Abstract
During the past two decades, apoptotic cell death has been the subject of an intense wave of investigation, leading to the discovery of multiple gene products that govern both its induction and execution. In parallel, it has progressively become evident that most, if not all, proteins that had initially been discovered for their essential role in apoptosis also mediate a wide range of non-apoptotic functions. On the one hand, apoptotic regulators and executioners are involved in non-lethal physiological processes as diverse as cell cycle progression, differentiation, metabolism, autophagy and inflammation. On the other hand, pro-apoptotic proteins can control other modalities of programmed cell death, in particular regulated necrosis. In this review, we summarize the unconventional roles of the apoptotic core machinery from a functional perspective and discuss their pathophysiological implications. EMBO reports advance online publication 9 March 2012; doi:10.1038/embor.2012.19
Keywords: AIF, apoptosome, BCL-2, caspase, death receptor, mitochondrial membrane permeabilization
See Glossary for abbreviations used in this article.
Glossary.
- AIF
apoptosis-inducing factor
- AMPA
2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl) propanoic acid
- ANT
adenine nucleotide translocase
- APAF-1
apoptotic peptidase activating factor 1
- ATR
ataxia telangiectasia and Rad3 related
- BAK
BCL-2 antagonist/killer
- BAX
BCL-2-associated X protein
- BCL
B-cell lymphoma
- BID
BH3 interacting domain death agonist
- BIM
BCL-2 interacting mediator of cell death
- BNIP3
BCL-2/adenovirus E1B 19 kDa interacting protein 3
- CDK2
cyclin-dependent kinase 2
- CDKN1
cyclin-dependent kinase inhibitor 1
- cIAP
cellular IAP
- CRADD
CASP2 and RIPK1 domain containing adaptor with death domain
- CrmA
cytokine response modifier A
- DIABLO
direct IAP binding protein with low pI
- ENDOG
endonuclease G
- EPO
erythropoietin
- ER
endoplasmic reticulum
- FADD
FAS-associated death domain protein
- FGF2
fibroblast growth factor 2
- HSP70
heat-shock 70 kDa protein
- IAP
inhibitor of apoptosis protein
- Ig
immunoglobulin
- IFNγ
interferon γ
- IL
interleukin
- IM
inner mitochondrial membrane
- IMS
mitochondrial intermembrane space
- IRE1α
inositol-requiring enzyme 1 α
- LATS1
large tumour suppressor, homologue 1
- MEF
mouse embryonic fibroblast
- MMP
mitochondrial membrane permeabilization
- MOMP
mitochondrial outer membrane permeabilization
- MPT
mitochondrial permeability transition
- MW
molecular weight
- NF-κB
nuclear factor-κB
- NOD
nucleotide-binding oligomerization domain containing
- NLRP
NLR family, pyrin domain containing
- OM
outer mitochondrial membrane
- PRR
pattern recognition receptor
- PTPC
permeability transition pore complex
- RIG-I
retinoic acid-inducible gene 1
- RIPK
receptor-interacting protein kinase
- ROS
reactive oxygen species
- Smac
second mitochondrial activator of caspases
- STAT1
signal transducer and activator of transcription 1
- TCR
T-cell receptor
- TLR4
Toll-like receptor 4
- TNFα
tumour necrosis factor α
- TNFR1
TNFα receptor 1
- TRADD
TNFR1-associated death domain protein
- TRAFs
TNFR-associated factors
- VDAC
voltage-dependent anion channel
- XIAP
X-linked IAP
Introduction
Apoptosis is a tightly regulated cell death modality that manifests with specific morphological features [1]. For some time, such a morphological uniformity has misled researchers to believe that most, if not all, instances of apoptosis would be executed by the same molecular mechanisms—with a prominent role for caspases, a peculiar class of cysteine proteases—and would have similar consequences at the organismal level (and hence fail to elicit inflammation), irrespective of the initiating stimulus. During the past two decades, along with an increasingly more refined understanding of apoptosis and its pathophysiological implications, it has become clear that the apparent morphological uniformity of this cell death mode conceals a consistent degree of biochemical and functional heterogeneity [2].
According to accepted models, apoptotic cell death can result from the activation of either of two major molecular cascades. The 'extrinsic pathway' transduces extracellular pro-apoptotic signals through plasma membrane receptors, most often resulting in the activation of the caspase cascade. The 'intrinsic pathway' monitors the intracellular microenvironment and relays this information to mitochondria, where the decision between life and death is taken. If pro-apoptotic signals, such as those dispatched by damaged DNA or in response to oxidative stress, predominate, mitochondrial membranes become permeabilized, leading to the execution of apoptosis through both caspase-dependent and caspase-independent mechanisms. MMP can be initiated at the OM by the pore-forming activity of pro-apoptotic members of the BCL-2 protein family such as BAX and BAK, a process that is referred to as MOMP [3]. Alternatively, MMP can originate from an abrupt increase in the permeability of the IM to small solutes, leading to osmotic swelling of the mitochondrial matrix and the consequent breakdown of the OM [4]. This latter phenomenon, which is known as MPT, has been ascribed to the activity of the PTPC—a multiprotein complex assembled at the junctions between the IM and OM [5]. However, the existence of the PTPC as a pre-assembled complex, its precise molecular identity and its true relevance for apoptotic signalling remain a matter of debate (see below). Of note, the signalling modules for extrinsic and intrinsic apoptosis are not entirely disjointed as, in some instances, the activation of extrinsic apoptosis can be relayed to MMP, which amplifies and accelerates the process [2].
An exhaustive compendium of the molecules and biochemical processes that are involved in the regulation and execution of apoptosis goes beyond the scope of this review and can be found elsewhere [4,6]. Here, we analyse the unconventional roles of the apoptotic core machinery—that is, the functions of pro- and anti-apoptotic molecules in non-apoptotic settings—and discuss their pathophysiological relevance.
Differentiation
Various components of the apoptotic machinery, in particular caspases, become activated during, and are required for, the terminal differentiation of cell types as diverse as haematopoietic, epithelial, sperm, muscle and trophoblast cells. These functions of the apoptotic apparatus are exerted at the cellular level and do not entail the induction of cell death, implying that they must be conceptually discriminated from the roles that apoptotic molecules play as bona fide cell death regulators during morphogenesis and tissue homeostasis.
Initially, caspases—in particular, caspase-3, -9 and -14—were thought to participate only in differentiation programmes that exhibit some of the morphological signs of apoptosis, as they involve the degradation of entire organelles and portions of the cells (for example, the maturation of erythrocytes, platelets, keratinocytes and the lens epithelium; [7]). However, the chemical or genetic inhibition of caspase-3 arrests the differentiation of erythroid precursors at an early stage, well before chromatin condensation and subsequent nuclear extrusion occur [8]. Moreover, caspase-8 can mediate the maturation of macrophages [9] and the differentiation of the placental trophoblast [10], two processes that are not associated with morphological features of apoptosis. Along similar lines, caspase-6 negatively regulates the differentiation of plasma cells, an effect that might be related to its ability to control the cell cycle of B-cell progenitors [11]. There are at least three distinct, non-exclusive mechanisms that might explain why caspase activation leads to apoptosis in some cases and to differentiation in others: (i) spatial restriction, as exemplified by the activation of caspase-9 during thrombopoiesis, which only occurs in perinuclear, granular structures [12]; (ii) temporal restriction, as illustrated by the transient wave of caspase-3 activation that occurs during early erythropoiesis [8]; and (iii) substrate specificity, as demonstrated in several models of cellular differentiation [9,13]. Interestingly, such specificity might result from the presence of chaperone proteins, such as HSP70, that protect differentiation-relevant proteins, like the transcription factor GATA-1, from caspase-mediated proteolysis during erythropoiesis [13]. Similarly, during monocyte differentiation, the protein acinus is cleaved by activated caspase-3, while poly(ADP-ribose) polymerase is not processed [8].
