Abstract
Our previous studies suggest that the α2,3sialylated T-antigen (NeuAcα2,3Galβ1,3GalNac-) and associated glycan structures are likely to be elevated during cancer. An easy and reliable strategy to label mucinous glycans that contain such carbohydrates can enable the identification of novel glycoproteins that are cancer associated. To this end, the present study demonstrates that the exchange sialylation property of mammalian ST3Gal-II can facilitate the labeling of mucin glycoproteins in cancer cells, tumor specimens and glycoproteins in cancer sera. Results show that: i) the radiolabeled mucin glycoproteins of each of the cancer cell lines studied (T47D, MCF7, LS180, LNCaP, SKOV3, HL60, DU4475 and HepG2) is distinct either in terms of the specific glycans presented or their relative distribution. While some cell lines like T47D had only one single sialylated O-glycan, other like LS180 and DU4475 contained a complex mixture of mucinous carbohydrates. ii) [14C]sialyl labeling of primary tumor cells identified a 25–35 kDa mucin glycoprotein unique to pancreatic tumor. Labeled glycoproteins for other cancers had higher molecular weight. iii) Studies of [14C] sialylated human sera showed larger mucin glycopeptides and 2fold larger mucin-type chains in human serum compared to [14C]sialyl labeled glycans of fetuin. Overall, the exchange sialylation property of ST3Gal-II provides an efficient avenue to identify mucinous proteins for applications in glycoproteomics and cancer research.
Keywords: Cancer-glycoproteins, 3′sialyl T-hapten, sialylation, radiosialyl tagging, lectin affinity binding, Tn epitope clustering
Cancer causes changes in a number of cellular processes that promote proliferation and tumorigenesis. Among these, cancer dramatically alters the cellular glycosylation machinery1. This results in an altered pattern of cell surface glycan expression during disease. Tumor associated antigens are also commonly found as sialylated mucin-type glycans2. In this regard, mucins are high molecular weight glycoproteins containing varying numbers of serine and threonine-rich tandem repeat regions. The O-glycan chains associated with these Ser/Thr units of mucins constitute 50–80% of its mass3. These glycans confer characteristic biochemical and biophysical properties to the mucins4,5. The number of tandem repeats have an effect on O-glycosylation and this may be associated with increased susceptibility to certain human diseases6–12. For example, the disialyl core NeuAcα2,3Galβ1,3(NeuAcα2,6)GalNAcα-O-Thr of a mucin type glycoprotein is a prototypic platelet aggregating factor on cancer cells that influences tumor metastasis13.
The identification of proteins bearing mucin-type O-linked glycans remains elusive due to the complex nature of their biosynthesis and the lack of tools14 comparable to those used to study N-linked glycosylation15–18. The large molecular size of mucins and the diversity of their carbohydrate structures on such molecules also contribute to difficulties in investigating the molecular features of mucins. These limitations highlight the importance of developing newer approaches for the proteomic analysis of mucin-type O-linked glycans. During the synthesis of O-glycans, the first step involves the attachment of GalNAc in α-linkage to hydroxyl groups of Ser/Thr by a family of ppGalNActransferases. This enzyme family has ~20 members, that display distinct substrate specificities and tissue distibutions19, 20. Since O-glycosylation is a post-protein folding event, only Ser/Thr residues that are exposed on the protein surface such as coil, turn and linker regions are expected to be glycosylated. The exact nature of the O-glycan formed is also a result of additional biosynthetic pathways and enzymes localized in the Golgi, especially glycosyltransferases and sulfotransferases21, 22. Among the glycosyltransferases, we have determined a dominant activity for α2,3 sialyltransferase towards Galβ1,3GalNAcα in various tumor tissues and cancer cell lines23, 24. Further α1,2-L-fucosyltransferase and Gal:3-0-sulfotransferase acting on Galβ1,3GalNAcα are not widely expressed by cancer cells23–25.
Since NeuAcα2,3Galβ1,3GalNAcα type structures could be dominant structures associated with cancerous mucins, it would be attractive to develop methods to identify such entities. In this context, we report here that the exchange sialylation properties of the mammalian sialyltransferase ST3Gal-II can aid the identification of such glycans. In previous studies, we have demonstrated that this enzyme in addition to its direct sialylation activity towards Galβ1,3GalNAcα26 can also catalyze the formation of CMP-NeuAc from 5′CMP in presence of a donor containing the NeuAcα2,3Galβ1,3GalNAc-unit27. This reaction, which proceeds in a direction opposite to the normal sialylation reaction, is termed ‘reverse sialylation’. In addition to this, we also reported that ST3Gal-II is capable of exchanging sialyl residues between CMP-NeuAc and NeuAcα2,3Galβ1,3GalNAc-unit of mucin glycoproteins28. This reaction process is termed ‘exchange sialylation’. In the present study, we demonstrate that the utilization of the exchange sialylation catalytic properties of ST3Gal-II enables the study of mucin type glycoproteins present in cancer cells, tumor tissues and cancer sera. While the present study demonstrates the labeling and preliminary characterization of primary mucinous cancer specimens, further studies on these radiosialyl labeled glycoproteins in the future would be able to determine the precise structure of these labeled glycans that are associated with cancer.
MATERIALS AND METHODS
Cancer cell lines
Cancer cell lines T47D, MCF-7, DU4475 (breast), LS180 (colon), LNCaP (prostate), SKOV3 (ovarian), HL60 (leukemic) and HepG2 (hepatic) were cultured as recommended by ATCC (Manassas, VA)25. All cell samples were homogenized with 0.1 M Tris-Maleate pH 7.2 containing 2% Triton X-100 using a Dounce glass, hand-operated homogenizer. The homogenate was centrifuged at 16,000g for 1h at 4°C. The cell extracts (1ml each) was incubated separately at 37°C for 24 h in 0.1M Na Cacodylate pH 6.0, 10μCi CMP-[9-3H] NeuAc and 100 mU of ST3Gal-II (reaction volume 1.4 ml). After incubation, the reaction mixtures were dialyzed in the cold room against 2L deionized distilled water with 4 changes for 72h, lyophilized to dryness, weighed and then picked up in 1.0 ml water. These [9-3H] sialyl labeled cell extracts were used in biochemical studies.
Tumor Specimens
A total of 10 tumor specimens from 10 different donors (pancreatic cancer:3; breast cancer:1; colon cancer:1; ovarian cancer:2 and prostate cancer:3) obtained during surgery at the Roswell Park Cancer Institute, were frozen within 1h of collection at −70°C. Tissues were homogenized at 4°C with 4 volumes of 0.1 M Tris-Maleate pH 7.2, 0.1% NaN3 using Kinematica. After adjusting the TritonX-100 concentration to 2%, these homogenates were mixed in the cold room for 1h using Speci-Mix (Thermolyne) and then centrifuged at 20,000g for 1h at 4°C. The clear fat-free supernatant was stored frozen at −20°C until use. The tumor extracts (0.1ml each) were incubated separately at 37°C for 20h in 0.1M Na cacodylate pH 6.0, 0.2 μCi CMP-[14C] NeuAc and 25mU ST3Gal II (reaction volume 0.16mL). After incubation, the reaction mixture was diluted with 1.0 mL water and dialyzed against water as described for the cell extracts.
In some cases, aliquots of these preparations were subjected to SDS-PAGE. SDS-PAGE was carried out using 4–20% polyacrylamide gradient gels. Following transfer to nitrocellulose membrane, radioactive glycoprotein bands were visualized using autoradiography.
