Abstract
Reverse speech has often been used as a control task in brain-mapping studies of language utilizing various non-invasive modalities. The rationale is that reverse speech is comparable to forward speech in terms of auditory characteristics, while omitting the linguistic components. Thus, it may control for non-language auditory functions. This finds some support in fMRI studies indicating that reverse speech resulted in less blood-oxygen-level-dependent (BOLD) signal intensity in perisylvian regions than forward speech. We attempted to externally validate a reverse speech control task using intracranial electrocorticography (ECoG) in eight patients with intractable focal epilepsy. We studied adolescent and adult patients who underwent extraoperative ECoG prior to resective epilepsy surgery. All patients received an auditory language task during ECoG recording. Patients were presented 115 audible question stimuli, including 30 reverse speech trials. Reverse speech trials more strongly engaged bilateral superior temporal sites than did the corresponding forward speech trials. Forward speech trials elicited larger gamma-augmentation at frontal lobe sites not attributable to sensorimotor function. Other temporal and frontal sites of significant augmentation showed no significant difference between reverse and forward speech. Thus, we failed to validate reported evidence of weaker activation of temporal neocortices during reverse compared to forward speech. Superior temporal lobe engagement may indicate increased attention to reverse speech. Reverse speech does not appear to be a suitable task for the control of non-language auditory functions on ECoG.
Keywords: high-frequency oscillations (HFOs), ripples, pediatric epilepsy surgery, video EEG monitoring
1. Introduction
Uniquely developed in humans (McNelly et al., 2009), understanding the cortical processes of language requires the direct study of human participants. Researchers have employed a broadening array of non-invasive modalities to study the complex structure and function of auditory language; including positron emission tomography (PET), functional magnetic resonance imaging (fMRI), electroencephalography (EEG), near-infrared spectroscopy (NIRS), and magnetoencephalography (MEG). Functional study generally relies upon performance of a well designed task to elicit cortical activity. Often, a control task is necessary to contrast with the primary task in order to isolate particular realms of function. However, validation of a particular task’s design and hypothetical effect is not simple, partly due to a lack of appropriate animal models of human language. Intuition about language is frequently the only guide in developing new tasks for non-invasive language study. Here, we use event-related electrocorticography (ECoG) as an external validating modality of the results obtained from non-invasive neuroimaging.
Intracranial ECoG, recorded in patients with focal epilepsy and/or brain tumor prior to surgical resection of diseased tissue, is a unique functional brain mapping tool. Based on the same principles as scalp EEG, the measure of interest in event-related ECoG mapping is the degree of augmentation of high frequency activity, compared to a baseline reference (Pfurtscheller and Lopes da Silva, 1999). The signals detected and routinely analyzed by ECoG methods fall within a class of electrophysiological activities known as local field potentials (LFP), which have been shown to correlate well with the blood-oxygen-level-dependent (BOLD) signal detected by fMRI (Logothetis, 2003). Indeed, task-related augmentation of broadband gamma-range signals in excess of 50-Hz have not only been shown to accurately localize cortical function (Crone et al., 2011; Jerbi et al., 2009; Miller et al., 2008) but have also been repeatedly shown to best predict the BOLD response, accounting for over 20-% of BOLD signal variability (Conner et al., 2011; Hermes et al., 2011). Low frequency alpha/beta activities are very minor predictors of the BOLD response (Hermes et al., 2011) that may describe electrocortical phenomena that are independent of that revealed by high frequency gamma components (Cardin et al., 2009; Conner et al., 2011; Engel and Fries, 2010). We utilize an auditory naming task to elicit high frequency gamma (50- to 150-Hz) activity in cerebral regions mediating language (Brown et al., 2008; Koga et al., 2011; Wu et al., 2011). Event-related ECoG may be useful in externally validating the language findings of non-invasive methods.
We are interested in contrasting our auditory naming task with a control task in order to segregate cortical gamma activity specific to auditory language from those attributable to a more broad involvement in auditory perception. The literature from functional studies points to a commonly used reverse speech control task (Gherri and Eimer, 2011; Moore-Parks et al., 2010; Perani et al., 1996; Redcay et al., 2008; Redcay and Courchesne, 2008; Sato et al., 2011). Generally, a reverse speech control task is a replica of the primary forward speech task that has been reversed in time. Such a control task is said to share auditory elements of the primary task (e.g. spectral details, intensity) but largely lack the intelligibility of language (e.g. syntax, semantics).
Previous fMRI studies report that forward speech induces stronger BOLD responses in bilateral superior temporal regions compared to reverse speech (Moore-Parks et al., 2010; Redcay et al., 2008; Redcay and Courchesne, 2008). This phenomenon has been observed across a wide age range, including children. These findings suggest that reverse speech may consistently control for non-language auditory activity that might otherwise confound temporal lobe activity related to the primary language task.
The aim of the present study is to externally validate the results of noninvasive language mapping modalities using event-related ECoG. Here, we test the hypothesis that forward speech will elicit larger augmentation of gamma activity compared to reverse speech in bilateral superior temporal regions. Analysis of the effects upon low frequency activity is included as a secondary measure.
2. Materials and Methods
2.1 Study Patients
Patients were selected by using the following inclusion criteria: (i) a history of intractable focal epilepsy scheduled for extraoperative subdural ECoG recording as part of presurgical evaluation at Children’s Hospital of Michigan or Harper University Hospital, Detroit, between December 2010 and July 2011, (ii) age of 8 years or older, and (iii) measurement of ECoG amplitude augmentations driven by a language task described in section 2.3. Exclusion criteria consisted of: (i) presence of massive brain malformations (such as large perisylvian polymicrogyria or hemimegalencephaly) which confound anatomical landmarks for the central sulcus and Sylvian fissure, (ii) history of hearing impairment, (iii) right language dominance as determined by Wada testing (i.e. intracarotid sodium amobarbital procedure) or left-handedness when Wada test results are not available (Knecht et al., 2000), (iv) multiple seizure foci involving both hemispheres, (v) Verbal Comprehension Index (VCI) or Verbal Intelligence Quotient (VIQ) less than 70, (vi) inability to complete the language task described in section 2.3 due to lack of adequate vocabulary or cooperation, and (vii) history of previous neurological surgery. We studied a consecutive series of eight patients satisfying all criteria (age range: 12 – 44 years; four females; Table 1). This study has been approved by the Institutional Review Board at Wayne State University, and written informed consent was obtained from all patients or their legal parent or guardian. Subdural platinum grid electrode (10-mm inter-contact distance; 4-mm diameter; Adtech, Racine, WI, USA) placement was as described previously by our team (Wu et al., 2011). Extraoperative video-ECoG recordings were obtained for 3 to 5 days, using a 192-channel Nihon Kohden Neurofax 1100A Digital System (Nihon Kohden America Inc., Foothill Ranch, CA, USA) at a sampling frequency of 1000-Hz as previously described (Wu et al., 2011). Total electrode contact number ranged from 100 to 120 (Table 1).
Table 1.
Patient Data
| Patient | Gender | Age at Surgery (years) |
Dominant Hand |
Age at Epilepsy (Onset) |
Antiepileptic mediations |
PSI† | VCI† | VIQ† | Schooling | Wada Test† (Language) |
Seizure type |
ECoG Electrode placement |
Seizure Onset Zone |
ECoG contacts (total) |
Histology |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 17 | Rt | 0 | TPM, OXC | 56 | 71 | N/A | Below Average, Normal 11th Grade |
Lt | Complex Focal Sz |
Rt FPTO | Rt PTO | 108 | Atrophy & Gliosis |
| 2 | Female | 15 | Rt | 13 | LEV | N/A | N/A | N/A | Above Average, Normal 10th Grade |
N/A | Focal Sz w/sGTC |
Lt FPTO | Lt Anterior T | 100 | Low Grade Tumor and Gliosis |
| 3 | Male | 44 | Both | 26 | LEV, OXC | N/A | N/A | N/A | Incomplete Graduate School |
Lt | Focal Sz w/sGTC |
Rt FPTO | Rt PO | 108 | Atrophy & Gliosis |
| 4 | Female | 37 | Rt | 19 | LAM, LAC | N/A | N/A | 72 | Completed High School |
Lt | Focal Sz w/sGTC |
Lt FPTO | Lt Mesial T | 112 | mild Gliosis |
| 5 | Female | 14 | Rt | 13 | LEV, OXC, LAC |
100 | 124 | N/A | Above Average, Normal 9th Grade |
N/A | Focal Sz w/sGTC |
Lt FPTO | Lt Lateral P | 120 | Low Grade Tumor |
| 6 | Male | 20 | Rt | 14 | LAC, CBZ | N/A | N/A | 82 | College Student | Lt | Focal Sz w/sGTC |
Lt FPTO | Lt Mesial T | 100 | mild Gliosis |
| 7 | Female | 14 | Rt | 7 | LAM | 100 | 79 | N/A | Normal 8th Grade | N/A | Focal Sz w/sGTC |
Rt FPTO | Rt Mesial T | 104 | Neuronal Loss & Gliosis |
| 8 | Male | 12 | Rt | 3 | VAL, OXC, LAC |
80 | 83 | N/A | Normal 6th Grade | Lt | Complex Focal Sz |
Lt FPTO | Lt Anterior & Inferior T |
116 | mild Gliosis |
LEV:Levetiracetam. LAM:Lamotrigine. LAC:Lacosamide. CBZ:Carbamazepine. OXC:Oxcarbazepine. TPM:Topiramate. VAL:Valproate. RT:Right. Lt:Left. Sz:Seizure. sGTC:Secondarily Generalized Tonic-Clonic Sz. F:Frontal. T:Temporal. O:Occipital. P:Parietal.
