Abstract
Objectives
To examine the feasibility of perflubutane-based ultrasound for grading hepatic fibrosis.
Methods
This prospective study included 202 subjects; main study (controls:33, F0–1:35, F2:26, F3:23, cirrhosis:29) and subsequent study (controls:16, F0–1:7, F2:20, F3:7, cirrhosis:6). Diagnostic abilities for assessing fibrosis grade were compared between contrast findings and FIB4 (age × AST/[platelet count × ALT0.5]).
Results
High-power emission produced an intrahepatic band-like structure, and the three-layer appearance was less frequent and monolayer appearance was more frequent in cirrhosis than controls/chronic hepatitis (P < 0.0001). Intensity difference at 15-min phase showed most significant correlation with fibrosis grade (ρ = 0.79, P < 0.0001), and the best areas under the receiver operating characteristic curves are 0.88 for marked fibrosis, 0.95 for advanced fibrosis and 0.97 for cirrhosis, which were significantly higher than those of FIB4, 0.85 for marked fibrosis, 0.89 for advanced fibrosis and 0.90 for cirrhosis. Sensitivity, specificity and efficiency of the intensity difference were 88%, 72% and 81% for marked fibrosis, 85%, 91% and 89% for advanced fibrosis and 97%, 90% and 91% for cirrhosis, respectively. The subsequent study validated the main study results; significant correlation between the intensity difference and the fibrosis grade (ρ = 0.73–0.77, P < 0.0001).
Conclusions
Perflubutane-based ultrasound accurately predicts the grade of hepatic fibrosis.
Key Points
• The behaviour of intrahepatic microbubbles depends on the severity of hepatic fibrosis.
• Layer enhancement pattern simply represents the degree of chronic liver disease.
• Parenchymal intensity change due to high-power emission predicts the hepatic fibrosis grade.
Keywords: Liver, Fibrosis, Cirrhosis, Ultrasound, Contrast agent
Introduction
Liver biopsy remains the gold standard for grading hepatic fibrosis, although many studies have been carried out to find alternative methods [1]. Liver biopsy, however, has some shortcomings; invasiveness in patients with impaired coagulation and the possibility of sampling error owing to the heterogeneous distribution of fibrosis [1, 2]. Furthermore, because repeated assessment of the grade of hepatic fibrosis may be required during the management of a prolonged clinical course, a non-invasive technique would be preferred to replace this invasive procedure.
Ultrasound has the advantages of being simple, non-invasive, and enables real-time observation. Recent studies with sulfur hexafluoride (Sonovue; Bracco, Milan, Italy) have shown the effectiveness of haemodynamic assessment of microbubbles based on time-intensity analysis to diagnose advanced stages of fibrosis, but failed to find a significant difference in the parameters between the different fibrosis grades [3–5].
Sonazoid™ (GE Healthcare, Oslo, Norway) is a second generation microbubble agent, with the feature of being captured in reticuloendothelial tissue, such as the liver and spleen [6]. As the behaviour of microbubbles depends on the acoustic power, instantaneous high-power emission (IHPE) after the injection of Sonazoid™ allows ultrasound to depict the difference in the parenchymal intensity change between cirrhosis and idiopathic portal hypertension, because an ultrasound beam with greater power than the threshold level destroys microbubbles immediately [7, 8]. Basically, as this novel technique estimates the amount of intrahepatic microbubbles by subtraction of the image before and after the microbubble breakdown, it may have the potential to assess the grade of hepatic fibrosis. The aim of this study was to determine the efficacy of contrast-enhanced ultrasound with Sonazoid™ as a non-invasive tool for the evaluation of the grade of hepatic fibrosis.
