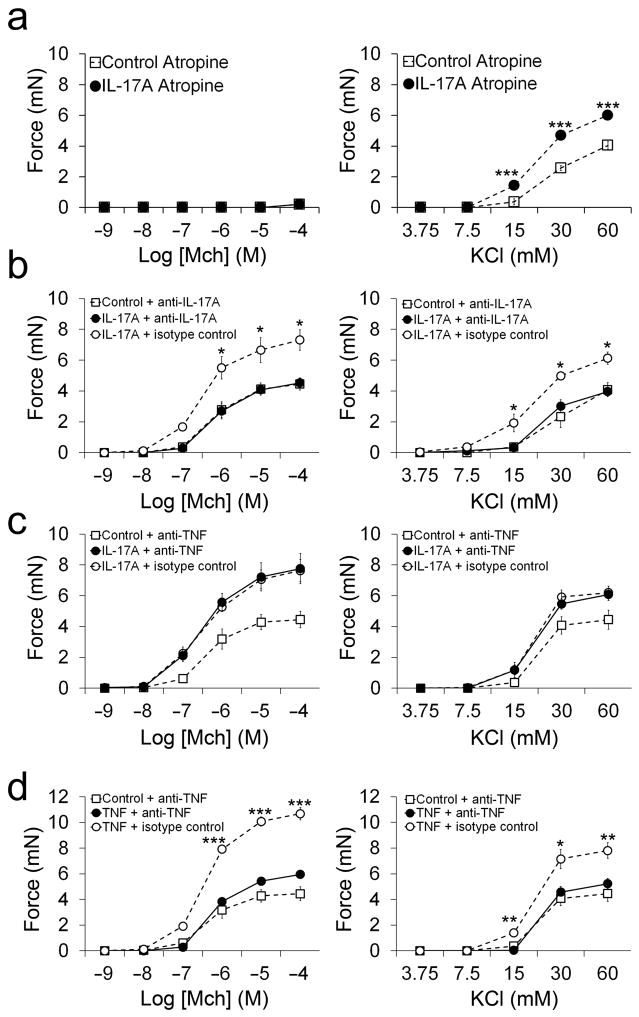
Figure 3. IL-17A-mediated enhanced ASM contraction is not due to contaminants or TNF-α release.
(a) Contractile force measurements from mouse tracheal rings treated for 12 hours with or without 100 ng ml−1 IL-17A in the presence of 1μM atropine and stimulated with methacholine MCh or KCl. (b) Contractile force measurements from tracheal rings stimulated with MCh or KCl after treatment for 12 hours with or without 100 ng ml−1 IL-17A in the presence of 40 μg ml−1 IL-17 neutralizing antibodies or isotype control antibodies. (c) Contractile force measurements from tracheal rings stimulated with MCh or KCl after treatment for 12 hours with or without 100 ng ml−1 IL-17A in the presence of 40 μg ml−1 TNF-α neutralizing antibodies or isotype control antibodies. (d) Contractile force measurements from tracheal rings stimulated with MCh or KCl after treatment for 12 hours with or without 100 ng ml−1 TNF-α in the presence of 40 μg ml−1 TNF-α neutralizing antibodies or isotype control antibodies. *P < 0.05, **P < 0.01 and ***P < 0.001 in IL-17A or TNF-α treated samples compared to control samples.
IL-17A stimulates contraction through NF-κB, RhoA and ROCK2
Contractile force in airway smooth muscle is generated through actin-myosin cross-bridging and is regulated by myosin light chain (MLC) phosphorylation36. To determine if IL-17A enhances airway smooth muscle contraction through regulation of MLC-phosphorylation, we measured MLC-P with an antibody specific to phosphorylated Ser19 of MLC. IL-17A dramatically increased MCh-induced MLC Ser19 phosphorylation (Fig. 4a,b). IL-17A is reported to function through activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)37,38. To determine if IL-17A enhanced airway smooth muscle contraction through activation of NF-κB, we measured NF-κB activation in tracheal airway smooth muscle after treatment with 100 ng ml−1 IL-17A. IL-17A rapidly decreased the cytosolic concentration of NF-κB and subsequently induced nuclear translocation of NF-κB, indicating that IL-17A activates NF-κB in tracheal rings at a concentration that also promotes enhanced contraction (Fig. 4c,d). To determine whether IL-17A induced activation of NF-κB was required for enhanced contraction, we treated tracheal rings with the NF-κB small molecule inhibitor, BAY 11-7082. BAY 11-7082 dose-dependently reduced the IL-17A-mediated enhanced contractile response to both MCh and KCl (Fig. 4e). NF-κB is known to regulate expression of the small GTPase, RhoA and its effector kinases ROCK1 and 239,40. The RhoA/ROCK signaling pathway is a critical mediator of airway smooth muscle contraction36,41. Treatment of tracheal rings with IL-17A increased expression of both RhoA and ROCK2, but not ROCK1 (Fig. 4f ,g). ROCK2 regulates MLC-phosphorylation through phosphorylation of the myosin-binding subunit of myosin phosphatase (MYPT1), which inhibits the activity of myosin phosphatase42. IL-17A treatment of airway smooth muscle stimulated MYPT1 