Serum Samples
Serum from each donor was examined initially for the incorporation of [14C] NeuAc into mucin glycoproteins; 20μL aliquots of sera were incubated separately with 0.04μCi CMP-[14C] NeuAc and 2 mU of cloned ST3Gal-II for 20h at 37°C. These incubated samples were diluted to 1 mL with water and then dialyzed against water (2L) with several changes in the cold room for 72 h. These dialyzed preparations contained significant amount of [14C] radioactivity in the range of 2–6 × 104. Based on these results a large scale study was undertaken as follows. Normal sera 0.6mL (a mixture of 0.15mL each from 4 donors) was incubated at 37°C for 22h in 0.1M Na cacodylate pH 6.0, 0.07 μCi CMP-[14C] NeuAc and 100mU ST3Gal-II (reaction volume 0.75mL). Ovarian cancer sera 1.8mL (a mixture of 0.36mL each from 5 donors) and pancreatic cancer sera 1.8mL (a mixture of 0.36mL each from 5 donors) were incubated separately 37°C for 22h in 0.1M Na cacodylate pH 6.0, 0.2 μCi CMP-[14C] NeuAc and 300mU ST3Gal-II (reaction volume 2.3mL). These samples were fractionated separately on Biogel P6 column (Fine Mesh; 1.0×116.0 cm). Fractions containing radioactivity excluded as first peak were pooled, lyophilized to dryness, weighed and dissolved in water to a concentration of 20mg per mL. In each case 0.7 mL of [14C] sialyl glycoprotein preparation (14mg) was subjected to exhaustive pronase digestion and 0.5 mL (10mg) was treated with alkaline borohydride.
Preparation of [14C] labeled sialyl fetuin
[14C] labeled sialyl fetuin was prepared by incubating 30mg fetuin (Sigma catalog F3004) with 1.5μCi CMP-[14C]NeuAc and 100mU ST3Gal-II in 0.8ml volume of 0.2M Na cacodylate pH 6.0 for 20h at 37°C. The reaction mixture was separated using Biogel-P2 (1.0×116.0 cm) chromatography. The first radioactive peak emerging at the void volume contained radiolabeled fetuin. This was lyophilized to dryness and used in the studies.
Proteolytic treatment and separation of glycopeptides
Pronase-digestion of [9-3H] sialyl mucin glycoproteins from cell extracts present in 0.2mL dialyzed sialyl extract was carried out in 1.0 ml of 0.1M Tris-HCl pH 7.0, 1 mM CaCl2, 1% ethanol and 0.1% NaN3 containing 10 mg Pronase CB (EMD-Chemicals) at 37 °C for 24h. After the treatment, the samples were kept frozen at −20°C before fractionation on Biogel P6 column. In the case of serum samples, 0.7mL of the three extensively dialyzed serum samples containing [14C] sialyl mucin glycoproteins were subjected to pronase (20mg) digestion as described above (reaction volume 1.4mL). After incubation at 37°C for 24h, 20mg pronase was again added and the incubation continued for another 24h.
Release of O-glycans from protein backbone and their separation
Mild alkaline borohydride treatment of [9-3H]/[14C] labeled sialyl mucin glycoproteins present in 0.2mL dialyzed cell extract and 0.5mL dialyzed serum was performed in Teflon lined screw-capped test tubes using 1.0M Na borohydride in 0.1 N NaOH in a total volume of 1.0 ml. Samples were incubated at 45°C for 24h, excess borohydride was destroyed by adding drops of acetic acid carefully, and storing frozen samples at −20°C before fractionation on Biogel P6 column. As anticipated, TLC of radio sialyl fetuin after this treatment showed one major component representing NeuAcα2,3Galβ1,3GalNAcα-ol and one minor component NeuAcα2, 3Galβ1,3(NeuAcα2,6)GalNAcα-ol.
Column chromatography
The following chromatography methods were applied for product separation: a) Biogel-P6 column (Fine Mesh; 1.0×116.0 cm) chromatography was carried out with 0.1 M pyridine acetate (pH5.4) as the eluent. Void volume of this column is 30mL; b) Lectin-agarose affinity chromatography using columns of 7ml bed volume of WGA- and VVL-agarose (Vector Lab, Burlingame, CA) under conditions recommended by supplier26, 27. Fractions of 1 mL were collected. After binding was allowed to occur till fraction 15 in all cases, the bound product was eluted. Product from WGA-agarose was eluted with 0.5 M GlcNAc and from VVL-agarose with 0.2 M GalNAc. c) Thin layer chromatography using Silica gel GHLF (250μm scored 20X20cm; Analtech Newark DE) was also used for further product separation26, 27. TLC was carried out on Silica gel GHLF (250μm scored 20X20cm; Analtech Newark DE). The solvent system 1-propanol/NH4OH/H2O (12/2/5 v/v) was used. The [9-3H] sialyl products were located by scraping 0.5cm width segments of silica gel and soaking in 2.0ml water in vials followed by liquid scintillation counting. The [14C] sialyl products were located by phosphorimaging.
RESULTS
Characterization of [9-3H] sialylated mucin-type glycoproteins from human cancer cell lines
The exchange sialylation reaction was applied to radiolabel mucin type glycoproteins from human cancer cells and the results are summarized in Table 1. The radiosialyl mucin glycoproteins were separated using WGA (Figure 1A) as well as VVL (Figure 1B) affinity chromatography. WGA binds terminal GlcNAc residues of glycans and also glycoproteins via sialyl residues. Using synthetic mucin core-2 compounds26, we have noted that WGA binds to mucin core-2 [9-3H]NeuAcα2,3Galβ1,3(GlcNAcβ1,6)GalNAcα- and its derivatives containing substituents on GlcNAc such as 6-O-Sulfo, β1,4 linked Gal or both and β1,4 linked (3-O-Sulfo)Gal. It does not bind to [9-3H]NeuAcα2,3 or α2,6Galβ1,4GlcNAcβ1,6(Galβ1,3)GalNAcα-. VVL on the other hand binds the Tn epitope (GalNAcα-O-Ser/Thr) of mucinous glycoproteins.
TABLE 1.
Characteristics of [9-3H]sialylated glycoproteins present in human cancer cell lysates
| Incorporation of [9-3H]NeuAc/mg wt* | [9-3H]sialyl Glycoproteins binding | Biogel P6 [9-3 H]Sialyl fractions from pronase digestion | |||
|---|---|---|---|---|---|
| Cancer cell line | CPM x 10−4 | WGA- Agarose % | VVL- Agarose % | P6 excluded | P6 included |
| T47D (breast) | 20.54 | 100 | 70 | Minor WB 95% VB 5% |
Major WB 40% VB 40% |
| MCF (breast) | 4.75 | >90 | 40 | Major | Major |
| LS180 (colon) | 8.31 | >90 | 40 | Major WB 95% VB 20% |
negligible |
| SKOV3 (ovary) | 8.48 | 60 | <10 | Major | Major |
| Hep G2 (hepatic) | 3.35 | 60 | <10 | Major | Major |
| HL60 (leukemia) | 3.10 | 60 | <10 | Major | Major |
| LNCap (prostate) | 8.89 | 50 | <10 | Major | Major |
Extensively dialyzed and then lyophilized material from [9-3H]sialylated cancer cell lysates
WB: WGA- agarose binding and VB: VVL-agarose binding
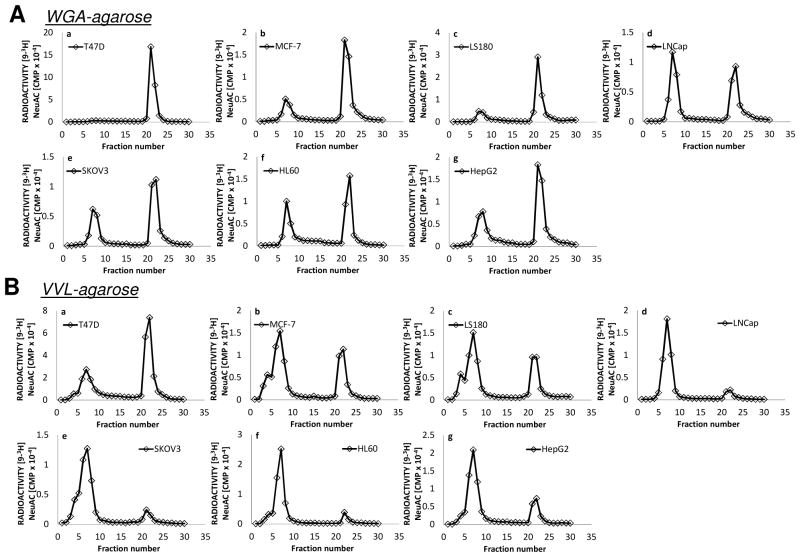
Fig 1. Lectin affinity chromatography of intact proteins.