Other apoptotic proteins that influence cell differentiation include APAF-1 and cytochrome c which underlie the activation of caspase-9 upon spatiotemporally restricted MMP also in non-apoptotic settings [7]. In addition, AIF, a caspase-independent cell death effector, and CRADD, an adaptor protein that transduces DNA-damage-elicited signals, both control the differentiation of adipocytes [14,15], though the underlying molecular mechanisms remain obscure. Of note, transgenic mice engineered for the overexpression of a dominant-negative variant of the TNFR1 functional interactor FADD or bearing a T-cell-specific Fadd gene knockout (the whole-body knockout is lethal, see below) exhibit retarded thymocyte development and reduced numbers of peripheral T cells [16,17]. It remains elusive whether such effects on T-cell differentiation are accounted for by the inactivation of the apoptotic or non-apoptotic functions of FADD.
Inflammation and immunity
One of the earliest recognized unconventional functions of the apoptotic apparatus is represented by the death-receptor-mediated activation of NF-κB-regulated inflammation [18]. Many other components of the apoptotic machinery, including several caspases, might participate in inflammatory and immune responses.
Ligand-bound death receptors, in particular TNFR1, have the potential to trigger a wide range of cellular responses ranging from cell death, through extrinsic apoptosis or regulated necrosis, to NF-κB activation. Depending on the cell type and specific context, NF-κB can transactivate genes with anti-apoptotic functions, such as BCL-2, or drive the production of pro-inflammatory mediators including TNFα and IFNγ [19]. Thus, it is not surprising that most, if not all, TNFR1 interactors, including caspase-8 and -10 [20], cIAP-1 and -2 (first characterized for their ability to bind, and hence inhibit, active caspase-3; [21]), TRADD, FADD and RIPK1 [22], influence inflammatory responses as they are required for optimal NF-κB activation. In addition, TRADD, RIPK1 and FADD participate in the intracellular signalling pathways elicited by PRRs such as RIG-I and TLR4 [23,24,25]. Similarly, XIAP, another member of the IAP family, is required for the full-blown activation of NF-κB by cytosolic PRRs of the NOD family [26], presumably due to its capacity to bind directly to RIPK2 [27]. Finally, a nuclear pool of TRADD reportedly binds to STAT1, thereby altering its DNA-binding activity in response to IFNγ [28].
Thus, several components of the extrinsic apoptotic pathway are involved in inflammation and innate immunity. This also applies to some caspases: caspase-1 and -5, which are responsible—within the supramolecular complex known as the inflammasome—for the proteolytic maturation of the pro-inflammatory cytokines IL-1β and IL-18 (Fig 1; [29,30]); caspase-11, which was originally described as an obligate co-activator of caspase-1 [31]; and caspase-12, which might be important for the attenuation of septic responses [32], as well as to the BH3-only protein BID, which can interact with cytosolic PRRs of the NOD family to facilitate the production of pro-inflammatory cytokines in response to NOD agonists [33]. Recent data suggest that the IL-1β-deficient phenotype of Casp1−/− mice described in 1995 [34,35] might be partly due to the concomitant, unwarranted deletion of Casp11 [36]. Accordingly, caspase-11 seems to be required for the caspase-1-mediated production of IL-1β by macrophages responding to bacterial products, but not to ATP and monosodium urate [36].
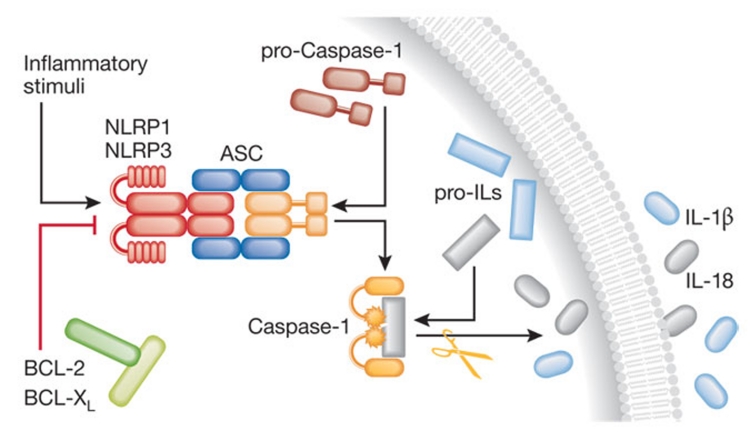
Figure 1. Pro-inflammatory functions of caspase-1.
Several stress conditions can trigger the assembly of the inflammasome, a multiprotein platform for the activation of caspase-1 consisting of specific PRRs such as NLRP1 and NLRP3 and the adaptor protein ASC. Active caspase-1 catalyses the proteolytic processing of pro-IL-1β and pro-IL-18 followed by the secretion of mature IL-1β and IL-18 into the extracellular milieu. BCL-2 and BCL-XL inhibit IL-1β and IL-18 secretion by binding to NLRP1. ASC, apoptosis-associated speck-like protein containing a CARD; BCL, B-cell lymphoma; IL, interleukin; NLRP, NLR family, pyrin domain containing; PRR, pattern recognition receptor.
The regulation of intrinsic apoptosis also impinges on immune functions. The BCL-2 protein family has a key role in both the induction (BH3-only members) and the regulation (pro- and anti-apoptotic multidomain members) of both MOMP- and MPT-driven MMP [37]. Moreover, BCL-2 and its anti-apoptotic homologue BCL-XL inhibit the inflammasome by interacting physically with NLRP1 [38,39]. The deletion of Endog, which codes for a mitochondrial endonuclease that participates in caspase-independent apoptosis [4], was initially thought to be embryonic-lethal in mice [40], owing to the unwarranted concomitant deletion of an adjacent gene. Subsequent studies with proper knockout models revealed that Endog−/− mice are viable and develop into adulthood with no obvious abnormalities [41]. B cells isolated from Endog−/− mice are deficient in Ig class switch, due to an impaired generation of double-strand breaks in the switch regions of Ig genes [42]. Although this aspect has not yet been investigated, it is tempting to speculate that Endog−/− mice might display increased susceptibility to some infectious diseases and a reduced propensity to develop autoimmune and allergic diseases.
Cell cycle control
Several constituents of the apoptotic apparatus, including components of both the extrinsic and the intrinsic pathway, have been shown to influence cell cycle progression, either by modulating proliferation in a rather general fashion or by controlling specific cell cycle phases (Fig 2).
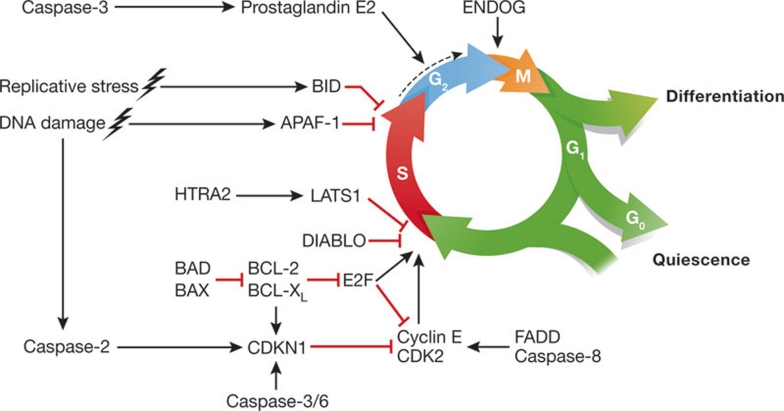
Figure 2. Cell cycle control by apoptotic regulators.