phosphorylation (Fig. 4f,g). To determine if the RhoA/ROCK2/MYPT1 pathway was required for IL-17A-mediated enhanced contraction, we blocked ROCK activity with the small molecule inhibitor Y-27632. Y-27632 inhibited IL-17A-mediated enhancement of tracheal ring contraction in a dose-dependent manner (Fig. 4h). To test whether stimulation of RhoA and ROCK2 expression was mediated through NF-κB, we also measured expression of these proteins following treatment of tracheal rings with BAY 11-7082. Stimulation of tracheal rings with MCh after BAY 11-7082 treatment in the presence of IL-17A blocked increased expression of RhoA and resulted in decreased phosphorylation of MYPT1 and MLC (Fig. 4i). Treatment with Y-27632 under similar conditions had no effect on nuclear NF-κB translocation or RhoA expression, but as expected blocked phosphorylation of MYPT1 and MLC (Fig. 4i). We also determined if IL-17A regulated airway smooth muscle contraction through modulation of calcium signaling. Treatment of lung slices with IL-17A had no effect on methacholine-induced calcium wave oscillation amplitude or frequency (Supplementary Fig. 3). Collectively, these data indicate that IL-17A enhances contractile responses of airway smooth muscle through activation of NF-κB and subsequent induction of RhoA and ROCK2 expression.
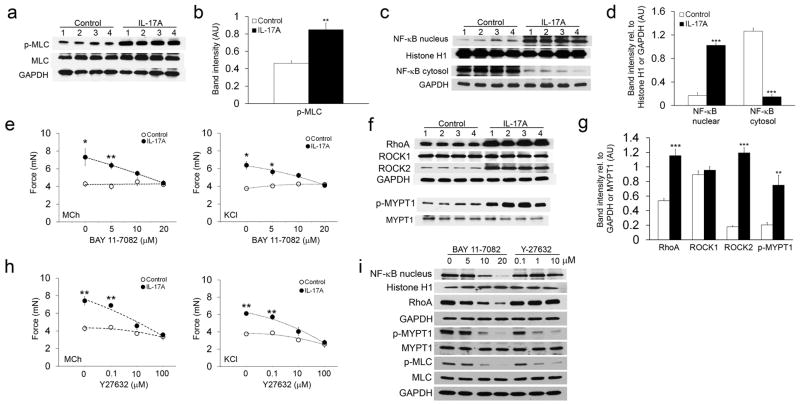
Figure 4. NF-κB/RhoA/ROCK2 signaling facilitates the effects of IL-17A on enhanced ASM contraction.
Tracheal smooth muscle samples were treated with 100 ng ml−1 IL-17A for 12 hours and 1 mM MCh for 15 minutes. Samples were then lysed, separated by SDS-PAGE and transferred to a membrane. Membranes were probed with antibodies to Ser-19 phosphorylated MLC, total MLC, and GAPDH (a), nuclear and cytosolic NF-κB, Histone H1, and GAPDH (c), RhoA, ROCK1, ROCK2, GAPDH, phosphorylated MYPT1, and total MYPT1 (f). Samples isolated from 4 mice are shown. All blots were stripped and re-probed to ensure equal amounts of protein were loaded in each well. Blots were analyzed with densitometry to determine the relative amounts of Ser-19 phosphorylated MLC (b), nuclear and cytosolic NF-κB (d), and RhoA, ROCK1, ROCK2, and phosphorylated MYPT1 (g). Tracheal rings were treated for 12 hours with or without 100 ng ml−1 IL-17A and BAY 11-7082 (0, 5, 10 and 20 μM; e) or Y27632 (0, 0.1, 10, and 100 μM; h). Contractile force measurements were then made in response to stimulation with 1 mM MCh or 60 mM KCl. (i) Tracheal smooth muscle samples were treated with 100 ng ml−1 IL-17A for 12 hours and 1 mM MCh for 15 minutes in the presence of the indicated doses of BAY 11-7082 or Y27632. Samples were then handled as described above to detect nuclear NF-κB, RhoA, phosphorylated MYPT1, total MYPT1, Ser-19 phosphorylated MLC, and total MLC. Blots were stripped and re-probed with either Histone H1 or GAPDH to ensure equal amounts of protein were loaded in each well. *P < 0.05, **P < 0.01 and ***P < 0.001 in IL-17A treated samples compared to control samples.
