Abstract
Bidirectional integration between sensory stimuli and contextual framing is fundamental to action control. Stimuli may entail context-dependent actions, while temporal or spatial characteristics of a stimulus train may establish a contextual framework for upcoming stimuli. Here we aimed at identifying core areas for stimulus–context integration and delineated their functional connectivity (FC) using meta-analytic connectivity modeling (MACM) and analysis of resting-state networks. In a multi-study conjunction, consistently increased activity under higher demands on stimulus–context integration was predominantly found in the right temporo-parietal junction (TPJ), which represented the largest cluster of overlap and was thus used as the seed for the functional connectivity analyses. The conjunction between task-dependent (MACM) and task-free (resting state) FC of the right TPJ revealed a shared network comprising bilaterally inferior parietal and frontal cortices, anterior insula, premotor cortex, putamen and cerebellum, i.e., a ‘ventral’ action / attention network. Stronger task-dependent (vs. task-free) connectivity was observed with the pre-SMA, dorsal pre-motor cortex, intraparietal sulcus, basal ganglia and primary sensory-motor cortex, while stronger resting-state (vs. task-dependent) connectivity was found with the dorsolateral prefrontal and medial parietal cortex.
Our data provide strong evidence that the right TPJ may represent a key region for the integration of sensory stimuli and contextual frames in action control. Task-dependent associations with regions related to stimulus processing and motor responses indicate that the right TPJ may integrate ‘collaterals’ of sensory processing and apply (ensuing) contextual frames, most likely via modulation of preparatory loops. Given the pattern of resting-state connectivity, internal states and goal representations may provide the substrates for the contextual integration within the TPJ in the absence of a specific task.
Keywords: fMRI, resting state, meta-analysis, connectivity modelling, right temporo-parietal junction
Introduction
Sensorimotor control is an integral part of our daily life and the essential prerequisite to interact with one’s environment, i.e. the internal and external milieu. Thus, the convergence and integration of both intero- and exteroceptive stimuli in the human brain is fundamental to allow for a comprehensive environmental picture (Berlucchi & Aglioti, 2010). In most functional neuroimaging experiments the selection of the adequate behavioral response is based on only a limited number of stimuli, i.e. the brain has to evaluate which stimuli are crucial to meet the task (Bays et al., 2010). This subset of bottom-up (sensory) input is subsequently weighted against top-down information such as contextual rules and goals. Fundamentally, top-down signals represent feedback from ‘higher’ (usually multimodal) brain regions to unimodal sensory or motor areas. Anatomically, such top-down feedback is implemented by diffuse connectivity into (primarily) dendritic terminals in cortical layers II-III, whereas bottom-up (feed-forward) connections primarily terminate in layer IV of a more circumscribed patch of the cortex. The result of this complex procedure consists of highly integrated data and constitutes the basis upon which the respective movements are planned. In the following, the term ‘contextual integration’ is used to denote the top-down modulation of sensorimotor processing by context-specific a-priori information. Context is here defined as any information affecting actions that is not provided by the given response stimulus itself but by the environment, ranging from explicit instructions about stimulus–response mappings to implicit expectations extracted from regularities in the stimulation sequence. The first aim of our study was to identify regions that are consistently (i.e. across different studies) activated by context-dependent sensorimotor control.
So far, we have only considered task-induced integration processes. However, the human brain is assumed to operate along a continuum between task-related performance and ‘mental rest’, i.e. ‘unconstrained’ cognition (Schilbach et al., 2008). This presumption is in line with several studies (Smith et al., 2009; Fox and Raichle, 2007) demonstrating that ‘physiological rest’ does not equate ‘mental rest’. Rather, it has been hypothesized (Schilbach et al., 2008) that the absence of an externally structured task entails a re-allocation of resources towards internally oriented, i.e. ‘conceptual’ (Binder et al., 1999), operations resulting in ‘mind-wandering’ (cf. Smallwood & Schooler, 2006). Thus, the second aim of our study was to assess the functional connectivity of the above-mentioned areas in both task-dependent and task-independent mental states. The third aim was to test for commonalities and differences in the functional connectivity pattern of these two fundamental states of brain function.
To date, a large number of functional neuroimaging studies have adopted task-based experimental designs to investigate the neural correlates of stimulus-response associations in humans (Egner, 2007) and non-human primates (Connolly et al., 2009). Despite the differences in experimental designs several studies have provided consistent evidence for an implementation of these processes in a bilateral fronto-parietal network. In line with data from single-cell recordings in non-human primates (Gottlieb & Snyder, 2010), the inferior parietal lobe (IPL) and adjacent intraparietal sulcus are conceptualized to evaluate and integrate incoming sensory input from different modalities. In this context, Spence & Driver (2004) claimed that the posterior parietal cortex plays a critical role in mediating the integration of spatial aspects of multimodal stimuli (e.g. visual, auditory or tactile) and their transformation into action-based representations. This is well in line with the presumption of IPL/IPS acting as a heteromodal integrative ‘hub’ committed to multi-sensory processing (Gottlieb, 2007, Toni et al., 2002). Such multimodal integration processes, however, may not be restricted to the posterior parietal cortex. Rather, there is evidence that multi-modal integration is also supported by regions within the (pre-)frontal and temporal cortex (Calvert et al., 2004, Driver & Noesselt, 2008). In particular, contextual information from the (pre-)frontal cortex enriches these integrative processes and permits a bidirectional coupling between stimulus and contextual framework (Koechlin & Jubault, 2006). Moreover, the function of (pre-)frontal areas in the system of sensorimotor control also comprises the exertion of ‘executive control’ on the (pre-) motor system (Koechlin & Summerfield, 2007). In particular, these regions were found to be involved in rule-based adjustment of motor plans, movement timing and action monitoring. Finally, the (pre-)motor areas are thought to select, initiate and execute the adequate motor program based on the highly integrated information from the parietal and (pre-)frontal cortex (Picard & Strick, 1996, Rizzolatti et al., 1998). Sensorimotor control thus depends on the integration of cognitive aspects with the monitoring of the internal and external milieu and the selection of appropriate responses based on these information.
In this context, the question arises as to which regions are consistently activated during the implementation of sensorimotor control, i.e. the association of a given stimulus with an arbitrary (instructed) response. Three recently published functional neuroimaging studies (Jakobs et al., 2009, Cieslik et al., 2010, Eickhoff et al., 2011) applied variations of a manual two-choice reaction time task with graduated levels of difficulty in stimulus-response mapping. Testing for neural effects of increasing demands on stimulus-response association in each study revealed a similar bilateral, though right-hemispherically dominant, fronto-parietal network. In order to statistically validate this prima facie evidence, i.e. to detect regions featuring a significant overlap across the abovementioned studies, we applied an image-based meta-analysis (IBMA) technique to investigate the multi-study conjunction of results. In this context, regions consistently activated across studies are assumed to implement higher-order processes in the cascade of stimulusresponse association.
However, even the common evidence provided by three studies might still reflect design-specific effects to a degree that precludes broad generalizations about this fundamental network. Thus, in the second part of the current study, we used meta-analytic connectivity modeling (MACM) to delineate the functional connectivity (FC) pattern of higher-order sensorimotor regions (i.e. consistently activated clusters observed in the IBMA) in the presence of an externally structured task. The basic idea behind this approach is to assess which brain regions are co-activated above chance with particular seed regions in functional neuroimaging experiments. Here, we used the BrainMap database (Laird et al., 2009a; www.brainmap.org) to identify co-activations with our seed regions (i.e. the results of the above-mentioned IBMA) across all studies listed in this database and subsequently performed an ALE (activation likelihood estimation) meta-analysis on these studies (Laird et al., 2009a; Eickhoff et al., 2009).