PSI:Processing Speed Index. VCI:Verbal Comprehension Index. VIQ:Verbal Intelligence Quotient. Neuropsychological testing was performed based on clinical necessity. Wada testing (i.e. intracarotid sodium amobarbital procedure) results are provided to indentify the language-dominant hemisphere. Due to use of an auditory language task, we include the measures VIQ and VCI, when available. We have also included PSI, when available, which has been suggested to correlate with the degree of dysfunction of cortical auditory information processing in patients with intractable epilepsy (Korostenskaja et al. 2010).
2.2 Coregistration of Electrodes on Individual Three-Dimensional MRI
MRI, including a volumetric-T1-weighted spoiled gradient echo image as well as fluid-attenuated inversion recovery image of the entire head, was obtained preoperatively using a previously described protocol (Nagasawa et al., 2010a). Planar X-ray images (lateral and antero-posterior) were acquired with subdural electrodes in place for localization on the brain surface; three metallic fiducial markers at anatomically well-defined locations aided coregistration with MRI. A three-dimensional MRI brain surface image was created with electrode sites delineated (Alkonyi et al., 2009; Muzik et al., 2007; von Stockhausen et al., 1997). Accuracy was confirmed by intraoperative digital photographs showing in situ electrode locations (Asano et al., 2005; Nagasawa et al., 2010a; Wu et al., 2011).
2.3 Auditory Naming Task
Language mapping by measurement of auditory naming-related gamma activity was performed using an auditory naming task similar to that previously reported (Brown et al., 2008). None of the patients had a seizure within two hours prior to or during task performance. While awake and comfortably seated on a bed in a room with unwanted noises minimized, patients received 85 question-and-answer trials. Question stimuli ranged from 1- to 2.5-s in duration. All questions were delivered via playback of an audio recording of the author’s (E.C.B.) voice using Presentation version 9.81 software (Neurobehavioral Systems Inc., Albany, CA, USA) and were designed to elicit 1 or 2 word answers with nouns; e.g. “What flies in the sky?”
In this study, we also delivered reverse speech trials during the task. To generate these reverse speech trials, a random set of 30 stimulus questions was selected. The audio recordings of these forward speech trials were duplicated and then reversed in time with Cool Edit Pro version 2.00 (Syntrillium Software Corp., Phoenix, AZ, USA). See Table 2 and supplemental Table S1 for details on presentation order for individual patients.
Table 2.
Stimulus Order and Behavioral Results
| Patient | Stimulus Order | Response Time Forward Speech Trials mean (95% CI) | Response Time Reverse Speech Trials mean (95% CI) | Correct Responses %Forward(%Reverse) |
|---|---|---|---|---|
| 1 | 30 Forward & 30 Reverse Stimuli pseudorandomly presented with 55 other Forward Stimuli | 1339 (996–1681) msec | 909 (771–1047) msec | 97% (97%) |
| 2 | Same as Patient 1 | 1573 (1177–1969) msec | 1257 (1136–1378) msec | 100% (100%) |
| 3 | Same as Patient 1 | 1831 (1402–2259) msec* | 984 (863–1105) msec* | 100% (97%) |
| 4 | Same as Patient 1 | 1878 (1268–2489) msec | 1104 (937–1271) msec | 90% (97%) |
| 5 | Same as Patient 1 | 1253 (1131–1375) msec* | 1057 (1014–1100) msec* | 100% (100%) |
| 6 | 30 Forward & 30 Reverse Stimuli pseudorandomly presented independent of other Stimuli | 1000 (870–1130) msec | 832 (721–942) msec | 100% (100%) |
| 7 | Similar to Patient 6 except different stimulus list | 2760 (1537–3982) msec | 1833 (640–3026) msec | 90% (100%) |
| 8 | Similar to Patient 7 except different stimulus order | 2104 (1608–2600) msec | 1430 (1056–1803) msec | 90% (93%) |
| Grand Average | N/A | 1707 (1509–1905) msec* | 1173 (1029–1336) msec* | 95.9% (98.0%) |
All response times averaged from Stimulus Onset analysis trials.
t-test indicates difference between forward and reverse speech trials; α = 0.05.
The audible session was recorded and integrated with ECoG as previously described (Brown et al., 2008; Wu et al., 2011). Subsequently, the onset and offset of auditory stimuli as well as the onset of the patient's vocalization of the response were marked for each trial. Cool Edit Pro was used to visually and audibly aid in the manual determination of these time-points. The response time was defined as the period between offset of stimulus presentation and onset of the respective overt response. Patients were instructed to answer “I don’t know” when they did not know the answer to or did not understand a stimulus.
2.4 Evaluation of ECoG Amplitude Changes
Each ECoG trace was transformed into the time-frequency domain, and we determined ‘when’ and ‘where’ gamma activity was augmented. The time-frequency analysis used in the present study was previously validated (Brown et al., 2008; Hoechstetter et al., 2004; Nagasawa et al., 2010a; Wu et al., 2011). In short, the primary measures of interest were the percent change in amplitude of gamma activity relative to that during the reference period (i.e.: the resting baseline) as well as statistical significance of task-related augmentation of gamma activity. The details of analytic methods are described below. The secondary measures include evaluation of low frequency alpha- and beta-oscillations, as described in the Supplementary Document.
2.4.1 Analysis of ECoG Amplitude Changes Relative to Stimulus Onset
A maximum of 60 trials were considered for analysis: 30 reverse speech trials and the 30 corresponding forward speech trials. Reverse and forward speech trial sets were analyzed separately. The inclusion criteria defining ECoG epochs suitable for this time-frequency analysis included: (i) a period of silence serving as a reference period of 400-ms duration was available between 600- to 200-ms prior to the onset of stimulus presentation. The exclusion criteria included: (i) ECoG trace was affected by movement artifacts, (ii) ECoG trace was affected by electrographic seizures, (iii) the corresponding forward or reverse speech trial was excluded due to failure to satisfy criteria, and (iv) ECoG trace from the superior temporal gyrus was affected by runs of interictal epileptiform discharges lasting 3 seconds or longer.
Time-frequency analysis was performed using BESA® EEG V.5.1.8 software (MEGIS Software GmbH, Gräfelfing, Germany). Each suitable ECoG trial was transformed into the time-frequency domain using a previously described complex demodulation technique (Hoechstetter et al., 2004; Papp and Ktonas, 1977; Wu et al., 2011). A given ECoG channel was assigned amplitude values as a function of frequency and time. For evaluation of high frequency gamma activity, time-frequency transformation was performed for frequencies between 10- and 200-Hz and latencies between −600-ms and +4,000-ms relative to the onset of stimulus presentation, in steps of 5-Hz and 10-ms as previously reported (Brown et al., 2008); see the Supplementary Document for the method used to evaluate low frequency alpha- and beta-range oscillations, presented herein as a secondary measure. At each time-frequency bin, we analyzed the percent change in amplitude (averaged across trials) relative to the grand mean amplitude of the reference period for each frequency epoch. Results are referred to as “event-related synchronization and desynchronization” (Pfurtscheller and Lopes da Silva, 1999) or “temporal spectral evolution” (TSE) (Salmelin and Hari, 1994).
To test for statistical significance in obtained TSE values, a two-step statistical analysis was performed using BESA software (Brown et al., 2008; Nagasawa et al., 2010a; Wu et al., 2011). Initially, a studentized bootstrap statistic (Davidson and Hinkley, 1999) was applied to obtain an uncorrected p-value independently for each time-frequency bin. In a second step, correction for multiple testing was performed, accounting for the partial correlation between neighboring TSE values. The following modified Bonferroni correction was used (Auranen, 2002; Simes, 1986): p-values derived for a particular channel were sorted in ascending order (pi, i = 1, …, N, where N is the number of bins) and the maximum index, m, for which pi < α*i/N was determined. The corrected significance level, α, was set to 0.05. All TSE values corresponding to indices i < m were considered statistically significant. This is less conservative than classical Bonferroni correction but well suited for multiple correlated items (Simes, 1986).
As described previously (Asano et al., 2009; Brown et al., 2008; Fukuda et al., 2010; Nagasawa et al., 2010b; Nagasawa et al., 2010a; Wu et al., 2011), an additional manual correction was employed. TSE values in a given electrode were declared significant only if, after the modified Bonferroni correction, a minimum of eight time-frequency bins contained within the gamma range from 50- to 150-Hz were arranged in a continuous array spanning (i) at least 20-Hz in width and (ii) at least 20-ms in duration; see the Supplementary Document for expanded explanation of the manual correction. All electrodes identified herein have exhibited statistically significant augmentation by this method for either the forward speech trials or the reverse speech trials. In all charts of the present study, a positive deflection indicates augmentation.
2.4.2 Analysis of ECoG Amplitude Changes Relative to Stimulus Offset
We maintained a pre-stimulus reference period that was jittered based upon stimulus duration. For each patient, we determined the longest stimulus duration, referred to here as tstim in milli-seconds. The inclusion criteria defining trials suitable for this time-frequency analysis included: (i) patient provides a correct response and (ii) a period of silence serving as a reference period lasting 400-ms immediately preceding the time point -tstim − 200-ms, with stimulus-offset defined as 0-ms. Time-frequency transformation was performed for latencies between -tstim − 200-ms and -tstim + 5,000-ms relative to the offset of stimulus presentation. The exclusion criteria, waveform evaluation, and statistics were as described in section 2.4.1 and Supplementary Document.
2.4.3 Analysis of ECoG Amplitude Changes Relative to Response Onset
We maintained a pre-stimulus reference period that was jittered based upon the combined stimulus and response-time duration. For each patient, we determined the longest stimulus + response time, referred to here as tresp in milliseconds. The inclusion criteria defining ECoG epochs suitable for this time-frequency analysis included: (i) patient provided a correct response, (ii) the response-time variability must be within 1000-ms across trials (Brown et al., 2008), and (iii) a period of silence serving as a reference period of 400-ms immediately preceding the time point -tresp − 200-ms, with response-onset defined as 0-ms. Time-frequency transformation was performed for latencies between -tresp − 200-ms and -tresp + 5,000-ms relative to the offset of stimulus presentation. The exclusion criteria, waveform evaluation, and statistics were as described in section 2.4.1 and Supplementary Document.