We explored if other cytokines produced by TH17 cells promote airway smooth muscle contraction. Incubation of tracheal rings with IL-17F or IL-22 for 12 hours at a range of doses (30–300 ng ml−1) had no significant effect on MCh or KCl-induced force generation (Fig. 5a,b). In contrast, incubation with IL-17A dose-dependently increased MCh or KCl-induced force generation (Fig. 5b). Treatment with 100 ng ml−1 IL-17F and IL-22 followed by stimulation with MCh also had no effect on MLC phosphorylation, while IL-17A at this dose triggered a substantial increase (Fig. 5c). Since IL-17A and F both bind to the IL-17RA/RC heterodimer, we were surprised to see that IL-17F administered at three times the amount required for maximal effects of IL-17A still had no effect on airway smooth muscle contraction. Therefore, we confirmed that IL-17A enhanced airway smooth muscle contraction through binding to IL-17RA/RC. Tracheal rings isolated from mice deficient in IL-17RC failed to develop enhanced contraction upon IL-17A treatment and stimulation with MCh and KCl (Fig. 5d). Mice deficient in IL-17RC were also protected from AHR in the OVA sensitization and challenge model of allergic asthma, which provides further evidence that IL-17A is a critical factor for the development of AHR in this model (Fig. 5e).
Figure 5. IL-17F and IL-22 do not enhance ASM contraction, and IL-17A requires IL-17RC.
(a) Contractile force measurements from tracheal rings treated for 12 hours with or without 100 ng ml−1 IL-17F or IL-22 and stimulated with MCh or KCl. (b) Dose-response curves of tracheal rings treated for 12 hours with or without increasing doses of IL-17A, IL-17F or IL-22 and stimulated with 1 mM MCh or 60 mM KCl. *P < 0.05 in IL-17A treated samples compared to control, IL-17F, or IL-22 treated samples. (c) Tracheal smooth muscle samples were treated with 100 ng ml−1 IL-17A, IL-17F, or IL-22 for 12 hours and 1 mM MCh for 15 minutes. Samples were then lysed, separated by SDS-PAGE, transferred to a membrane, and probed with antibodies to Ser-19 phosphorylated MLC, total MLC, and GAPDH. (d) Contractile force measurements from tracheal rings isolated from control or Il17rc−/− mice treated for 12 hours with or without 100 ng ml−1 IL-17A and stimulated with increasing doses of MCh or KCl. *P < 0.05 and **P < 0.01 in IL-17A treated control samples compared to Il17rc−/− mice. (e) Pulmonary resistance measurements after administration of acetylcholine in control and Il17rc−/− mice immunized and intranasally challenged with OVA or saline. *P < 0.05 in OVA treated control mice compared to OVA treated Il17rc−/− mice.
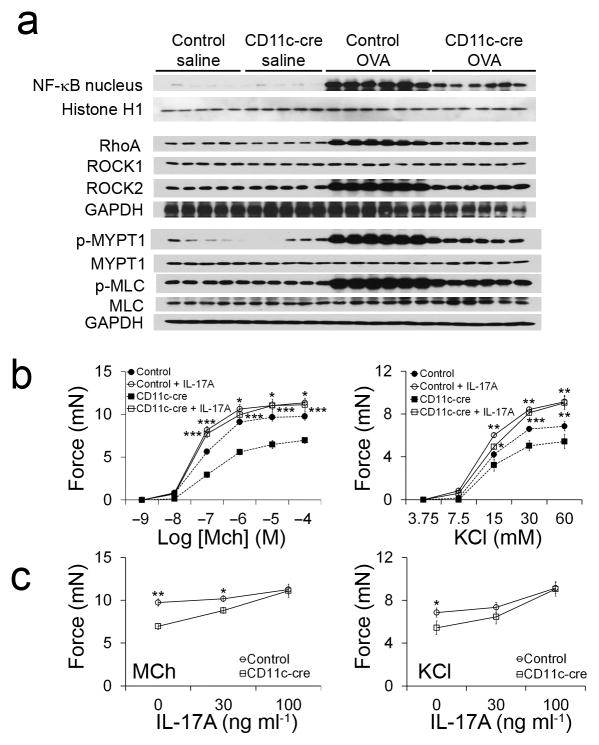
To determine whether mice lacking integrin αvβ8 on dendritic cells were protected from AHR through impaired activation of an IL-17A induced NF-κB/RhoA/ROCK2 signaling cascade, we studied the activation of this pathway in OVA challenged control and Itgb8f/f x CD11c-cre mice. OVA sensitization and challenge induced a robust increase in NF-κB (nuclear), RhoA, ROCK2, p-MYPT1, and p-MLC in airway smooth muscle dissected from control mice, but had substantially reduced effects on each of these endpoints in airway smooth muscle from Itgb8f/f x CD11c-cre mice (Fig. 6a). Tracheal rings from OVA sensitized and challenged Itgb8f/f x CD11c-cre mice also had impaired contractile responses to both MCh and KCl (Fig. 6b). To test if a lack of IL-17A was responsible for the impaired contractile responses, we incubated tracheal rings from OVA sensitized and challenged control or Itgb8f/f x CD11c-cre mice with IL-17A. Pre-incubation of tracheal rings from OVA sensitized and challenged Itgb8f/f x CD11c-cre mice with IL-17A restored methacholine-induced force generation in a dose-dependent fashion to levels seen in control mice (Fig. 6c). Pre-incubation of tracheal rings from OVA sensitized and challenged control mice had only a minimal additional effect on contractility, probably because these tissues had already been exposed to IL-17A in vivo. These data suggest that mice lacking integrin αvβ8 on dendritic cells are protected from AHR as a consequence of reduced exposure of airway smooth muscle to IL-17A.