Materials and methods
Enrolment of the subjects
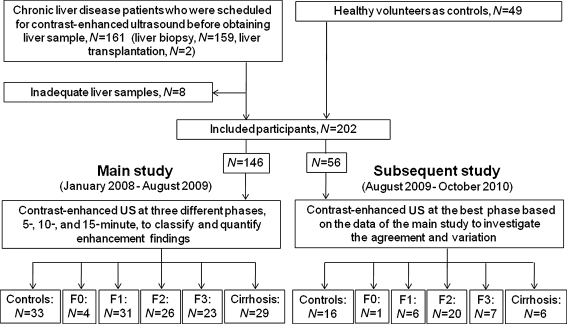
This prospective study was performed in Chiba University Hospital after approval by the ethics committee. The study was composed of two sub-studies; the main study (January 2008 to August 2009) aimed to classify the contrast-enhanced patterns of liver parenchyma, and to determine the relationship between contrast-enhanced findings and the grade of hepatic fibrosis, and the subsequent study (August 2009 to October 2010) investigated the agreement and variation of the data in the main study. The study enrolled the following subjects: chronic liver disease (CLD) patients who were scheduled for contrast-enhanced ultrasound before providing a liver sample (liver biopsy or liver transplantation) and healthy volunteers without signs of hepatic disease as controls (Fig. 1). However, we excluded patients with hepatic tumours diagnosed by ultrasound or egg allergy, which is a contraindication for Sonazoid™. Written informed consent was obtained from all participants. Blood samples were collected from all CLD patients within the 3-day period before the ultrasound examinations, and FIB4 (age × AST/[platelet count × ALT0.5]) was calculated as an indirect marker of fibrosis [9].
Fig. 1.
The main study (January 2008 to August 2009) classified and quantitated the enhancement findings and the subsequent study (August 2009 to October 2010) investigated agreements and variations in the findings
Ultrasound examinations
Ultrasound examinations (AplioXG, Toshiba, Tokyo, Japan; 3.75 MHz convex probe) were performed under the supine position after more than four hours of fasting. We screened the abnormalities such as intrahepatic arterio-portal/portal-venous communications and portal vein thrombosis, because these might affect the contrast-enhanced findings. The settings were changed for the contrast-enhanced study; harmonic mode with a low mechanical index (MI, 0.25), a depth to cover the whole right lobe of the liver, a focus point 8 cm below the skin surface and a dynamic range of 55 dB. The perfluorobutane microbubble agent (Sonazoid™, 0.0075 mL/kg) was injected manually into the antecubital vein, followed by a 3 ml flush of normal saline. All the cine images were stored digitally on the hard disk of the ultrasound system. Clinical symptoms, including blood pressure and oxygen saturation before and after the contrast-enhanced ultrasound examinations were monitored to screen adverse events.
Main study
The liver parenchyma was examined via a right inter-costal approach at three different phases, 5, 10 and 15 min after injection of the agent, using IHPE at maximum acoustic power level (MI, 1.4–1.6; 1.5 s at 20 Hz), according to the previous study [8]. A different imaging plane was selected carefully for the observation of the next phase, because microbubble breakdown caused by the previous IHPE might affect the subsequent contrast enhancement. All the ultrasound examinations were performed by H.I., a hepatologist with seven years’ experience with ultrasound at the time of the initial case. Second contrast-enhanced ultrasound examination was performed to evaluate inter-operator agreement in the subjects who agreed to it and whose examination could be scheduled within 1 week after the initial examination. This was carried out by M.T., a hepatologist with more than eight years’ experience with ultrasound.
Subsequent study
It was the final step of our study to investigate the agreement and variation of IHPE data. The ultrasound observations were carried out for three different imaging planes in one of the three phases, which provided the enhancement findings closest to the fibrosis grade based on the data from the main study. All the ultrasound examinations were also performed by H.I.
Analysis of contrast-enhanced ultrasound data
Parenchymal enhancement
The initial review was performed by H.I. for the post-IHPE sonograms. Layer appearance was defined when the parenchymal enhancement showed band-like structure which appeared horizontally on the sonogram. Then the findings were reviewed by two reviewers (T.S. and H.K., hepatologists with 6 years’ experience with ultrasound) who examined the inter-reviewer agreement with no prior knowledge of the pathological data or any other information of the patients, and provided a final result for layer appearances in this study by consensual decision-making.