2.5 Categorization of Electrode Sites with Significant Gamma-Augmentation
The anatomical localization of language functions can be highly variable even in patients selected for left hemispheric language dominance (Berger et al., 1989; Duchowny et al., 1996; Hamberger et al., 2007; Ojemann et al., 1989; Ojemann et al., 2003). Therefore, we have chosen to leverage the excellent temporal resolution of ECoG measures. We categorized electrode sites based solely on the temporal characteristics of gamma-augmentations. A given electrode is defined as an ‘Auditory’ site if (i) significant gamma-augmentation begins within 300-ms following stimulus onset (Flinker et al., 2010) and (ii) ends prior to 300-ms following stimulus offset during either forward or reverse speech trial sets. Thus, Auditory sites are those that are temporally ‘locked’ to the stimuli; a stimulus refers to the entire auditory question. All other electrodes with significant gamma-augmentation were treated as ‘Non-Auditory’ sites. These Non-Auditory sites were further sub-categorized based upon the temporal domain in which peak gamma augmentation occurred. That is, ‘Late Stimulus’ sites exhibited peak augmentation during the stimulus, ‘Pre-Response’ sites exhibited peak augmentation after stimulus-offset but prior to response-onset, and ‘Post-Response’ sites exhibited peak augmentation following response-onset.
2.6 Comparing Forward Speech to Reverse Speech
We compared forward and reverse speech stimulus sets with identical durations and overall auditory characteristics (e.g. same voice and volume) but differing only by relative reversal in time. Additional analyses of latency to gamma-augmentation and degree of early stimulus gamma-augmentation were performed only upon Auditory sites as described in the Supplementary Document. Statistics were generated using IBM SPSS Statistics version 19 software (SPSS Inc., Chicago, IL, USA). For behavioral and latency measures, a t-test was performed to obtain a 95% confidence interval (C.I.) of the means. For comparison of signal changes at temporal lobe sites, we focused on the first 2.5-s following stimulus-onset, since the longest included stimuli were 2.5-s in duration. We averaged the percent gamma-augmentation, relative to the reference, across the frequency range 50- to 150-Hz, as described in the Supplementary Document. We utilized the related measures of (i) peak augmentation/attenuation, determined using Microsoft Excel 2007 (Microsoft Corp., Redmond, WA, USA), and (ii) area under the gamma-augmentation curve (AUC), determined using the trapezoidal numerical integration function (trapz) in MatLab (The MathWorks Inc., Natick, MA, USA); this procedure for calculating the AUC was slightly modified for low frequency alpha- and beta-attenuations, 8- to 24-Hz, as described in the Supplementary Document. The AUC takes both augmentation amplitude as well as duration into account, making for a more complete measure of ‘activity’. For comparison of signal changes at frontal lobe sites, we focused on results of response-onset analysis from 2-s prior to response-onset to 1-s after; peak augmentation/attenuation but not AUC was considered for frontal lobe sites due to variability of response-times and response durations. The Wilcoxon Signed Ranks Test was applied across electrode sites in order to test the hypothesis that forward and reverse speech stimuli induce differential cortical activity. Alongside uncorrected statistical results of ECoG signal comparisons is provided the median difference between the forward and reverse speech trials; forward minus reverse.
3. Results
3.1 Behavioral Data
All subjects satisfying the criteria described in section 2.1 were able to complete the task. Appropriate responses were recorded for both trial types.
Behavioral results are summarized in Table 2. For trials included in stimulus-onset analysis, the grand-mean response-time across subjects was longer for forward speech trials (1707-ms; 95-% C.I.: 1509- to 1905-ms) compared to that for reverse speech trials (1173-ms; 95-% C.I.: 1029- to 1336-ms).
On average, 95.9-% (95-% C.I.: 91.7- to 100-%) of the forward speech naming questions were answered correctly while the response “I don’t know” or equivalent was appropriately elicited by 98.0-% (95-% C.I.: 95.9- to 100-%) of the corresponding reverse speech stimuli. Patient 3 chose to utter “gibberish” and patient 4 chose to utter “nothing” in response to reverse speech trials. These alternative responses to reverse speech stimuli were considered equivalent to the response “I don’t know”. All other patients consistently articulated “I don’t know” in response to reverse speech trials.
3.2 Temporal Lobe
Across all eight patients, the temporal lobe yielded a total of 34 sites with significant gamma-augmentation. Of these sites, 26 were classified as Auditory, 7 as Late Stimulus, 1 as Pre-Response, and 0 as Post-Response. The time-frequency analysis of two representative Auditory sites can be found in Figure 1. Results from gamma-range analyses are summarized in Figure 2 and supplemental Table S2.
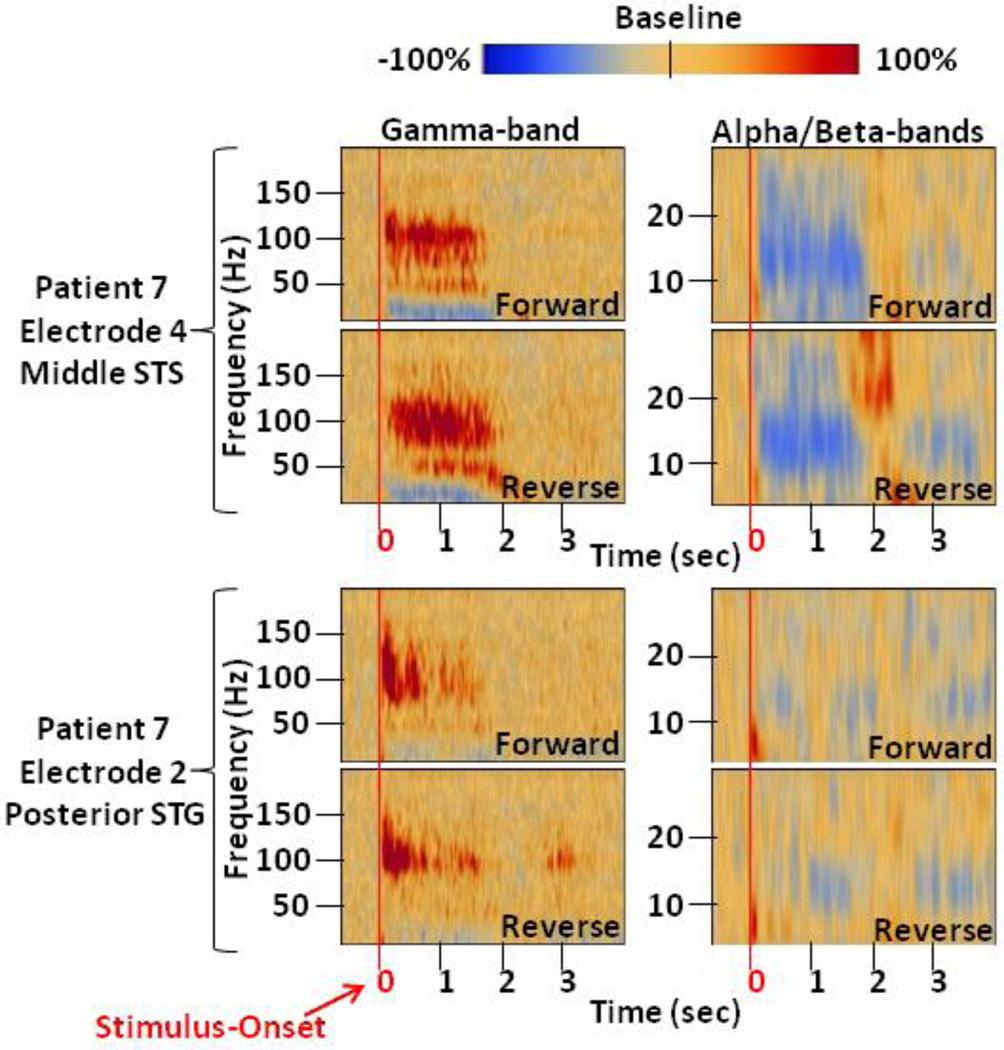
Figure 1. Time-Frequency Analysis at Two Auditory Sites.
Depicted here are the time-frequency results for both forward and reverse speech trials at two electrode sites of Patient 7 that were classified as Auditory. Similar to our previous studies (Brown et al., 2008), task-related gamma-augmentations exhibit a broadband nature (Crone et al., 2011) that is largely contained within the range from 50- to 150-Hz. Alpha/beta-attenuations can be seen to approximately correspond with gamma-augmentation. Within the auditory category, we observed two subtypes of temporal profiles: (i) those with gamma-augmentation that extended throughout the stimulus duration (top electrode) and (ii) those with gamma-augmentation primarily during the very early portions of the stimulus (bottom electrode). These were not separated in comparison analyses. The baseline reference extends over a 400-ms silent period occurring prior to stimulus onset. Stimulus questions range from 1- to 2.5-s in duration. STG = Superior Temporal Gyrus. STS = Superior Temporal Sulcus.