Figure 6. Impaired IL-17A signaling mediates the protection of Itgb8f/f x CD11c-cre mice from AHR.
(a) Western blots were prepared as described in Figure 3 with tracheal smooth muscle samples isolated from control and Itgb8f/f x CD11c-cre mice immunized with saline or OVA. Lysates from 5 individual mice in the saline immunized groups and lysates from 6 individual mice in the OVA immunized groups are shown. Blots were stripped and re-probed with either Histone H1 or GAPDH to ensure equal amounts of protein were loaded in each well. (b) Contractile force measurements from tracheal rings isolated from control and Itgb8f/f x CD11c-cre mice immunized with saline or OVA and treated for 12 hours with or without 100 ng ml−1 IL-17A followed by stimulation with MCh or KCl. *P < 0.05, **P < 0.01 and ***P < 0.001 in IL-17A treated samples from control mice compared to control samples (above) or IL-17A treated samples from Itgb8f/f x CD11c-cre mice compared to control samples (below). (c) Contractile force measurements in tracheal rings isolated from OVA immunized control and Itgb8f/f x CD11c-cre mice and treated with the indicated concentrations of IL-17A for 12 hours followed by stimulation with 1 mM MCh or 60 mM KCl. *P < 0.05 and **P < 0.01 in IL-17A treated samples from control mice compared to IL-17A treated samples from Itgb8f/f x CD11c-cre mice.
DISCUSSION
A growing body of evidence implicates TH17 cells and their associated cytokines in allergic asthma, especially in severe cases where inflammation is predominantly driven by neutrophils3,4. The data from this study indicate a novel mechanism for TH17 cells in asthma pathogenesis. We show for the first time that IL-17A enhances the contractile response of airway smooth muscle to MCh and KCl. Furthermore, we show that activation of latent TGF-β by integrin αvβ8 on dendritic cells is required for TH17 cell development in the lung in response to sensitization and challenge with OVA and Alum. Interestingly, mice lackingαvβ8 on dendritic cells were protected from AHR without changes in inflammatory cell numbers, goblet cell differentiation, or lymphocyte recall responses, and were also protected from AHR in the house dust mite asthma model. Overall, these findings suggest that IL-17A can contribute to AHR through direct effects on airway smooth muscle, without grossly modulating airway inflammation or mucus metaplasia.
We performed a number of experiments to test if enhanced contraction provoked by IL-17A was due direct or indirect interactions between IL-17A and IL-17 receptors on airway smooth muscle. We depleted epithelium from tracheal rings by mechanical brushing to distinguish effects of IL-17A on epithelium and airway smooth muscle. Increased contraction was still observed in epithelium-denuded tracheal rings, which indicates that the effects of IL-17A on airway contractility do not depend on its known effects on airway epithelial cells. In line with previous reports, we saw that epithelial removal in and of itself was sufficient to enhance contractile responses of tracheal rings to MCh and KCl34,35, and we show here that this effect is potentiated further by pretreatment with IL-17A. We considered the possibility that a contaminant (e.g., LPS) in our IL-17A preparations might inadvertently stimulate contractile responses. However, enhanced contractile responses were completely prevented by adding IL-17A blocking antibodies, indicating that this result was due to the cytokine and not to contaminating factors. We also tested the specificity of IL-17A for its cognate receptor on airway smooth muscle. The IL-17 cytokine family signals through heterodimeric complexes of the IL-17 Receptor A (IL-17RA)-IL-17RE receptors, and IL-17A and F are known to bind the IL-17RA/RC heterodimer in other tissues28. We found that both of the IL-17RA/RC isoforms are expressed in airway smooth muscle and that enhanced contraction triggered by IL-17A was completely prevented in tracheal rings isolated from IL-17RC-deficient mice, and that these mice were protected from AHR induced by OVA sensitization and challenge. Collectively, these data indicate that IL-17A acts through IL-17RA/RC receptors expressed by airway smooth muscle to increase contractile responses. It was somewhat surprising that IL-17F, which like IL-17A can ligate IL-17RA/RC heterodimers, did not affect airway smooth muscle contractility over a range of concentrations that were sufficient to see the effects of IL-17A. We suspect that this result reflects the lower affinity of IL-17F for these receptors 43.