As mentioned above, regions participating in stimulus–context integration are also engaged in task-free brain states. Thus, it may be speculated that a shared procedure is based upon a subset of regions, which are activated irrespective of the current mental state. To test this hypothesis, we investigated ‘resting-state’ FC using functional imaging data from 100 healthy volunteers. The time-series of each seed region was cross-correlated with the time-series of all other gray-matter voxels in the brain. Consistent functional coupling across mental states (i.e. overlap of regions co-activated across studies with our seed and regions with significant intrinsic connectivity to our seed) would indicate that the seed and target regions participate in very much the same networks during task-dependent stimulus–context integration and task-free, unstructured processing. In contrast, divergent results would delineate networks that depend on the mental state and thus allow for a differentiation of internally and externally driven FC networks (Eickhoff & Grefkes, 2011).
Material & Methods
I. Image-based meta-analysis
We performed an IBMA by multi-study conjunction over three recently published fMRI studies (Jakobs et al., 2009; Cieslik et al., 2010; Eickhoff et al., 2011). Regions consistently activated by higher demands on sensorimotor integration were identified by first computing the respective contrasts in each study, thresholded at p<0.05 (cluster-level FWE-corrected; cluster-forming threshold at voxel-level p<0.001; Worsley et al., 1996). In particular, the minimal number of voxels required to meet the threshold criterion ranged from 305 to 315 voxels [voxel size 1.5 mm3 isotropic; Jakobs et al., 2009: 308 voxels; Cieslik et al., 2010: 305 voxels; Eickhoff et al., 2011: 315 voxels]. Hence, the comparability across studies was ensured by enclosing similar numbers of subjects and applying the same pre-processing algorithms. Regions consistently engaged (across studies) by increasing demands for stimulus–context integration in sensorimotor control were then identified by means of conjunction analysis. Subsequently, all findings were anatomically localized using version 1.5 of the SPM Anatomy toolbox (www.fz -juelich.de/ime/spm_anatomy_toolbox, Eickhoff et al., 2005, 2006, 2007).
Each of the three included studies applied a manual reaction-time task requiring participants to respond as fast and correctly as possible to visually presented stimuli by pressing a button with either their left or right index finger.
In the first study (Cieslik et al., 2010), 24 participants were instructed to react to lateralized stimuli (red dots) briefly presented (200 ms) in a randomized order. Before each task block, subjects were instructed to respond with either the corresponding (spatially congruent response) or the contralateral (spatially incongruent response) index finger. Activation related to increased integration demands was then assessed by contrasting incongruent with congruent trials independently of the stimulus- or response side.
In the second study (Jakobs et al., 2009), 26 participants responded to centrally presented visual stimuli (arrows), which were either pointing uniformly to one side or in a randomized order to either side (random hands condition; 50% chance for each side) with the corresponding index finger. Increasing demands on stimulus–context integration were delineated by contrasting random hands with unilateral conditions.
In the third study (Eickhoff et al., 2011) left- or right-pointing arrows were centrally presented to 20 participants. This time, however, arrow direction was non-uniformly distributed, with 80% pointing to one side. This laterality bias was randomly varied between blocks of trials. Moreover, in some blocks this bias was covertly reversed in the middle of the block. Increased integration demands were assessed by testing for activity that was parametrically related to the acquisition and adaptation of response biases in line with the probabilistic structure of the stimuli.
The respective contrasts reflecting increased demands for stimulus–context integration in sensorimotor control were thresholded at a cluster-level FWE-corrected p<0.05. The ensuing activation maps where then subjected to a conjunction analysis, i.e. we performed the conjunction against the (conservative) conjunction null hypothesis using the minimum statistic (Nichols et al., 2005). In practice, this was implemented by first applying a voxel-level cluster-forming threshold to all of the three analyses. Subsequently, each of the three excursion sets was filtered for cluster extent to threshold at cluster-level FWE-corrected p<0.05 (cluster-forming threshold at voxel-level p<0.001, i.e., T>3.09). Finally, we computed the intersection between the three thresholded and filtered SPM{T}-maps. This procedure exactly conforms to the conjunction-null minimum statistics, as the intersection only becomes non-zero (and hence significant) if each of the three individual analyses was significant. This IBMA provided four regions of overlapping activation. The right TPJ showed a cluster size of 104 voxels. Additionally, we observed three smaller clusters (right IPS, bilateral dPMC) with an average cluster size just over 20 voxels. Given this dramatic difference in cluster extent, we decided to exclude these considerably smaller regions and focus our analysis on the predominant finding, which survived conservative thresholding. Hence, the only region of spatially extended overlap between significant activation in all three individual analyses (i.e. the right TPJ) represented the seed for the subsequent connectivity modeling.
II. Task-based FC: Meta-analytic connectivity modeling
FC of the seed(s) during the performance of structured tasks was defined by delineating the co-activation pattern of the seed based on the activations reported in published functional imaging results. The concept behind this approach is predicated on the notion that FC is reflected in the correlation of activity in spatially distinct brain regions. That is, regions that are functionally connected should co-activate above chance in functional neuroimaging studies and vice versa. In this context, it should be noted that there are major conceptual differences between anatomical, functional and effective connectivity: (1) Anatomical connectivity denotes the presence of fiber connections linking two areas in the brain, i.e. the existence of a structural connection between their neurons. In contrast, (2) FC is correlative in nature, i.e. solely based on the likelihood of observing activation in a target region, given that activation is present within the seed area. In meta-analytic connectivity modeling (MACM), as performed in the current study, the unit of observation is not a specific point in an acquired time series but a particular neuroimaging experiment. MACM thus extends the scale on which FC is evaluated beyond data points in a time series (single study) to a whole set of neuroimaging experiments (MACM across studies). Here, FC is expressed as coherent activation across experiments and should delineate networks that are conjointly recruited by a broad range of tasks. Finally, (3) effective connectivity is defined as the causal influence one area exerts over another and may be tested with approaches such as dynamic causal modeling or structural equation modeling.
Here, analysis of task-based functional connectivity was performed by MACM using the BrainMap database (Laird et al., 2009a, www.brainmap.org). This database contained, at the time of analysis, the location of reported activation foci and associated meta-data of approximately 10,000 neuroimaging experiments. Of these, only fMRI studies that reported functional mapping data from healthy participants were considered. Studies investigating age, gender, disease, or drug effects were excluded. No further constraints (e.g., on acquisition or analysis details, experimental design, or stimulation procedures) were applied. Comparability with respect to the location of significant activation was ensured given the high standardization in the publication of neuroimaging data, i.e. the ubiquitous adherence to standard coordinate systems, such that all experiments contained in the database refer to activation coordinates within the same standard space. Using this broad pool of neuroimaging results, MACM can then be used to test for associations between activation probabilities of different areas. Importantly, this inference is performed independently of the paradigms used or other experimental factors, but rather is solely based on the likelihood of observing activation in a target region given that activation is present within the seed area. This completely data-driven approach thus avoids selection biases that may result from adhering to current cognitive ontologies, which might not always overlap with the organizational modes of brain function.
In practice, MACM was performed in two steps: First, we identified all experiments in the BrainMap database that featured at least one focus of activation within the volume of the respective seed region (i.e. the cluster obtained from the IBMA). Second, quantitative meta-analysis (see below) was employed to test for the across-study convergence of the activity foci reported in these experiments. As all experiments entering this analysis were selected by the fact that they feature activation in the seed, highest convergence will be observed in the seed region. Significant convergence of other activity foci, however, indicates consistent co-activation, i.e., task-based FC with the seed. Thus, it has to be noted that the functional connectivity pattern as observed in the MACM analysis is not specific to a distinct task or paradigm but rather reflects regional coupling that is present across a broad range of different tasks and paradigms.