Several pro- and anti-apoptotic proteins have been shown to influence cell cycle progression. This can occur in a rather generalized fashion (as exemplified by the caspase-3-dependent release of prostaglandin E2 by dying cells) or involve specific cell cycle phases (as exemplified by the fact that cells overexpressing DIABLO are selectively arrested at the G1–S boundary). Moreover, apoptotic regulators can control the cell cycle under physiological circumstances (as suggested by the fact that ENDOG-deficient cells accumulate at the G2–M transition in the absence of any other stimulus) or in response to stress (as exemplified by caspase-2 and APAF-1, the depletion of which affects the cell cycle arrest induced by DNA damage). APAF-1, apoptotic peptidase activating factor 1; BAX, BCL-2-associated X protein; BCL, B-cell lymphoma; BID, BH3 interacting domain death agonist; CDK2, cyclin-dependent kinase 2; CDKN1, cyclin-dependent kinase inhibitor 1; DIABLO, direct IAP binding protein with low pI; ENDOG, endonuclease G; FADD, FAS-associated death domain protein; LATS1, large tumour suppressor, homologue 1.
Executioner caspases including caspase-3 and -6 reportedly restrain the proliferation of B cells [11,43], perhaps linked to changes in the expression of cell cycle inhibitors such as CDKN1 or to alterations in the requirements for B cells to trespass the G0–G1 boundary. In addition, caspase-3 might cause the release of prostaglandin E2 by tumour cells succumbing to chemotherapy, in turn stimulating the proliferation of residual, therapy-resistant, cancer cells [44]. Such a caspase-3-mediated autocrine circuit might have important therapeutic implications, as demonstrated by the fact that caspase-3 activation inversely correlates with disease-free and overall survival in cohorts of head and neck carcinoma, and breast cancer patients, respectively [44]. Intriguingly, the contribution of caspases to cell cycle regulation might be phylogenetically ancient, as suggested by the critical role of Leishmania major metacaspase in the correct segregation of the nucleus and the kinetoplast [45].
FADD and caspase-8 are required for the proper entry of activated lymphocytes into the S phase of the cell cycle [46,47]. T cells expressing a dominant-negative variant of FADD exhibit limited proliferation rates in response to TCR stimulation, correlating with defects in Ca2+ signalling [47], reduced phosphorylation of the S6 kinase, impaired expression of cyclin E and activation of CDK2 [46]. Many mitochondrial components of the apoptotic apparatus can participate in the regulation of the cell cycle. For instance, HTRA2—a mitochondrial protease that, upon MMP, promotes both caspase-dependent and -independent apoptosis—reportedly cleaves LATS1 in non-apoptotic circumstances, thereby generating LATS1 fragments that inhibit the G1–S transition [48]. Along similar lines, the ectopic overexpression of DIABLO (another mitochondrial activator of caspases that is released after MMP) has been demonstrated to arrest leukaemic cells at the G1–S boundary [49]. This contrasts with the observation that mice lacking the murine orthologue of DIABLO (Smac) are viable and normally develop into fertile adults [50], casting some doubts on the actual pathophysiological relevance of the apoptosis-unrelated roles of DIABLO. Finally, ENDOG not only seems to be required for DNA recombination and repair in both mammalian and yeast cells [51], but also might be necessary for proliferation, as ENDOG-depleted cells accumulate at the G2–M transition [52].
Both BCL-2 and BCL-XL function as negative regulators of the cell cycle, through several mechanisms. BCL-2 delays the G1–S transition by (i) inhibiting CDK2 [53]; (ii) upregulating CDKN1 [54]; and/or (iii) interfering with the transcriptional activity of E2F [54,55]. Similar cell cycle inhibitory functions have been ascribed to BCL-XL [56,57], while pro-apoptotic BCL-2-like proteins such as BAX and BAD stimulate cell cycle progression [53,56]. Of note, the cell cycle regulatory functions of anti-apoptotic BCL-2 family members seem to be evolutionarily conserved [57,58] and mediated by a specific protein domain that does not participate in the regulation of apoptosis [59].
The cytosolic adaptor APAF-1 is involved in the regulation of the cell cycle in response to DNA damaging agents, as demonstrated by the fact that APAF-1-depleted human cancer cells fail to accumulate in the S phase upon cisplatin- and irradiation-induced DNA damage [60] and that the absence of APAF-1 facilitates DNA-damage-induced chromosomal instability [61]. Bid−/− murine cells have also been ascribed with defects in the DNA damage response [62], but these results have been questioned by subsequent studies [63]. A nuclear pool of BID might be required for the proper activation of ATR in response to replicative stress [64]. Moreover, caspase-2—a DNA-damage-responsive caspase that can catalyse the proteolytic activation of BID [65]—appears to operate, independently of its catalytic functions and of any of its known interactors, as a translational regulator of CDKN1, mediating cytoprotective cell cycle arrest following DNA damage [66]. These observations suggest that components of the apoptotic machinery regulate the cell cycle not only in physiological settings but also in the context of stress responses.
Metabolism and autophagy
Some pieces of the molecular apparatus for apoptosis, notably those that operate in close connection with mitochondria, have been discovered to modulate various aspects of the bioenergetic and biosynthetic metabolism of the cell.
Due to its essential role as an electron shuttle between complexes III and IV of the mitochondrial respiratory chain, cytochrome c, which upon MMP drives the apoptosome-mediated activation of caspase-9 [67], is the most representative metabolism-relevant component of the apoptotic machinery (Fig 3; [68,69]). AIF, a phylogenetically ancient protein of the IMS, exhibits NADH oxidase enzymatic activity [70]. Besides its role as a caspase-independent cell death effector [71], mammalian AIF is required for the assembly or stabilization of respiratory complex I (Fig 3; [70]), and the whole-body knockout of Aif1 results in early embryonic death [72]. Yeast cells lacking aif1 proliferate slowly when cultured on non-fermentable energy sources and are characterized by a respiratory defect in complex III [70]. Similarly, Drosophila melanogaster larvae lacking the fly orthologue of AIF manifest respiratory deficits in complexes I and IV, resulting in premature death by day 8 after egg laying [73]. In humans, distinct loss-of-function mutations of AIF either cause prenatal ventriculomegaly linked to decreased activities of respiratory complexes I and IV [74] or a severe mitochondrial encephalomyopathy that manifests postnatally [75]. In mice, the muscle- and liver-specific deletion of Aif1 can result in increased glucose tolerance, enhanced insulin sensitivity, reduced fat mass and resistance against obesity induced by high caloric intake [76]. Altogether, these observations underscore an evolutionarily conserved, pathophysiologically relevant role of AIF in the regulation of mitochondrial metabolism.
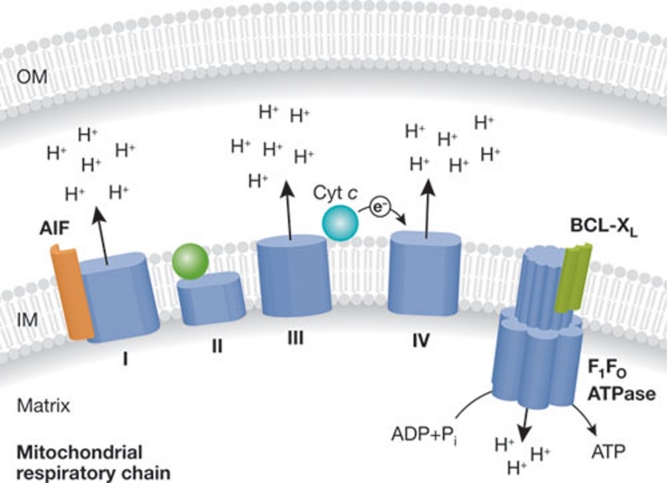
Figure 3. Metabolic roles of BCL-XL, cytochrome c and AIF.
Both cytochrome c and AIF are essential for the function of the respiratory chain, and thus for mitochondrial ATP generation through the F1Fo ATPase. In particular, cytochrome c mediates the transport of electrons between complex III and complex IV, while mammalian AIF is critical for the structural stability of complex I. BCL-XL binds to the F1Fo ATPase, thereby stimulating its enzymatic activity. AIF, apoptosis-inducing factor; BCL, B-cell lymphoma; IM, inner mitochondrial membrane; OM, outer mitochondrial membrane.