The NF-κB transcription factor is a major target of pro-inflammatory cytokines and we found in this study that IL-17A causes a robust activation of NF-κB upon MCh stimulation of airway smooth muscle. Since our primary phenotype after IL-17A treatment was enhanced contraction, we looked for NF-κB target genes that would modulate airway smooth muscle contraction. IL-17A treatment increased RhoA and ROCK2 expression, which are two prominent regulators of myosin light chain (MLC) phosphorylation and subsequent smooth muscle contraction36. Several groups have reported in studies of smooth muscle that activation of RhoA stimulates ROCK2 to phosphorylate myosin light chain phosphatase (MYPT)44–46. Phosphorylation deactivates MYPT and results in a net increase in MLC phosphorylation. Accordingly, our data show that IL-17A-induced expression of RhoA and ROCK2 correlated with enhanced phosphorylation of MLC and MYPT1. Phosphorylation of these proteins and expression of RhoA and ROCK2 was blocked with BAY 11-7082, a small molecule inhibitor of NF-κB, indicating that these proteins are target genes of NF-κB. This pathway was also stimulated in airway smooth muscle of OVA sensitized and challenged mice, and was impaired in mice lacking integrin αvβ8 on dendritic cells. Furthermore, reduced tracheal ring contraction in OVA sensitized and challenged mice lacking integrin αvβ8 on dendritic cells could be completely restored by ex vivo IL-17A treatment. Together these data suggest that protection from AHR in mice lacking integrin αvβ8 on dendritic cells results from impaired IL-17A signaling. Since these mice had a near-absence of pulmonary IL-17A producing αβ T cells (TH17), but minimal changes in IL-17A producing γδ T cells, our data suggest a model whereby IL-17A produced by TH17 cells acts on airway smooth muscle to induce AHR.
TH17 cells and IL-17A have been implicated in several mouse models of asthma, although some of these data are conflicting. Adoptive transfer of antigen-specific TCR transgenic T cells polarized in vitro to the Th17 phenotype into antigen challenged mice enhances AHR, as does pulmonary administration of an IL-17A overexpressing adenovirus47. Furthermore, as we observed in this study with IL-17RC-deficient mice, IL-17RA-deficient mice have been reported to show dramatic protection from AHR in an OVA sensitization and challenge model of allergic asthma48. However, this same study found that neutralization of IL-17A at the time of OVA challenge exacerbated AHR and local administration of IL-17A reduced eosinophilia and AHR. Another study using a similar model showed impaired induction of AHR in IL-17A-deficient mice crossed to OVA-specific TCR transgenic mice49. Several other studies that either neutralized IL-17A with antibodies or administered exogenous IL-17A have shown mixed effects on allergen-induced AHR, but the preponderance of evidence supports an important role for IL-17A in allergic airway inflammation50–53. It should be noted that the relative contributions of various cells and cytokines to models of allergic asthma can differ among inbred strains of mice, which might be one explanation for the apparently conflicting reports about the contributions of IL-17 to allergic asthma. Since all of the studies described in the current manuscript were performed in C57BL/6 mice, we cannot be certain that the same effects would be seen in other strains (e.g. Balb/c) in which TH2 cytokines have more potent effects. IL-17A was recently shown to act synergistically with IL-13 to enhance AHR in the A/J mouse strain, whereas the C3H/HeJ strain is naturally protected from AHR through decreased production of IL-17A5. Indeed, we and others have previously reported that inhibition of IL-13 can potently inhibit AHR in models of allergic asthma and that over-expression or administration of IL-13 is sufficient to induce AHR, in part through direct effects of IL-13 on airway epithelial cells. Based on the results of this study, we suspect transgenic or intranasal administration of IL-13 results in higher concentrations of IL-13 than those released in the context of allergen challenge, and that under those circumstances IL-17 is not required for the development of AHR. Although inhibitor studies confirm that released IL-13 is important in the development of allergen-induced AHR, our results suggest that the small amounts of IL-13 released in response to allergen challenge are not in themselves sufficient to induce AHR, but require the additional effect of IL-17.
The results of this study provide several novel insights into how TH17 cells and TH17 cytokines contribute to the development of allergic asthma, and thus identify a number of new potential therapeutic targets. Our results identify the integrin, αvβ8, as a critical upstream activator that is essential for TH17 cell generation in allergic airway disease. We also show that loss of this integrin on dendritic cells dramatically inhibits TH17 cell generation without effects on the IL-17A producing γδ T cells, suggesting it might be possible to inhibit this TH17 induction without eliminating the key role played by IL-17A producing γδ T cells in pulmonary defense against bacterial and fungal pathogens. Furthermore, our findings are the first demonstration that IL-17A, but not IL-17F or IL-22, can contribute to allergic asthma by directly enhancing the contractile responses of airway smooth muscle cells, through a pathway that involves activation of NF-κB and induction of RhoA and ROCK2 expression. The αvβ8 integrin, IL-17A and NF-κB and its downstream effectors could thus all be attractive targets for improved treatment of allergic asthma.
METHODS
Mice
We generated mice lacking integrin β8 on DCs (Itgb8f/f x CD11c-cre) and mice deficient in IL-17RC (Il17rc−/−) as previously described26,54. All mice were on the C57BL/6 background and experiments were approved by the Institutional Animal Care and Use Committee of UCSF.