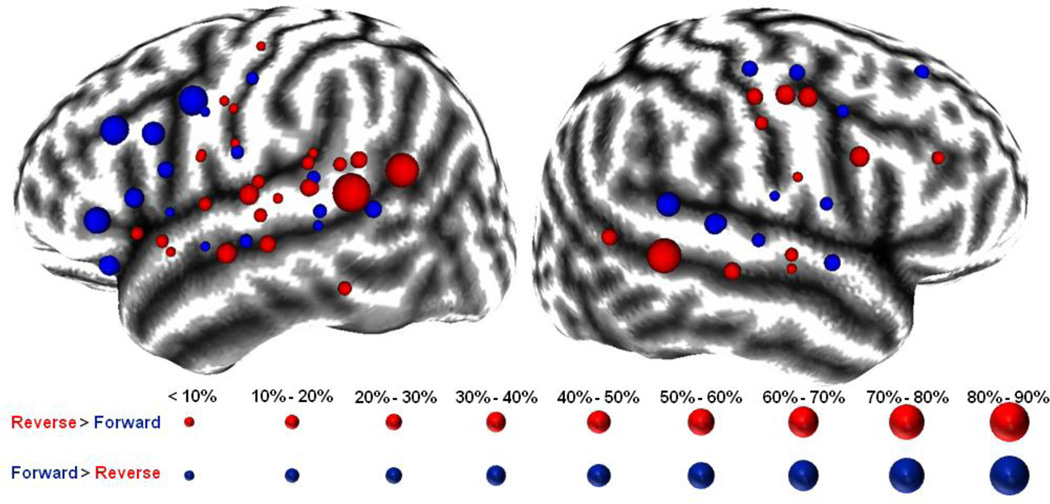
Figure 2. Relative Gamma-Augmentations across All Patients.
We created a combined image across all patients with a previously described landmark constrained conformal cortical mapping approach using in-house neuroimaging software (Muzik et al., 2007). The size of provided electrode locations depicts the difference between forward and reverse speech trials in peak gamma-augmentations, as averaged across the frequency range 50- to 150-Hz, in percent above baseline. Red electrodes are those for which reverse speech elicited the larger peak gamma-augmentation while blue electrodes are those for which that of forward speech was larger. As can be seen, especially in the left hemisphere, electrodes for which reverse speech elicits a larger peak gamma-augmentation tend to cluster in the superior temporal lobe while those for which forward speech elicits a larger peak gamma-augmentation tend to cluster in the inferior-lateral frontal lobe. Only electrodes of the frontal and temporal lobes for which forward or reverse speech elicited significant gamma augmentation are displayed on this MNI152 template brain atlas.
3.2.1 Auditory Temporal Lobe Sites
Of the 26 temporal lobe sites classified as Auditory, 8 sites (2 right hemisphere and 6 left) were located over the posterior superior temporal gyrus, 6 sites (2 right hemisphere and 4 left) over the posterior superior temporal sulcus, 7 sites (2 right hemisphere and 5 left) over the middle portion of the superior temporal gyrus, and 5 sites (1 right hemisphere and 4 left) over the middle portion of the superior temporal sulcus. On stimulus-onset analysis, the reverse speech trials were associated with a gamma-band ECoG waveform possessing an AUC larger than that associated with the corresponding forward speech trials (p < 0.001; median difference [forward – reverse] = −22.80-%-s). All 8 patients exhibited at least one temporal lobe Auditory site with a gamma-augmentation of larger AUC during reverse speech compared to forward speech. No significant difference was found between the peak gamma-augmentations associated with reverse and forward speech trials (p = 0.096; −11.44-%). Analysis of the low frequency alpha and beta range yielded no significant differences in either AUC (p = 0.809; −0.07-%-s) or peak-attenuations (p = 0.341; 1.26-%). Representative results obtained from patient 5 can be found on electrodes 1 through 9 in Figure 3. Results from the remaining 17 electrodes are depicted graphically in the supplemental Figure S1.
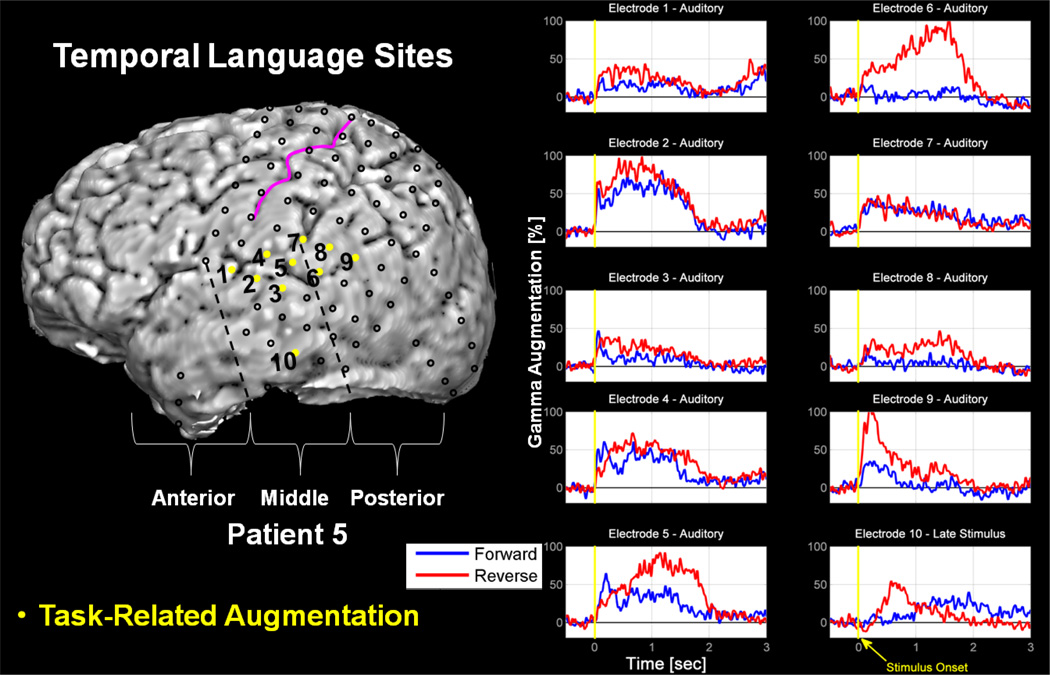
Figure 3. Reverse versus Forward Speech at Temporal Sites in Patient 5.
Complete analysis revealed 10 temporal lobe electrode sites with significant gamma-augmentation. Electrodes 1 thru 9 are situated over or near to the superior temporal gyrus and classified as Auditory, based upon temporal characteristics. Electrode 10 is situated over the middle portion of middle temporal sulcus and classified as Late Stimulus, based upon temporal characteristics. Both quantitatively and qualitatively, it is clear that the reverse speech trials more strongly engaged these language-related temporal lobe sites. The pink curve in the figure denotes the central sulcus. The vertical, dashed black lines depict our method of dividing the temporal lobe into ‘anterior’, ‘middle’, and ‘posterior’ portions; each line is drawn down perpendicular to the axis of the temporal lobe from the inferior points of the pre- and post-central sulci, respectively. Results shown are those of stimulus-onset analysis. A seizure onset zone was not resolved in this patient. The tumor in this patient lies in the parietal lobe near the postcentral sulcus.
In evaluating only the very early portion of the stimulus-onset response (<300ms following stimulus onset), a significant difference was found between forward and reverse speech for neither the AUC (p = 0.409; 1.13-%-s) nor the peak gamma-augmentation (p = 0.209; 8.24-%). The difference in the latency to gamma-augmentation between forward and reverse speech trials did not reach significance (95-% C.I. [forward – reverse]: −65.36- to 23.46-ms); excluding only 4 Auditory sites that were significant for only one trial type, 3 of which significant augmentation occurred with reverse speech but not forward.
3.2.2 Non-Auditory Temporal Lobe Sites
Of the 8 temporal lobe sites classified as Non-Auditory (7 Late Stimulus, 1 Pre-Response, 0 Post-Response), 1 site was located over the right anterior superior temporal gyrus, 5 sites (2 right hemisphere and 3 left) over the middle portion of the superior temporal gyrus, 1 site over the left posterior superior temporal gyrus, and 1 site over the middle portion of the left middle temporal sulcus. Stimulus-onset analysis of gamma activity revealed no significant difference between AUC measures for reverse and forward speech (p = 0.674; 19.83-%-s). Moreover, no significant difference was found between the peak gamma-augmentations for reverse and forward speech (p = 0.674; −6.55-%). Analysis of the low frequency alpha and beta range yielded a significantly increased peak attenuation during reverse speech trials (p = 0.036; 2.41-%) that was not corroborated by AUC measures (p = 0.123; 2.60-%-s). A representative result from patient 5 can be found on electrode 10 in Figure 3. Results from the remaining 7 electrodes are depicted graphically in the supplemental Figure S1.
Patient 6 did have a statistically significant Pre-Response gamma-augmentation at 1 site over the medial temporal region during forward but not reverse speech, indicating that only the forward speech trials engaged the medial temporal region in this patient. This medial temporal site was not included in the comparison analysis because there remained doubt that the signal was neocortical in origin.
3.3 Frontal Lobe Augmentations
Across all eight patients, a total of 31 sites with significant gamma-augmentation were noted in the frontal lobe. Of these, 4 were classified as Auditory, 4 as Late Stimulus, 10 as Pre-Response, and 13 as Post-Response. Results from gamma-range analyses are summarized in Figure 2 and Supplementary Table S3.
3.2.1 Pre-Response Frontal Lobe Sites
Of the 10 frontal lobe sites classified as Pre-Response, 3 sites (1 right hemisphere and 2 left) were located over the inferior frontal sulcus, 3 sites (1 right hemisphere and 2 left) over the inferior frontal gyrus, 3 sites (1 right hemisphere and 2 left) over the precentral gyrus, and 1 site over the left precentral sulcus. Response-onset analysis based on the Wilcoxon Signed Ranks test showed that forward speech trials elicit a larger peak gamma-augmentation in these frontal Pre-Response sites as compared to the corresponding reverse speech trials (p = 0.028; 25.49-%). Similarly, the low frequency alpha and beta range exhibited a greater peak-attenuation during forward speech trials compared to reverse speech trials (p = 0.037; −11.97-%). Representative results from patient 2 can be found in Figure 4. Results from the remaining 4 electrodes are depicted graphically in the supplemental Figure S2.