For the meta-analysis in the second step, the revised version of the activation likelihood estimation (ALE) approach was used (Laird et al., 2009a; Eickhoff et al., 2009). This algorithm aims at identifying areas where the convergence of activations across different experiments is higher than expected under conditions of random spatial associations between them. The key idea behind ALE is to treat reported activation foci not as points but centers for 3-D Gaussian probability distributions reflecting the associated spatial uncertainty. For each experiment included, the probability distributions of all reported foci are combined into a modeled activation (MA) map (Turkeltaub et al., 2011). Taking the union across these MA maps for all experiments yielded voxelwise ALE scores describing the convergence of results at each particular location of the brain. To distinguish ‘true’ convergence across studies from random convergence (i.e. noise), ALE scores are compared to an empirical null distribution reflecting a random spatial association between experiments (Eickhoff et al., 2011). Hereby, a random-effects inference is invoked, focusing on the above-chance convergence between studies, not the clustering of foci within a particular study. The p-value of an observed ALE score is given by the proportion of equal or higher values obtained under the null distribution. The ALE maps, reflecting the across-study convergence of co-activations with the seed region, were thresholded at cluster level–corrected p<0.05 (cluster-forming threshold: p<0.001 at voxel level) and converted to Z-scores for visualization.
III. Task-independent connectivity: Resting-state correlations
Resting-state fMRI images were acquired in 100 healthy volunteers (50 female, mean age 45.2 years) without any record of neurological or psychiatric disorders. All subjects gave written informed consent to the study protocol, which had been approved by the local ethics committee of the University of Bonn. Before the imaging session, subjects were instructed to keep their eyes closed and just let their mind wander without thinking of anything in particular but not to fall asleep (which was confirmed in post-scan debriefing). For each subject 300 resting state EPI images were acquired using blood-oxygen-level-dependent (BOLD) contrast [gradient-echo EPI pulse sequence, TR = 2.2s, TE = 30ms, flip angle = 90°, in plane resolution = 3.1 × 3.1mm2, 36 axial slices (3.1mm thickness) covering the entire brain.
The first four scans served as dummy images allowing for magnetic field saturation and were discarded prior to further processing using SPM8 (www.fil.ion.ucl.ac.uk/spm). The EPI images were first corrected for head movement by affine registration using a two-pass procedure in which the images were first aligned to the initial volumes and subsequently to the mean of all volumes after the first pass. The mean EPI image for each subject was then spatially normalized to the MNI single subject template (Holmes et al., 1998) using the ‘unified segmentation’ approach (Ashburner & Friston, 2005) and the ensuing deformation was applied to the individual EPI volumes. Finally, images were smoothed by a 5-mm FWHM Gaussian kernel to improve signal-to-noise ratio and compensate for residual anatomical variations.
The time-series data of each voxel were processed as follows (cf. Fox et al., 2009; Weissenbacher et al., 2009): In order to reduce spurious correlations, variance that could be explained by the following nuisance variables was removed: (i) The six motion parameters derived from the image realignment (ii) the first derivative of the realignment parameters (iii) mean grey matter, white matter and CSF signal per time-point as obtained by averaging across voxels attributed to the respective tissue class in the SPM 8 segmentation and (iv) coherent signal changes across the whole brain as reflected by the first five components of a principal component analysis (PCA) decomposition of the whole-brain time-series. All of these nuisance variables entered the model as first-order and - except for the PCA components - also as second-order terms. We note that the above approach, in particular the removal of variance related to the most dominant signal components, may remove some signal of interest but should increase specificity of the ensuing results (Bellec et al., 2006; Fox and Raichle, 2007). Data was then band-pass filtered preserving frequencies between 0.01 and 0.08Hz, since meaningful resting-state correlations will predominantly be found in these frequencies given that the BOLD-response acts as a low-pass filter (Biswal et al., 1995; Fox and Raichle, 2007; Greicius et al., 2003).
As for the MACM analysis, seed regions of interest were provided by the clusters obtained from the IBMA. Time-courses were extracted for all voxels within the particular cluster that were located in the grey matter of the individual subject as indicated by a segmentation of the individual EPI image (Ashburner and Friston, 2005). Of the 104 voxels in the right TPJ cluster, the number of voxels more likely representing grey matter than any other tissue class was on average (across subjects) 90.7 (SD=10.6; range: 71-104). The time-course of the seed region was then expressed as the first eigenvariate of the individual voxels. Linear (Pearson) correlation coefficients between the time series of the seed regions (clusters obtained from the IBMA) and all other grey matter voxel in the brain were computed to quantify resting-state FC. These voxel-wise correlation coefficients were then transformed into Fisher‘s Z-scores and tested for consistency by a one-sample T-test across subjects. The results of this random-effects analysis were then thresholded at a cluster-level corrected threshold of p<0.05 (cluster-forming threshold: p<0.001 at voxel-level).
IV. Conjunction and differences between MACM & resting-state FC
In order to delineate areas showing task-dependent and task-independent FC with the seed region(s) obtained from the IBMA, we performed a conjunction analysis between the MACM and resting state analyses using the strict minimum statistics (Nichols et al., 2005). That is, for each seed region, we identified those voxels that showed significant FC with this seed in the analysis of interactions in the task-dependent as well as in the analysis of interactions in the task-independent state. In practice such consistent connectivity was delineated by computing the intersection of the (cluster-level FWE-corrected) connectivity maps from the two analyses detailed above.
Comparison between task-dependent and task-independent FC was performed by computing the voxel-wise contrast between the Z-scores obtained from the MACM and resting-state analyses. Difference Z-scores were deemed significant if they corresponded to a p<0.001. Finally, results from the difference analysis were masked with the respective main effect, that is, voxels showing stronger connectivity in MACM vs. resting-state analyses were only retained if they indeed showed a significant task-driven connectivity with the seed (as revealed by the MACM analysis).
Results
I. Image-based meta-analysis
In each of the three included studies, increased demands on sensorimotor control recruited a widespread bilateral though right-dominant fronto-parietal network (Fig. 1A-C). The IBMA then indicated four regions of overlapping activation. Among these, a cluster in the right TPJ represented the most extensive region of overlap with a cluster size of 104 voxels. Additionally, we observed three considerably smaller clusters (right IPS, bilateral dPMC) with an average cluster size just over 20 voxels. Given this clear difference in cluster extent, we decided to focus the subsequent connectivity analyses on the predominant cluster found in the right TPJ (Fig. 1D). Thus, the right TPJ (area PFm, Caspers et al., 2006, 2008; MNI peak coordinates: 58/-46/27 [cluster-size 104 voxels/ 351mm3], see supplementary Fig. S1) was used as the sole seed region for the analysis of task-depended and task-independent FC via MACM and resting-state correlations.
Figure 1.
In three recently published neuroimaging studies (A: Eickhoff et al., 2011, B: Jakobs et al., 2009, C: Cieslik et al., 2010) we applied variations of a manual two-choice reaction time task to investigate neural correlates of increasing demands on sensorimotor control. In each of these studies, we observed activation of a similar bilateral, though right-hemispheric dominant fronto-parietal network. Significant activations are projected onto rendered surfaces of the MNI single-subject template brain. The subsequent image-based meta-analysis revealed a single focus of convergent activation in the right temporo-parietal junction (D) which was thus used as seed region for the analysis of functional connectivity.