Allergen challenge models
We sensitized equal numbers of male and female 6–8 week old mice on days 0, 7, and 14 by intraperitoneal injection of 50 μg ovalbumin (OVA, Sigma-Aldrich) emulsified in 1 mg of aluminum potassium sulfate. One week after the lastsensitization, mice were intranasally challengedon 3 consecutive days with 100 μg OVA in 40 μL saline. Alternatively, 40 μL dust mite fecal pellet preparation (2.5 mg/ml, Greer Laboratories) or saline was administered intranasally on day 0, 7, 14 and 21. 24 hours after the last OVA challenge and 72 hours after the last HDM challenge, mice were attached to a pulmonary mechanics analyzer (FlexiVent; SIRAQ Inc, Canada), ventilated and paralyzed, and airway mechanics were measured in response to acetylcholine administered via tail vein.
We lavaged lungs five times with 0.8 ml of phosphate-buffered saline (PBS) and then inflated them with 10% buffered formalin to 25 cm H2O of pressure. Paraffin-embedded 5-μm sections were stained with Periodic acid-Schiff (PAS) to evaluate mucus production. Antigen- specific lymphocyte proliferation was assessed by 3H-thymidine uptake.
Flow Cytometry
Single-cell suspensions were generated from whole lungs and incubated for 4 hours at 37o C in complete RPMI containing 50 ng/mL PMA (Sigma), 1 μM ionomycin (Sigma), and 2 μM monensin (eBioscience). Cells were then stained and analyzed as previously described25.
Tracheal ring contraction
Excised tracheas from male mice were cut into 3 mm rings, and placed in DMEM supplemented with 10mM HEPES and antibiotics. Rings were incubated with or without cytokines or antibodies for 12 hours in a 37o C incubator with 5% CO2. Rings were suspended in a 15-ml organ bath filled with oxygenated modified Krebs-Henseleit solution, connected to a force-displacement transducer (FT03, Grass Instrument Co., West Warwick, RI) and a resting tension of 0.5 g was applied. After a 15 minute equilibration period, concentration-response curves to methacholine (MCh) and potassium chloride (KCl) were constructed.
We obtained human lung from a 66 year-old male lung transplant donor in accordance with procedures approved by the UCSFCommittee on Studies Involving Human Beings. Bronchi of 5 to 8 mm diameter were dissected free and cut into 4-mm-thick rings. We assessed contraction as above except a resting tension of 2 g was applied and rings were first contracted with 120 mM KCl after equilibration for 2 hours.
Airway contraction in lung slices
We prepared lung slices from 6–10 week old male mice based on the method developed by Bai and Sanderson55. Lungs were inflated with 1.0 ml of 2% agarose (37° C, low-melt preparative agarose) through a tracheal catheter. Air (0.2 ml) was injected to flush the agarose out of the airways. Lungs were cooled to 4° C and serial 140 μm sections were cut with a vibratome (model EMS-4000, EMS). Slices were maintained in DMEM supplemented with antibiotics at 37° C and 10% CO2 for up to 3 days. Images of whole lung slices were acquired using the area scan function and 5X objective on a temperature-controlled Leica DM6000 inverted microscope at 37° C.
For measurement of calcium oscillations, lung slices were loaded with Oregon green 488 BAPTA-AM (20 μM in HBSS containing 0.1% Pluronic F-127 and 100 μM sulfobromophthalein) for 45 min at 30° C and de-esterified for 30 min at 30° C in HBSS containing 100 μM sulfobromophthalein. Fluorescence imaging was performed with a NIKON spinning disk confocal microscope at 20 frames/second. Changes in fluorescence intensity from selected regions of interest (5 X 5 pixels) were analyzed with ImageJ software.
Western Blots
Smooth muscle was dissected from tracheas and homogenized in lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, 1% (v/v) Nonidet P40, 10 mM NaF, 1 mM Na3VO4, and complete Mini protease inhibitor cocktail (Roche). TKM buffer (50 mM Tris-HCl, pH 7.4, 25 mM KCl, 5 mM MgCl2) with 0.25 M Sucrose was used for nuclear extracts. Samples were separated by 10% SDS-PAGE, transferred to a polyvinylidene difluoride membrane (Millipore). Membranes were blocked for 30 min with 5% skim milk or BSA, incubated for 2 hours with primary antibodies, incubated for 1 hour with peroxidase-conjugated secondary antibody and developed with Plus-ECL reagent (PerkinElmer).
Statistical Analysis
The statistical significance of differences between paired groups was calculated with a two-tailed Student's t-test. One-way ANOVA was used for comparison of multiple groups using SigmaStat 3.11, and when differences were statistically significant (P≤0.05), this was followed with a Bonferroni t-test for subsequent pairwise analysis.
Supplementary Material
Acknowledgments
This work was supported by US National Institutes of Health (NIH) grants HL64353, HL53949, HL083950, AI024674, and U19 AI077439 (to D. Sheppard), a NIH Ruth L. Kirschstein National Research Service Award HL095314 (to A. Melton), an American Lung Association of California Research Training Fellowship Award (to A. Melton), and funds from the University of California, San Francisco (UCSF) Strategic Asthma Basic Research Center. De-identified human lung was kindly provided by M. Matthay (UCSF) and P. Wolters (UCSF). IL-17RC knockout mice were kindly provided by W. Ouyang (Genentech).