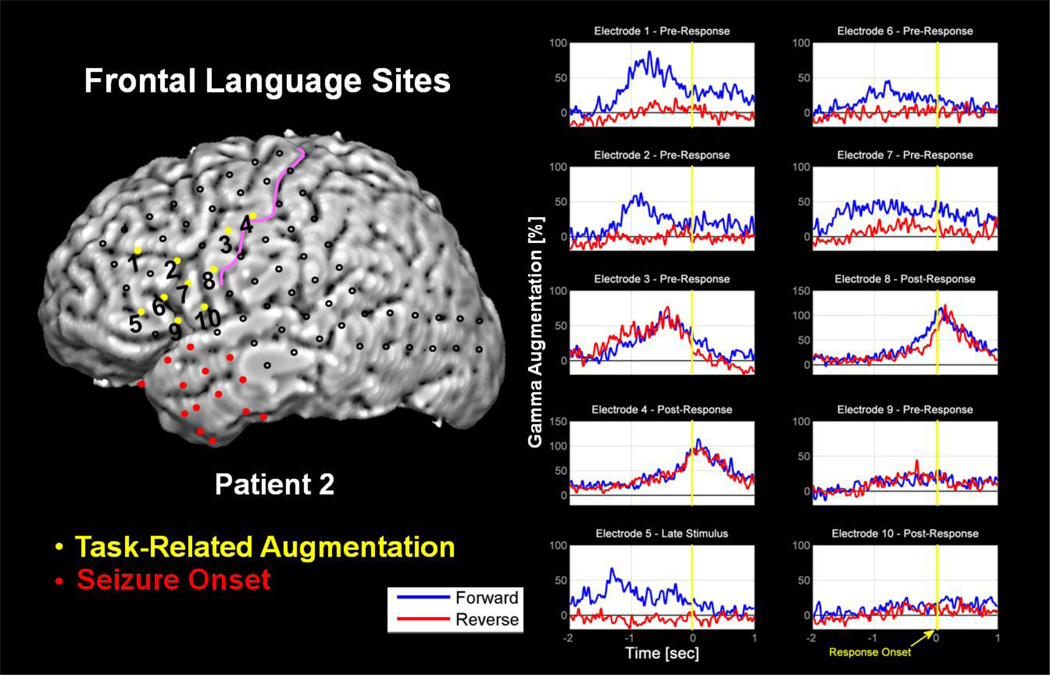
Figure 4. Reverse versus Forward Speech at Frontal Sites in Patient 2.
Complete analysis revealed 10 frontal lobe electrode sites with significant gamma-augmentation. Electrodes 4, 8, and 10 were classified as Post-Response, based upon temporal characteristics, each located over a portion of the precentral gyrus. Electrode 5 was classified as Late Stimulus, based upon temporal characteristics, and is located over the inferior frontal gyrus. Electrodes 1–3, 6, 7, and 9 were classified as Pre-Response, based upon temporal characteristics. Electrodes 1 and 2 are located over portions of the inferior frontal sulcus. Electrodes 6 and 9 are situated over portions of the inferior frontal gyrus. Electrode 7 is situated over the precentral sulcus. Electrode 3 is situated over the precentral gyrus. Both quantitatively and qualitatively, it is clear that the forward speech trials more strongly engage sites classified a Pre-Response; especially those more anterior to the precentral sulcus. Also evident is the finding that sites classified as Post-Response are similarly activated by the forward and reverse speech tasks. Results shown are those of response-onset analysis. Red colored electrodes depict the seizure onset zone. The pink curve in the figure denotes the central sulcus.
3.2.2 Late Stimulus Frontal Lobe Sites
Of the 4 frontal lobe sites classified as Late Stimulus, 1 site was located over the right middle frontal gyrus and 3 sites (1 right hemisphere and 2 left) over the inferior frontal gyrus. Response-onset analysis based on the Wilcoxon Signed Ranks test showed no difference in the peak-augmentation of gamma activity between Forward and Reverse Speech trials (p = 0.144; 34.85-%). Analysis of the low frequency alpha and beta range yielded no significant difference in peak-attenuations (p = 0.465; 4.51-%). A representative result from patient 2 can be found in Figure 4. Results from the remaining 3 electrodes are depicted graphically in the supplemental Figure S2.
3.2.3 Post-Response Frontal Lobe Sites
Of the frontal lobe sites classified as Post-Response, all 13 sites (5 right hemisphere and 8 left) were located over the precentral gyrus or sulcus. Response-onset analysis failed to reveal a difference between peak gamma-augmentations at these sites between the forward speech trials and the corresponding reverse speech trials (p = 0.594; 2.04-%). Similarly, the low frequency alpha and beta yielded no significant difference in peak-attenuation between forward and reverse speech trials (p = 0.055; 4.64-%). Representative results derived from patient 2 can be found in Figure 4. Results from the remaining 10 electrodes are depicted graphically in the supplemental Figure S2.
3.3.4 Auditory Frontal Lobe Sites
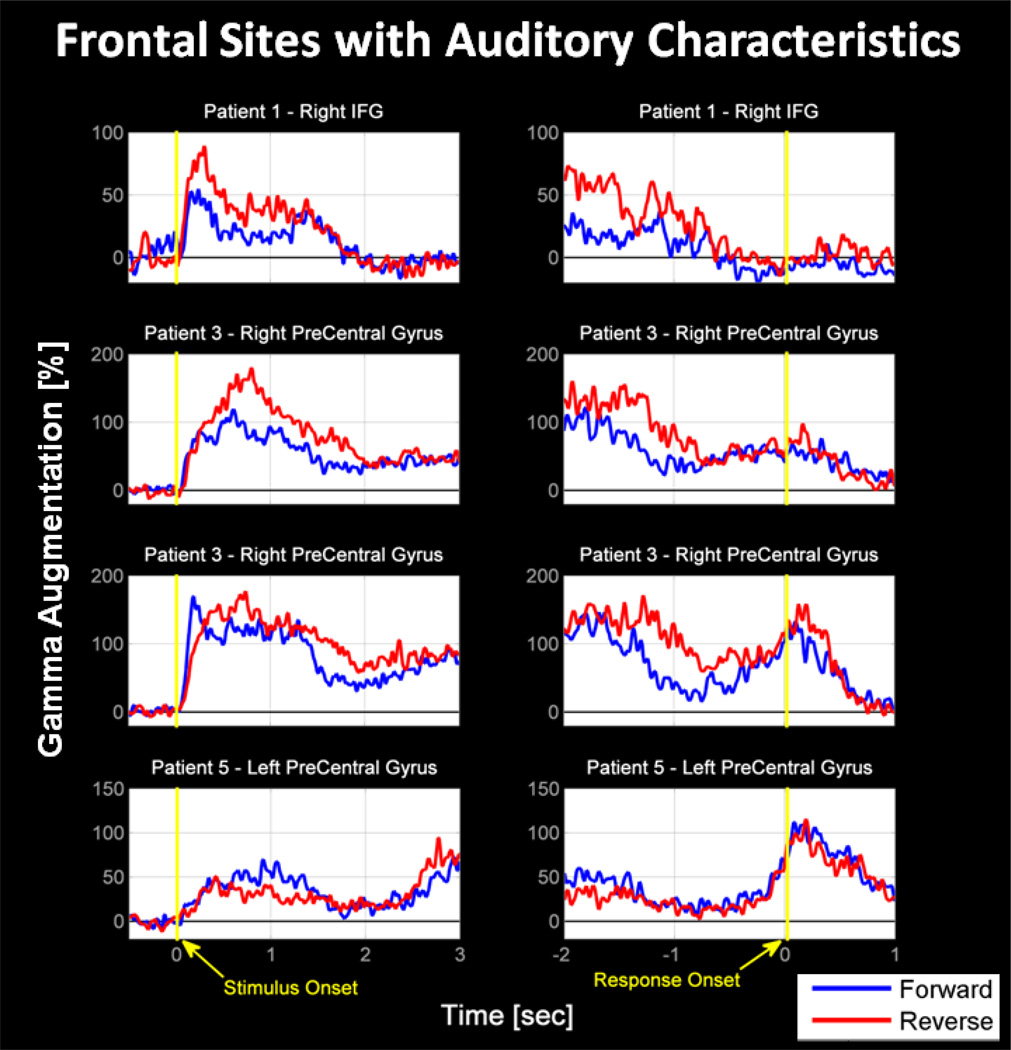
Four frontal lobe sites showed very early gamma-augmentation during questions and were classified as Auditory, one over the right inferior frontal gyrus in patient 1, 2 over the right precentral gyrus in patient 3, and 1 over the left precentral gyrus in patient 5. Plots of the activities of these sites can be seen in Figure 5. The frontal Auditory site found in patient 1 showed augmentation exclusively during the stimulus, whereas those of patients 3 & 5 had augmentations during both the stimulus and response. Due to the small number of frontal electrodes with Auditory activity, the apparent heterogeneity within the group, and their analysis extending beyond the scope of this study, a comparison test of significance between the tasks was not performed.
Figure 5. Unique Frontal Sites with Auditory Activity.
Four electrode sites of the frontal lobe were unexpectedly classified as Auditory. These were observed in the right inferior frontal gyrus of patient 1 and the left precentral gyrus of patients 3 and 5; patient 3 having two such sites. In patient 1, this Auditory frontal gamma-augmentation occurred only during the stimulus. There was no significant gamma-augmentation during the response. In patients 3 and 5, augmentation occurred both during the stimulus as well as during the response. We believe that these electrodes may indicate the location of the frontal eye-field, which has recently been implicated in auditory functions (Kirchner et al., 2009).
3.4 Correction for Multiple Comparisons
The above analyses included a total of 27 statistical comparisons between forward and reverse speech trial types. After applying the conservative Bonferroni correction for multiple comparisons, only the increase in AUC of gamma-augmentations during reverse speech trials compared to forward speech at temporal lobe Auditory sites remained significant (corrected p < 0.05).
