Abstract
A series of carbocyclization cascades of allyl ketenimines initiated through a thermal aza-Claisen rearrangement of N-phosphoryl-N-allyl ynamides is described. Interceptions of the cationic intermediate via Meerwein-Wagner rearrangements and polyene-type cyclizations en route to fused bi- and tricyclic frameworks are featured.
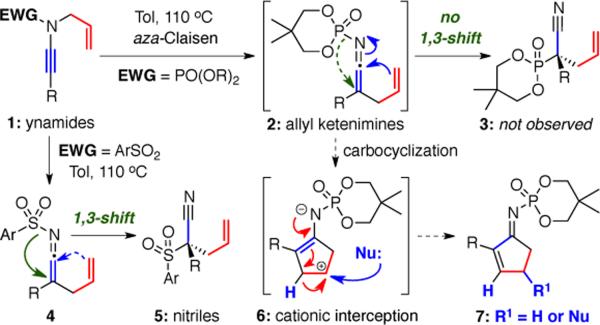
We recently reported a new class of ynamides1–3 bearing a phosphoryl group as the required electron-withdrawing component.4 Most notably, it was demonstrated that N-phosphoryl-N-allyl ynamides 1 [for EWG = PO(OR)2] could undergo a thermal 3-aza-Claisen rearrangement5,6 to generate allyl ketenimine7 intermediates 2 in situ without suffering a subsequent facile 1,3-shift observed in related N-sulfonyl systems [1➡4 when EWG = ArSO2], leading to an effective formation of structurally unique quaternary nitriles 58,9 (Scheme 1). While such a 1,3-sulfonyl shift can be of immense interest,10 it precluded us from developing a useful carbocyclization of allyl ketenimines 4.8,9 Consequently, in lieu of a 1,3-phosphoryl shift, we envisioned that carbocyclizations of 2 could take place to afford cyclopentenyl zwitter ionic intermediates 6, which would allow us to construct cyclopentenimine derivatives 7 via a 1,2-H shift, or more powerfully, nucleophilic trapping11–13 of 6. We report herein our success in intercepting these intermediates.
Scheme 1.
A Carbocyclization of Allyl Ketenimines
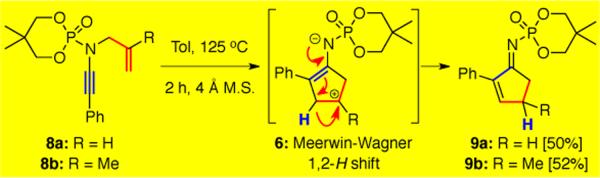
Our first success with an ynamide-initiated thermal carbocyclization was the rearrangement of ynamide 8a to α,β-unsaturated cyclopentenimine 9a in 50% yield (Scheme 2). We were fascinated with this discovery, as it implied that a 1,2-H shift through zwitter ionic intermediate 6 may be in operation. Furthermore, ynamide 8b also underwent the tandem aza-Claisen rearrangement–carbocyclization to give 9b, presumably through a tertiary carbocation intermediate.
Scheme 2.
