Abstract
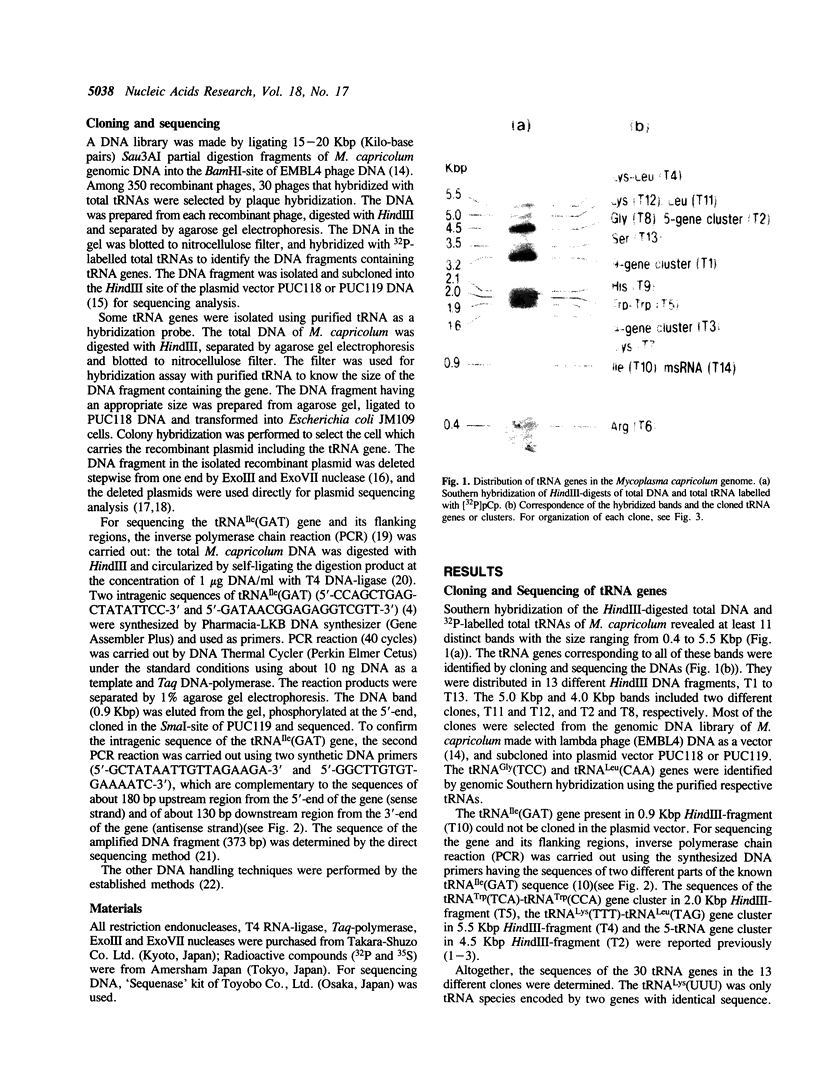
The genes for presumably all the tRNA species in Mycoplasma capricolum, a derivative of Gram-positive eubacteria, have been cloned and sequenced. There are 30 genes encoding 29 tRNA species. This number is the smallest in all the known genetic systems except for mitochondria. The sequences of 9 tRNA genes of them have been previously reported (1-3). Twenty-two genes are organized in 5 clusters consisting of nine, five, four and two genes (2 sets), respectively. The other eight genes exist as a single transcription unit. All the tRNAs are encoded each by a single gene, except for the occurrence of two tRNA(Lys)(TTT) genes. The arrangement of tRNA genes in the 9-gene cluster, the 5-gene cluster, the 4-gene cluster and one of the 2-gene clusters reveals extensive similarity with a part of the 21-tRNA gene cluster and/or the 16-tRNA gene cluster in Bacillus subtilis, respectively. The results suggest that the present M. capricolum tRNA genes have evolved from large tRNA gene clusters in the ancestral Gram-positive bacterial genome common to M. capricolum and B. subtilis, by discarding genes for redundant as well as non-obligate tRNAs, so that all the codons may be translated by as small a number of tRNAs as possible.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andachi Y., Yamao F., Iwami M., Muto A., Osawa S. Occurrence of unmodified adenine and uracil at the first position of anticodon in threonine tRNAs in Mycoplasma capricolum. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7398–7402. doi: 10.1073/pnas.84.21.7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andachi Y., Yamao F., Muto A., Osawa S. Codon recognition patterns as deduced from sequences of the complete set of transfer RNA species in Mycoplasma capricolum. Resemblance to mitochondria. J Mol Biol. 1989 Sep 5;209(1):37–54. doi: 10.1016/0022-2836(89)90168-x. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Collins F. S., Weissman S. M. Directional cloning of DNA fragments at a large distance from an initial probe: a circularization method. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6812–6816. doi: 10.1073/pnas.81.21.6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England T. E., Bruce A. G., Uhlenbeck O. C. Specific labeling of 3' termini of RNA with T4 RNA ligase. Methods Enzymol. 1980;65(1):65–74. doi: 10.1016/s0076-6879(80)65011-3. [DOI] [PubMed] [Google Scholar]