4. Discussion
4.1 Primary Findings
We failed to prove the hypothesis that forward speech elicits larger augmentation of gamma activity in bilateral superior temporal regions than does reverse speech. On the contrary, we rejected the null hypothesis in favor of an opposing alternative hypothesis: reverse speech more strongly engages bilateral superior temporal regions than forward speech. It is unlikely that this finding is due to a greater cognitive demand imposed by reverse speech trials or to Type I error. Rather, our subjects were instructed to provide a generic response to the reverse speech trials while the forward speech trials required a unique and appropriate response. Supporting this notion is the fact that the forward speech trials were associated with longer response times and greater peak augmentation at frontal lobe Pre-Response sites. Thus, we have demonstrated a double dissociation between Auditory sites of the superior temporal region, responding more strongly to the reverse speech trials, and Pre-Response sites of the frontal lobe, responding more strongly to the forward speech trials. Taken together, although forward speech trials imposed a greater cognitive demand, reverse speech appeared to more strongly engage bilateral superior temporal cortices. Enhanced gamma-augmentation at Auditory sites of the superior temporal gyrus during reverse speech trials may indicate increased attention (Crone et al., 2011; Deco and Thiele, 2009). Relevant psychoacoustic reasons for why reverse speech may be a poor control for non-language auditory functions can be found in the perceptually unusual time reversal of a distinctly human voice. The idea that temporally reversing speech signals completely removes intelligibility is flawed in that the amplitude envelope and all spectral detail are otherwise intact. The authors and our patients can attest to our ability to identify reverse speech trials as originating from a human voice; one of our patients spontaneously tried to identify the reverse speech trials as being French. Indeed, it has been previously shown that even speech signals that are severely degraded in spectral detail and amplitude envelope dynamics can carry a surprising degree of intelligibility (Shannon et al., 1995). It may be more accurate to speak of the perceptual effect created by the reversing of speech signals as that of 'confused intelligibility' rather than 'removed intelligibility'; the result does not lack intelligibility per se, although it cannot be understood without prior experience and practice (Cowan et al., 1982).
In the present study, the first sound in most of the forward speech stimuli was the same (/w/), and on the majority of occasions the first word was the interrogative 'what'; as seen in supplemental Table S1. In contrast, the sounds ending the forward speech stimuli were highly variable. Therefore, reverse speech stimuli began with a wide and unpredictable range of speech sounds. This creates the potential for the occurrence of a confounder which may explain our results; i.e. increased novelty of the early portion of the reverse speech trials may enhance the gamma-augmentations at Auditory sites. To search for the occurrence of such an undesirable phenomenon, we employed additional analyses at Auditory sites to address possible differences in the latency to gamma-augmentation and early gamma-augmentation peak (<300ms post-stimulus-onset) between forward and reverse speech trials, as described in the Supplementary Document. If variability in the first sound was seriously confounding our results, we would expect to find that reverse speech would have a significantly earlier onset of gamma-augmentation following stimulus onset or elicit larger gamma-augmentation during this very early period of the stimulus. However, our analysis yielded no significant difference between the latency to gamma-augmentation or early gamma-augmentation peak between forward and reverse speech trials. These analyses failed to account for our findings simply by variability in the first sound between stimuli and suggest that the confounding effects of such a phenomenon may be modest, if any.
Our ECoG study compared the effects of forward speech stimuli to that of corresponding reverse speech stimuli based on the frequency range from 50- to 150-Hz, as in our previous studies (Brown et al., 2008; Wu et al., 2011). This frequency range is largely out of reach of noninvasive EEG or MEG methods (Dalal et al., 2009) and is measured on a finer spatial scale with ECoG; each macro-electrode records the activities from on the order of 100,000 neurons (Modolo et al., 2010). It completely contains the recently proposed χ-band (76- to 150-Hz), which has been suggested to be the optimal range for spectral-band-based features in brain mapping (Miller et al., 2008). Additionally, our frequency range of interest is related to the LFP spectrum, defined as neurophysiological activity below 300-Hz. LFPs have been shown to “reflect cooperative activity in neuronal populations” (Logothetis, 2003). LFPs and related electrophysiological measures are known to be selectively sensitive to activity involving pyramidal cells, which are almost ideally vertically oriented in an ‘open-field’ geometrical arrangement; the apical dendrite more superficial relative to the soma, creating a cortical array of ‘dendrite-to-soma dipoles’ (Buzsáki, 2004; Logothetis, 2003). Further, computational modeling and experimental data have shown that the dominant mechanism of generation of gamma activity above 50Hz involves reciprocal interactions between pyramidal cells and certain classes of locally-projecting, inhibitory interneurons (Whittington et al., 2011). Specifically, fast-spiking inhibitory interneurons generate high frequency oscillations in concert with rhythmic feedback from pyramidal cells in order to ‘gate’ synaptic inputs and synchronize pyramidal cell output (Cardin et al., 2009). In short, our ECoG methods represent a validated, reliable, and direct measure of task-related cortical activity.
4.2 Secondary Findings
In addition to gamma range high-frequency activity, we considered low frequency oscillations across the alpha and beta ranges. Attenuations at these frequencies were originally described as being closely related to gamma-augmentations (Crone et al., 1998; Crone et al., 2011; Pfurtscheller and Lopes da Silva, 1999). However, the underlying functional meaning of amplitude changes in these low frequency components is less well understood compared to that of high frequency gamma (Engel and Fries, 2010). More recently, activity at these low frequencies has generally been found to be more spatially distributed and less dynamic than those of the gamma range (Crone et al., 2006; Crone et al., 2011; Fukuda et al., 2010; Hermes et al., 2011) and such low frequency changes may be functionally independent of those in the gamma range (Cardin et al., 2009; Conner et al., 2011). Indeed, at temporal lobe sites classified as Auditory, we found that low frequency oscillations in the alpha/beta range exhibited similar attenuations between forward and reverse speech trials even though the gamma-band was more strongly augmented during reverse speech stimuli.
Regarding the beta range, emerging theories suggest a role in maintenance of the ‘status quo’ (Engel and Fries, 2010). This might explain the enhanced attenuation of low frequency oscillations we observed at Non-Auditory sites of the temporal lobe during reverse speech trials, where gamma augmentations were not seen to differ from those of forward speech: reverse speech trials may have required additional flexibility in language comprehension processes in an attempt to decode meaning from an unusual but clearly human-speech-related stimulus. Relevant to the double-dissociation we observed, the opposite occurred at frontal lobe sites classified as Pre-Response where forward speech trials elicited both increased gamma-augmentation as well as alpha/beta-attenuation: forward speech trials may have required additional cognitive flexibility in preparing a unique and relevant response. Finally, the lack of a difference in low frequency attenuations at Auditory sites of the superior temporal lobes suggests that the enhanced gamma-augmentation observed during reverse speech trials is not simply due to increased novelty of the reverse speech stimuli, which would be expected to increase relative attenuation, nor to auditory imagery of the repetitive response “I don’t know”, which would represent a type of ‘status-quomaintenance’ and elicit a decreased relative attenuation.
4.3 ECoG and fMRI
Task-related measures obtained using methods such as fMRI are currently widely employed and reported in the neuroscience literature. Such methods have the strong benefit of being noninvasive, enabling data collection from normal subjects. Additionally, fMRI possesses a spatial resolution exceeding that of all other functional mapping modalities as well as the ability to obtain measures from both superficial as well as deep brain structures. The disadvantages of fMRI include poor temporal resolution and reliance upon indirect measures of cortical activity, i.e. the BOLD effect which is more directly related to cortical blood flow and metabolism than to the electrophysiological activity of neurons. Nevertheless, it is thought that “the BOLD-fMRI signal will always reflect the input and intracortical processing taking place in an imaged cortical area” (Logothetis, 2003). PET and NIRS rely upon biophysical mechanisms that are similar but not identical to that of BOLD-fMRI (Perani et al., 1996; Sato et al., 2011).
Without doubt, our results derived from invasive ECoG measurements are inconsistent with the broader literature based on non-invasive methods, such as fMRI, PET, and NIRS. As non-invasive neuroimaging represents an important tool in the study of brain function, an appraisal of possible reasons for the observed discrepancy is warranted. One possibility may be differences in the applied tasks between studies. However, the difference between forward and reverse speech observed in the non-invasive neuroimaging literature does not appear to be highly task-specific, as indicated by the following three complimentary studies: In one fMRI study involving passive listening, simple or complex audio stories represented the forward speech trials while time reversal of the simple stories generated the reverse speech trials (Redcay et al., 2008). Another fMRI study described a lexical-semantic decision task as the forward speech trials, during which both child and adult subjects were to determine the appropriateness of a noun that followed a descriptive sentence (Moore-Parks et al., 2010). To their reverse speech control trials, subjects were to always select the answer ‘incorrect’ in response; this is similar to our requirement to always respond with “I don’t know” when a stimulus is incomprehensible. In an NIRS study, a female Japanese voice was used to read children’s stories to Japanese infants and compare the oxygenated hemoglobin ([Oxy-Hb]) response to the same voice played in reverse (Sato et al., 2011). In all the above studies, a larger response to forward speech was demonstrated in temporal neocortices. Differences in task details do not appear important when comparing forward to reverse speech.