Footnotes
Conflict of interest: The authors declare that they have no competing interests as defined by Nature Publishing Group, or other interests that might be perceived to influence the results and discussion reported in this paper.
AUTHOR CONTRIBUTIONS
M.K. designed the study, generated all of the tracheal ring contraction and Western Blot data, performed analyses and wrote the manuscript, A.C.M., designed the study, generated all of the flow cytometry and recall data, performed analyses and wrote the manuscript, C.C., generated all of the lung slice data, performed analyses and wrote the manuscript, K.E.H., X.R., Y.W., X.B., performed the asthma physiology studies, M.B.E. designed and constructed the muscle bath and provided expertise in the methodology and modifications for the analysis of mouse tracheal ring contraction, J.T.L. and K.A. provided a substantial intellectual contribution, X.H. and D.S. oversaw the design and interpretation of all studies described and oversaw writing of the manuscript.
References
- 1.Lloyd CM, Hessel EM. Functions of T cells in asthma: more than just T(H)2 cells. Nat Rev Immunol. 2010;10:838–848. doi: 10.1038/nri2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grunig G, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alcorn JF, Crowe CR, Kolls JK. TH17 cells in asthma and COPD. Annu Rev Physiol. 2010;72:495–516. doi: 10.1146/annurev-physiol-021909-135926. [DOI] [PubMed] [Google Scholar]
- 4.Souwer Y, Szegedi K, Kapsenberg ML, de Jong EC. IL-17 and IL-22 in atopic allergic disease. Curr Opin Immunol. 2010;22:821–826. doi: 10.1016/j.coi.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Lajoie S, et al. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nature immunology. 2010;11:928–935. doi: 10.1038/ni.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 7.Finkelman FD, Hogan SP, Hershey GK, Rothenberg ME, Wills-Karp M. Importance of cytokines in murine allergic airway disease and human asthma. J Immunol. 2010;184:1663–1674. doi: 10.4049/jimmunol.0902185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bullens DM, et al. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res. 2006;7:135. doi: 10.1186/1465-9921-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Faria IC, de Faria EJ, Toro AA, Ribeiro JD, Bertuzzo CS. Association of TGF-beta1, CD14, IL-4, IL-4R and ADAM33 gene polymorphisms with asthma severity in children and adolescents. J Pediatr (Rio J) 2008;84:203–210. doi: 10.2223/JPED.1783. [DOI] [PubMed] [Google Scholar]
- 10.Pulleyn LJ, Newton R, Adcock IM, Barnes PJ. TGFbeta1 allele association with asthma severity. Hum Genet. 2001;109:623–627. doi: 10.1007/s00439-001-0617-y. [DOI] [PubMed] [Google Scholar]
- 11.Silverman ES, et al. Transforming growth factor-beta1 promoter polymorphism C-509T is associated with asthma. Am J Respir Crit Care Med. 2004;169:214–219. doi: 10.1164/rccm.200307-973OC. [DOI] [PubMed] [Google Scholar]
- 12.Zeyrek D, et al. Association of interleukin-1beta and interleukin-1 receptor antagonist gene polymorphisms in Turkish children with atopic asthma. Allergy Asthma Proc. 2008;29:468–474. doi: 10.2500/aap.2008.29.3154. [DOI] [PubMed] [Google Scholar]
- 13.Settin A, Zedan M, Farag M, Ezz El Regal M, Osman E. Gene polymorphisms of IL-6(-174) G/C and IL-1Ra VNTR in asthmatic children. Indian J Pediatr. 2008;75:1019–1023. doi: 10.1007/s12098-008-0161-z. [DOI] [PubMed] [Google Scholar]
- 14.Mangan PR, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 15.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 16.Ivanov II, et al. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 17.Yang XO, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 18.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atarashi K, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 20.Veldhoen M, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 21.Ivanov II, et al. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Volpe E, et al. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 24.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 25.Melton AC, et al. Expression of alphavbeta8 integrin on dendritic cells regulates Th17 cell development and experimental autoimmune encephalomyelitis in mice. J Clin Invest. 2010;120:4436–4444. doi: 10.1172/JCI43786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Travis MA, et al. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–365. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fossiez F, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 29.Blaho VA, Buczynski MW, Dennis EA, Brown CR. Cyclooxygenase-1 orchestrates germinal center formation and antibody class-switch via regulation of IL-17. J Immunol. 2009;183:5644–5653. doi: 10.4049/jimmunol.0901499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin Y, et al. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity. 2009;31:799–810. doi: 10.1016/j.immuni.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wills-Karp M, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 32.Tliba O, et al. IL-13 enhances agonist-evoked calcium signals and contractile responses in airway smooth muscle. British journal of pharmacology. 