Feasibility of the Carbocyclization
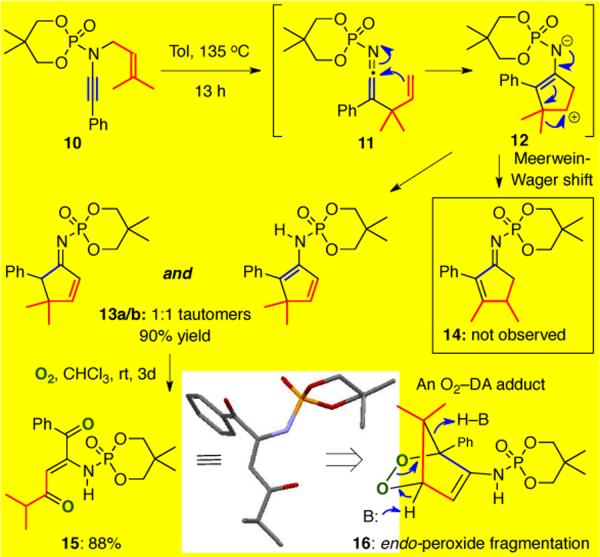
While it is also possible that deprotonation could lead to 1-amido dienes that then tautomerized to the observed cyclopentenimines, the idea of a 1,2-H shift was enticing. We therefore wondered if other Meerwein-Wagner shifts could occur following the initial aza-Claisen rearrangement and carbocyclization (Scheme 3). To explore this possibility, N-prenyl ynamide 10 was heated to 135 °C, with the hopes of demonstrating a 1,2-methyl shift through the formation cyclopentenimine 14. Unfortunately, 14 was not observed. Instead, a 1:1 tautomeric mixture of 13a and 13b was isolated in 90% yield caused by deprotonation α to the enamide instead of the desired methyl shift. Remarkably, when air was bubbled through the tautomeric mixture at rt in CHCl3, a [4+2] cycloaddition of 2-amido diene 13b with O2 ensued to first give endo-peroxide 16 that subsequently fragmented to the isolated ene-dione 15. While this could be a radical fragmentation, although the reaction conditions involved no base, it is very likely another example of a Kornblum-DeLaMare process.14,15
Scheme 3.
Attempts at a 1,2-Alkyl Shift: An Unexpected [4+2]
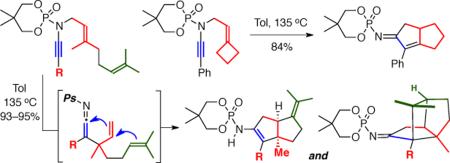
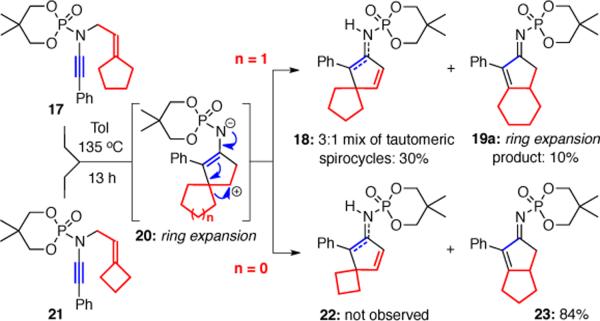
The idea of pursuing [4+2] cycloadditions with in situ generated 2-amido dienes was intriguing, however we were still very interested in intercepting the zwitter ionic intermediates through either Meerwein-Wagner rearrangements or nucleophilic trappings. To explore the former, we prepared ynamides 17 and 21 bearing a tethered methylcyclopentylidine and methylcyclobutylidine, respectively, reasoning that the added ring strain should favor ring expansion through zwitter ions 20 (Scheme 4). When 17 was heated to 135 °C, spirocycles 18 resulting from elimination dominated, however bicycle 19a was also isolated in 10% yield representing a successful ring-expansion. Moreover, ynamide 21 with increased ring strain yielded ring-expansion product 23 in 84% yield and spirocycle 22 was not observed.
Scheme 4.
Ring-Expansion Through Meerwein-Wagner Shift
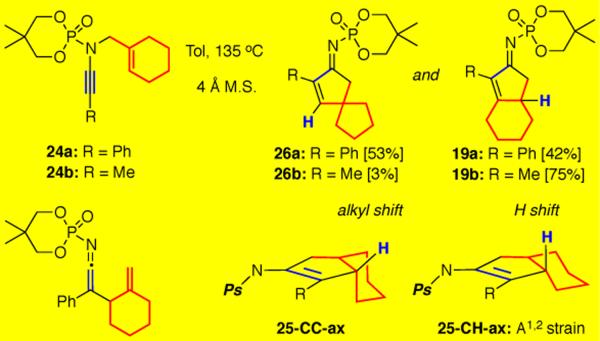
We also, rather unexpectedly, discovered a Meerwein-Wagner ring-contraction when pursuing the carbocyclization of ynamide 24a bearing a tethered methylcyclohexene (Scheme 5). In addition to the anticipated 5,6-fused bicycle 19a resulting from a 1,2-H shift, 5,5-spirocycle 26a was isolated as the major product in 53% yield. It is possible that A1,2 strain promoted the CC bond of the fused cyclohexane ring to adopt a pseudo-axial position in 25-CC-ax, thereby allowing the 1,2-alkyl shift to compete. This notion was furthered by our experimentation with methyl- terminated ynamide 24b, where the expected 1,2-H shift dominated to give 19b in 75% yield and only 3% of the spirocycle 26b.