This is not the first instance in which a discrepancy between results of electrophysiological and fMRI studies has been reported. In monkeys, the visual area known as V4, along with V1, V2, V3, and V5/MT, was shown on fMRI to be strongly activated by a visual motion processing task (Tolias et al., 2001). However, electrophysiological methods consistently showed that V1, V2, V3, and V5/MT but not V4 were involved in motion processing (Logothetis, 2003). Logothetis has proposed that this discrepancy is due to fMRI’s sensitivity to the metabolic activity of all classes of cortical interneurons, not just the fast-spiking inhibitory type, in addition to that of pyramidal cells. It is speculated that distant brain regions may mediate “some kind of modulatory function” that is insufficient to drive pyramidal cells, creating the opportunity for local intracortical processing to proceed undetected by electrophysiology (Logothetis, 2003). Such ‘modulatory function’ has yet to be clearly described. We speculate that the ECoG data presented here indicates a stronger engagement of temporal language neocortex by a reverse speech stimulus, perhaps indicating increased attention (Crone et al., 2011; Deco and Thiele, 2009) in an attempt to decode meaning from an incomprehensible but distinctly human sound; human speech can be accurately recognized even with severely degraded detail in both amplitude envelope and spectral information (Shannon et al., 1995), both of which are left intact by reverse speech tasks, albeit with reversed temporal dynamics. Using the same reasoning as Logothetis, the increased activation to forward speech observed on fMRI may be caused by an enhanced ‘modulation’ (rather than engagement) of superior temporal language areas in response to what is being perceived and decoded as normal human language. This ‘modulation’ of temporal language cortices during processing of the native language may involve interneuronal cell types beyond fast-spiking inhibitory interneurons or, potentially, cortical astrocytes, which have been shown to be intimately associated with both normal synaptic functions (the ‘tripartite synapse’) and the regulation of cortical blood flow (Iadecola and Nedergaard, 2007; Wieronska and Pilc, 2009). Understanding of this ‘modulation’ clearly requires further multimodal investigation.
4.4 Studying Language Function in Patients with Epilepsy
One inherent limitation in ECoG studies is that all subjects are undergoing a surgical procedure for the treatment of a neurological disorder, typically epilepsy or brain tumor. Thus, patients in ECoG studies are often assumed to represent an ‘abnormal’ population. Methods do exist by which the degree of departure from normality can be estimated. For example, Processing Speed Index values < 90, approximately, may indicate a > 1 standard deviation departure from normal electrographic indicators of auditory information processing in patients with intractable epilepsy (Korostenskaja et al., 2010). Age at onset of epilepsy, whether prior to or after age 14, is another measure that has been shown to be important in predicting brain dysfunction (Kaaden and Helmstaedter, 2009). We have also included information regarding VCI, VIQ, Wada test results, as well as qualitative educational information, when available, in Table 1. Our patient selection methods imposed an exclusion criterion when the VCI or VIQ was known to be less than 70, indicative of a severe cognitive impairment in the realm of verbal functions. As can be observed from the behavioral and neuropsychological data provided, there is little reason to believe that our study cohort deviates significantly from ‘normal’ individuals with intact language function. All patients were able to satisfactorily complete our auditory naming task along with the corresponding reverse speech trials. Additionally, it has been shown that patients with epilepsy have a BOLD effect in response to auditory language tasks similar to that observed in normal subjects, especially when atypical language lateralization is not present (Carpentier et al., 2001). Like normal subjects, epileptic patients show increased BOLD response in superior temporal regions in response to forward speech compared to reverse speech (Gaillard et al., 2004). The findings from this study can be considered to represent ‘approximately normal’ brain function and may be applied to the broader population.
5. Conclusion
The purpose for the use of a reverse speech task in neuroimaging studies of language is to control for auditory and motor processing (Moore-Parks et al., 2010). Although the non-invasive neuroimaging literature is quite consistent across modalities in showing that the superior temporal regions are more strongly engaged by forward speech compared to reverse speech, we have observed the opposite effect using invasive ECoG methods. Whether this indicates a true discrepancy between metabolic and electrophysiologic measures is a question that must be reserved for future studies combining non-invasive neuroimaging with invasive electrophysiology for simultaneous recordings. While reverse speech may ideally control for the physical aspects of spoken language, more needs to be learned about the functional cortical events related to hearing reverse speech before it can be effectively incorporated into studies of language as a control or contrast task for auditory processing. Further studies using other auditory baselines such as spectrally-rotated speech, signal-correlated noise, noise-vocoded speech, 'musical rain', or other stimuli of non-human origin are warranted to determine a control task to better segregate neural activation for general auditory perception from that specific to linguistic function (Mottonen et al., 2006; Scott et al., 2009).
Supplementary Material
Acknowledgements
This work was supported by NIH grants NS47550 and NS64033 (to E. Asano). We are grateful to Katsuaki Kojima, M.D., Harry T. Chugani, M.D., Carol Pawlak, R.EEG./EP.T., Ruth Roeder, R.N., M.S., Sarah Minarik, R.N., B.S.N., Alanna Marie Carlson, M.A., Elizabeth Bohme, M.A., and the staff of the Division of Electroneurodiagnostics at Children‘s Hospital of Michigan, Wayne State University’s School of Medicine for the collaboration and assistance in performing the studies described above. Cynthia L. Arfken, PhD, Associate Professor of Psychiatry and Behavioral Neurosciences at Wayne State University, graciously provided statistical consultation. We would also like to acknowledge the insightful comments and constructive critiques provided by three anonymous NeuroImage reviewers. The first author thanks the Translational Neuroscience Program and the MD/PhD Program of Wayne State University’s School of Medicine for providing an environment in which to perform translational research and grow as an investigator.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alkonyi B, Juhász C, Muzik O, Asano E, Saporta A, Shah A, Chugani HT. Quantitative brain surface mapping of an electrophysiologic/metabolic mismatch in human neocortical epilepsy. Epilepsy Res. 2009;87:77–87. doi: 10.1016/j.eplepsyres.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano E, Juhász C, Shah A, Muzik O, Chugani DC, Shah J, Sood S, Chugani HT. Origin and propagation of epileptic spasms delineated on electrocorticography. Epilepsia. 2005;46:1086–1097. doi: 10.1111/j.1528-1167.2005.05205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano E, Nishida M, Fukuda M, Rothermel R, Juhász C, Sood S. Differential visually-induced gamma-oscillations in human cerebral cortex. Neuroimage. 2009;45:477–489. doi: 10.1016/j.neuroimage.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auranen T. Master's Thesis. Espoo, Finland: Helsinki University of Technology, Department of Electrical and Communications Engineering; 2002. Nonparametric statistical analysis of time-frequency representations of magnetoencephalographic data. [Google Scholar]
- Berger MS, Kincaid J, Ojemann GA, Lettich E. Brain mapping techniques to maximize resection, safety, and seizure control in children with brain tumors. Neurosurgery. 1989;25:786–792. doi: 10.1097/00006123-198911000-00015. [DOI] [PubMed] [Google Scholar]
- Brown EC, Rothermel R, Nishida M, Juhász C, Muzik O, Hoechstetter K, Sood S, Chugani HT, Asano E. In vivo animation of auditory-language-induced gamma-oscillations in children with intractable focal epilepsy. Neuroimage. 2008;41:1120–1131. doi: 10.1016/j.neuroimage.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Large-scale recording of neuronal ensembles. Nat. Neurosci. 2004;7:446–451. doi: 10.1038/nn1233. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier A, Pugh KR, Westerveld M, Studholme C, Skrinjar O, Thompson JL, Spencer DD, Constable RT. Functional MRI of language processing: dependence on input modality and temporal lobe epilepsy. Epilepsia. 2001;42:1241–1254. doi: 10.1046/j.1528-1157.2001.35500.x. [DOI] [PubMed] [Google Scholar]
- Conner CR, Ellmore TM, Pieters TA, DiSano MA, Tandon N. Variability of the relationship between electrophysiology and BOLD-fMRI across cortical regions in humans. J. Neurosci. 2011;31:12855–12865. doi: 10.1523/JNEUROSCI.1457-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N, Leavitt LA, Massaro DW, Kent RD. A fluent backward talker. J Speech Hear Res. 1982;25:48–53. doi: 10.1044/jshr.2501.48. [DOI] [PubMed] [Google Scholar]
- Crone NE, Korzeniewska A, Franaszczuk PJ. Cortical gamma responses: searching high and low. Int J Psychophysiol. 2011;79:9–15. doi: 10.1016/j.ijpsycho.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone NE, Miglioretti DL, Gordon B, Sieracki JM, Wilson MT, Uematsu S, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. I. Alpha and beta event-related desynchronization. Brain. 1998;121:2271–2299. doi: 10.1093/brain/121.12.2271. [DOI] [PubMed] [Google Scholar]
- Crone NE, Sinai A, Korzeniewska A. High-frequency gamma oscillations and human brain mapping with electrocorticography. Prog Brain Res. 2006;159:275–295. doi: 10.1016/S0079-6123(06)59019-3. [DOI] [PubMed] [Google Scholar]
- Dalal SS, Baillet S, Adam C, Ducorps A, Schwartz D, Jerbi K, Bertrand O, Garnero L, Martinerie J, Lachaux JP. Simultaneous MEG and intracranial EEG recordings during attentive reading. Neuroimage. 2009;45:1289–1304. doi: 10.1016/j.neuroimage.2009.01.017. [DOI] [PubMed] [Google Scholar]