Intensity analysis
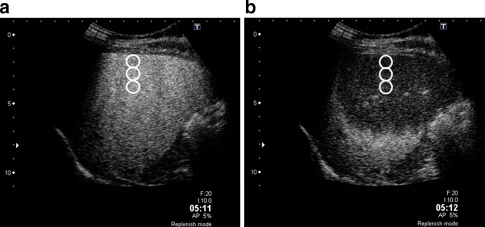
The analysis was carried out using image analysing software (ImageLab-avi; Toshiba, Tokyo, Japan) with reference to the methodology described in the literature [8]. Two images, before and after IHPE, were prepared for each subject and three round-shaped regions of interests (ROIs, 10 mm in diameter) were placed manually and longitudinally at the centre of each image, from 10 to 30 mm below the liver surface. The difference of signal intensity (dB) before and after IHPE was calculated (Fig. 2). Considering the variance caused by the measurement processes, the average difference in signal intensities obtained from three measurements was used as intensity difference data.
Fig. 2.
Contrast-enhanced images of a 56-year-old woman with chronic hepatitis, in the 5-min phase. a Before instantaneous high-power emission (IHPE): the liver parenchyma showed homogeneous enhancement. Three regions of interests (ROIs, white circles) were placed longitudinally in the centre of the image from 10 to 30 mm in depth. b After IHPE: the liver parenchyma around the liver surface appeared as a hypo-enhancement area because of the breakdown of microbubbles by IHPE. The difference in signal intensity (dB) between the two images was calculated and the average difference in the signal intensities was defined as “intensity difference”
Histological assessment
Liver samples were obtained within a week of the ultrasound examination. Paraffin-embedded specimens were stained with haematoxylin–eosin for assessment of cell morphology and Azan stain for assessment of fibrosis. Fibrosis grade and activity grade were assessed according to the METAVIR scoring system by the consensus reading of two expert hepatologists (F.I. and K.F., each with pathological examination experience of more than 20 years). Fibrosis was graded on a scale of 0–4 (F0, F1, F2, F3, cirrhosis) and activity grade was scored on a scale of 0–3 (A0–1, A2, A3). In this study, fibrosis grade was also evaluated quantitatively as a “fibrosis-ratio”; a digital image (40×) of an Azan-stained specimen was loaded into the image analysis software (Photoshop; Adobe systems, San Jose, CA, USA), using an off-line personal computer, and the collagen-fibre area, stained by aniline blue, and the entire tissue area were measured as pixel numbers using image binarisation techniques. The average ratio between them obtained by three-time measurements was defined as the fibrosis ratio (%) and the fibrosis ratio of controls was defined as 0% for data analysis [10].
Statistical analysis
The Spearman rank correlation was used for the correlation between discrete and continuous variables and Pearson’s correlation coefficient was used for the correlation among continuous variables. The Chi-squared test was used to compare the layer appearances among controls and patients with chronic hepatitis and cirrhosis. The correlation between the fibrosis ratio and the intensity difference data at each phase in the main study and in the subsequent study were compared using Fisher’s z-transformation. For the comparison of other parameters in more than two groups, analysis of variance with Scheffe post hoc test was used. Receiver operating characteristic curves were applied to determine the best cut-off values of the intensity difference with the best sensitivity and specificity in discriminating fibrosis stages. Diagnostic accuracy of the intensity difference was assessed by areas under the receiver operating characteristic curves (Az), 95% confidence interval, sensitivity, specificity, positive and negative predictive values, and efficiency for the prediction of significant fibrosis. Intra- or inter-observer variability and variations in intensity difference in the subsequent study were calculated by the coefficient of variation obtained by standard deviation/mean × 100. Inter-operator and inter-reviewer agreement was assessed by Kappa value calculation. Agreement grade was defined as <0.2 for poor, 0.2–0.4 for moderate, 0.4–0.6 for fair, 0.6–0.8 for good and 0.8–1.0 for excellent. Probability values below 0.05 were considered to be significant. All statistical analyses were performed using the SPSS package (version 17.0 J; SPSS, Chicago, IL, USA). Az values were obtained using ROCKIT1.1B2.