Lectin-agarose affinity chromatography of [9-3H] sialylated glycoproteins of lysates from human cancer cell lines a: T47D; b: MCF-7; c: LS180; d: LNCaP; e: SKOV3; f: HL60; g: HepG2. A column of 7ml bed volume of WGA-agarose (top half of figure, A) or VVL-agarose (bottom half of figure, B) was employed using 10mM Hepes pH7.5 containing 0.1mM CaCl2, 0.01mM MnCl2 and 0.1% NaN3 as the running buffer. An aliquot (either 50 or 100 μL) of [9-3H] sialyl dialyzed cell extract preparation was diluted to 1 mL with the running buffer and applied to the affinity column. Fractions of 1.0ml were collected. After fraction 15, the bound material was eluted with 0.5M GlcNAc or 0.2M GalNAc in the same buffer.
WGA-agarose affinity chromatography of [9-3H] sialylated glycoproteins of cell lysates indicated that breast cancer cell line T47D contained exclusively WGA binding mucin glycoproteins (Fig 1Aa). The other breast cancer cell line MCF-7 and the colon cancer cell line LS180 contained mostly WGA binding mucin glycoproteins (Fig 1Ab, 1Ac), though a fraction of the product did not bind WGA. The prostate cancer cell line LNCaP contained almost equal amounts of WGA binding and non-binding mucin glycoproteins (Fig 1Ad). The ovarian cancer cell line SKOV3, the hepatic cancer cell line HepG2 and the leukemia cancer cell line HL60 contained about 60% WGA binding mucin glycoproteins (Fig 1Ae–1Ag). Overall, the WGA binding properties of T47D, MCF7 and LS180 glycoproteins were different from that of the other cell lines.
VVL-agarose affinity chromatography of [9-3H] sialylated glycoproteins obtained from these same cell lysates indicated that T47D contained about 70% VVL binding mucin glycoproteins (Fig 1Ba) followed by MCF-7 and LS180, which contained about 40% VVL binding mucin glycoproteins (Fig 1Bb, 1Bc). LNCaP, SKOV3, HL60 and HepG2 contained more than 90% VVL non-binding mucin glycoproteins (Fig 1Bd–1Bg). The results demonstrate additional differences in the expression of the GalNAcα-O-Ser/Thr (Tn–epitope) in the cancer cells including the possible existence of clustered Tn epitopes inT47D, MCF-7 and LS180.
Analysis of [9-3H] sialylated glycopeptides and glycans
Besides evaluating full glycoproteins, additional analysis of individual mucinous glycans can be performed following pronase digestion of peptide backbone and alkaline borhydride treatment to release O-glycans.
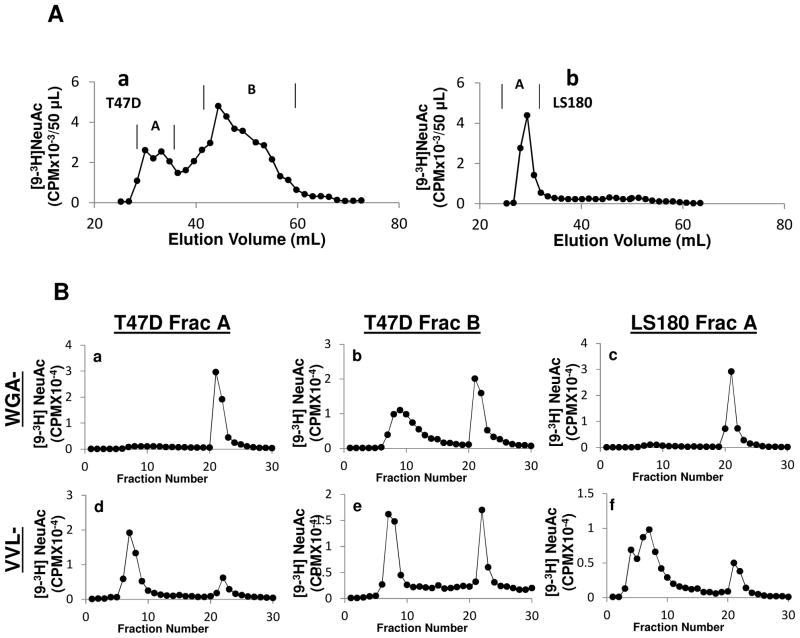
Upon pronase digestion, as seen, T47D gave rise to one excluded and another major glycopeptide fraction that was included in the column (Fig 2Aa). LS180, on the other hand, contained mostly Biogel P6 excluded mucin glycopeptides (Fig 2Ab). The other cell lines gave rise to a similiar Biogel P6 pattern consisting of 2–3 component glycopeptide fractions (Sup Fig 1). The distinction between the glycans of LS180 and T47D was further revealed by resolving various fractions from the Biogel P6 column using WGA- and VVL- affinity chromatography (Fig. 2B). As seen, most of the high molecular weight fraction from T47D (fraction A) bound WGA-agarose (Fig 2Ba) but not VVL-agarose (Fig 2Bd). On the other hand, the glyopeptide fraction B from T47D displayed equal binding to WGA and VVL (Fig 2Bb, 2Be). The fraction of LS180 that was excluded in the Biogel P6 column (fraction A) behaved similar to fraction A of T47D in that it contained mostly WGA-agarose binding material (Fig 2Bc) whereas VVL-agarose binding material was only 20% (Fig 2Bf).
Fig 2. Analysis of pronase-digested fragments.
A. Fractionation of pronase-digested [9-3H] sialylated glycoproteins from human cancer cell lines (T47D in subpanel a and LS180 in subpanel b) using Biogel P6 size exclusion chromatography. Here, [9-3H] sialyl mucin glycoproteins from cell extracts were digested in 1.0 ml of 0.1M Tris-HCl pH 7.0, 1 mM CaCl2, 1% ethanol and 0.1% NaN3 containing 10 mg Pronase CB (EMD-Chemicals) at 37 °C for 24h. A Biogel P6 column (Fine Mesh; 1.0×116.0 cm) was used with 0.1 M pyridine acetate (pH5.4) as the eluent at room temperature. B. Fractions containing radioactivity in panel A peaks were pooled, lyophilized to dryness and dissolved in a small volume of water. Lectin affinity chromatography of these fractions was performed as described in Fig 1. Subpanels a–c: WGA-agarose chromatography and subpanels d–f: VVL-agarose chromatography. Both fractions A and B for T47D and fraction A of LS180 were resolved as indicated in figures.