II. FC analysis by coordinate-based meta-analysis (MACM)
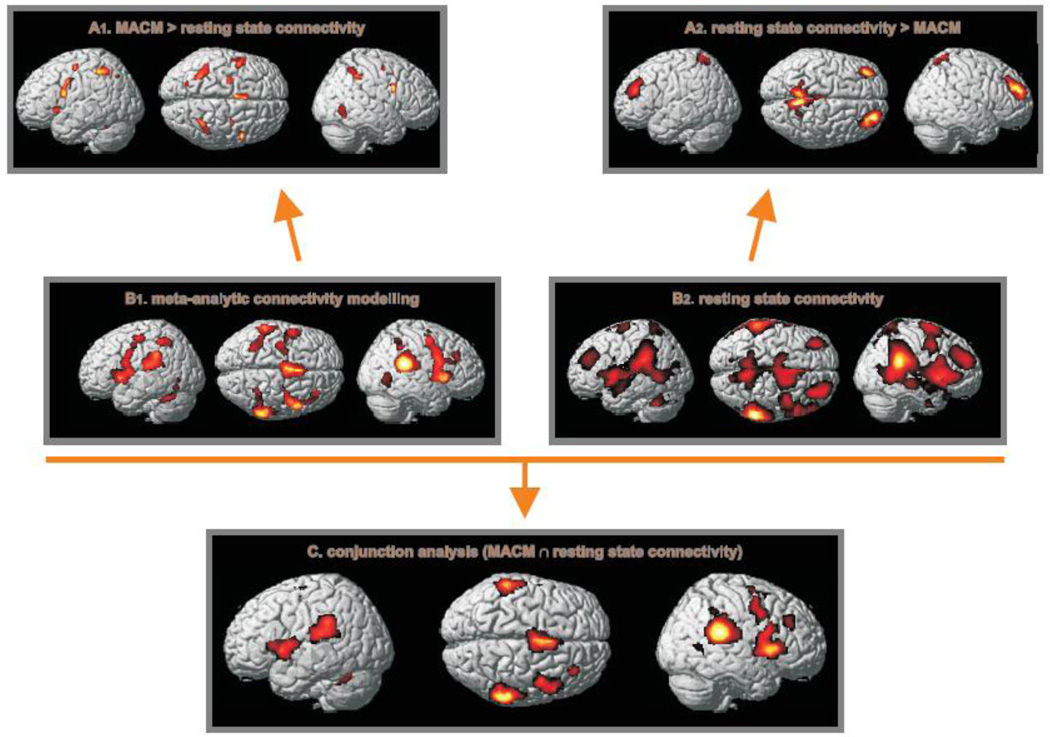
In addition to the ‘shared’ network as described below, task-dependent FC (Fig. 2B1), as revealed by MACM, involved the bilateral pre-supplementary motor area (pre-SMA, area 6, Geyer, 2004), ventral and dorsal premotor cortex (vPMC/ dMPC, area 6, Geyer, 2004) and the thalamus. Furthermore, left-hemispheric putamen, ventral premotor cortex (vPMC, area 6, Geyer, 2004), M1 (Area 4p; Geyer et al., 1996) and S1 (Areas 3b, 3a, 2; Geyer et al., 1999, 2000; Grefkes et al., 2001) as well as right-hemispheric pallidum and caudate nucleus revealed significant co-activation. An co-activations were also found in the region of the right areas 44/45 (Amunts et al., 1999) and bilateral (anterior [hIP2]) intraparietal sulcus (Choi et al., 2006) extending into the superior (area 7PC, Scheperjans et al., 2008a,b) and inferior (Area PFcm, Caspers et al., 2006, 2008) parietal lobe. When assessing the paradigm classes of those experiments that featured activation in the right TPJ seed and hence contributed to the MACM analysis, we observed that several different kinds of paradigms/tasks were associated with right TPJ activation (see supplementary Fig. S2). A strong predominance of any particular kind of task, however, was not found, with strong contributions of somatosensory, visual, cognitive, and motor tasks.
Figure 2.
The conjunction analysis (C) across task-dependent connectivity (MACM, B1) and that obtained for the task-free state (B2) revealed a shared network comprising bilaterally inferior parietal cortex, area 44, insula and supplementary motor area (SMA), right premotor and middle cingulate cortex, middle temporal gyrus, putamen and OP4 as well as the left cerebellum. In both task-driven and task-free states the right TPJ thus entertains close functional connectivity with a bilateral though right-dominant ‘ventral’ action-control/ attention network (Corbetta & Shulman, 2002). Functional connectivity specific for the task-dependent mental state (MACM, B1) additionally involved bilateral (pre-)SMA, dorsal premotor cortex (dMPC), intraparietal sulcus (IPS), basal ganglia and left sensorimotor cortex (M1/S1). In the resting-state analysis (Fig. 2B2), reflecting TPJ connectivity in the absence of a structured task, we observed additional bilateral activation of the dorsolateral prefrontal cortex (DLPFC), insula and middle cingulate cortex, right superior parietal and premotor cortex as well as left SII.
III. FC analysis of resting-state imaging data
In the resting state (Fig. 2B2), reflecting rTPJ connectivity in the absence of a structured task, we observed, in addition to the shared network described below, significant correlations with the dorsolateral prefrontal cortex (DLPFC), insula (Id1, Kurth et al., 2010b), middle cingulate cortex (Palomero-Gallagher et al., 2008, 2009) and inferior/ superior parietal lobe. Additional lefthemispheric correlation was found in the operculum (OP1, OP4; Eickhoff et al., 2006a,b), cerebellum (lobule VII, crus I & II; Diedrichsen et al., 2009), dPMC, precuneus and temporal pole, whereas additional right-hemispheric correlation occurs in the area 45 (Amunts et al., 1999) and inferior temporal gyrus.
VI. Conjunction across MACM & resting-state FC analyses
The conjunction (Fig. 2C) across both individual FC analyses (Fig. 2B1 & 2B2) revealed a shared network comprising bilaterally the inferior parietal cortex (areas PF, PFm) extending into the TPJ, inferior frontal area 44, the anterior dorsal insula and the SMA (area 6, Geyer, 2004). Right hemispheric activation was observed in the dPMC and middle cingulate cortex, the posterior DLPFC (cf. Rottschy et al., in press), the middle temporal gyrus, putamen and OP 4. Activation restricted to the left hemisphere was found only in the cerebellum (lobule VI). In summary, in both task-dependent and task-free states, the right TPJ entertains close FC with a bilateral, though right-dominant network resembling the ‘ventral action-control/ attention network’ as described by Corbetta and Shulman (2002).
V. Difference between MACM & resting-state FC analyses
Figure 2A1 illustrates the pattern of FC that was specific for the task-dependent state as revealed by the contrast of ‘MACM > resting state connectivity’. We observed significantly stronger task-dependent FC of the right TPJ with the medial motor cortex (SMA/pre-SMA), bilateral area 44, the dorsal premotor cortex, intraparietal sulcus/ superior parietal lobe (7A, 7PC, hIP3) and thalamus as well as left-hemispheric regions of more pronounced connectivity of the putamen, the insula lobe and the cerebellum (lobule VI). In the right hemisphere the dPMC and V5 (hOC5, Malikovic et al., 2007) featured stronger task-dependent than task-independent FC with the right TPJ.
The reversed contrast (‘resting state > MACM connectivity’, Fig. 2A2) revealed areas featuring stronger FC with the seed in the task-free ‘resting’ state. Such a pattern was significantly seen bilaterally in the medial superior parietal lobe (5Ci, 7A, 5M, Scheperjans et al., 2005), dorsolateral prefrontal cortex and anterior/ middle cingulate cortex as well as in the anterior superior temporal sulcus.
Discussion
We demonstrated that across three studies, increased demand on stimulus–context integration in sensorimotor control was consistently associated with increased activation of the right TPJ (Fig. 1D). Subsequently, whole-brain functional connectivity of this region was delineated via meta-analytic connectivity modeling (task-dependent FC) and analysis of resting-state images from 100 healthy volunteers (task-independent FC).
Convergent functional coupling across approaches, i.e. independent of the presence or absence of an externally structured task, was observed in a shared network with right-hemispheric dominance. Herein, the inferior parietal cortex, area 44, the anterior dorsal insula and SMA (Fig. 2C) were found bilaterally. Dorsal premotor cortex, middle temporal gyrus, middle cingulate cortex, putamen and parietal opercular area OP4 on the right hemisphere as well as the left-hemispheric cerebellum featured unilateral convergent functional coupling with the right TPJ.
Stronger task-independent FC with the seed (‘resting state > MACM connectivity’, Fig. 2A2) was observed bilaterally in the medial superior parietal lobe and adjacent posterior cingulate cortex, dorsolateral prefrontal cortex and anterior/middle cingulate cortex.
The reversed contrast, i.e. FC specific for the task-dependent state (‘MACM connectivity > resting state’, Fig. 2A1), revealed significantly stronger coupling of the right TPJ with bilateral area 44 and thalamus. In addition, we observed differential co-activation on left-hemispheric superior parietal lobe, pre-SMA, insula, putamen and cerebellum (lobule VI) as well as of the dorsal premotor cortex and V5 (hOC5) in the right hemisphere.