Results
Clinical data of the subjects
The study had 202 participants after the exclusion of 8 CLD patients because of inadequate biopsy specimens; 146 subjects for the main study and 56 subjects for the subsequent study (Table 1). Liver samples were obtained from all subjects; percutaneous needle biopsy (16-/18-gauge needle; BARD, Tempe, AZ, USA) in 144 patients without ascites, transjugular liver biopsy (18-gauge needle; Cook, Bloomington, IN, USA) in 12 patients with ascites, and total hepatectomy in two patients. Biopsy samples showed 20 ± 3.3 (mean ± SD, 11–28) mm in length and 21 ± 5.7 (mean ± SD, 13–66) mm2 in area. The fibrosis ratio was 3.3 ± 1.6% (0.5–6.4) for F1, 5.4 ± 2.7% (1.8–15) for F2, 11.5 ± 3.1% (6.2–18) for F3 and 22.2 ± 4.6% (12–35) for cirrhosis. Significant correlation was observed between the fibrosis ratio and the grade of fibrosis by Spearman’s correlation coefficient (ρ = 0.954, P < 0.0001)
Table 1.
Clinical and biochemical data of all subjects
| Main study | Subsequent study | |||
|---|---|---|---|---|
| Controls, n = 33 | CLD, n = 113 | Controls, n = 16 | CLD, n = 40 | |
| Age (years) | 46 ± 16 (26–82) | 55 ± 12 (23–78) | 62 ± 18 (29–86) | 52 ± 15 (24–74) |
| Gender (male/female) | 21/12 | 37/76 | 9/7 | 18/22 |
| BMI (kg/m2) | 21.7 ± 2.4 (16–26) | 22.7 ± 3.9 (16–37) | 21.8 ± 2.4 (17–25) | 24.0 ± 3.7 (16–35) |
| Presence of ascites (%) | 0 (0) | 12 (11) | 0 (0) | 2 (5) |
| AST (IU/L) | 18.0 ± 3.5 (13–24) | 54.8 ± 51.4 (16–447) | 19.9 ± 5.5 (13–32) | 71.4 ± 53.5 (19–236) |
| ALT (IU/L) | 13.4 ± 5.3 (9–30) | 61 ± 89 (10–867) | 14.6 ± 3.3 (10–19) | 91.7 ± 101 (16–526) |
| Total bilirubin (mg/dL) | 0.87 ± 0.33 (0.4–1.3) | 1.0 ± 1.4 (0.4–15) | 0.6 ± 0.14 (0.5–0.8) | 1.2 ± 2.2 (0.7–1.8) |
| Albumin (g/dL) | 4.5 ± 0.36 (3.9–5.0) | 4.0 ± 0.54 (1.7–5.4) | 4.2 ± 0.3 (3.9–4.8) | 4.3 ± 0.4 (3.2–5.2) |
| Platelets (109/L) | 244 ± 44 (163–340) | 177 ± 70 (44–428) | 227 ± 43 (162–336) | 182 ± 59 (48–320) |
| FIB4 | 1.0 ± 0.61 (0.4–2.6) | 3.0 ± 2.5 (0.40–15) | 1.5 ± 0.7 (0.5–3.0) | 2.8 ± 2.7 (0.7–16) |
| Aetiology, HCV/HBV/AIH/PBC /NASH/Alcohol/ Cryptogenic | – | 62/13/10/13/3/5/7 | – | 15/5/5/5/6/2/2 |
| Activity grade, A0-1/A2/A3 | – | 53/50/10 | – | 21/9/10 |
| Grade of fibrosis, F0/F1/F2/F3/ Cirrhosis | – | 4/31/26/23/29 | – | 1/6/20/7/6 |
| Child-Pugh class, A/B/C | – | 19/9/1 | – | 5/0/1 |
CLD, chronic liver disease; BMI, body mass index; AST, aspartate transaminase; ALT, alanine transaminase; FIB4, age × AST/(Platelet Count/ALT0.5); HCV, hepatitis C virus; HBV, hepatitis B virus; AIH, autoimmune hepatitis; PBC, primary biliary cirrhosis; NASH, non-alcoholic steatohepatitis
Main study results
Parenchymal enhancement after IHPE
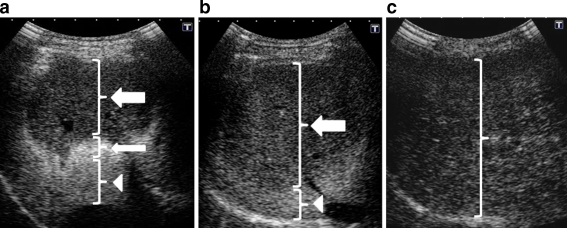