Another point at which apoptotic cell death and metabolism intersect is represented by the PTPC [4]. By assuming a high conductance state, the PTPC mediates MPT (thus initiating MMP) in response to selected intracellular stress conditions, such as the overgeneration of ROS and cytosolic Ca2+ overloads [4]. In addition, most, if not all, structural components and functional interactors of the PTPC mediate important metabolic functions. For example, ANT catalyses the exchange of ATP with ADP between the mitochondrial matrix and the cytosol [77]. VDAC operates as a voltage-regulated channel for the transport of anionic solutes across the outer mitochondrial membrane [78]. Cyclophilin D functions as a peptidyl-prolyl cis–trans isomerase, assisting the folding of proteins within the mitochondrial matrix [79]. The peripheral benzodiazepine receptor regulates the flux of cholesterol across mitochondrial membranes, thus controlling steroidogenesis [80]. Hexokinase converts glucose into glucose-6-phosphate, the starting block of glucose metabolism [81]. Finally, creatine kinase catalyses the ATP-dependent transformation of creatine into phosphocreatine, constituting a highly diffusible intracellular energy store [82]. Murine hepatocytes lacking Ant1 and Ant2 respond almost normally to inducers of MPT [83]. Similarly, Vdac1−/− Vdac2−/− Vdac3−/− MEFs undergo MPT that is indistinguishable from their normal counterparts [84]. These observations suggest that the PTPC, or at least some of its constituents, might be dispensable for MPT-driven cell death. However, many PTPC components exist in multiple, at least in part, functionally redundant isoforms, which complicates the generation of reliable gene knockout models [7]. One notable exception is provided by cyclophilin D, which, unexpectedly, is fully dispensable for embryonic and post-embryonic development but required for the induction of necrotic cell death in response to several distinct triggers, in vitro and in vivo [85]. Thus, the actual pathophysiological relevance of both the apoptotic and non-apoptotic functions of the PTPC remain to be elucidated.
Both pro- (for example, BAX) and anti-apoptotic (for example, BCL-2 and BCL-XL) BCL-2 family members operate at the ER to modulate the release of Ca2+ transients [86], which has direct implications for mitochondrial bioenergetics and for the function of a plethora of cytosolic Ca2+-regulated enzymes [87]. At the IM of neurons, BCL-XL interacts physically with and stimulates the F1Fo ATPase, the enzymatic complex that exploits the electrochemical gradient generated by respiratory complexes I–IV to synthesize ATP (Fig 3; [88]). In addition, anti-apoptotic proteins from the BCL-2 family bind to and inhibit the essential autophagic modulator Beclin 1 [89]. Conversely, multiple BH3-only proteins including BAD, BNIP3, BIM, BID and PUMA stimulate autophagy, owing to their ability to competitively displace Beclin 1 from inhibitory interactions with anti-apoptotic BCL-2 proteins [89]. Thus, by modulating autophagy, BCL-2 family members influence the maintenance of organellar homeostasis as well as the preservation of ATP and metabolic substrates during stress responses [90]. In addition, both pro- and anti-apoptotic BCL-2 proteins participate in the regulation of mitochondrial morphology and dynamics [91]. This intimate connection has important implications not only for apoptosis [92], but also for the bioenergetic functions of mitochondria in a variety of pathophysiological scenarios [93].
Regulation of non-apoptotic cell death
Recently, non-apoptotic instances of programmed cell death, in particular regulated necrosis, have spurred great interest [94]. Although the molecular characterization of this cell death subroutine is only in its infancy, it has become increasingly clear that the molecular machineries for apoptosis and regulated necrosis are interconnected in a complex network of reciprocal control.
One of the best-characterized pathways of regulated necrosis, which has been named necroptosis, is elicited by the ligation of TNFR1 in conditions in which caspases, in particular caspase-8, are inhibited [94]. Necroptosis relies on the activation and mutual interaction of two homologue kinases, RIPK1 and RIPK3, and is executed by a bioenergetic catastrophe that involves, among other processes, ROS overgeneration and enhanced glycogenolytic and glutaminolytic fluxes [94]. As early as in 1998, when the existence of regulated necrosis was first intuited, the inhibition of caspases by Z-VAD-fmk or by the overexpression of the viral serpin CrmA was found to enhance the sensitivity of murine fibrosarcoma cells to TNFα-induced cell death [95]. This discovery ignited an intense wave of research that, throughout the following decade, led to the discovery of the molecular mechanisms whereby multiple components of the apoptotic apparatus inhibit non-apoptotic cell death modalities, in particular regulated necrosis.
In line with the original observations by Vercammen and colleagues [95], caspase-8 has been shown to tonically inhibit regulated necrosis by mediating the proteolytic cleavage of RIPK1 and RIPK3 [96,97]. Casp8−/− mice do not survive embryogenesis beyond day 11.5 [98]. Strikingly, this embryonic lethal phenotype fully depends on RIPK3, as Casp8−/− Ripk3−/− mice develop into fertile adults [99]. Of note, Casp8−/− Ripk3−/− mice accumulate abnormal T cells in peripheral lymphoid organs, thus far resembling Fas−/− mice [99]. These results suggest that caspase-8 is required for the proper development of the T-cell compartment by virtue of its apoptotic functions, whereas it is indispensable for embryonic development owing to its role as an anti-necrotic factor (Fig 4).
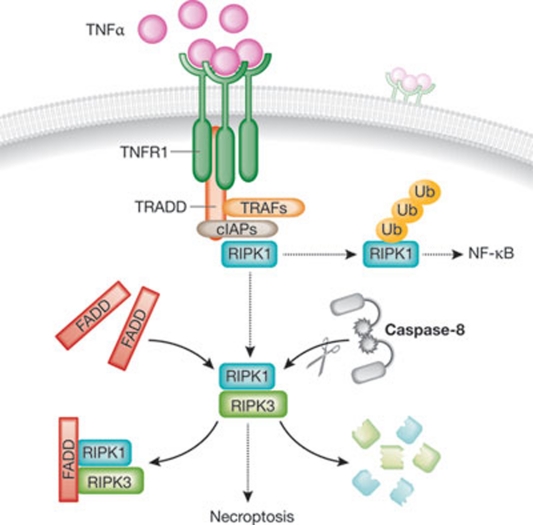
Figure 4. Anti-necrotic functions of caspase-8 and FADD.
In response to TNFα, the intracellular tails of TNFR1 trimers drive the assembly of a supramolecular complex including, among other factors, TRADD, TRAFs, cIAPs and RIPK1. Upon ubiquitination, RIPK1 can stimulate the canonical pathway of activation of the anti-apoptotic and pro-inflammatory transcription factor NF-κB. Alternatively, deubiquitinated RIPK1 can interact with RIPK3 and trigger necroptosis. The pro-apoptotic proteins FADD and caspase-8, the latter by cleaving RIPK1 and RIPK3, tonically inhibit this pro-necrotic signalling cascade. cIAP, cellular inhibitor of apoptosis protein; FADD, FAS-associated death domain protein; NF-κB, nuclear factor-κB; RIPK; receptor-interacting protein kinase; TNFα, tumour necrosis factor α; TNFR1, TNFα receptor 1; TRADD, TNFR1-associated death domain protein; TRAF, TNFR-associated factor.