Concepts of functional connectivity
Functional connectivity is defined as the ‘temporal coincidence of spatially distant neurophysiological events’ and may be assessed with cross-correlation of e.g. spiking patterns or field potentials in neurophysiological experiments (Schlögl & Supp, 2006). Currently, however, most FC analyses are based on (resting-state) fMRI. In their seminal study, Biswal et al. (1995) cross-correlated the time courses of resting-state fMRI signals from different brain regions, noting that FC may be inferred from significant correlation in the signal fluctuations across distant brain regions. Here, we assessed correlation (across scans, i.e., time) between the BOLD-signal time course of the right TPJ and time courses of all other locations in the brain. Using this approach, we aimed at identifying regions that are significantly (functionally) coupled with the seed region in the task-independent state.
Large-scale databases such as BrainMap (Laird et al., 2009a, www.brainmap.org) and algorithms for meta-analytic connectivity modeling (MACM; Laird et al., 2009a; Eickhoff et al., 2009) constitute the basis for comprehensive task-based FC analyses: MACM is based on the idea of assessing which brain regions co-activate above chance with the seed across a large range of functional tasks. In contrast to the more traditional definition of FC as coherent fluctuations across time, in MACM the unit of observation is thus the neuroimaging experiments. The strength of MACM is the delineation of networks that are conjointly recruited across a broad range of tasks, reflecting robust patterns of coordinated activity in response to an external structured task.
These two approaches hence provide complementary methods of investigating FC: Task-independent FC was assessed by correlating resting state MRI time-series, while task-dependent FC was revealed by investigation of significant co-activations of the seed region across different neuroimaging experiments (Eickhoff & Grefkes, 2011). Together, these allow a comprehensive assessment of the FC of the right TPJ across fundamentally different mental states.
Right TPJ in and beyond stimulus–context integration
In the present study, we defined the location of a seed region in the right TPJ based on a multi-study conjunction of experiments on stimulus–context integration and then assessed its co-activation patterns across a wide range of tasks as well as resting-state BOLD signal correlations. Therefore, the functional connectivity analyses did not pertain specifically to stimulus–context integration but rather provided an across-task and no-task assessment of the interactions of the seed (Eickhoff & Grefkes 2011). That is, our results reflect general interactions of the seed region, not those specific to stimulus–context integration. Nevertheless, the approach taken to identify the seed has important implications for the interpretation of our connectivity data, as a rather broadly defined macroanatomical region like the TPJ may contain several different functional modules. That is, different areas within a region like the TPJ may hold different functions and hence potentially also connectivity patterns. In fact, when considering the current literature on the TPJ, it may be noted, that this region is not only implicated in action control and stimulus–context integration but also in several other tasks, some of which seem to hold psychological similarities (e.g. stimulus-driven attention, visual search; Corbetta & Shulman 2002; Mavritsaki et al. 2010; Menon et al. 2010), while others appear completely unrelated (e.g. social cognition, mentalizing, perspective taking; Decety & Lamm, 2007; David et al. 2008; Vogeley et al. 2001). There is thus good evidence for a functional heterogeneity within the TPJ. Given this very likely differentiation, we would thus argue that the tasks used to define a seed should have an important influence on the subsequent connectivity analyses even though these consider across-task and no-task interactions. To illustrate this point, it may be assumed that a seed in the right TPJ defined by a conjunction across social-cognition tasks would have had a different location within this region and most likely also a different pattern of MACM and resting-state connectivity. While our approach hence does not definitively associate a brain region and its connectivity to a particular cognitive function such as stimulus–context integration, the functional context established by the definition of the seed nevertheless provides its precise allocation to a (functional) module and hence an important constraint to the interpretation of the observed connectivity patterns. This holds in particular for regions that are as broadly defined and functionally heterogeneous as the TPJ.
Right TPJ and the concept of predictive coding
The performed multi-study conjunction indicated that the right TPJ (area PFm) as the most extensive region of overlap between three neuroimaging contrasts probing increased demands for sensorimotor control and stimulus-response integration. Previous studies point to a key role of this region also in other ‘higher cognitive functions’, such as attention (Corbetta & Shulman, 2002) or visuomotor integration (Mooshagian et al., 2008). This raises the question as to how these operations can be integrated in a comprehensive theoretical construct.
Predictive coding (Rao & Ballard, 1999, Summerfield & Mangels, 2006, Kilner et al., 2007a) is a hypothesis on the fundamental nature of neuronal information processing. Within this model the brain is conceptualized as a Bayesian machine, i.e. perception is based upon generative models enabling probabilistic inference on sensory inputs and the underlying causes. This is enabled by a hierarchical organization of brain regions with reciprocal connections between them. The prediction error, i.e. difference between sensory input and internal prediction, is computed at each level and passed to higher levels via forward connections. Its size reflects the accuracy of the predictions and potential necessity for adjustment. Feedback connections pass predictions back to the lower level. The objective of these computations is the (unconscious and highly automated) minimization of the environmental entropy (i.e. average uncertainty) to optimize predictions about incoming information. So far, evidence for predictive coding has mainly been discussed in the context of sensory paradigms (Behrens et al., 2007, Summerfield & Mangels, 2006). However, observations of faster reaction times and reduced error rates for predominant relative to deviant cues have raised the notion of predictive motor coding. Mechanistically, predictions about prospective sensory input should entail the a priori preparation of an adequate motor program. If an upcoming stimulus matches the prediction the prepared motor program simply has to be released, instead of being chosen de novo from the motor repertoire, providing more efficient reactions.
As each of the three studies used to define the seed region presented lateralized visual stimuli and required lateralized responses, it may be argued that the observed effects may be attributable to increased attention and spatial (re-)orientation (Corbetta & Shulman, 2002, Thiel et al., 2004). In particular, stimuli appeared more frequently in the unexpected (and hence unattended; Shulman et al., 2009) location in ‘high-demand’ conditions. This in well in line with a study of Downar et al. (2000) observing an involvement of (predominantly) the right temporoparietal junction in multimodal change detection, i.e. detection of ‘salient’ stimuli. We would argue that these interpretations (re-orientation or attentional demands) may be reconciled with predictive coding concepts. Under this theoretical framework, stimulus-driven re-orienting may be understood as the upstream effects of high prediction errors, which trigger a reactive orientation towards the site of the unpredicted stimulus. In a Bayesian system of sensory inference attention may thus be conceptualized as inference on the precision of predictions (Feldman & Friston, 2010). If a prediction error is high, attentional re-orientation is instantiated.
Lesions of (especially) the right (Vallar et al., 1993) TPJ have been conjectured to clinically manifest themselves as a lack of awareness of space on the contralesional side of the body, i.e., neglect (Mavritsaki et al., 2010). In accordance with the theoretical framework outlined above, a unilateral deficit in evaluating upstreaming stimuli may result in persistent ‘attention’ to only one (i.e. the ipsilesional) side of the environment. However, in their study Karnath and colleagues (2001) emphasized that ‘the superior temporal cortex rather than the IPL or TPO junction is the substrate of spatial neglect in both monkeys and humans.’ Thus, the putative involvement of (r)TPJ lesions in neglect is still a matter of debate.
In summary, based on the assumption that probabilistic inference is an integral part of sensory processing and motor preparation, the concept of predictive coding may provide a theoretical framework for the computational processes underlying stimulus-response integration for sensorimotor control. Based on the current multi-study conjunction we would argue that the right TPJ might be a key structure for implementing attentional (re-)orientation by inference on prediction errors within this framework.
Core network of consistent functional connectivity
The term “core network“ denotes regions featuring convergent functional coupling with the right TPJ in the task-driven and endogenously controlled state (Fig. 2C). Its nodes are thus part of very much the same networks as the seed irrespective of the current mental state. In this context, it has to be noted, that close resemblance between ‘resting-state networks’ and those jointly engaged in task-based studies has been reported and hence the notion of "rest" in the absence of a specific task has evolved into a concept of an unconstrained sampling of different brain networks with preponderance for introspective aspects (Raichle et al., 2001, Schilbach et al., 2008, Smith et al., 2009).