Three-layer appearances were found on the post-IHPE sonograms by the initial review: a three-layer appearance showing a hypo-enhancement band near the liver surface with a hyper-enhancement band in the middle and hypo-enhancement band at the bottom, a two-layer appearance showing hypo-enhancement band as the first layer and hyper-enhancement band as the second layer, and a monolayer appearance, showing only hypo-enhancement parenchyma (Fig. 3). The width of band-like structure in the three-layer appearance was 49.1 ± 10.4 (20–80) mm in the first layer, 12.9 ± 2.8 (8–18) mm in the second layer and 29.2 ± 12.1 (10–62) mm in the third layer. The deepest level of band-like structure was 63 ± 8.1 (42–90) mm in the first layer and 76 ± 9.9 (52–125) mm in the second layer. Similarly, the width of band-like structure of two-layer appearance showed 67.5 ± 4.2 (60–70) mm in the first layer, and the deepest level of that was 79 ± 5.5 (70–85) mm in the first layer. There was no significant difference in the width or the deepest level of band-like structure between control, chronic hepatitis and cirrhosis groups.
Fig. 3.
Three patterns of parenchymal enhancement after IHPE in the 15-min phase. a A 60-year-old woman, control subject: Three-layer appearance with a hypo-enhancement band near the liver surface (thick arrow), a hyper-enhancement band in the middle (thin arrow) and a hypo-enhancement band in the bottom (arrowhead). b A 58-year-old woman, with hepatitis C-related chronic hepatitis, F3: Two-layer appearance with a hypo-enhancement band as the first layer (arrow) and a hyper-enhancement band as the second layer (arrowhead). c A 56-year-old woman with hepatitis C-related cirrhosis: Monolayer appearance with a single hypo-enhancement layer
The three-layer appearance was significantly less frequent, and the monolayer appearance was significantly more frequent, in cirrhosis than controls/chronic hepatitis at all phases (P < 0.0001). However, the layer-appearance did not differ significantly between the controls, F1, F2 and F3. Inter-operator agreement for the layer appearance after IHPE was excellent (kappa = 0.824) and inter-reviewer agreement of review results for layer appearance was also excellent (kappa = 0.912).
Intensity analysis
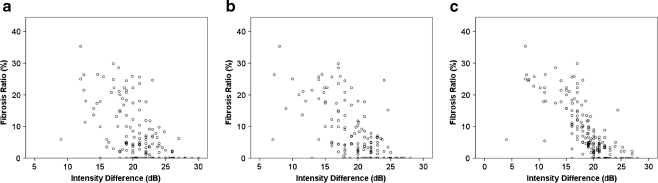
Significant correlations were found between the intensity difference and fibrosis grade (Spearman’s correlation coefficients: 5-minute phase, ρ = 0.47, P < 0.0001; 10-minute phase, ρ = 0.64, P < 0.0001, 15-minute phase, ρ = 0.79, P < 0.0001)/fibrosis ratio (Fig. 4). The intensity difference in the 15-min phase showed a closer correlation with the fibrosis ratio than the 5-min (P = 0.0009) and 10-min phase (P = 0.041). Inter-observer variability for the IHPE technique was 8.7% in the 5-min phase, 7.9% in the 10-min phase and 8.7% in the 15-min phase. The Az value of the intensity difference for cirrhosis at the 15-min phase was significantly higher than those of FIB4 (Table 2). Efficiency of diagnosis in the 15-min phase was 81% for marked fibrosis, 89% for advanced fibrosis and 91% for cirrhosis (Table 3).