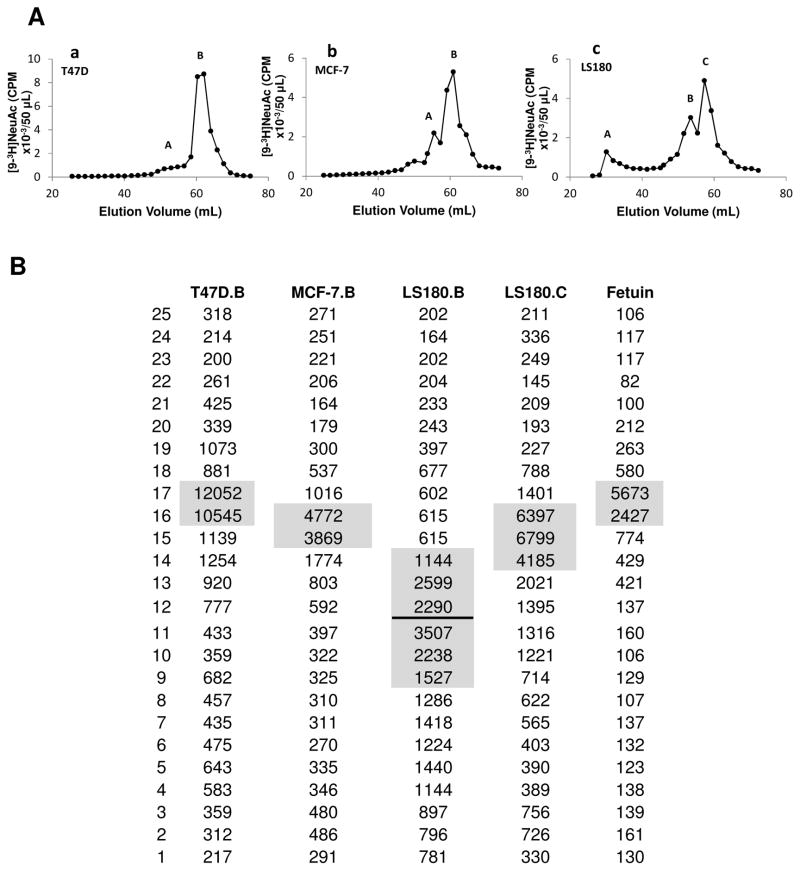
Mild alkaline borhydride treatment of [9-3H] sialylated mucin-type glycoproteins of cell lysates followed by Biogel P6 column chromatography showed that T47D, MCF-7, LS180 and also HepG2, contained similar O-glycan components, one major and one minor component except for an excluded fraction in the case of LS180 (Fig 3Aa, 3Ab, 3Ac; Sup Fig 2D). LNCaP, SKOV3 and HL60 gave rise to 3–4 components (Sup Fig 2A–2C). Thin layer chromatography of the Biogel P6 fractions obtained from alkaline borohydride treated [9-3H] sialylated glycoproteins of cell lysates indicated that T47D and MCF-7 contained one distinct major component whereas LS180 contained three distinct major components (Fig 3B). The glycan from T47D and MCF7 that eluted at 60–65mL moved similarly on the TLC plate as the major tritium labeled sialylated O-glycan of fetuin (NeuAcα2,3Galβ1,3GalNAc-ol) which also eluted at 60–65mL on the Biogel P6 column.
Fig 3. Mild alkaline borohydride treatment of glycoproteins.
A: Mild alkaline borohydride treatment of [9-3H] labeled sialyl mucin glycoproteins was performed in Teflon lined screw-capped test tubes using 1.0M Na borohydride in 0.1 N NaOH in a total volume of 1.0 ml. Samples were incubated at 45°C for 24h, excess borohydride was destroyed by adding drops of acetic acid carefully, and product was fractionated on Biogel P6 column as described in Fig 2 A. Results are presented for T47D (subpanel a), MCF-7 (subpanel b) and LS180 (subpanel c). B: Thin layer chromatography (TLC) analysis of Biogel P6 fractions obtained in panel A [T47D fraction B; MCF-7 fraction B; LS180 fractions B, C] and also the major O-glycan of fetuin. TLC was carried out on Silica gel GHLF (250μm scored 20X20cm; Analtech Newark DE) with 1-propanol/NH4OH/H2O (12/2/5 v/v) as solvent. The [9-3H] sialyl products were located by scraping 0.5cm width segments of silica gel and soaking in 2.0ml water in vials followed by liquid scintillation counting.
While detailed structural information is not revealed in this study, the data from Fig. 1–3 demonstrate that each of the cancer cell lines examined has a distinct pattern of O-linked glycosylation, either in terms of the identity of the underlying glycan or its relative abundance.
[14C] radiolabeling of mucin type carbohydrate chains using ST3Gal II
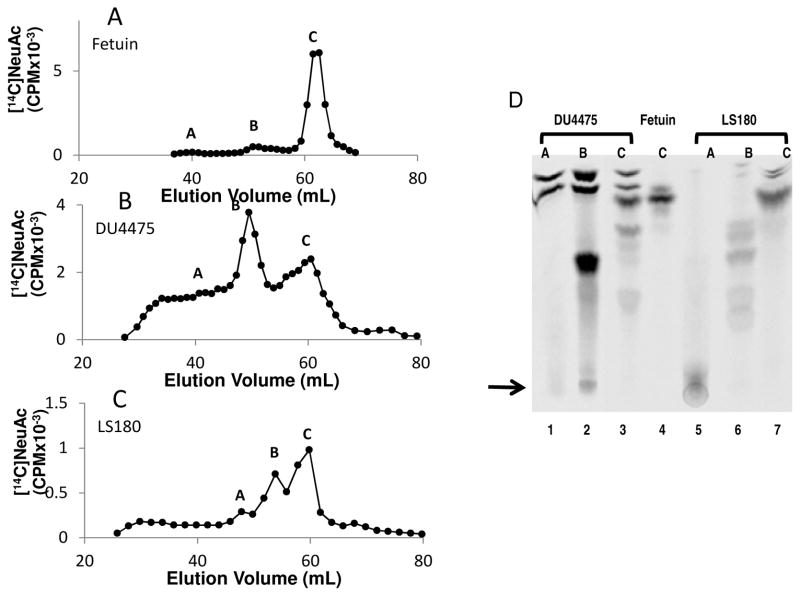
C-14 labeling of mucins is preferred when carbohydrate complexity increases since autoradiography is more straightforward using C-14 compounds compared to tritiated molecules. We demonstrate this by analyzing the mucin type glycoproteins from cell lysates of the breast cancer cell line DU4475 and colon cancer cell line LS180. Both glycoproteins were labelled using CMP [14C] NeuAc and ST3Gal-II. Bovine fetal protein fetuin was used as a standard during chromatography analysis. Radiolabeled samples were subjected to mild alkaline borohydride treatment and then fractionated on Biogel P6 column (Fig 4A–4C). The chromatogram of DU44745 (Fig 4B) and LS180 (Fig 4C) are more complex as compared to T47D (Fig. 3Aa). In this regard, DU4475 and LS180 exhibited larger mucin carbohydrate chains compared to T47D. Fractions separated by Biogel P6 were further subjected to TLC followed by autoradiography (Fig. 4D). While fetuin (Fig 4D Lane 4) contains one dominant glycan (NeuAcα2,3Galβ1,3GalNAc-ol), both DU4475 (Lanes 1–3) and LS180 (Lanes 5–7) contained multiple O-glycans with different mobilities. In this regard, DU4475 and LS180 are known to express Gal:3-O-sulfotransferase that act on mucin core 2 LacNAc type 2 chains 23,25. Thus, complex mucin type structures are anticipated in these cells.
Fig 4. Analysis of C-14 labeled glycans.
[14C] sialyl mucin glycoproteins from various cells were subjected to mild alkaline borohydride treatment as described in Fig 3. Biogel P6 column chromatographic patterns are presented for A: [14C] sialyl Fetuin, B: [14C] sialyl DU4475 mucin glycoproteins and C: [14C] sialyl LS180 mucin glycoproteins. D. Thin layer chromatography of fractions obtained from panels A–C are provided for [14C] sialyl DU4475 Biogel P6 fractions A, B and C (lanes 1, 2, 3 respectively), [14C] sialyl Fetuin O-glycan (lane 4) and [14C] sialyl LS180 Biogel P6 fractions A, B and C (lanes 5, 6, 7 respectively). The arrow indicates the origin of the TLC. Mobile phase moves from bottom to top. The [14C] sialyl products were located by phosphorimaging of TLC plates.