- Davidson AC, Hinkley DV. Studentized bootstrap method. Cambridge: Cambridge University Press; 1999. Bootstrap Methods and their Application, Chapter 4.4.1; pp. 161–175. [Google Scholar]
- Deco G, Thiele A. Attention: oscillations and neuropharmacology. Eur. J. Neurosci. 2009;30:347–354. doi: 10.1111/j.1460-9568.2009.06833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchowny M, Jayakar P, Harvey AS, Resnick T, Alvarez L, Dean P, Levin B. Language cortex representation: effects of developmental versus acquired pathology. Ann. Neurol. 1996;40:31–38. doi: 10.1002/ana.410400108. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P. Beta-band oscillations--signalling the status quo? Curr. Opin. Neurobiol. 2010;20:156–165. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Flinker A, Chang EF, Kirsch HE, Barbaro NM, Crone NE, Knight RT. Single-trial speech suppression of auditory cortex activity in humans. J. Neurosci. 2010;30:16643–16650. doi: 10.1523/JNEUROSCI.1809-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Juhász C, Hoechstetter K, Sood S, Asano E. Somatosensory-related gamma-, beta- and alpha-augmentation precedes alpha- and beta-attenuation in humans. Clin. Neurophysiol. 2010;121:366–375. doi: 10.1016/j.clinph.2009.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard WD, Balsamo L, Xu B, McKinney C, Papero PH, Weinstein S, Conry J, Pearl PL, Sachs B, Sato S, Vezina LG, Frattali C, Theodore WH. fMRI language task panel improves determination of language dominance. Neurology. 2004;63:1403–1408. doi: 10.1212/01.wnl.0000141852.65175.a7. [DOI] [PubMed] [Google Scholar]
- Gherri E, Eimer M. Active listening impairs visual perception and selectivity: an ERP study of auditory dual-task costs on visual attention. J. Cogn. Neurosci. 2011;23:832–844. doi: 10.1162/jocn.2010.21468. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, McClelland S, III, McKhann GM, II, Williams AC, Goodman RR. Distribution of auditory and visual naming sites in nonlesional temporal lobe epilepsy patients and patients with space-occupying temporal lobe lesions. Epilepsia. 2007;48:531–538. doi: 10.1111/j.1528-1167.2006.00955.x. [DOI] [PubMed] [Google Scholar]
- Hermes D, Miller KJ, Vansteensel MJ, Aarnoutse EJ, Leijten FS, Ramsey NF. Neurophysiologic correlates of fMRI in human motor cortex. Hum. Brain. Mapp. 2011 doi: 10.1002/hbm.21314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoechstetter K, Bornfleth H, Weckesser D, Ille N, Berg P, Scherg M. BESA source coherence: a new method to study cortical oscillatory coupling. Brain Topogr. 2004;16:233–238. doi: 10.1023/b:brat.0000032857.55223.5d. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat. Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Jerbi K, Ossandon T, Hamame CM, Senova S, Dalal SS, Jung J, Minotti L, Bertrand O, Berthoz A, Kahane P, Lachaux JP. Task-related gamma-band dynamics from an intracerebral perspective: review and implications for surface EEG and MEG. Hum. Brain Mapp. 2009;30:1758–1771. doi: 10.1002/hbm.20750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaaden S, Helmstaedter C. Age at onset of epilepsy as a determinant of intellectual impairment in temporal lobe epilepsy. Epilepsy Behav. 2009;15:213–217. doi: 10.1016/j.yebeh.2009.03.027. [DOI] [PubMed] [Google Scholar]
- Kirchner H, Barbeau EJ, Thorpe SJ, Regis J, Liegeois-Chauvel C. Ultra-rapid sensory responses in the human frontal eye field region. J. Neurosci. 2009;29:7599–7606. doi: 10.1523/JNEUROSCI.1233-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht S, Deppe M, Drager B, Bobe L, Lohmann H, Ringelstein E, Henningsen H. Language lateralization in healthy right-handers. Brain. 2000;123:74–81. doi: 10.1093/brain/123.1.74. [DOI] [PubMed] [Google Scholar]
- Koga S, Rothermel R, Juhász C, Nagasawa T, Sood S, Asano E. Electrocorticographic correlates of cognitive control in a stroop task-intracranial recording in epileptic patients. Hum. Brain Mapp. 2011;32:15080–11591. doi: 10.1002/hbm.21129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korostenskaja M, Pardos M, Fujiwara H, Kujala T, Horn P, Rose D, Byars A, Brown D, Seo JH, Wang Y, Vannest J, Xiang J, Degrauw T, Naatanen R, Lee KH. Neuromagnetic evidence of impaired cortical auditory processing in pediatric intractable epilepsy. Epilepsy Res. 2010;92:63–73. doi: 10.1016/j.eplepsyres.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Logothetis NK. The underpinnings of the BOLD functional magnetic resonance imaging signal. J. Neurosci. 2003;23:3963–3971. doi: 10.1523/JNEUROSCI.23-10-03963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNelly K, Dapretto M, Bookheimer S. Language and the developing brain: insights from neuroimaging. First ed. New York, NY: Cambridge University Press; 2009. [Google Scholar]
- Miller KJ, Shenoy P, den Nijs M, Sorensen LB, Rao RN, Ojemann JG. Beyond the gamma band: the role of high-frequency features in movement classification. IEEE Trans. Biomed. Eng. 2008;55:1634–1637. doi: 10.1109/TBME.2008.918569. [DOI] [PubMed] [Google Scholar]
- Modolo J, Bhattacharya B, Edwards R, Campagnaud J, Legros A, Beuter A. Using a virtual cortical module implementing a neural field model to modulate brain rhythms in Parkinson's disease. Front. Neurosci. 2010;4:pii. 45. doi: 10.3389/fnins.2010.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore-Parks EN, Burns EL, Bazzill R, Levy S, Posada V, Muller RA. An fMRI study of sentence-embedded lexical-semantic decision in children and adults. Brain Lang. 2010;114:90–100. doi: 10.1016/j.bandl.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottonen R, Calvert GA, Jaaskelainen IP, Matthews PM, Thesen T, Tuomainen J, Sams M. Perceiving identical sounds as speech or non-speech modulates activity in the left posterior superior temporal sulcus. Neuroimage. 2006;30:563–569. doi: 10.1016/j.neuroimage.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Muzik O, Chugani DC, Zou G, Hua J, Lu Y, Lu S, Asano E, Chugani HT. Multimodality data integration in epilepsy. Int. J. Biomed. Imaging. 2007;2007:13963. doi: 10.1155/2007/13963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T, Rothermel R, Juhasz C, Fukuda M, Nishida M, Akiyama T, Sood S, Asano E. Cortical gamma-oscillations modulated by auditory-motor tasks-intracranial recording in patients with epilepsy. Hum. Brain Mapp. 2010a;31:1627–1642. doi: 10.1002/hbm.20963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T, Rothermel R, Juhasz C, Nishida M, Sood S, Asano E. Cortical gamma-oscillations modulated by visuomotor tasks: Intracranial recording in patients with epilepsy. Epilepsy Behav. 2010b;18:254–261. doi: 10.1016/j.yebeh.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J. Neurosurg. 1989;71:316–326. doi: 10.3171/jns.1989.71.3.0316. [DOI] [PubMed] [Google Scholar]
- Ojemann SG, Berger MS, Lettich E, Ojemann GA. Localization of language function in children: results of electrical stimulation mapping. J. Neurosurg. 2003;98:465–470. doi: 10.3171/jns.2003.98.3.0465. [DOI] [PubMed] [Google Scholar]
- Papp N, Ktonas P. Critical evaluation of complex demodulation techniques for the quantification of bioelectrical activity. Biomed. Sci. Instrum. 1977;13:135–145. [PubMed] [Google Scholar]
- Perani D, Dehaene S, Grassi F, Cohen L, Cappa SF, Dupoux E, Fazio F, Mehler J. Brain processing of native and foreign languages. Neuroreport. 1996;7:2439–2444. doi: 10.1097/00001756-199611040-00007. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin. Neurophysiol. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Redcay E, Courchesne E. Deviant functional magnetic resonance imaging patterns of brain activity to speech in 2–3-year-old children with autism spectrum disorder. Biol. Psychiatry. 2008;64:589–598. doi: 10.1016/j.biopsych.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E, Haist F, Courchesne E. Functional neuroimaging of speech perception during a pivotal period in language acquisition. Dev. Sci. 2008;11:237–252. doi: 10.1111/j.1467-7687.2008.00674.x. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Hari R. Spatiotemporal characteristics of sensorimotor neuromagnetic rhythms related to thumb movement. Neuroscience. 1994;60:537–550. doi: 10.1016/0306-4522(94)90263-1. [DOI] [PubMed] [Google Scholar]
- Sato H, Hirabayashi Y, Tsubokura H, Kanai M, Ashida T, Konishi I, Uchida-Ota M, Konishi Y, Maki A. Cerebral hemodynamics in newborn infants exposed to speech sounds: A whole-head optical topography study. Hum. Brain Mapp. 2011 doi: 10.1002/hbm.21350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SK, McGettigan C, Eisner F. A little more conversation, a little less action--candidate roles for the motor cortex in speech perception. Nat. Rev. Neurosci. 2009;10:295–302. doi: 10.1038/nrn2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon RV, Zeng FG, Kamath V, Wygonski J, Ekelid M. Speech recognition with primarily temporal cues. Science. 1995;270:303–304. doi: 10.1126/science.270.5234.303. [DOI] [PubMed] [Google Scholar]
- Simes RJ. An improved Bonferroni procedure for multiple tests of significance. Biometrika. 1986;73:751–754. [Google Scholar]
- Tolias AS, Smirnakis SM, Augath MA, Trinath T, Logothetis NK. Motion processing in the macaque: revisited with functional magnetic resonance imaging. J. Neurosci. 2001;21:8594–8601. doi: 10.1523/JNEUROSCI.21-21-08594.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Stockhausen HM, Thiel A, Herholz K, Pietrzyk U. A convenient method for topographical localization of intracranial electrodes with MRI and a conventional radiograph. Neuroimage. 1997;5:S514. [Google Scholar]
- Whittington MA, Cunningham MO, Lebeau FE, Racca C, Traub RD. Multiple origins of the cortical gamma rhythm. Dev. Neurobiol. 2011;71:92–106. doi: 10.1002/dneu.20814. [DOI] [PubMed] [Google Scholar]
- Wieronska JM, Pilc A. Metabotropic glutamate receptors in the tripartite synapse as a target for new psychotropic drugs. Neurochem. Int. 2009;55:85–97. doi: 10.1016/j.neuint.2009.02.019. [DOI] [PubMed] [Google Scholar]
- Wu HC, Nagasawa T, Brown EC, Juhasz C, Rothermel R, Hoechstetter K, Shah A, Mittal S, Fuerst D, Sood S, Asano E. Gamma-oscillations modulated by picture naming and word reading: Intracranial recording in epileptic patients. Clin. Neurophysiol. 2011;122:1929–1942. doi: 10.1016/j.clinph.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.