Fig. 4.
Correlation between fibrosis ratio and the intensity difference in the 5-, 10- and 15-min phases. a 5-min phase: Pearson’s correlation coefficient was r = 0.56 (P < 0.0001). b 10-min phase: Pearson’s correlation coefficient was r = 0.67 (P < 0.0001). c 15-min phase: Pearson’s correlation coefficient was r = 0.76 (P < 0.0001). The intensity difference in the 15-min phase showed the closest correlation with the fibrosis ratio among the three phases
Table 2.
Comparison of Az values for the diagnosis of marked fibrosis, advanced fibrosis and cirrhosis between the intensity difference in the 15-min phase and FIB4
| For marked fibrosis (≥F2) | For advanced fibrosis (≥F3) | For cirrhosis | |
|---|---|---|---|
| The intensity difference in the 15-min phase | 0.88 (0.81–0.92) | 0.95 (0.91–0.98) | 0.97 (0.93–0.99) |
| FIB4 | 0.85 (0.78–0.91) | 0.89 (0.82–0.94) | 0.90 (0.81–0.96) |
| P-values | 0.15 | 0.057 | 0.017 |
Az, area under the receiver operating characteristic curves; marked fibrosis, ≥F2; advanced fibrosis, ≥F3; FIB4, age × AST/(platelet count/ALT0.5); AST (IU/L), aspartate transaminase; ALT (IU/L), alanine transaminase
Table 3.
Diagnostic accuracy of the intensity difference in the 15-min phase
| Diagnostic accuracy (%) | |||
|---|---|---|---|
| For marked fibrosis (≥F2) | For advanced fibrosis (≥F3) | For cirrhosis | |
| Sensitivity | 88 | 85 | 97 |
| Specificity | 72 | 91 | 90 |
| Positive predictive value | 78 | 85 | 70 |
| Negative predictive value | 84 | 91 | 99 |
| Efficiency | 81 | 89 | 91 |
There was no significant relationship between the intensity difference (5-/10-/15-min) and the activity grade (P = 0.078, P = 0.053, P = 0.15, respectively). In patients with cirrhosis, there were no significant correlations between the intensity difference in the 5-/10-/15-min phases and the Child-Pugh class (P = 0.18, P = 0.079, P = 0.099), T-BIL (P = 0.082, P = 0.11, P = 0.054), and albumin (P = 0.53, P = 0.52, P = 0.95).
Subsequent study results
The subsequent study was carried out at the 15-min phase, whose findings were closest to the fibrosis grade. The layer appearance in three different imaging planes was identical in 52 of the 56 subjects (93%).
Variations of intensity difference in the three different imaging planes were 6.3 ± 2.9% (4–13.4) in controls, 6.1 ± 2.2% (2.8–9.4) in F1, 5.1 ± 3.1% (1.5–10.4) in F2, 7.0 ± 2.4% (3.3–10.6) in F3, and 12 ± 4.6% (6.6–18) in cirrhosis. The intensity difference in each imaging plane showed significant correlation with the grade of fibrosis (Spearman’s correlation coefficients: first imaging plane, ρ = 0.76, P < 0.0001; second imaging plane, ρ = 0.73, P < 0.0001, third imaging plane, ρ = 0.77, P < 0.0001) and with the fibrosis-ratio (Pearson’s correlation coefficient: first imaging plane, r = 0.81, P < 0.0001; second imaging plane, r = 0.73, P < 0.0001, third imaging plane, r = 0.80, P < 0.0001). Fisher’s z transformation revealed that there was no significant difference between the intensity difference at the 15-min phase of the main study and that in each of the three imaging planes of the subsequent study; first imaging plane (P = 0.73), second imaging plane (P = 0.37) and third imaging plane (P = 0.87).