SDS-PAGE analysis of 14C sialyl mucin glycoproteins from tumor specimens
In order to demonstrate the utility of this methodology with primary human samples, we radiolabeled using CMP[14C]-NeuAc and ST3Gal-II, the mucin glycoproteins present in three pancreatic, one breast, one colon, two ovarian and three prostate tumor specimens. Labeled glycoproteins isolated from these tumor specimen were subjected to SDS-PAGE. Each tumor was observed to exhibit radiolabled mucins with distinct molecular mass. The three specimens of pancreatic tumor (Sup Fig. 3 Lanes 1–3) exhibited two distinct bands in the molecular weight range 25–35KD. Higher molecular mass glycoproteins are seen in colon and ovarian tumors (Sup Fig. 3 Lanes 5,6). Taken together, the data demonstrate the ability to label mucins on primary tissue using the exchange sialylation reaction.
Mucin type glycoproteins in human serum
The exchange sialylation properties of ST3Gal-II was utilized for [14C]sialyl labeling of mucin glycoproteins present in pooled mixtures of four normal, five ovarian cancer and five pancreatic cancer sera. The [14C]sialyl labeled mucin glycoproteins were isolated by Biogel P6 chromatography. In parallel, [14C]sialyl fetuin was prepared from fetuin through exchange sialylation by ST3Gal-II. Exhaustive pronase digestion (Sup Fig. 4A–4D) as well as mild alkaline borohydride (Sup Fig. 4E–4H) products were separated using Biogel P6 column chromatography. In the case of pronase digested samples, the major glycopeptides from sera elute at 35–45 mL (Sup Fig. 4A–4C) whereas the two glycopeptide fractions from feuin elute at 45–50 mL and 55–60mL (Sup Fig. 4D). These results indicate that the sialylated mucin glycopeptides from serum are larger than fetuin mucin glycopeptides. The distribution of radioactivity in Biogel P6 fractions after alkaline borohydride treatment (Sup Fig. 4E–4H) expressed as percent is as follows: fractions 40–60 mL and 61–70 mL: 39.0 & 61.0 in ovarian sera; 36.7 & 63.3 in pancreatic sera, 39.4 and 60.6 in normal sera and fetuin 17.4 & 82.6. Even though the majority of glycans are small in the human sera as well as in fetuin, the proportion of large mucin chains (fractions 40–60) are considerably larger (36.7–39.4% vs 17.4%; more than two- fold) in human sera compared to fetuin. The studies in Sup Fig. 4 demonstrate not only the utility of exchange sialylation in identifying mucin glycoproteins in serum but also open up the research area for further efforts for identifying distinct cancer associated antigen in patient serum.
DISCUSSION
Among the various posttranslational modification reactions of proteins, glycosylation is the most abundant. Glycosylation can alter the charge, conformation and stability of proteins, and induce heterogenous profiles as a consequence of the production of variable glycoforms. Cancer associated glycans are often found on a class of proteins called mucins29, which are large, highly O-glycosylated proteins involved in the protection and control of signalling at epithelial surface. Mucins themselves exert profound tumor-promoting effects. This is in part mediated through interactions with their glycan moities4, 30, 31. The following sections summarize our experimental finding in the context of existing knowledge regarding various cancers.
Ovarian Cancer
CA125 is a mucin commonly employed as a diagnostic marker for epithelial ovarian cancer32. CA125 is highly enriched in serine and threonine residues and recently designated as MUC16 mucin33. Its carbohydrate content based on its mass is estimated to be 24–28% with the majority being O-linked glycans34–36. Core 1 and core 2 type glycans are the major O-linked glycans expressed on CA12537. The ovarian cancer cell line SKOV3 examined in this study has not been shown to express CA125 but it does express MUC1 and MUC238. These mucins are apparently the source of large O-glycans obtained in the present study as evident from the Biogel P6 chromatography elution profiles of pronase digested fractions (Sup Fig. 1C) and alkaline borohydride released glycans (Sup Fig. 2B).
Leukemic cells
P-selectin glycoprotein ligand (PSGL-1) is a disulfide-bonded homodimeric mucin-type glycoprotein on leukocytes that interacts with both P- and E-selectin. A majority of the O-glycans in PSGL-1 are disialylated or neutral forms of core 2 tetrasaccharide39. A comparison of PSGL-1 and CD43 expressed by the leukemia cell line HL60 cells indicates they are differently O-glycosylated39. CD43 lacks the fucosylated glycans found on PSGL-1 and is enriched with the non-fucosylated, disialylated core 2 tetrasaccharide. The present study showed that [9-3H] sialyl mucin glycoproteins in the HL60 cell lysate when subjected to pronase digestion gave large size glycopeptides fractions (Sup Fig. 1D) and upon alkaline borohydride treatment gave rise to large size O-glycans as evident from the elution profile obtained on Biogel P6 chromatography (Sup Fig. 2C).
Breast Cancer
Cancer associated mucins show antigenic differences from normal mucins. Analysis of the O-glycans attached to the mucin produced by the normal lactating breast and by breast cancer cell lines has shown that the oligosaccharides attached to the normal mucin are core2 based structures whereas short core-1 based structures dominated in the cancer associated mucin40. The composition as well as the number of O-glycans added to MUC1 was found altered in breast cancer40. A two-fold reduction in the actual number of O-glycans attached to MUC1 was observed in normal cells as compared with T47D cells41. Further MUC1 purified from the serum of an advanced breast cancer patient contained 83% mostly sialylated core1 glycans and 17% core2 glycans42. MUC6 has been detected in breast carcinoma43. Freire et al44 showed that MUC6 expressed by MCF-7 breast cancer cells was aberrantly glycosylated because it contained the Tn antigen. They demonstrated the feasibility of MUC6-Tn glycoconjugates as an attractive target to be used in cancer immunotherapy45. In the present study [9-3H]sialylated glycoproteins of cell lysates through the enzymatic exchange sialylation showed the predominance of clustered Tn epitopes in breast cancer cell lines T47D and MCF-7 as evident from VVL-agarose affinity chromatography of the mucin glycoproteins before (Fig. 1Ba, 1Bb) as well as VVL-agarose chromatography after pronase digestion (Fig 2Be). These results are consistent with a recent study demonstrating that the GSTA region of the MUC1 tandem repeat contains a highly immunodominant epitope when presented with immature short O-glycans such as GalNAcα-O-Ser/Thr (Tn) and NeuAcα2,6 GalNAc α-O-Ser/Thr (STn); the cancer specific expression of this glycopeptide epitope makes it a prime candidate for immunodiagnostic and therapeutic measures46. The present study further showed that the breast cancer cell line DU4475 expresses complex, large mucin carbohydrate chains as evident from pronase digestion (Fig. 4B) and TLC of Biogel P6 fractions obtained alkaline borohydride treatment (Fig 4D, Lanes 2,3). We found earlier that DU4475 expresses Gal:3-O-sulfotransferase specific for mucin core2 LacNAc type 2 chains25, 27.
Colon cancer
Expression of MUC1 in colorectal carcinomas was found to be associated with tumor progression47, 48. As anticipated, in the present study the colon cancer cell line LS180 has been shown to synthesize complex sialylated mucin carbohydrate structures (Fig 2Ab and Fig 3Ac). LS180 is known from our earlier study25, 27 to express Gal:3-O-sulfotransferase specific for mucin core 2 LacNAc type 2 chains. The present study has shown further that LS180 synthesizes Tn epitopes in clusters on the mucin polypeptide chain (Fig 1Bc and Fig 3B Lanes 3,4; Fig 4C and 4D Lanes 5, 6).