Similarly to their Casp8−/− counterparts, neither Fadd−/− nor Ripk1−/− mice are viable. Fadd−/− mice die in utero at embryonic day 15.5, displaying widespread necrosis and signs of cardiac failure and abdominal haemorrhage [100,101]. Ripk1−/− mice appear normal at birth but succumb at 1–3 days of age, manifesting extensive apoptosis in the lymphoid and adipose compartments [102]. Notably, Fadd−/− Ripk1−/− double knockout mice are born at Mendelian frequencies, yet fail to survive beyond 3 weeks of age [101]. Moreover, lymphocytes isolated from Fadd−/− Ripk1−/− mice maintain some of the developmental defects that characterize their Ripk1−/− counterparts [101]. Thus, while Fadd seems to be dispensable for the phenotypic manifestations of the Ripk1 knockout, at both the organismal and cellular levels, Ripk1 is required for the embryonic lethality induced by the absence of Fadd (Fig 4). The intestinal epithelial-cell-specific knockout of Fadd has also been associated with spontaneous enteritis and colitis in mice, a pathology that failed to develop if Ripk3 was simultaneously knocked out [103]. Similarly, the absence of Fadd in epidermal keratinocytes drives Ripk3-dependent necrosis and skin inflammation in mice [104]. Taken together, these observations demonstrate that the anti-necrotic activities of apoptotic regulators are particularly relevant in vivo, in both developmental and pathological scenarios.
Others
In addition to the structured functional profiles discussed above, some apoptotic regulators and executioners exert highly specific, often cell-type-dependent and context-dependent, activities, as illustrated here by a few examples.
Caspase-1 has been shown to mediate the proteolysis-independent secretion of multifunctional proteins including pro-IL-1α and FGF2 [105]. In neurons, excessive but non-apoptotic caspase-3 activity triggers the calcineurin-mediated dephosphorylation and subsequent removal of AMPA-type receptors, leading to the degeneration of dendritic spines and alterations in glutamatergic signalling [106]. BCL-2 family members including BAK and BAX stimulate the unfolded protein response by interacting physically with the ER stress sensor IRE1α [107]. FADD participates in the induction of neuroplasticity and in the development of the abstinence syndrome [108]. HTRA2 is important for the maintenance of neuronal homeostasis, and loss-of-function mutations of HTRA2 have been associated with familial variants of Parkinson disease [109]. Finally, signals transduced by the death receptor FAS have been implicated in the paracrine crosstalk between the implanting embryo and endometrial cells [110]. Thus, the unconventional functions of the apoptotic apparatus are relevant in a plethora of distinct pathophysiological settings.
Concluding remarks
As we have discussed in this review, during the past two decades the molecular machinery for apoptosis has been demonstrated to mediate a wide array of non-apoptotic effects. These range from general bioenergetic functions, such as those mediated by cytochrome c, the depletion of which is incompatible with mammalian life, to more specific, often cell-type-restricted activities. These observations suggest that the apoptotic machinery has not evolved 'from scratch' but rather by taking advantage of pre-existing proteins with non-lethal functions. In this hypothetical scenario, one might wonder which proteins would have been selected by evolution to mediate apoptosis. On the basis of our current knowledge of the apoptotic apparatus, it is tempting to speculate that two types of proteins have mainly been co-opted to acquire functions in cell death regulation. First, proteins with peculiar structural features and/or that are normally segregated in non-accessible cell compartments. This is the case with IMS proteins such as cytochrome c and AIF, which receive their prosthetic groups—haem and FAD, respectively—and acquire their final conformation only once their precursors have been imported into mitochondria. Second, proteins that play a role in the management of stress. These factors—including BCL-2 family members, which regulate autophagy and the unfolded protein response, APAF-1, which controls DNA damage responses, and others (see above)—might have initially evolved as stress-response proteins and subsequently acquired the capacity to regulate the demise of severely damaged cells.
One particular type of stress that is intimately linked to cell death is represented by viral infection. On the one hand, viruses must divert the cell metabolism to their own advantage, while avoiding the premature death of their hosts. On the other hand, at late stages of viral infection, viruses often actively induce cell death, to facilitate the dissemination of new infectious particles. Viral genomes encode a plethora of apoptotic regulators, including anti-apoptotic orthologues of BCL-2 and inhibitors of caspases that operate similarly to IAPs [111]. Moreover, as viral genomes are sometimes very small, they might have favoured the selection of proteins with multiple functions. Thus, at least some of the proteins that regulate both lethal and vital aspects of the cellular metabolism might have evolved in viruses and have subsequently been acquired by host cells during host–pathogen co-evolution.
One intriguing question is how apoptotic regulators might get activated to mediate non-apoptotic functions without affecting cell death. In addition to what was mentioned above for caspases, there are many scenarios that might at least partly explain this. First, activation threshold: while generalized MMP—that is, MMP near-to-simultaneously involving most mitochondria within one single cell—is lethal, MMP affecting single mitochondria is a relatively common process that entails their autophagic removal [90]. In this scenario, IMS proteins might be released in insufficient levels to trigger cell death, yet in amounts high enough to exert non-apoptotic functions. Second, post-translational modifications: several apoptotic regulators, including members of the BCL-2 protein family, are subjected to post-translation control, for instance being (de)activated upon (de)acetylation or (de)phosphorylation (for example, BAD and BCL-2; [112,113]), or as a result of de(oligomerization) reactions (for example, BAX; [114]). Thus, distinct sets of post-translational modifications might activate the same protein to mediate distinct effects. In cases in which apoptotic and non-apoptotic functions have been mapped to different domains of the same protein [39,59], post-translational modifications might simply determine the predominant activity by exposing or concealing the corresponding domains. Third, subcellular localization: multiple apoptotic regulators are localized, either physiologically or upon translocation in response to specific triggers, to distinct subcellular compartments. For instance, under physiological conditions, BCL-2 can be found to variable extents at the ER, at the OM and in the nucleus [115], whereas AIF can be detected in extra-mitochondrial sites only upon MMP [71]. In this scenario, the same protein might exhibit context-dependent activities, perhaps linked to localization-specific post-translational changes or interactors.
By identifying and thoroughly characterizing further non-apoptotic functions of apoptosis-regulatory proteins, future studies will surely provide additional insights into these questions (see also Sidebar A).
Sidebar A | In need of answers.
One of the main questions that permeate cell death research since the discovery of the role of cytochrome c in intrinsic apoptosis is to which extent the different—death-related and death-unrelated—functions of apoptotic proteins contribute to the phenotypic effects of their pharmacological or genetic inhibition? The fact that most, if not all, proteins involved in the regulation and execution of apoptosis exert several non-apoptotic functions has provided an additional layer of complexity to cell death research, at least at two distinct levels. First, appropriate knockout models could not be generated for all proteins with a key role in metabolism at the organismal level, including cytochrome c and AIF. Second, the phenotypic manifestations of knockout models could not be appropriately interpreted for components of the apoptotic machinery that are dispensable for mammalian development, such as HTRA2. Appropriate genetic models in which the apoptotic and non-apoptotic functions of cytochrome c and AIF can be discriminated from one another have been generated [116,117], but these remain notable exceptions. Most often, the molecular determinants that underlie the pleiotropic functions of apoptotic proteins have not yet been entirely understood, de facto precluding similar developments. The generation of an in-depth molecular understanding of the apoptotic machinery should allow for the establishment of genetic models in which the multifaceted activities of apoptotic regulators are uncoupled, in turn providing deeper insights into the actual requirement for apoptosis in embryonic and post-embryonic development.

Lorenzo Galluzzi, Guido Kroemer, Christina Trojel-Hansen & Oliver Kepp
Acknowledgments
The authors are supported by the Ligue contre le Cancer (équipe labellisée), AXA Chair for Longevity Research, Cancéropôle Ile-de-France, Institut National du Cancer (INCa), Fondation Bettencourt-Schueller, Fondation de France, Fondation pour la Recherche Médicale, Agence National de la Recherche and the European Commission (Apo-Sys, ArtForce, ChemoRes. Death-Train) and the LabEx Immuno-Oncology.
Footnotes
The authors declare that they have no conflict of interest.