Discussion
As shown in the results, the coefficient of correlation between the fibrosis grade and parenchymal enhancement showed time-dependent change and the closest relationship was detected on the 15-min phase image. This might be explained by the duration time of stability of microbubble distribution in the hepatic sinusoid after the contrast agent injection, i.e. the ratio between the static microbubbles captured in the liver and dynamic microbubbles that are not captured but circulating in the intrahepatic vessel might vary greatly among individual subjects in the 5-min and 10-min phases. Signals originating mainly from captured microbubbles may be suitable for grading hepatic fibrosis.
Kupffer cell may be implicated in the development of hepatic fibrosis [11], and phagocytosis of microbubbles by Kupffer cells is one of the underlying mechanisms for parenchymal enhancement by Sonazoid™. However, in the present study, no significant correlation was found between parenchymal intensity and hepatic function reserve, although previous studies have reported that Kupffer cell function is closely related to hepatocyte function [12]. It suggests that Sonazoid™-induced enhancement might reflect the grade of hepatic fibrosis independently of Kupffer cell function, although continuous study would be necessary to clarify in vivo behaviour of the microbubbles.
Nevertheless, as Sonovue does not have an accumulation property in the liver, previous studies with this agent focused on the haemodynamic evaluation; movement of microbubble in the liver, such as transit time between portal vein and hepatic vein or hepatic vein arrival time [3–5]. However, their results do not seem to separate different fibrosis stages; only discriminate advanced fibrosis (F3–F4) from mild fibrosis (F0–F2), probably because of the variability of the haemodynamic-based study, difficulty of the observation of dynamic microbubble or different property of microbubble.
Unexpectedly, the authors found a unique parenchymal enhancement pattern, a layered appearance. We speculate that an acoustic reaction of the microbubbles against the high power emission accounts for the layer structure. At first, the proximal hypo-intensity band may have a smaller number of microbubbles because of breakdown after high-power emission. If there were numbers of microbubbles in the liver parenchyma, as in the controls, some of them may act as a shelter to protect the distal microbubbles from the ultrasound beam. Consequently, reflection of the ultrasound beam by microbubbles may generate the intermediate hyper-intensity band. Furthermore, reflection of the ultrasound beam at the intermediate band may decrease exposure of microbubbles in the distal parenchyma to the ultrasound beam, which would generate the distal band, resulting in the three-layer appearance. Meanwhile in cirrhosis, because the total amount of intrahepatic microbubbles may be much lower than in the controls, most of the intrahepatic microbubbles could be destroyed by the high power emission. Less reflection of the ultrasound beam results in neither the intermediate nor the distal band being generated and a monolayer appearance. Our results have shown that the layer appearance is significantly effective for differentiating between controls and patients with chronic hepatitis and cirrhosis, although the layer appearance was difficult to differentiate among F1, 2, 3 and 4. The authors strongly recommend that this easy and simple technique should be applied to estimate the degree of liver fibrosis generally while performing the contrast enhanced study.
Diagnostic abilities for moderate or severe grades of fibrosis in the other imaging techniques are almost similar to the results of our technique; the Az value for F3 and/or cirrhosis is 0.84/0.95 by transient elastography [13] and 0.9244 for F2 [14], and 0.962 for F1 and 0.994 for F2 [15] by magnetic resonance elastography. However, the latter has limitations requiring large-scale expensive equipment and inconvenient procedures. Meanwhile, the authors emphasise that contrast-enhanced ultrasound suffers from a drawback of “a requirement of the agent injection”. It adds a certain complexity to the simple procedure, and a usage of the agent might make the results variable. Furthermore, it has a risk of causing side-effects though the severe events might be rare. Transient elastography and blood sample marker may have the advantage of simplicity in the acquisition and analysis of the data. In any event, diagnostic accuracy for the mild grade of fibrosis should be improved in the future, and contrast-enhanced ultrasound is expected to play a major role because of its simple and low-cost procedure.