Pancreatic cancer
Inflamation has been shown to be involved in pancreatic cancer development and progression49, 50 and could provide the conditions necessary to stimulate changes in the glycosyltransferases and other factors that produce cancer associated glycans. Over expression of MUC 1 in advanced pancreatic cancer51 and MUC1-based immunotherapeutic treatment strategies have been reported52. Human pancreatic ribonuclease (RNase1) has a molecular weight of 31 kDa53. High levels of RNase 1 have been detected in the sera of most patients with pancreatic adenocarcinoma. RNase1 contains three N-glycosylation sites occupied54 and two unoccupied O-glycosylation sites Asn-XX-Ser/Thr55. It is interesting to note that the present study identified in the three pancreatic tumor specimens examined, two [14C] sialyl mucin glycoproteins in the range of 25–35 kDa (Sup Fig. 3) suggesting their similarity to pancreatic RNase which might have acquired sialylated O-glycan chains.
Prostate cancer
MUC1 is a tumor associated antigen that is highly related to tumor progression in prostate cancer patients56. A disialyl core type 1 linked to Thr was identified in MUC1 tandem repeat57. Mono and disialyl core-type 1 O-linked carbohydrate chains were identified in haptoglobin of prostate cancer sera58. In the present study, alkaline borohydride treated [9-3H]sialyl mucin glycoprotein of the prostate cancer cell line LNCaP gave rise to two distinct peaks A and B (Sup Fig 2A), the structure of B being NeuAcα2,3Galβ1,3GalNAcOH.
Human sera
The molecular size of [14C] sialyl mucin glycopeptides from human sera was quite larger than that of [14C] sialyl fetuin glycopeptides (Sup Fig. 4A–4D). This would suggest that [14C] sialyl O-glycan chains are either large or in cluster or in very close proximity to N-linked carbohydrates in the polypeptide chain not cleavable by pronase, in contrast to fetuin carbohydrate chains. In support of these findings, we found the large size [14C] sialyl mucin type chains resulting from alkaline borohydride treatment as 38% from sera as opposed to 17% from fetuin (Sup Fig. 4E–4H).
CONCLUSION
Overall, the present study examined cancer cell lines, tumor specimens and sera by utilizing the exchange sialylation catalytic properties of ST3Gal-II for radiosialyl labeling and showed distinct differences in mucin glycoproteins as well as the mucin glycopeptide species arising upon pronase digestion from various cancer cell lines. Further, it indicated not only differences in the distribution of mucin carbohydrate chains but also the different structures of sialylated mucin carbohydrate chains among the various human cancer cell lines studied. The data from mild alkaline borohydride treatment were also supportive of the above contention. SDS-PAGE of [14C]sialylated glycoproteins in the extracts of various tumor specimens further showed unique differential pattern of mucin glycoproteins in various cancers. The mucin glycopeptides as well as the mucin-type carbohydrate chains arising from human cancer sera were found to be distinctively different both qualitatively and quantitatively from that of fetuin. In essence the specific sialyl labeling technique by exploiting the exchange sialylation capability of ST3Gal-II would be very valuable for studying mucin type structures in health and diseases. Hence, ST3Gal-II could serve as a versatile tool in glycoproteomics.
Supplementary Material
Acknowledgments
The study was supported by NIH Grants CA121294, HL63014 and DOD grant W81XWH-06-1-0013.
Abbreviations
- CMP
Cytidine 5′ monophosphate
- NeuAc
N-acetylneuraminic acid (sialic acid)
- PSGL-1
P-selectin glycoprotein ligand-1
- T
Galβ1,3GalNAcα-O-Ser/Thr
- Tn
GalNAcα-O-Ser/Thr
- VVL
Vicia villosa lectin
- WGA
Wheat germ agglutinin
- RM
Reaction Mixture
- SDS
Sodium dodecyl sulfate
- TLC
Thin layer chromatography
- PAGE
Polyacrylamide gel electrophoresis
- KDa
Kilo Daltons
Footnotes
SUPPORTING INFORMATION AVAILABLE
This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Baldus SE, Engelmann K, Hanisch FG. MUC1 and MUCs, A family of human mucins with impact in cancer biology. Crit Rev Clin Lab Sci. 2004;41:89–231. doi: 10.1080/10408360490452040. [DOI] [PubMed] [Google Scholar]
- 2.Kawa S, Kato M, Oguchi H, Hsue GL, Kobayashi T, Koiwai T, Tokoo M, Furuta S, Ichikawa T, Kanai M. Clinical evaluation of pancreatic cancer-associated mucin expressing CA19-9, CA50, Span-1, sialyl SSEA-1, and Dupan-2. Scand J Gastroenterol. 1992;27:635–643. doi: 10.3109/00365529209000132. [DOI] [PubMed] [Google Scholar]
- 3.Holingsworth MA, Swanson BJ. Mucins in Cancer: Protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 4.Silverman HS, Sutton-Smith M, McDermott K, Heal P, Leir S-H, Morris HR, Hollingsworth MA, Dell A, Harris A. The contribution of tandem repeat number to the o-glycosylation of mucins. Glycobiology. 2003;13:265–277. doi: 10.1093/glycob/cwg028. [DOI] [PubMed] [Google Scholar]
- 5.Hanisch FG, Muller S. MUC1: The polymorphic appearance of a human mucin. Glycobiology. 2000;10:439–449. doi: 10.1093/glycob/10.5.439. [DOI] [PubMed] [Google Scholar]
- 6.Carvalho F, Seruca R, David L, Amorim A, Seixas M, Bennett E, Clausen H, Sutton-Smith M. MUC1 gene polymorphism and gastric cancer - an epidemiological study. Glycoconj J. 1997;14:107–111. doi: 10.1023/a:1018573201347. [DOI] [PubMed] [Google Scholar]
- 7.Swallow DM, Vinall LE, Gum JR, Kim YS, Yang H, Rotter JI, Mirza M, Lee JC, Lennard-Jones JE. Ulcerative colitis is not associated with differences in MUC2mucin allele length. J Med Genet. 1999;36:859–860. [PMC free article] [PubMed] [Google Scholar]
- 8.Kyo K, Parkes M, Takei Y, Nishimori H, Vyas P, Satsangi J, Simmons J, Nagawa H, Baba S, Jewel D, et al. Association of ulcerative colitis with rare VNTR alleles of the human intestinal mucin gene MUC3. Hum Mol Genet. 1999;8:307–311. doi: 10.1093/hmg/8.2.307. [DOI] [PubMed] [Google Scholar]
- 9.Kyo K, Muto T, Nagawa H, Lathrop GM, Nakamura Y. Association of distinct variants of the intestinal mucin gene MUC3A with ulcerative colitis and Crohn’s disease. J Hum Genet. 2001;46:5–20. doi: 10.1007/s100380170118. [DOI] [PubMed] [Google Scholar]
- 10.Kirkbride H, Bolscher J, Nazmi J, Vinall L, Nash M, Moss F, Mitchell D, Swallow D. Genetic polymorphism of MUC7: allele frequencies and association with asthma. J Hum Genet. 2001;9:347–354. doi: 10.1038/sj.ejhg.5200642. [DOI] [PubMed] [Google Scholar]
- 11.Vinall L, Fowler J, Jones A, Kirkbride H, de Bolos C, Laine A, Porchet N, Gum J, Kim Y, Moss F, et al. Polymorphism of human mucin genes in chest disease: possible significance of MUC2. Am J Respir Cell Mol Biol. 2000;23:678–686. doi: 10.1165/ajrcmb.23.5.4176. [DOI] [PubMed] [Google Scholar]