The human insula (most notably the anterior dorsal portion) activates in a broad range of tasks across diverse functional domains, such as emotion processing, interoception, (working) memory and attention (Craig, 2009, Kurth et al., 2010a). Thus, the insula is regarded as integration area, mediating dynamic information flow between large-scale brain networks (Menon & Uddin, 2010; Dosenbach et al. 2006) as well as providing a link between the processing of external information and monitoring the internal milieu (Craig, 2009). In the current study, consistent co-activation of the anterior dorsal insula may therefore originate from its function as a integrative hub controlling the flow of information and implementing task-sets, i.e., high level priors.
Several neuroimaging studies provide evidence for a role of the inferior parietal cortex (IPL) in the multi-modal integration of stimuli (Renier et al., 2009) as well as movement planning and execution (Iacoboni, 2006). The FC with the (right) TPJ reflects the dense anatomical connectivity between these (Lewis & Van Essen, 2000). Most likely, the IPL might implement the planning, selection and preparation of movement routines that is controlled by the predictions (and associated errors) provided by the right TPJ.
It should be noted that this ‘core network’ (right TPJ, anterior insula, IPL) resembles the so-called ‘ventral attention network’ (Corbetta & Shulman, 2002), typically activated during the detection of salient and behaviorally relevant stimuli, i.e. stimulus-driven reorienting, and acts as a ‘circuit breaker’ for ongoing processes in the dorsal attention network (Corbetta et al., 2008). The close similarity between the ‘core network’ and the ‘ventral attention network’ thus fits well with the Bayesian framework of stimulus-driven reorienting as outlined above. In line with the FC data provided by Fox et al. (2006) we would thus argue for an important role of the TPJ within the ventral attention network, potentially reflecting a computational core in a predictive coding system.
Stronger couplings in the task-dependent state
In a system of Bayesian inference, minimization of prediction errors requires the supply with bottom-up (sensory) information. In this context, bilateral activation of the thalamus may be reconciled with its putative function as ‘input gate’ routing upstreaming information to sensorimotor and association cortices (Johansen-Berg et al., 2005) with collaterals to the TPJ as a predictive integrator. This interpretation may particularly hold for the right hemisphere where activation in the thalamus was observed in those parts that were shown to connect to the temporal cortex (including the TPJ) (Behrens et al., 2003). On the left hemisphere, in contrast, predominant activation in regions projecting to the prefrontal cortex (probably mediodorsal nucleus) may reflect the role of the thalamus as a cortico-cortical integration hub (Cappe et al., 2009), such as the possible involvement of the mediodorsal thalamus in sending prospective motor information to the DLPFC (Watanabe & Funahashi, 2011).
Regions of the posterior parietal cortex, in particular the superior parietal lobe and intraparietal sulcus (SPL/IPS), are involved in stimulus–context integration and stimulus-response matching (Wolfensteller et al., 2010). Thus, functional coupling with these regions may indicate pre-processing of incoming information by these, i.e. ‘outsourcing’ of lower-level integration processes. In other words, there may be parallel processing of the stimuli themselves (in the SPL/IPS) and their match with current predictive codes (in the right TPJ), allowing inference on both stimuli and predictions. Formally, this would entail a functional hierarchy between the SPL/IPS and the higher-level right TPJ.
Subsequently, the frontal areas may utilize this prediction to adjust behavioral plans and goals (Koechlin & Summerfield, 2007), linking predictive coding on sensory information with predictive motor coding. In the current study, bilateral co-activation of area 44 may indicate this region as an important node for the "action" stream, which is consistent with previous evidence implicating this region in behavioral planning and executive top-down influences on premotor areas (Koechlin & Jubault, 2006). The coupling between the right TPJ and area 44 may hence correspond to an alignment between predictions and the preparation of adequate behavioral responses. Such response patterns may be pre-selected and hence prepared in the likewise co-activated premotor areas, i.e., the dPMC and the pre-SMA. While FC analyses may not reveal the directionality of interactions, based on previous evidence, we would propose the following relationship between these regions: Whereas area 44 provides the link between the sensory and motor domain, the pre-SMA may subsequently control the implementation of motor preparation in the dPMC. This view would be in line with observations that the pre-SMA is involved in executive motor control, e.g., modifications of movement plans by inhibition or switching of responses (Picard & Strick, 1996). In contrast, the dPMC features close interactions with the motor output system (Dum & Strick, 2005, Chouinard & Paus, 2006) and therefore is a putative recipient of the generated motor plans. In the proposed model, the dPMC would thus constitute the lowest stage of the motor stream, implementing the actual preparation of motor responses.
In summary, we thus propose that the task-based FC data, in synopsis with previous evidence from humans and non-human primates, may indicate interaction of the right TPJ with a "sensory stream" of predictive coding consisting of the thalamus as the sensory gateway and the SPL/IPS for stimulus processing on one hand as well as with a ‘motor stream’ comprising area 44, pre-SMA and dPMC, itself potentially organized in a hierarchical fashion reflecting a progression from more abstract motor plans to the preparation of a particular action (i.e. the specification of free parameters in motor commands, such as direction, extend and force of a given movement).
Stronger couplings in the task-independent state
Patterns of neural activation in the absence of an externally structured task reflect the brain’s ’physiological baseline’ (Gusnard & Raichle, 2001) but may not be equated with 'mental rest' due to the high spatio-temporal structuring of ongoing activity that seems to reflect task-relevant networks (cf. Fox and Raichle, 2007, Smith et al., 2009). Rather than being at rest, the brain should thus be in a state of unconstrained cognition (Schilbach et al., 2008), i.e. implementing a broad variety of (predominantly internally oriented) operations. In the current study, we observed increased connectivity of the right TPJ in this task-independent (compared to stimulus-driven) brain state with a bilateral network comprising the anterior cingulate and dorsolateral prefrontal cortices as well as the precuneus and adjacent posterior cingulate cortex (PrC/PPC).
Interestingly, medial parietal and cingulate cortices were reported to show highest levels of glucose consumption in the endogenously controlled state (Gusnard & Raichle, 2001) and de-activate upon commencement of structured tasks (Schilbach et al., 2008), supporting the notion of a 'default mode network'. Nevertheless, activity within these regions is not restricted to the ’physiological baseline’. Rather they been observed in a broad range of internally directed cognitive tasks including episodic memory and first-person perspective taking (Vogeley et al., 2001) as well as the processing of self-relevant information and intentions, including intentions to actions (cf. Cavanna & Trimble, 2006). It may hence be assumed that in the mode of unconstrained cognition these midline regions may gather and integrate information about past self-referential events. Hereby, they could provide personal experience as an important backdrop for mental operations in the absence of externally structured tasks or sensory information. In contrast, the (anterior) dorsolateral prefrontal cortex has been conceptualized as a key node for the generation and representation of internal goal and task-set representation, i.e., overarching plans (Koechlin & Summerfield, 2007). Though speculative, we would thus propose, that the right TPJ may provide the computational link between autobiographic memories (past, PrC/PPC), self-reference (present, ACC) and goal-representations (future, DLPFC) by evaluating predictive codes. In Bayesian framework, this would thus represent the basis of forming predictions about future long-term goals based on previous experience. How does this relate to the apparent role of the right TPJ during stimulus-driven task, namely optimizing short-term representations of the sensory environment for motor preparation? We would conclude, that by interaction with domain-specific brain regions the right TPJ and anterior insula forming the 'core network' may implement the governance of predictive coding across a wide range of mental states, irrespective of the domain (perceptual, motor or cognitive) and time course (short-term or long-term).