The major limitation of our study is the observation of the limited area of the right lobe. One of the reasons for the selection of this area is that we thought contrast enhancement should be analysed in the area corresponding to parenchyma from which the liver sample is taken, as most of the biopsy procedures were performed in the right lobe. However, assessment of intensity in a much wider area of the liver should be made in the future to overcome the heterogeneous distribution of fibrosis.
In conclusion, the features of microbubbles exposed to high-power ultrasound emission seem to play a role in predicting the grade of hepatic fibrosis. Although further study would be needed to validate the results, our non-invasive and repeatedly available technique may have the potential to improve patient care in the long-term management of CLD.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 2.Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614–2618. doi: 10.1111/j.1572-0241.2002.06038.x. [DOI] [PubMed] [Google Scholar]
- 3.Staub F, Tournoux-Facon C, Roumy J, et al. Liver fibrosis staging with contrast-enhanced ultrasonography: prospective multicenter study compared with METAVIR scoring. Eur Radiol. 2009;19:1991–1997. doi: 10.1007/s00330-009-1313-x. [DOI] [PubMed] [Google Scholar]
- 4.Ridolfi F, Abbattista T, Marini F, et al. Contrast-enhanced ultrasound to evaluate the severity of chronic hepatitis C. Dig Liver Dis. 2007;39:929–935. doi: 10.1016/j.dld.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Orlacchio A, Bolacchi F, Petrella MC, et al. Liver contrast enhanced ultrasound perfusion imaging in the evaluation of chronic hepatitis C fibrosis: preliminary results. Ultrasound Med Biol. 2011;37:1–6. doi: 10.1016/j.ultrasmedbio.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Marelli C. Preliminary experience with NC100100, a new ultrasound contrast agent for intravenous injection. Eur Radiol. 1999;9:S343–S346. doi: 10.1007/PL00014070. [DOI] [PubMed] [Google Scholar]
- 7.Maruyama H, Matsutani S, Saisho H, Mine Y, Yuki H, Miyata K. Extra-low acoustic power harmonic images of the liver with perflutren: novel imaging for real-time observation of liver perfusion. J Ultrasound Med. 2003;22:931–938. doi: 10.7863/jum.2003.22.9.931. [DOI] [PubMed] [Google Scholar]
- 8.Maruyama H, Ishibashi H, Takahashi M, Imazeki F, Yokosuka O. Effect of Signal Intensity from the Accumulated Microbubbles in the Liver for Differentiation of Idiopathic Portal Hypertension from Liver Cirrhosis. Radiology. 2009;252:587–594. doi: 10.1148/radiol.2522081899. [DOI] [PubMed] [Google Scholar]
- 9.Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 10.Goodman ZD, Stoddard AM, Bonkovsky HL, et al. Fibrosis progression in chronic hepatitis C: morphometric image analysis in the HALT-C trial. Hepatology. 2009;50:1738–1749. doi: 10.1002/hep.23211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Leclercq I, Brymora JM, et al. Kupffer cells mediate leptin-induced liver fibrosis. Gastroenterology. 2009;137:713–723. doi: 10.1053/j.gastro.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuckerman E, Slobodin G, Sabo E, Yeshurun D, Naschitz JE, Groshar D. Quantitative liver-spleen scan using single photon emission computerized tomography (SPECT) for assessment of hepatic function in cirrhotic patients. J Hepatol. 2003;39:326–332. doi: 10.1016/S0168-8278(03)00296-4. [DOI] [PubMed] [Google Scholar]
- 13.Friedrich-Rust M, Ong MF, Martens S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960–974. doi: 10.1053/j.gastro.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 14.Asbach P, Klatt D, Schlosser B, et al. Viscoelasticity-based staging of hepatic fibrosis with multifrequency MR elastography. Radiology. 2010;257:80–86. doi: 10.1148/radiol.10092489. [DOI] [PubMed] [Google Scholar]
- 15.Huwart L, Sempoux C, Vicaut E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology. 2008;135:32–40. doi: 10.1053/j.gastro.2008.03.076. [DOI] [PubMed] [Google Scholar]