- 12.Kaneko MK, Kato Y, Kameyama A, Ito H, Kuno A, Hirabayashi J, Kubota T, Amano K, Chiba Y, Hasegawa Y, Sasagawa I, Mishima K, Narimatsu H. Functional glycosylation of human pedoplanin: Glycan structure of platelet aggregation-inducing factor. FEBS Lett. 2007;581:331–336. doi: 10.1016/j.febslet.2006.12.044. [DOI] [PubMed] [Google Scholar]
- 13.Harris A, Reid C. Cystic fibrosis and mucins. J Med Genet. 1998;35:82–83. doi: 10.1136/jmg.35.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hang HC, Yu C, Kato DL, Bertozzi CR. A metabolic labeling approach toward proteomic analysis of mucin-type o-linked glycosylation. Proc Natl Acad Sci (USA) 2003;100:14846–14851. doi: 10.1073/pnas.2335201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zang H, Li XJ, Martin DB, Aebersold R. Identification and quantification of N-linked glycoproteins using hydrazied chemistry, stable isotope labeling and mass spectrometry. Nat Biotechnol. 2003;21:660–666. doi: 10.1038/nbt827. [DOI] [PubMed] [Google Scholar]
- 16.Kaji H, Saito H, Yamauchi Y, Shinkawa T, Taoka M, Hirabayashi J, Kasai K, Takahashi N, Isobe T. Lectin affinity capture, isotope-coded tagging and mass spectrometry to identify N-linked glycoproteins. Nat Biotechnol. 2003;21:667–672. doi: 10.1038/nbt829. [DOI] [PubMed] [Google Scholar]
- 17.Xiong L, Regnier FEJ, Chromatogr B. Use of a lectin affinity selector in the search for unusual glycosylation in proteomics. Analyt Technol Biomed Life Sci. 2002;782:405–418. doi: 10.1016/s1570-0232(02)00671-2. [DOI] [PubMed] [Google Scholar]
- 18.Plummer TH, Elder JH, Alexander S, Phelan AW, Tarentino AL. Demonstration of peptide: N-glycosidase F activity in endo-beta-N-acetylglucosaminidase F preparations. J Biol Chem. 1984;259:10700–10704. [PubMed] [Google Scholar]
- 19.Wopereis S, Lefeber DJ, Morava E, Wevers RA. Mechanisms in protein o-glycan biosynthesis and clinical and molecular aspects of protein o-glycan biosynthesis defects: a review. Clin Chem. 2006;52:574–600. doi: 10.1373/clinchem.2005.063040. [DOI] [PubMed] [Google Scholar]
- 20.Tarp MA, Clausen H. Mucin type o-glycosylation and its potential use in drug and vaccine development Biochim. Biophys Acta. 2008;1780:546–563. doi: 10.1016/j.bbagen.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Brockhausen I. Pathways of O-glycan biosynthesis in cancer cells Biochim. Biophys Acta. 1999;1473:67–95. doi: 10.1016/s0304-4165(99)00170-1. [DOI] [PubMed] [Google Scholar]
- 22.Van den Steen P, Rudd PM, Dwek RA, Opdenakker G. Concepts and principles of o-linked glycosylation. Crit Rev Biochem Mol Biol. 1998;33:151–208. doi: 10.1080/10409239891204198. [DOI] [PubMed] [Google Scholar]
- 23.Chandrasekaran EV, Xue J, Neelamegham S, Matta KL. The pattern of glycosyl- and sulfotransferase activities in cancer cell lines: a predictor of individual cancer-associated distinct carbohydrate structures for the structural identification of signature glycans. Carbohydr Res. 2006;341:983–994. doi: 10.1016/j.carres.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 24.Chandrasekaran EV, Xue J, Piskorz C, Locke RD, Toth K, Slocum HK, Matta K. L Potential tumor markers for human gastric cancer: an elevation of glycan: sulfotransferases and a concomitant loss of α1,2-fucosyltransferase activities. J Cancer Res Clin Oncol. 2007;133:599–611. doi: 10.1007/s00432-007-0206-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chandrasekaran EV, Jain RK, Rhodes JM, Chawda R, Piskorz C, Matta KL. Characterization of distinct Gal: 3-O-sulfotransferase activities in human tumor epithelial cell lines and of calf lymph node GlcNAc : 6-O-sulfotransferase activity. Glycoconj J. 1999;16:523–536. doi: 10.1023/a:1007074005371. [DOI] [PubMed] [Google Scholar]
- 26.Chandrasekaran EV, Xue J, Xia J, Chawda R, Piskorz C, Locke RD, Neelamegham S, Matta KL. Analysis of the specificity of sialyltransferases toward mucin core-2, globo and related structures. Identification of the sialylation sequence and the effects of sulfate, fucose, methyl and fluoro substituents of the carbohydrate chain in the biosynthesis of selectin and siglec ligands and noval sialylation by cloned 2, 3(O)sialyltransferase. Biochemistry. 2005;44:15619–15635. doi: 10.1021/bi050246m. [DOI] [PubMed] [Google Scholar]
- 27.Chandrasekaran EV, Xue J, Xia J, Locke RD, Matta KL, Neelamegham S. Reversible sialylation: Synthesis of cytidine 5 –monophospho-N-acetylneuraminic acid from cytidine 5 –monophosphate with 2,3-sialyl o-glycan-, glycolipid-, and macromolecule-based donors yields diverse sialylated products. Biochemistry. 2008;47:320–330. doi: 10.1021/bi701472g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chandrasekaran EV, Xue J, Xia J, Locke RD, Patil SA, Neelamegham S, Matta KL. Mammalian sialyltransferase ST3Gal-II: Its exchange sialylation catalytic properties allow labeling of sialyl residues in mucin type sialylated glycoproteins and specific gangliosides. 2011 doi: 10.1021/bi 200301w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burdick MD, Harris A, Reid CJ, Iwamura T, Hollingsworth MA. Oligosaccharides expressed on MUC1 produced by pancreatic and colon tumor cell lines. J Biol Chem. 1997;272:24198–24202. doi: 10.1074/jbc.272.39.24198. [DOI] [PubMed] [Google Scholar]
- 30.Moniaux N, Andrianifahanana M, Brand RE, Batra SK. Multiple roles of mucins in pancreatic cancer, a lethal and challenging malignancy. Br J Cancer. 2004;91:1633–1638. doi: 10.1038/sj.bjc.6602163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andrianifahanana M, Moniaux N, Batra SK. Regulation of mucin expression: Mechanistic aspects and implications for cancer and inflammatory diseases. Biochem Biophys Acta. 2006;1765:189–222. doi: 10.1016/j.bbcan.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Duffy MJ, Bonfrer JM, Kulpa J, Rustin GJ, Soletormos G, Torre GC, Tuxen MK, Zwirner MCA. 125 in ovarian cancer: European Group on Tumor Markers guidelines for clinical use. Int J Gynecol Cancer. 2005;15:679–691. doi: 10.1111/j.1525-1438.2005.00130.x. [DOI] [PubMed] [Google Scholar]
- 33.Yin BW, Lloyd KO. Molecular cloning of the CA125 ovarian cancer antigen: Identification as a new mucin, MUC16. J Biol Chem. 2001;276:27371–27375. doi: 10.1074/jbc.M103554200. [DOI] [PubMed] [Google Scholar]
- 34.Davis HM, Zurawski VR, Jr, Bast RC, Jr, Klug TL. Characterization of the CA 125 Antigen Associated with Human Epithelial Ovarian Carcinomas. Cancer Res. 1986;46:6143–6148. [PubMed] [Google Scholar]