Scheme 5.
Ring-Contraction Through Meerwein-Wagner Shift
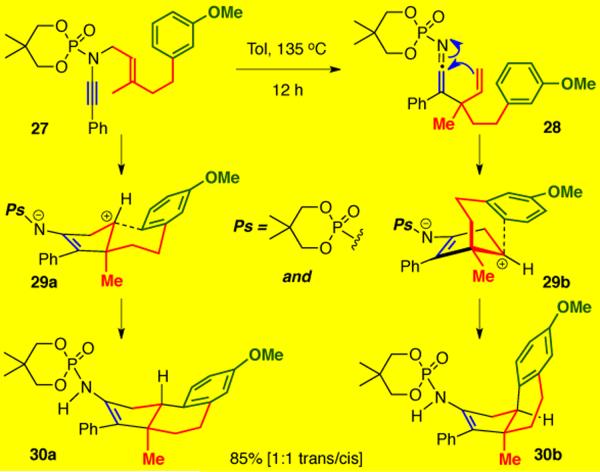
After successfully completing several examples of intercepting the zwitter ionic intermediates via Meerwein-Wagner rearrangements, we wanted to explore the possibility of using the aza-Claisen rearrangement to initiate a carbocyclization cascade with tethered carbon nucleophiles. Gratifyingly, ynamide 27 featuring a m-methoxyphenyl moiety tethered to the allyl fragment cleanly underwent the required 3-aza-Claisen rearrangement followed by carbocyclization and Friedel-Craft electrophilic aromatic substitution to give 30a and 30b as a 1:1 mixture of trans and cis isomers without any competing alkyl shifts (Scheme 6).
Scheme 6.
Friedel-Craft Trapping of Cationic Intermediate
The ability to intercept these cationic intermediates with aryl nucleophiles was exciting and propelled us into exploring other nucleophilic trappings. Inspired by the abundance of beautiful work using terpenes in cationic polyene cascades16,17 we decided to investigate the possibility of an ynamide-initiated carbocyclization cascade with terpene-derived ynamides (Scheme 7).
Scheme 7.
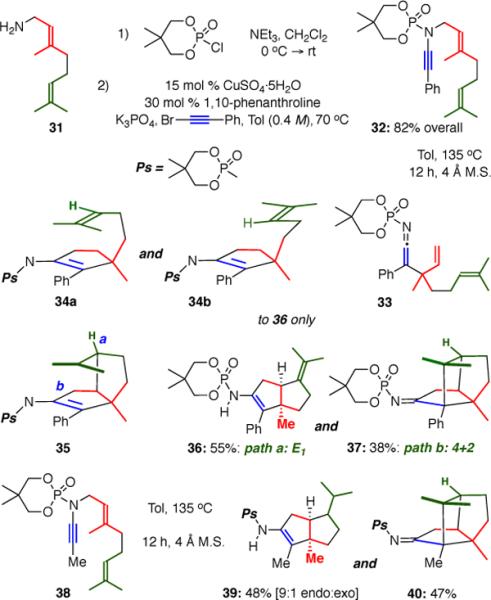
Ynamide Initiated Cationic Polyene Cascade
Starting from commercially available geranylamine 31, a simple two-step protocol involving phosphorylation and Cu-catalyzed amidative cross-coupling gave ynamide 32 in 82% overall yield. When 32 was heated to 135 °C for 12 hours, 5,5-cis-fused bicycle 36 bearing an exocyclic olefin was isolated in 55% yield as a single diastereomer. Of note, the cis-fused bicyclic core in 36 is prominent in triquinane18 natural products. In addition to 36, tricycle 37 featuring four contiguous stereocenters and three all-carbon quaternary centers was isolated in 38% yield as a single diastereomer. The geometry of both products was determined by NOE analysis.