References
- Kroemer G et al. (2009) Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ 16: 3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L et al. (2012) Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ 19: 107–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait SW, Green DR (2010) Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol 11: 621–632 [DOI] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Brenner C (2007) Mitochondrial membrane permeabilization in cell death. Physiol Rev 87: 99–163 [DOI] [PubMed] [Google Scholar]
- Brenner C, Grimm S (2006) The permeability transition pore complex in cancer cell death. Oncogene 25: 4744–4756 [DOI] [PubMed] [Google Scholar]
- Wajant H (2002) The Fas signaling pathway: more than a paradigm. Science 296: 1635–1636 [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Joza N, Tasdemir E, Maiuri MC, Hengartner M, Abrams JM, Tavernarakis N, Penninger J, Madeo F, Kroemer G (2008b) No death without life: vital functions of apoptotic effectors. Cell Death Differ 15: 1113–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zermati Y, Garrido C, Amsellem S, Fishelson S, Bouscary D, Valensi F, Varet B, Solary E, Hermine O (2001) Caspase activation is required for terminal erythroid differentiation. J Exp Med 193: 247–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordet O, Rebe C, Plenchette S, Zermati Y, Hermine O, Vainchenker W, Garrido C, Solary E, Dubrez-Daloz L (2002) Specific involvement of caspases in the differentiation of monocytes into macrophages. Blood 100: 4446–4453 [DOI] [PubMed] [Google Scholar]
- Black S, Kadyrov M, Kaufmann P, Ugele B, Emans N, Huppertz B (2004) Syncytial fusion of human trophoblast depends on caspase 8. Cell Death Differ 11: 90–98 [DOI] [PubMed] [Google Scholar]
- Watanabe C, Shu GL, Zheng TS, Flavell RA, Clark EA (2008) Caspase 6 regulates B cell activation and differentiation into plasma cells. J Immunol 181: 6810–6819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Botton S, Sabri S, Daugas E, Zermati Y, Guidotti JE, Hermine O, Kroemer G, Vainchenker W, Debili N (2002) Platelet formation is the consequence of caspase activation within megakaryocytes. Blood 100: 1310–1317 [DOI] [PubMed] [Google Scholar]
- Ribeil JA et al. (2007) Hsp70 regulates erythropoiesis by preventing caspase-3-mediated cleavage of GATA-1. Nature 445: 102–105 [DOI] [PubMed] [Google Scholar]
- Felmer R, Horvat S, Clinton M, Clark AJ (2003) Overexpression of Raidd cDNA inhibits differentiation of mouse preadipocytes. Cell Prolif 36: 45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen SM, Yu Y, Wu ZX, Zheng Y, Chen GQ, Wang LS (2011) Apoptosis-inducing factor is a target gene of C/EBPa and participates in adipocyte differentiation. FEBS Lett 585: 2307–2312 [DOI] [PubMed] [Google Scholar]
- Walsh CM, Wen BG, Chinnaiyan AM, O'Rourke K, Dixit VM, Hedrick SM (1998) A role for FADD in T cell activation and development. Immunity 8: 439–449 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Rosenberg S, Wang H, Imtiyaz HZ, Hou YJ, Zhang J (2005) Conditional Fas-associated death domain protein (FADD): GFP knockout mice reveal FADD is dispensable in thymic development but essential in peripheral T cell homeostasis. J Immunol 175: 3033–3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Neriah Y, Karin M (2011) Inflammation meets cancer, with NF-κB as the matchmaker. Nat Immunol 12: 715–723 [DOI] [PubMed] [Google Scholar]
- Perkins ND (2007) Integrating cell-signalling pathways with NF-κB and IKK function. Nat Rev Mol Cell Biol 8: 49–62 [DOI] [PubMed] [Google Scholar]
- Shikama Y, Yamada M, Miyashita T (2003) Caspase-8 and caspase-10 activate NF-κB through RIP, NIK and IKKα kinases. Eur J Immunol 33: 1998–2006 [DOI] [PubMed] [Google Scholar]
- Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, Gillard JW, Jaquith JB, Morris SJ, Barker PA (2008) cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell 30: 689–700 [DOI] [PubMed] [Google Scholar]
- Ermolaeva MA, Michallet MC, Papadopoulou N, Utermohlen O, Kranidioti K, Kollias G, Tschopp J, Pasparakis M (2008) Function of TRADD in tumor necrosis factor receptor 1 signaling and in TRIF-dependent inflammatory responses. Nat Immunol 9: 1037–1046 [DOI] [PubMed] [Google Scholar]
- Balachandran S, Venkataraman T, Fisher PB, Barber GN (2007) Fas-associated death domain-containing protein-mediated antiviral innate immune signaling involves the regulation of Irf7. J Immunol 178: 2429–2439 [DOI] [PubMed] [Google Scholar]
- Michallet MC et al. (2008) TRADD protein is an essential component of the RIG-like helicase antiviral pathway. Immunity 28: 651–661 [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang H, Li J, Rosenberg S, Zhang EC, Zhou X, Qin F, Farabaugh M (2011) RIP1-mediated regulation of lymphocyte survival and death responses. Immunol Res 51: 227–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauler LD, Duckett CS, O'Riordan MX (2008) XIAP regulates cytosol-specific innate immunity to Listeria infection. PLoS Pathog 4: e1000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg A, Correa RG, Garrison JB, Le Negrate G, Welsh K, Huang Z, Knoefel WT, Reed JC (2009) XIAP mediates NOD signaling via interaction with RIP2. Proc Natl Acad Sci USA 106: 14524–14529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesemann DR, Qin H, Kokorina N, Benveniste EN (2004) TRADD interacts with STAT1α and influences interferon-γ signaling. Nat Immunol 5: 199–207 [DOI] [PubMed] [Google Scholar]
- Bian ZM, Elner SG, Khanna H, Murga-Zamalloa CA, Patil S, Elner VM (2011) Expression and functional roles of caspase-5 in inflammatory responses of human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 52: 8646–8656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F, Tschopp J (2004) Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell 117: 561–574 [DOI] [PubMed] [Google Scholar]
- Wang S, Miura M, Jung YK, Zhu H, Li E, Yuan J (1998) Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell 92: 501–509 [DOI] [PubMed] [Google Scholar]
- Saleh M et al. (2004) Differential modulation of endotoxin responsiveness by human caspase-12 polymorphisms. Nature 429: 75–79 [DOI] [PubMed] [Google Scholar]
- Yeretssian G, Correa RG, Doiron K, Fitzgerald P, Dillon CP, Green DR, Reed JC, Saleh M (2011) Non-apoptotic role of BID in inflammation and innate immunity. Nature 474: 96–99 [DOI] [PubMed] [Google Scholar]
- Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA (1995) Altered cytokine export and apoptosis in mice deficient in interleukin-1β converting enzyme. Science 267: 2000–2003 [DOI] [PubMed] [Google Scholar]
- Li P et al. (1995) Mice deficient in IL-1β-converting enzyme are defective in production of mature IL-1β and resistant to endotoxic shock. Cell 80: 401–411 [DOI] [PubMed] [Google Scholar]
- Kayagaki N et al. (2011) Non-canonical inflammasome activation targets caspase-11. Nature 479: 117–121 [DOI] [PubMed] [Google Scholar]
- Youle RJ, Strasser A (2008) The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9: 47–59 [DOI] [PubMed] [Google Scholar]
- Bruey JM et al. (2007) Bcl-2 and Bcl-XL regulate proinflammatory caspase-1 activation by interaction with NALP1. Cell 129: 45–56 [DOI] [PubMed] [Google Scholar]
- Faustin B, Chen Y, Zhai D, Le Negrate G, Lartigue L, Satterthwait A, Reed JC (2009) Mechanism of Bcl-2 and Bcl-X(L) inhibition of NLRP1 inflammasome: loop domain-dependent suppression of ATP binding and oligomerization. Proc Natl Acad Sci USA 106: 3935–3940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J et al. (2003) Endonuclease G is required for early embryogenesis and normal apoptosis in mice. Proc Natl Acad Sci USA 100: 15782–15787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David KK, Sasaki M, Yu SW, Dawson TM, Dawson VL (2006) EndoG is dispensable in embryogenesis and apoptosis. Cell Death Differ 13: 1147–1155 [DOI] [PubMed] [Google Scholar]