2003;140:1159–1162. doi: 10.1038/sj.bjp.0705558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parris JR, Cobban HJ, Littlejohn AF, MacEwan DJ, Nixon GF. Tumour necrosis factor-alpha activates a calcium sensitization pathway in guinea-pig bronchial smooth muscle. J Physiol. 1999;518 ( Pt 2):561–569. doi: 10.1111/j.1469-7793.1999.0561p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barnes PJ, Cuss FM, Palmer JB. The effect of airway epithelium on smooth muscle contractility in bovine trachea. Br J Pharmacol. 1985;86:685–691. doi: 10.1111/j.1476-5381.1985.tb08946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barnett K, Jacoby DB, Nadel JA, Lazarus SC. The effects of epithelial cell supernatant on contractions of isolated canine tracheal smooth muscle. Am Rev Respir Dis. 1988;138:780–783. doi: 10.1164/ajrccm/138.4.780. [DOI] [PubMed] [Google Scholar]
- 36.Somlyo AP, Somlyo AV. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol. 2000;522(Pt 2):177–185. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sonder SU, et al. IL-17-induced NF-{kappa}B activation via CIKS/Act1: physiologic significance and signaling mechanisms. J Biol Chem. 2011 doi: 10.1074/jbc.M110.199547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao Z, et al. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3:811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 39.Goto K, et al. The proximal STAT6 and NF-kappaB sites are responsible for IL-13- and TNF-alpha-induced RhoA transcriptions in human bronchial smooth muscle cells. Pharmacol Res. 2010;61:466–472. doi: 10.1016/j.phrs.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimada H, Rajagopalan LE. Rho kinase-2 activation in human endothelial cells drives lysophosphatidic acid-mediated expression of cell adhesion molecules via NF-kappaB p65. J Biol Chem. 2010;285:12536–12542. doi: 10.1074/jbc.M109.099630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiba Y, Misawa M. The role of RhoA-mediated Ca2+ sensitization of bronchial smooth muscle contraction in airway hyperresponsiveness. J Smooth Muscle Res. 2004;40:155–167. doi: 10.1540/jsmr.40.155. [DOI] [PubMed] [Google Scholar]
- 42.Khromov A, Choudhury N, Stevenson AS, Somlyo AV, Eto M. Phosphorylation-dependent autoinhibition of myosin light chain phosphatase accounts for Ca2+ sensitization force of smooth muscle contraction. J Biol Chem. 2009;284:21569–21579. doi: 10.1074/jbc.M109.019729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ely LK, Fischer S, Garcia KC. Structural basis of receptor sharing by interleukin 17 cytokines. Nat Immunol. 2009;10:1245–1251. doi: 10.1038/ni.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patil SB, Bitar KN. RhoA- and PKC-alpha-mediated phosphorylation of MYPT and its association with HSP27 in colonic smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2006;290:G83–95. doi: 10.1152/ajpgi.00178.2005. [DOI] [PubMed] [Google Scholar]
- 45.Ohama T, Hori M, Sato K, Ozaki H, Karaki H. Chronic treatment with interleukin-1beta attenuates contractions by decreasing the activities of CPI-17 and MYPT-1 in intestinal smooth muscle. J Biol Chem. 2003;278:48794–48804. doi: 10.1074/jbc.M310166200. [DOI] [PubMed] [Google Scholar]
- 46.Chen XY, Dun JN, Miao QF, Zhang YJ. Fasudil hydrochloride hydrate, a Rho-kinase inhibitor, suppresses 5-hydroxytryptamine-induced pulmonary artery smooth muscle cell proliferation via JNK and ERK1/2 pathway. Pharmacology. 2009;83:67–79. doi: 10.1159/000178814. [DOI] [PubMed] [Google Scholar]
- 47.McKinley L, et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181:4089–4097. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schnyder-Candrian S, et al. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med. 2006;203:2715–2725. doi: 10.1084/jem.20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakae S, et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 50.Wilson RH, et al. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med. 2009;180:720–730. doi: 10.1164/rccm.200904-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oda N, et al. Interleukin-17F induces pulmonary neutrophilia and amplifies antigen-induced allergic response. Am J Respir Crit Care Med. 2005;171:12–18. doi: 10.1164/rccm.200406-778OC. [DOI] [PubMed] [Google Scholar]
- 52.Hellings PW, et al. Interleukin-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthma. Am J Respir Cell Mol Biol. 2003;28:42–50. doi: 10.1165/rcmb.4832. [DOI] [PubMed] [Google Scholar]
- 53.Song C, et al. IL-17-producing alveolar macrophages mediate allergic lung inflammation related to asthma. J Immunol. 2008;181:6117–6124. doi: 10.4049/jimmunol.181.9.6117. [DOI] [PubMed] [Google Scholar]
- 54.Zheng Y, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 55.Bai Y, Sanderson MJ. Modulation of the Ca2+ sensitivity of airway smooth muscle cells in murine lung slices. Am J Physiol Lung Cell Mol Physiol. 2006;291:L208–221. doi: 10.1152/ajplung.00494.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.