The divergence in reaction pathways leading to the bi- and tricylic products was interesting. As one might imagine, after the initial carbocyclization, the second olefin may add to the carbocation through the facial approach shown in either 34a or 34b, which followed by elimination would give 36. Mechanistically more intriguing is the formal [4+2] cycloaddition to afford 37, which must arise through the olefin approach shown in 34a, followed by enamide addition to the resulting tertiary carbocation.
The described carbocyclization cascade worked equally well with methyl-terminated ynamide 38. After 12 hours, 5,5-cis-fused bicycle 39 was isolated as a 9:1 mixture of endo and exo olefin isomers, as well as tricycle 40 as a single diastereomer.
We have showcased here a tandem aza-Claisen–carbocyclization for the synthesis of α,β-unsaturated cyclopentenimines from N-phosphoryl-N-allyl ynamides. Furthermore, we have demonstrated the ability to intercept the zwitter ionic intermediates through Meerwein-Wagner ring expansions and contractions, as well as with tethered carbon-nucleophiles to afford bi- and tricyclic scaffolds. Applications of these carbocyclization cascades in total synthesis are currently underway.
Supplementary Material
Acknowledgement
We thank NIH [GM066055] for funding. KAD thanks the American Chemical Society for a Division of Medical Chemistry Predoctoral Fellowship. We thank Dr. Vic Young and Mr. Gregory T. Rohde of the University of Minnesota for providing X-ray structural analysis. The authors would also like to thank one of the referees for providing valuable mechanistic suggestions.
Footnotes
Supporting Information Available: Experimental procedures as well as NMR spectra, and characterizations are available for all new compounds and free of charge via Internet http://pubs.acs.org.
References
- 1.(a) Evano G, Coste A, Jouvin K. Angew. Chem. Int. Ed. 2010;49:2840. doi: 10.1002/anie.200905817. [DOI] [PubMed] [Google Scholar]; (b) DeKorver KA, Li H, Lohse AG, Hayashi R, Lu Z, Zhang Y, Hsung RP. Chem. Rev. 2010;110:5064. doi: 10.1021/cr100003s. [DOI] [PMC free article] [PubMed] [Google Scholar]; For current leading reviews on ynamides, see
- 2.(a) Tracey MR, Hsung RP, Antoline JA, Kurtz KCM, Shen L, Slafer BW, Zhang Y. In: Science of Synthesis, Houben-Weyl Methods of Molecular Transformations. Weinreb Steve M., editor. Chapter 21.4. Georg Thieme Verlag KG; Stuttgart, Germany: 2005. [Google Scholar]; (b) Mulder JA, Kurtz KCM, Hsung RP. Synlett. 2003:1379. [Google Scholar]; For leading reviews on the synthesis of ynamides, see:
- 3.(a) Lu Z, Kong W, Yuan Z, Zhao X, Zhu G. J. Org. Chem. 2011;76:8524. doi: 10.1021/jo2015278. [DOI] [PubMed] [Google Scholar]; (b) Davies PW, Cremonesi A, Dumitrescu L. Angew Chem., Int. Ed. 2011;50:8931. doi: 10.1002/anie.201103563. [DOI] [PubMed] [Google Scholar]; (c) Greenaway RL, Campbell CD, Holton OT, Russell CA, Anderson EA. Chem.–Eur. J. 2011;17:14366. doi: 10.1002/chem.201102880. [DOI] [PubMed] [Google Scholar]; (d) Aikawa K, Hioki Y, Shimizu N, Mikami K. J. Am. Chem. Soc. 2011;133:20092. doi: 10.1021/ja2085299. [DOI] [PubMed] [Google Scholar]; (e) Schotes C, Mezzetti A. J. Org. Chem. 2011;76:5862. doi: 10.1021/jo200776c. [DOI] [PubMed] [Google Scholar]; (f) Dassonneville B, Witulski B, Detert H. Eur. J. Org. Chem. 2011:2836. [Google Scholar]; (g) Nissen F, Detert H. Eur. J. Org. Chem. 2011:2845. [Google Scholar]; (h) Mukherjee A, Dateer RB, Chaudhuri R, Bhunia S, Narayan Karad S, Liu R-S. J. Am. Chem. Soc. 2011;133:15372. doi: 10.1021/ja208150d. [DOI] [PubMed] [Google Scholar]; (i) Kitamura T, Hasan Morshed M, Tsukada S, Miyazaki Y, Iguchi N, Inoue D. J. Org. Chem. 2011;76:8117. doi: 10.1021/jo2015467. [DOI] [PubMed] [Google Scholar]; (j) Vasu D, Hung H-H, Bhunia S, Gawade SA, Das A, Liu R-S. Angew. Chem., Int. Ed. 2011;50:6911. doi: 10.1002/anie.201102581. [DOI] [PubMed] [Google Scholar]; (k) Smith DL, Goundry WRF, Lam HW. Chem. Commun. 2012;48:1505. doi: 10.1039/c1cc13595c. [DOI] [PubMed] [Google Scholar]; (l) Dateer RB, Shaibu BS, Liu R-S. Angew Chem., Int. Ed. 2012;51:113. doi: 10.1002/anie.201105921. [DOI] [PubMed] [Google Scholar]; (m) Beveridge RE, Batey RA. Org. Lett. 2012;14:540. doi: 10.1021/ol2031608. [DOI] [PubMed] [Google Scholar]; (n) Cao J, Xu Y, Kong Y, Cui Y, Hu Z, Wang G, Deng Y, Lai G. Org. Lett. 2012;14:38. doi: 10.1021/ol2027762. [DOI] [PubMed] [Google Scholar]; (o) Laouiti A, Rammah MM, Rammah MB, Marrot J, Couty F, Evano G. Org. Lett. 2012;14:6. doi: 10.1021/ol2032152. [DOI] [PubMed] [Google Scholar]; (p) Jouvin K, Heimburger J, Evano G. Chem. Sci. 2012;3:756. [Google Scholar]; For ynamide papers published since May 2011, see:
- 4.DeKorver KA, Walton MC, North TD, Hsung RP. Org. Lett. 2011;13:4862. doi: 10.1021/ol201947b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Majumdar KC, Bhayyacharyya T, Chattopadhyay B, Nandi RK. Synthesis. 2009:2117. [Google Scholar]; (b) Nubbemeyer U. Top Curr. Chem. 2005;244:149. [Google Scholar]; For leading reviews on aza-Claisen rearrangements, see:
- 6.(a) DeKorver KA, North TD, Hsung RP. Synlett. 2010:2397. doi: 10.1055/s-0030-1258544. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhang Y, DeKorver KA, Lohse AG, Zhang Y-S, Huang J, Hsung RP. Org. Lett. 2009;11:899. doi: 10.1021/ol802844z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Krow GR. Angew. Chem., Int. Ed. Engl. 1971;10:435. [Google Scholar]; (b) Gambaryan NP. Usp. Khim. 1976;45:1251. [Google Scholar]; (c) Dondoni A. Heterocycles. 1980;14:1547. [Google Scholar]; (d) Barker MW, McHenry WE, Patai S. In: The Chemistry of Ketenes, Allenes and Related Compounds. Wiley-Interscience, editor. Chichester, UK: 1980. pp. 701–720. Part 2. [Google Scholar]; (e) Alajarin M, Vidal A, Tovar F. Targets Heterocycl. Syst. 2000;4:293. [Google Scholar]; For reviews on the chemistry of ketenimines, see:
- 8.DeKorver KA, Johnson WL, Zhang Y, Hsung RP, Dai H, Deng J, Lohse AG, Zhang Y-S. J. Org. Chem. 2011;76:5092. doi: 10.1021/jo200780x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeKorver KA, Hsung RP, Lohse AG, Zhang Y. Org. Lett. 2010;12:1840. doi: 10.1021/ol100446p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Fleming FF, Liu W. Eur. J. Org. Chem. 2009:699. [Google Scholar]; (b) Fleming FF, Wei G, Steward OW. J. Org. Chem. 2008;73:3674. doi: 10.1021/jo8001062. [DOI] [PubMed] [Google Scholar]; (c) Mermerian AH, Fu GC. Angew. Chem. Int. Ed. 2005;44:949. doi: 10.1002/anie.200461886. [DOI] [PubMed] [Google Scholar]; For leading references on synthetic applications of nitriles, see:
- 11.(a) Sosa JR, Tudjarian AA, Minehan TG. Org. Lett. 2008;10:5091. doi: 10.1021/ol802147h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tudjarian AA, Minehan TG. J. Org. Chem. 2011;76:3576. doi: 10.1021/jo200271s. [DOI] [PMC free article] [PubMed] [Google Scholar]; For related carbocyclizations of ynol ethers, see:
- 12.Hanessian S, Tremblay M, Marzi M, Del Valle JR. J. Org. Chem. 2005;70:5070. doi: 10.1021/jo050326w. [DOI] [PubMed] [Google Scholar]
- 13.(a) Giese S, Kastrup L, Stiens D, West FG. Angew. Chem., Int. Ed. 2000;39:1970. doi: 10.1002/1521-3773(20000602)39:11<1970::aid-anie1970>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]; (b) Giese S, West FG. Tetrahedron. 2000;56:10221. [Google Scholar]; (c) Wang Y, Schill BD, Arif AM, West FG. Org. Lett. 2003;5:2747. doi: 10.1021/ol034985b. [DOI] [PubMed] [Google Scholar]; (d) Huang J, Leboeuf D, Frontier AJ. J. Am. Chem. Soc. 2011;133:6307. doi: 10.1021/ja111504w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Grant TN, Rieder CJ, West FG. Chem. Commun. 2009:5676. doi: 10.1039/b908515g. [DOI] [PubMed] [Google Scholar]; For related examples of intercepted Nazarov cyclizations, see:
- 14.Kornblum N, DeLaMare HE. J. Am. Chem. Soc. 1951;73:880. [Google Scholar]
- 15.(a) Tang Y, Cole KP, Buchanan GS, Li G, Hsung RP. Org. Lett. 2009;11:1591. doi: 10.1021/ol900237e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Buchanan GS, Cole KP, Tang Y, Hsung RP. J. Org. Chem. 2011;76:7027. doi: 10.1021/jo200936r. [DOI] [PMC free article] [PubMed] [Google Scholar]; For recent examples, see:
- 16.(a) Johnson WS. Acc. Chem. Res. 1968;1:1. [Google Scholar]; (b) Johnson WS. Angew. Chem. Int. Ed. 1976;15:9. doi: 10.1002/anie.197600091. [DOI] [PubMed] [Google Scholar]; (c) Johnson WS, Telfer SJ, Cheng S, Schubert U. J. Am. Chem. Soc. 1987;109:2517. [Google Scholar]; For reviews on polyene cyclizations, see:
- 17.(a) Stork G, Burgstahler AW. J. Am. Chem. Soc. 1955;77:5068. [Google Scholar]; (b) Eschenmoser A, Ruzicka L, Jeger O, Arigoni D. Helv. Chim. Acta. 1955;38:1890. [Google Scholar]; (c) Stadler PA, Eschenmoser A, Schinz H, Stork G. Helv. Chim. Acta. 1957;40:2191. [Google Scholar]; Also see:
- 18.Mehta G, Srikrishna A. Chem. Rev. 1997;97:671. doi: 10.1021/cr9403650. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.