- Zan H, Zhang J, Al-Qahtani A, Pone EJ, White CA, Lee D, Yel L, Mai T, Casali P (2011) Endonuclease G plays a role in immunoglobulin class switch DNA recombination by introducing double-strand breaks in switch regions. Mol Immunol 48: 610–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo M et al. (2003) Caspase-3 regulates cell cycle in B cells: a consequence of substrate specificity. Nat Immunol 4: 1016–1022 [DOI] [PubMed] [Google Scholar]
- Huang Q et al. (2011) Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat Med 17: 860–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambit A, Fasel N, Coombs GH, Mottram JC (2008) An essential role for the Leishmania major metacaspase in cell cycle progression. Cell Death Differ 15: 113–122 [DOI] [PubMed] [Google Scholar]
- Arechiga AF et al. (2007) A Fas-associated death domain protein/caspase-8-signaling axis promotes S-phase entry and maintains S6 kinase activity in T cells responding to IL-2. J Immunol 179: 5291–5300 [DOI] [PubMed] [Google Scholar]
- Hueber AO, Zornig M, Bernard AM, Chautan M, Evan G (2000) A dominant negative Fas-associated death domain protein mutant inhibits proliferation and leads to impaired calcium mobilization in both T-cells and fibroblasts. J Biol Chem 275: 10453–10462 [DOI] [PubMed] [Google Scholar]
- Kuninaka S et al. (2007) Serine protease Omi/HtrA2 targets WARTS kinase to control cell proliferation. Oncogene 26: 2395–2406 [DOI] [PubMed] [Google Scholar]
- Jia L, Patwari Y, Kelsey SM, Srinivasula SM, Agrawal SG, Alnemri ES, Newland AC (2003) Role of Smac in human leukaemic cell apoptosis and proliferation. Oncogene 22: 1589–1599 [DOI] [PubMed] [Google Scholar]
- Okada H et al. (2002) Generation and characterization of Smac/DIABLO-deficient mice. Mol Cell Biol 22: 3509–3517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttner S et al. (2007) Endonuclease G regulates budding yeast life and death. Mol Cell 25: 233–246 [DOI] [PubMed] [Google Scholar]
- Huang KJ, Ku CC, Lehman IR (2006) Endonuclease G: a role for the enzyme in recombination and cellular proliferation. Proc Natl Acad Sci USA 103: 8995–9000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Gomez G, Berns A, Brady HJ (1998) A link between cell cycle and cell death: Bax and Bcl-2 modulate Cdk2 activation during thymocyte apoptosis. EMBO J 17: 7209–7218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vairo G, Soos TJ, Upton TM, Zalvide J, DeCaprio JA, Ewen ME, Koff A, Adams JM (2000) Bcl-2 retards cell cycle entry through p27(Kip1), pRB relative p130, and altered E2F regulation. Mol Cell Biol 20: 4745–4753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind EF, Wayne J, Wang QZ, Staeva T, Stolzer A, Petrie HT (1999) Bcl-2-induced changes in E2F regulatory complexes reveal the potential for integrated cell cycle and cell death functions. J Immunol 162: 5374–5379 [PubMed] [Google Scholar]
- Janumyan YM, Sansam CG, Chattopadhyay A, Cheng N, Soucie EL, Penn LZ, Andrews D, Knudson CM, Yang E (2003) Bcl-xL/Bcl-2 coordinately regulates apoptosis, cell cycle arrest and cell cycle entry. EMBO J 22: 5459–5470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly LA, Huang DC, Strasser A (1996) The cell death inhibitor Bcl-2 and its homologues influence control of cell cycle entry. EMBO J 15: 6979–6990 [PMC free article] [PubMed] [Google Scholar]
- Quinn L, Coombe M, Mills K, Daish T, Colussi P, Kumar S, Richardson H (2003) Buffy, a Drosophila Bcl-2 protein, has anti-apoptotic and cell cycle inhibitory functions. EMBO J 22: 3568–3579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DC, O'Reilly LA, Strasser A, Cory S (1997) The anti-apoptosis function of Bcl-2 can be genetically separated from its inhibitory effect on cell cycle entry. EMBO J 16: 4628–4638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zermati Y et al. (2007) Nonapoptotic role for Apaf-1 in the DNA damage checkpoint. Mol Cell 28: 624–637 [DOI] [PubMed] [Google Scholar]
- Mouhamad S, Galluzzi L, Zermati Y, Castedo M, Kroemer G (2007) Apaf-1 deficiency causes chromosomal instability. Cell Cycle 6: 3103–3107 [DOI] [PubMed] [Google Scholar]
- Kamer I, Sarig R, Zaltsman Y, Niv H, Oberkovitz G, Regev L, Haimovich G, Lerenthal Y, Marcellus RC, Gross A (2005) Proapoptotic BID is an ATM effector in the DNA-damage response. Cell 122: 593–603 [DOI] [PubMed] [Google Scholar]
- Kaufmann T, Tai L, Ekert PG, Huang DC, Norris F, Lindemann RK, Johnstone RW, Dixit VM, Strasser A (2007) The BH3-only protein bid is dispensable for DNA damage- and replicative stress-induced apoptosis or cell-cycle arrest. Cell 129: 423–433 [DOI] [PubMed] [Google Scholar]
- Liu Y, Bertram CC, Shi Q, Zinkel SS (2011) Proapoptotic Bid mediates the Atr-directed DNA damage response to replicative stress. Cell Death Differ 18: 841–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Srinivasula SM, Druilhe A, Fernandes-Alnemri T, Alnemri ES (2002) Caspase-2 induces apoptosis by releasing proapoptotic proteins from mitochondria. J Biol Chem 277: 13430–13437 [DOI] [PubMed] [Google Scholar]
- Sohn D, Budach W, Janicke RU (2011) Caspase-2 is required for DNA damage-induced expression of the CDK inhibitor p21(WAF1/CIP1). Cell Death Differ 18: 1664–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G (2006) Mechanisms of cytochrome c release from mitochondria. Cell Death Differ 13: 1423–1433 [DOI] [PubMed] [Google Scholar]
- Hosler JP, Ferguson-Miller S, Mills DA (2006) Energy transduction: proton transfer through the respiratory complexes. Annu Rev Biochem 75: 165–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Li Y, Shelton JM, Richardson JA, Spencer E, Chen ZJ, Wang X, Williams RS (2000) Cytochrome c deficiency causes embryonic lethality and attenuates stress-induced apoptosis. Cell 101: 389–399 [DOI] [PubMed] [Google Scholar]
- Vahsen N et al. (2004) AIF deficiency compromises oxidative phosphorylation. EMBO J 23: 4679–4689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joza N et al. (2001) Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature 410: 549–554 [DOI] [PubMed] [Google Scholar]
- Brown D, Yu BD, Joza N, Benit P, Meneses J, Firpo M, Rustin P, Penninger JM, Martin GR (2006) Loss of Aif function causes cell death in the mouse embryo, but the temporal progression of patterning is normal. Proc Natl Acad Sci USA 103: 9918–9923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joza N et al. (2008) The molecular archaeology of a mitochondrial death effector: AIF in Drosophila. Cell Death Differ 15: 1009–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger I, Ben-Neriah Z, Dor-Wolman T, Shaag A, Saada A, Zenvirt S, Raas-Rothschild A, Nadjari M, Kaestner KH, Elpeleg O (2012) Early prenatal ventriculomegaly due to an AIFM1 mutation identified by linkage analysis and whole exome sequencing. Mol Genet Metab (in the press) [DOI] [PubMed] [Google Scholar]
- Ghezzi D, Sevrioukova I, Invernizzi F, Lamperti C, Mora M, D'Adamo P, Novara F, Zuffardi O, Uziel G, Zeviani M (2010) Severe X-linked mitochondrial encephalomyopathy associated with a mutation in apoptosis-inducing factor. Am J Hum Genet 86: 639–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pospisilik JA et al. (2007) Targeted deletion of AIF decreases mitochondrial oxidative phosphorylation and protects from obesity and diabetes. Cell 131: 476–491 [DOI] [PubMed] [Google Scholar]