Supplementary Material
Acknowledgements
This work was partly funded by the Human Brain Project (R01-MH074457; A.R.L., S.B.E., P.T.F), the Initiative and Networking Fund of the Helmholtz Association within the Helmholtz Alliance on Systems Biology (Human Brain Model; K.Z., S.B.E.) and the DFG (IRTG 1328, S.B.E.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amunts K, Schleicher A, Bürgel U, Mohlberg H, Uylings HB, Zilles K. Broca's region revisited: cytoarchitecture and intersubject variability. J Comp Neurol. 1999;412(2):319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bays PM, Singh-Curry V, Gorgoraptis N, Driver J, Husain M. Integration of goal- and stimulus-related visual signals revealed by damage to human parietal cortex. J Neurosci. 2010;30(17):5968–5978. doi: 10.1523/JNEUROSCI.0997-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Walton ME, Rushworth MF. Learning the value of information in an uncertain world. Nat Neurosci. 2007;10(9):1214–1221. doi: 10.1038/nn1954. [DOI] [PubMed] [Google Scholar]
- Bellec P, Perlbarg V, Jbabdi S, Pélégrini-Issac M, Anton JL, Doyon J, Benali H. Identification of large-scale networks in the brain using fMRI. Neuroimage. 2006;29(4):1231–1243. doi: 10.1016/j.neuroimage.2005.08.044. [DOI] [PubMed] [Google Scholar]
- Berlucchi G, Aglioti SM. The body in the brain revisited. Exp Brain Res. 2010;200(1):25–35. doi: 10.1007/s00221-009-1970-7. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci. 1999;11(1):80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Calvert G, Spence C, Stein BE. Handbook of multisensory processes. MIT press; 2004. [Google Scholar]
- Cappe C, Rouiller EM, Barone P. Multisensory anatomical pathways. Hear Res. 2009;258(1–2):28–36. doi: 10.1016/j.heares.2009.04.017. [DOI] [PubMed] [Google Scholar]
- Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K. The human inferior parietal cortex: cytoarchitectonic parcellation and interindividual variability. Neuroimage. 2006;33(2):430–448. doi: 10.1016/j.neuroimage.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Caspers S, Eickhoff SB, Geyer S, Scheperjans F, Mohlberg H, Zilles K, Amunts K. The human inferior parietal lobule in stereotaxic space. Brain Struct Funct. 2008;212(6):481–495. doi: 10.1007/s00429-008-0195-z. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Zilles K, Mohlberg H, Schleicher A, Fink GR, Armstrong E, Amunts K. Cytoarchitectonic identification and probabilistic mapping of two distinct areas within the anterior ventral bank of the human intraparietal sulcus. J Comp Neurol. 2006;495(1):53–69. doi: 10.1002/cne.20849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard PA, Paus T. The primary motor and premotor areas of the human cerebral cortex. Neuroscientist. 2006;12(2):143–152. doi: 10.1177/1073858405284255. [DOI] [PubMed] [Google Scholar]
- Cieslik EC, Zilles K, Kurth F, Eickhoff SB. Dissociating bottom-up and top-down processes in a manual stimulus-response compatibility task. J Neurophysiol. 2010;104(3):1472–1483. doi: 10.1152/jn.00261.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly PM, Bennur S, Gold JI. Correlates of perceptual learning in an oculomotor decision variable. J Neurosci. 2009;29(7):2136–2150. doi: 10.1523/JNEUROSCI.3962-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- David N, Aumann C, Santos NS, Bewemick BH, Eickhoff SB, Newen A, Shah NJ, Fink GR, Vogeley K. Differential involvement of the posterior temporal cortex in mentalizing but not perspective taking. Soc Cogn Affect Neurosci. 2008;3(3):279–289. doi: 10.1093/scan/nsn023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Lamm C. The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. Neuroscientist. 2007;13(6):580–593. doi: 10.1177/1073858407304654. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. Neuroimage. 2009;46(1):39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50(5):799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. A multimodal cortical network for the detection of changes in the sensory environment. Nat Neurosci. 2000;3(3):277–283. doi: 10.1038/72991. [DOI] [PubMed] [Google Scholar]
- Driver J, Noesselt T. Multisensory interplay reveals crossmodal influences on 'sensory-specific' brain regions, neural responses, and judgments. Neuron. 2008;57(1):11–23. doi: 10.1016/j.neuron.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Frontal lobe inputs to the digit representations of the motor areas on the lateral surface of the hemisphere. J Neurosci. 2005;25(6):1375–1386. doi: 10.1523/JNEUROSCI.3902-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner T. Congruency sequence effects and cognitive control. Cogn Affect Behav Neurosci. 2007;7(4):380–390. doi: 10.3758/cabn.7.4.380. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25(4):1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Schleicher A, Zilles K, Amunts K. The human parietal operculum. I. Cytoarchitectonic mapping of subdivisions. Cereb Cortex. 2006a;16(2):254–267. doi: 10.1093/cercor/bhi105. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Amunts K, Mohlberg H, Zilles K. The human parietal operculum. II. Stereotaxic maps and correlation with functional imaging results. Cereb Cortex. 2006b;16(2):268–279. doi: 10.1093/cercor/bhi106. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K. Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. Neuroimage. 2006;32(2):570–582. doi: 10.1016/j.neuroimage.2006.04.204. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, Amunts K. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage. 2007;36(3):511–521. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30(9):2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Pomjanski W, Jakobs O, Zilles K, Langner R. Neural correlates of developing and adapting behavioral biases in speeded choice reactions--an FMRI study on predictive motor coding. Cereb Cortex. 2011;21(5):1178–1191. doi: 10.1093/cercor/bhq188. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Grefkes C. Approaches for the integrated analysis of structure, function and connectivity of the human brain. Clin EEG Neurosci. 2011;42(2):107–121. doi: 10.1177/155005941104200211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman H, Friston KJ. Attention, uncertainty, and free-energy. Front Hum Neurosci. 2010;4:215. doi: 10.3389/fnhum.2010.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 2006;103(26):10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101(6):3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer S, Ledberg A, Schleicher A, Kinomura S, Schormann T, Bürgel U, Klingberg T, Larsson J, Zilles K, Roland PE. Two different areas within the primary motor cortex of man. Nature. 1996;382(6594):805–807. doi: 10.1038/382805a0. [DOI] [PubMed] [Google Scholar]
- Geyer S, Schleicher A, Zilles K. Areas 3a, 3b, and 1 of human primary somatosensory cortex. Neuroimage. 1999;10(1):63–83. doi: 10.1006/nimg.1999.0440. [DOI] [PubMed] [Google Scholar]
- Geyer S, Schormann T, Mohlberg H, Zilles K. Areas 3a, 3b, and 1 of human primary somatosensory cortex. Part 2. Spatial normalization to standard anatomical space. Neuroimage. 2000;11(6 Pt 1):684–696. doi: 10.1006/nimg.2000.0548. [DOI] [PubMed] [Google Scholar]
- Geyer S. The microstructural border between the motor and the cognitive domain in the human cerebral cortex. Adv Anat Embryol Cell Biol. 2004;174:I–VIII. 1–89. doi: 10.1007/978-3-642-18910-4. [DOI] [PubMed] [Google Scholar]
- Gottlieb J. From thought to action: the parietal cortex as a bridge between perception, action, and cognition. Neuron. 2007;53(1):9–16. doi: 10.1016/j.neuron.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Gottlieb J, Snyder LH. Spatial and non-spatial functions of the parietal cortex. Curr Opin Neurobiol. 2010;20(6):731–740. doi: 10.1016/j.conb.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefkes C, Geyer S, Schormann T, Roland P, Zilles K. Human somatosensory area 2: observer-independent cytoarchitectonic mapping, interindividual variability, and population map. Neuroimage. 2001;14(3):617–631. doi: 10.1006/nimg.2001.0858. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2(10):685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr. 1998 Mar–Apr;22(2):324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- Iacoboni M. Visuo-motor integration and control in the human posterior parietal cortex: evidence from TMS and fMRI. Neuropsychologia. 2006;44(13):2691–2699. doi: 10.1016/j.neuropsychologia.2006.04.029. [DOI] [PubMed] [Google Scholar]