- 35.Lloyd KO, Yin BW, Kudryashov V. Isolation and characterization of ovarian cancer antigen CA 125 using a new monoclonal antibody (VK-8): identification as a mucin-type molecule. Int J Cancer. 1997;7:842–850. doi: 10.1002/(sici)1097-0215(19970529)71:5<842::aid-ijc24>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 36.O’Brien TJ, Beard JB, Underwood LJ, Dennis RA, Santin AD, York L. The CA 125 Gene: An Extracellular Superstructure Dominated by Repeat Sequences. Tumour Biol. 2001;22:348–366. doi: 10.1159/000050638. [DOI] [PubMed] [Google Scholar]
- 37.Wong NK, Easton RL, Panico M, Sutton-Smith M, Morrison JC, Attanzio FA, Morris HR, Clark GF, Dell A, Patankar M. Characterization of the Oligosaccharides Associated with the Human Ovarian Tumor Marker CA125. J Biol Chem. 2003;278:28619–28634. doi: 10.1074/jbc.M302741200. [DOI] [PubMed] [Google Scholar]
- 38.Dong Y, Walsh MD, Cummings MC, Wright RG, Khoo SK, Parsons PG, McGuckan MA. Expression of MUC1 and MUC2 mucins in epithelial ovarian tumors. J Pathol. 1997;183:311–317. doi: 10.1002/(SICI)1096-9896(199711)183:3<311::AID-PATH917>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 39.Wilkins PP, McEver RP, Cummings RD. Structures of the O-Glycans on P-selectin Glycoprotein Ligand-1 from HL 60 Cells. J Biol Chem. 1996;271:18732–18742. doi: 10.1074/jbc.271.31.18732. [DOI] [PubMed] [Google Scholar]
- 40.Muller S, Goletz S, Packer N, Gooley A, Lawson AM, Hanisch F-G. Localization of o-glycosylation sites on glycopeptides fragments from lactation associated MUC1: All putative sites within the tandem repeat are glycosylation targets in vivo. J Biol Chem. 1997;272:24780–24793. doi: 10.1074/jbc.272.40.24780. [DOI] [PubMed] [Google Scholar]
- 41.Muller S, Alving K, Peter-Katalinic J, Zachara N, Gooley A, Hanisch F-G. High density o-glycosylation on tandem repeat peptide from secretory MUC1 of T47D breast cancer cells. J Biol Chem. 1999;274:18165–18172. doi: 10.1074/jbc.274.26.18165. [DOI] [PubMed] [Google Scholar]
- 42.Storr S, Royle L, Chapman CJ, Hamid UMA, Robertson J, Murray A, Dwek RA, Rudd PM. The o-linked glycosylation of secretory/shed MUC1 from an advanced breast cancer patient’s serum. Glycobiology. 2008;18:456–462. doi: 10.1093/glycob/cwn022. [DOI] [PubMed] [Google Scholar]
- 43.Pereira MB, Dias AJ, Reis CA, Schmitt FC. Immunohistochemical study of the expression of MUC5AC and MUC6 in breast carcinomas and adjacent breast tissues. J Clin Pathol. 2001;54:210–213. doi: 10.1136/jcp.54.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freire T, Bay B, von Mendsdorff-Pouilly S, Osinaga E. Molecular basis of incomplete o-glycan synthesis in MCF-7 breast cancer cells: putative role of MUC6 in Tn antigens expression. Cancer Res. 2005;65:7880–7887. doi: 10.1158/0008-5472.CAN-04-3746. [DOI] [PubMed] [Google Scholar]
- 45.Freire T, Lo-Man R, Piller F, Piller V, Leclerc C, Bay S. Enzymatic large scale synthesis of MUC6-Tn glycoconjugates for antitumor vaccination. Glyobiology. 2006;16:390–401. doi: 10.1093/glycob/cwj082. [DOI] [PubMed] [Google Scholar]
- 46.Tarp MA, Sorensen AL, Mandel U, Paulsen H, Burchell J, Taylor-Papadimitriou J, Clausen H. Identification of novel cancer specific immunodominant glycopeptides epitope in the MUC1 tandem repeat. Glycobiol. 2007;17:197–209. doi: 10.1093/glycob/cwl061. [DOI] [PubMed] [Google Scholar]
- 47.Baldus SE, Monig SP, Huxel S, Landsberg S, Hanisch FG, Engelmann K, Schneider PM, Thiele J, Holsher AH, Dienes HP. MUC1 and nuclear beta-catenin are coexpressed at the invasion front of colorectal carcinomas are both correlated with tumor prognosis. Clin Cancer Res. 2004;10:2790–2796. doi: 10.1158/1078-0432.ccr-03-0163. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki H, Shoda J, Kawamoto T, Shinozaki E, Miyahara N, Hotta S, Lizuka Y, Nakahara A, Tanaka N, Yanaka A, Irimura T. Expression of MUC1 recognized by monoclonal antibody MY.1E12 is a useful biomarker for tumor aggressiveness of advanced colon carcinoma. Clin Exp Metastas. 2004;21:321–329. doi: 10.1023/b:clin.0000046133.35133.cc. [DOI] [PubMed] [Google Scholar]
- 49.Farrow B, Evers BM. Inflammation and the development of pancreatic cancer. Surg Oncol. 2002;10:153–169. doi: 10.1016/s0960-7404(02)00015-4. [DOI] [PubMed] [Google Scholar]
- 50.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hinoda Y, Ikematsu Y, Horinochi M, Sato S, Yamamoto K, Nakano T, Fukui M, Suchiro Y, Hamanaka Y, Nishikawa Y, Kida H, Waki S, Oka M, Imai K, Yonezawa S. Increased expression of MUC1 in advanced pancreatic cancer. J Gastroenterol. 2003;38:1162–1166. doi: 10.1007/s00535-003-1224-6. [DOI] [PubMed] [Google Scholar]
- 52.Qu CF, Li Y, Song YJ, Rizvi SMA, Raja C, Zhang D, Samra J, Smith R, Perkins AC, Apostolidis C, Allen BJ. MUC1 expression in primary and metastatic pancreatic cancer cells for in vitro treatment by (213) Bi-C595 radioimmunoconjugate. Br J Cancer. 2004;91:2086–2093. doi: 10.1038/sj.bjc.6602232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weickmann JL, Elson M, Glitz DG. Purification and characterization of human pancreatic ribonuclease. Biochemistry. 1981;20:1272–1278. doi: 10.1021/bi00508a035. [DOI] [PubMed] [Google Scholar]
- 54.Ribó M, Beintema JJ, Osset M, Fernández E, Bravo J, de Llorens R, Cuchillo CM. Heterogeneity in the glycosylation pattern of human pancreatic ribonuclease. Biol Chem Hoppe Seyler. 1994;375:357–363. [PubMed] [Google Scholar]
- 55.Beintema JJ, Wietzes P, Weickmann JL, Glitz DG. The amino acid sequence of human pancreatic ribonuclease. Anal Biochem. 1984;136:48–64. doi: 10.1016/0003-2697(84)90306-3. [DOI] [PubMed] [Google Scholar]
- 56.Li Y, Cozzi PJ. MUC is a promising therapeutic target for prostate cancer therapy. Curr Cancer Drug Targets. 2007;7:259–271. doi: 10.2174/156800907780618338. [DOI] [PubMed] [Google Scholar]
- 57.Takeuchi H, Kato K, Denda-Nagai K, Hanisch FG, Clausen H, Irimura T. The epitope recognized by the unique anti-MUC1 monoclonal antibody MY.1E12 involves sialylα2,3galactosylβ1,3N-acetylgalactosaminide linked to a distinct threonine residue in the MUC1 tandem repeat. J Immunol Methods. 2002;270:199–209. doi: 10.1016/s0022-1759(02)00298-3. [DOI] [PubMed] [Google Scholar]
- 58.Fujimura T, Shinohara Y, Tissot B, Pang PC, Kurogochi M, Saito S, Arai Y, Sadilek M, Murayama K, Dell A, Nishimura SI, Hakomori S-I. Glycosylation status of haptoglobin in sera of patients with prostate cancer vs. benign prostate disease or normal subjects. Int J Cancer. 2008;122:39–49. doi: 10.1002/ijc.22958. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.