- Belzacq AS, Brenner C (2003) The adenine nucleotide translocator: a new potential chemotherapeutic target. Curr Drug Targets 4: 517–524 [DOI] [PubMed] [Google Scholar]
- Colombini M (1980) Structure and mode of action of a voltage dependent anion-selective channel (VDAC) located in the outer mitochondrial membrane. Ann NY Acad Sci 341: 552–563 [DOI] [PubMed] [Google Scholar]
- Gothel SF, Marahiel MA (1999) Peptidyl-prolyl cis-trans isomerases, a superfamily of ubiquitous folding catalysts. Cell Mol Life Sci 55: 423–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos V (2003) Peripheral benzodiazepine receptor: structure and function in health and disease. Ann Pharm Fr 61: 30–50 [PubMed] [Google Scholar]
- Wilson JE (2003) Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol 206: 2049–2057 [DOI] [PubMed] [Google Scholar]
- Schlattner U, Tokarska-Schlattner M, Wallimann T (2006) Mitochondrial creatine kinase in human health and disease. Biochim Biophys Acta 1762: 164–180 [DOI] [PubMed] [Google Scholar]
- Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC (2004) The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature 427: 461–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD (2007) Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol 9: 550–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines CP et al. (2005) Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434: 658–662 [DOI] [PubMed] [Google Scholar]
- Rong Y, Distelhorst CW (2008) Bcl-2 protein family members: versatile regulators of calcium signaling in cell survival and apoptosis. Annu Rev Physiol 70: 73–91 [DOI] [PubMed] [Google Scholar]
- Rutter GA, Rizzuto R (2000) Regulation of mitochondrial metabolism by ER Ca2+ release: an intimate connection. Trends Biochem Sci 25: 215–221 [DOI] [PubMed] [Google Scholar]
- Alavian KN et al. (2011) Bcl-xL regulates metabolic efficiency of neurons through interaction with the mitochondrial F1FO ATP synthase. Nat Cell Biol 13: 1224–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiuri MC, Zalckvar E, Kimchi A, Kroemer G (2007) Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 8: 741–752 [DOI] [PubMed] [Google Scholar]
- Green DR, Galluzzi L, Kroemer G (2011) Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science 333: 1109–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland SG, Conradt B (2010) New role of the BCL2 family of proteins in the regulation of mitochondrial dynamics. Curr Opin Cell Biol 22: 852–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suen DF, Norris KL, Youle RJ (2008) Mitochondrial dynamics and apoptosis. Genes Dev 22: 1577–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannella CA (2008) Structural diversity of mitochondria: functional implications. Ann NY Acad Sci 1147: 171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G (2010) Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol 11: 700–714 [DOI] [PubMed] [Google Scholar]
- Vercammen D, Beyaert R, Denecker G, Goossens V, Van Loo G, Declercq W, Grooten J, Fiers W, Vandenabeele P (1998) Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med 187: 1477–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Yang Y, Mei Y, Ma L, Zhu DE, Hoti N, Castanares M, Wu M (2007) Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal 19: 2056–2067 [DOI] [PubMed] [Google Scholar]
- He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X (2009) Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-α. Cell 137: 1100–1111 [DOI] [PubMed] [Google Scholar]
- Varfolomeev EE et al. (1998) Targeted disruption of the mouse caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity 9: 267–276 [DOI] [PubMed] [Google Scholar]
- Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES (2011) RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471: 368–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh WC et al. (1998) FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science 279: 1954–1958 [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhou X, McQuade T, Li J, Chan FK, Zhang J (2011) Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature 471: 373–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P (1998) The death domain kinase RIP mediates the TNF-induced NF-κB signal. Immunity 8: 297–303 [DOI] [PubMed] [Google Scholar]
- Welz PS, Wullaert A, Vlantis K, Kondylis V, Fernandez-Majada V, Ermolaeva M, Kirsch P, Sterner-Kock A, van Loo G, Pasparakis M (2011) FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature 477: 330–334 [DOI] [PubMed] [Google Scholar]
- Bonnet MC, Preukschat D, Welz PS, van Loo G, Ermolaeva MA, Bloch W, Haase I, Pasparakis M (2011) The adaptor protein FADD protects epidermal keratinocytes from necroptosis in vivo and prevents skin inflammation. Immunity 35: 572–582 [DOI] [PubMed] [Google Scholar]
- Keller M, Ruegg A, Werner S, Beer HD (2008) Active caspase-1 is a regulator of unconventional protein secretion. Cell 132: 818–831 [DOI] [PubMed] [Google Scholar]
- D'Amelio M et al. (2011) Caspase-3 triggers early synaptic dysfunction in a mouse model of Alzheimer's disease. Nat Neurosci 14: 69–76 [DOI] [PubMed] [Google Scholar]
- Hetz C et al. (2006) Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1α. Science 312: 572–576 [DOI] [PubMed] [Google Scholar]
- Ramos-Miguel A, Esteban S, Garcia-Sevilla JA (2010) The time course of unconditioned morphine-induced psychomotor sensitization mirrors the phosphorylation of FADD and MEK/ERK in rat striatum: role of PEA-15 as a FADD-ERK binding partner in striatal plasticity. Eur Neuropsychopharmacol 20: 49–64 [DOI] [PubMed] [Google Scholar]
- Plun-Favreau H et al. (2007) The mitochondrial protease HtrA2 is regulated by Parkinson's disease-associated kinase PINK1. Nat Cell Biol 9: 1243–1252 [DOI] [PubMed] [Google Scholar]
- Fluhr H, Wenig H, Spratte J, Heidrich S, Ehrhardt J, Zygmunt M (2011) Non-apoptotic Fas-induced regulation of cytokines in undifferentiated and decidualized human endometrial stromal cells depends on caspase-activity. Mol Hum Reprod 17: 127–134 [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Brenner C, Morselli E, Touat Z, Kroemer G (2008a) Viral control of mitochondrial apoptosis. PLoS Pathog 4: e1000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassik MC, Scorrano L, Oakes SA, Pozzan T, Korsmeyer SJ (2004) Phosphorylation of BCL-2 regulates ER Ca2+ homeostasis and apoptosis. EMBO J 23: 1207–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Minemoto Y, Zhang J, Liu J, Tang F, Bui TN, Xiang J, Lin A (2004) JNK suppresses apoptosis via phosphorylation of the proapoptotic Bcl-2 family protein BAD. Mol Cell 13: 329–340 [DOI] [PubMed] [Google Scholar]
- Cartron PF, Priault M, Oliver L, Meflah K, Manon S, Vallette FM (2003) The N-terminal end of Bax contains a mitochondrial-targeting signal. J Biol Chem 278: 11633–11641 [DOI] [PubMed] [Google Scholar]
- Lu QL, Hanby AM, Nasser Hajibagheri MA, Gschmeissner SE, Lu PJ, Taylor-Papadimitriou J, Krajewski S, Reed JC, Wright NA (1994) Bcl-2 protein localizes to the chromosomes of mitotic nuclei and is correlated with the cell cycle in cultured epithelial cell lines. J Cell Sci 107: 363–371 [DOI] [PubMed] [Google Scholar]
- Cheung EC et al. (2006) Dissociating the dual roles of apoptosis-inducing factor in maintaining mitochondrial structure and apoptosis. EMBO J 25: 4061–4073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Z et al. (2005) Specific ablation of the apoptotic functions of cytochrome C reveals a differential requirement for cytochrome C and Apaf-1 in apoptosis. Cell 121: 579–591 [DOI] [PubMed] [Google Scholar]