- Jakobs O, Wang LE, Dafotakis M, Grefkes C, Zilles K, Eickhoff SB. Effects of timing and movement uncertainty implicate the temporo-parietal junction in the prediction of forthcoming motor actions. Neuroimage. 2009;47(2):667–677. doi: 10.1016/j.neuroimage.2009.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Behrens TE, Sillery E, Ciccarelli O, Thompson AJ, Smith SM, Matthews PM. Functional-anatomical validation and individual variation of diffusion tractography-based segmentation of the human thalamus. Cereb Cortex. 2005;15(1):31–39. doi: 10.1093/cercor/bhh105. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Ferber S, Himmelbach M. Spatial awareness is a function of the temporal not the posterior parietal lobe. Nature. 2001;411(6840):950–953. doi: 10.1038/35082075. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Friston KJ, Frith CD. The mirror-neuron system: a Bayesian perspective. Neuroreport. 2007a;18(6):619–623. doi: 10.1097/WNR.0b013e3281139ed0. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Jubault T. Broca's area and the hierarchical organization of human behavior. Neuron. 2006;50(6):963–974. doi: 10.1016/j.neuron.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Summerfield C. An information theoretical approach to prefrontal executive function. Trends Cogn Sci. 2007;11(6):229–235. doi: 10.1016/j.tics.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010a;214(5–6):519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth F, Eickhoff SB, Schleicher A, Hoemke L, Zilles K, Amunts K. Cytoarchitecture and probabilistic maps of the human posterior insular cortex. Cereb Cortex. 2010b;20(6):1448–1461. doi: 10.1093/cercor/bhp208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Kurth F, Fox PM, Uecker AM, Turner JA, Robinson JL, Lancaster JL, Fox PT. ALE Meta-Analysis Workflows Via the Brainmap Database: Progress Towards A Probabilistic Functional Brain Atlas. Front Neuroinformatics. 2009a;3:23. doi: 10.3389/neuro.11.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JW, Van Essen DC. Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. J Comp Neurol. 2000;428(1):112–137. doi: 10.1002/1096-9861(20001204)428:1<112::aid-cne8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Malikovic A, Amunts K, Schleicher A, Mohlberg H, Eickhoff SB, Wilms M, Palomero-Gallagher N, Armstrong E, Zilles K. Cytoarchitectonic analysis of the human extrastriate cortex in the region of V5/MT+: a probabilistic, stereotaxic map of area hOc5. Cereb Cortex. 2007;17(3):562–574. doi: 10.1093/cercor/bhj181. [DOI] [PubMed] [Google Scholar]
- Mavritsaki E, Allen HA, Humphreys GW. Decomposing the neural mechanisms of visual search through model-based analysis of fMRI: top-down excitation, active ignoring and the use of saliency by the right TPJ. Neuroimage. 2010;52(3):934–946. doi: 10.1016/j.neuroimage.2010.03.044. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooshagian E, Kaplan J, Zaidel E, Iacoboni M. Fast visuomotor processing of redundant targets: the role of the right temporo-parietal junction. PLoS One. 2008;3(6):e2348. doi: 10.1371/journal.pone.0002348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25(3):653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Palomero-Gallagher N, Mohlberg H, Zilles K, Vogt B. Cytology and receptor architecture of human anterior cingulate cortex. J Comp Neurol. 2008;508(6):906–926. doi: 10.1002/cne.21684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero-Gallagher N, Vogt BA, Schleicher A, Mayberg HS, Zilles K. Receptor architecture of human cingulate cortex: evaluation of the four-region neurobiological model. Hum Brain Mapp. 2009;30(8):2336–2355. doi: 10.1002/hbm.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex. 1996 May–Jun;6(3):342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RP, Ballard DH. Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects. Nat Neurosci. 1999;2(1):79–87. doi: 10.1038/4580. [DOI] [PubMed] [Google Scholar]
- Renier LA, Anurova I, De Volder AG, Carlson S, VanMeter J, Rauschecker JP. Multisensory integration of sounds and vibrotactile stimuli in processing streams for "what" and "where". J Neurosci. 2009;29(35):10950–10960. doi: 10.1523/JNEUROSCI.0910-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G, Matelli M. The organization of the cortical motor system: new concepts. Electroencephalogr Clin Neurophysiol. 1998;106(4):283–296. doi: 10.1016/s0013-4694(98)00022-4. [DOI] [PubMed] [Google Scholar]
- Scheperjans F, Grefkes C, Palomero-Gallagher N, Schleicher A, Zilles K. Subdivisions of human parietal area 5 revealed by quantitative receptor autoradiography: a parietal region between motor, somatosensory, and cingulate cortical areas. Neuroimage. 2005;25(3):975–992. doi: 10.1016/j.neuroimage.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Scheperjans F, Hermann K, Eickhoff SB, Amunts K, Schleicher A, Zilles K. Observer-independent cytoarchitectonic mapping of the human superior parietal cortex. Cereb Cortex. 2008a;18(4):846–867. doi: 10.1093/cercor/bhm116. [DOI] [PubMed] [Google Scholar]
- Scheperjans F, Eickhoff SB, Hömke L, Mohlberg H, Hermann K, Amunts K, Zilles K. Probabilistic maps, morphometry, and variability of cytoarchitectonic areas in the human superior parietal cortex. Cereb Cortex. 2008b;18(9):2141–2157. doi: 10.1093/cercor/bhm241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L, Eickhoff SB, Rotarska-Jagiela A, Fink GR, Vogeley K. Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the "default system" of the brain. Conscious Cogn. 2008;17(2):457–467. doi: 10.1016/j.concog.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Schlögl A, Supp G. Analyzing event-related EEG data with multivariate autoregressive parameters. Prog Brain Res. 2006;159:135–147. doi: 10.1016/S0079-6123(06)59009-0. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Astafiev SV, Franke D, Pope DL, Snyder AZ, McAvoy MP, Corbetta M. Interaction of stimulus-driven reorienting and expectation in ventral and dorsal frontoparietal and basal ganglia-cortical networks. J Neurosci. 2009;29(14):4392–4407. doi: 10.1523/JNEUROSCI.5609-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood J, Schooler JW. The restless mind. Psychol Bull. 2006;132(6):946–958. doi: 10.1037/0033-2909.132.6.946. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106(31):13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence C, Driver J. Crossmodal space and crossmodal attention. Oxford University Press; 2004. [Google Scholar]
- Summerfield C, Mangels JA. Dissociable neural mechanisms for encoding predictable and unpredictable events. J Cogn Neurosci. 2006;18(7):1120–1132. doi: 10.1162/jocn.2006.18.7.1120. [DOI] [PubMed] [Google Scholar]
- Thiel CM, Zilles K, Fink GR. Cerebral correlates of alerting, orienting and reorienting of visuospatial attention: an event-related fMRI study. Neuroimage. 2004;21(1):318–328. doi: 10.1016/j.neuroimage.2003.08.044. [DOI] [PubMed] [Google Scholar]
- Toni I, Shah NJ, Fink GR, Thoenissen D, Passingham RE, Zilles K. Multiple movement representations in the human brain: an event-related fMRI study. J Cogn Neurosci. 2002;14(5):769–784. doi: 10.1162/08989290260138663. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallar G, Antonucci G, Guariglia C, Pizzamiglio L. Deficits of position sense, unilateral neglect and optokinetic stimulation. Neuropsychologia. 1993;31(11):1191–1200. doi: 10.1016/0028-3932(93)90067-a. [DOI] [PubMed] [Google Scholar]
- Vogeley K, Bussfeld P, Newen A, Herrmann S, Happé F, Falkai P, Maier W, Shah NJ, Fink GR, Zilles K. Mind reading: neural mechanisms of theory of mind and self-perspective. Neuroimage. 2001;14(1 Pt 1):170–181. doi: 10.1006/nimg.2001.0789. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Funahashi S. Thalamic mediodorsal nucleus and working memory. Neurosci Biobehav Rev. 2011 doi: 10.1016/j.neubiorev.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage. 2009;47(4):1408–1416. doi: 10.1016/j.neuroimage.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Wolfensteller U, von Cramon DY. Bending the rules: strategic behavioral differences are reflected in the brain. J Cogn Neurosci. 2010;22(2):278–291. doi: 10.1162/jocn.2009.21245. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4(1):58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.