- Gafny R., Hyman H. C., Razin S., Glaser G. Promoters of Mycoplasma capricolum ribosomal RNA operons: identical activities but different regulation in homologous and heterologous cells. Nucleic Acids Res. 1988 Jan 11;16(1):61–76. doi: 10.1093/nar/16.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green C. J., Vold B. S. Sequence analysis of a cluster of twenty-one tRNA genes in Bacillus subtilis. Nucleic Acids Res. 1983 Aug 25;11(16):5763–5774. doi: 10.1093/nar/11.16.5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori H., Osawa S. Origin and evolution of organisms as deduced from 5S ribosomal RNA sequences. Mol Biol Evol. 1987 Sep;4(5):445–472. doi: 10.1093/oxfordjournals.molbev.a040455. [DOI] [PubMed] [Google Scholar]
- Hori H., Sawada M., Osawa S., Murao K., Ishikura H. The nucleotide sequence of 5S rRNA from Mycoplasma capricolum. Nucleic Acids Res. 1981 Oct 24;9(20):5407–5410. doi: 10.1093/nar/9.20.5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwami M., Muto A., Yamao F., Osawa S. Nucleotide sequence of the rrnB 16S ribosomal RNA gene from Mycoplasma capricolum. Mol Gen Genet. 1984;196(2):317–322. doi: 10.1007/BF00328065. [DOI] [PubMed] [Google Scholar]
- Kawauchi Y., Muto A., Osawa S. The protein composition of Mycoplasma capricolum. Mol Gen Genet. 1982;188(1):7–11. doi: 10.1007/BF00332989. [DOI] [PubMed] [Google Scholar]
- Kilpatrick M. W., Walker R. T. The nucleotide sequence of glycine tRNA from Mycoplasma mycoides sp. capri. Nucleic Acids Res. 1980 Jun 25;8(12):2783–2786. doi: 10.1093/nar/8.12.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komine Y., Adachi T., Inokuchi H., Ozeki H. Genomic organization and physical mapping of the transfer RNA genes in Escherichia coli K12. J Mol Biol. 1990 Apr 20;212(4):579–598. doi: 10.1016/0022-2836(90)90224-A. [DOI] [PubMed] [Google Scholar]
- Korneluk R. G., Quan F., Gravel R. A. Rapid and reliable dideoxy sequencing of double-stranded DNA. Gene. 1985;40(2-3):317–323. doi: 10.1016/0378-1119(85)90055-1. [DOI] [PubMed] [Google Scholar]
- Loughney K., Lund E., Dahlberg J. E. tRNA genes are found between 16S and 23S rRNA genes in Bacillus subtilis. Nucleic Acids Res. 1982 Mar 11;10(5):1607–1624. doi: 10.1093/nar/10.5.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A., Yamao F., Osawa S. The genome of Mycoplasma capricolum. Prog Nucleic Acid Res Mol Biol. 1987;34:29–58. doi: 10.1016/s0079-6603(08)60492-4. [DOI] [PubMed] [Google Scholar]
- Ogasawara N., Moriya S., Yoshikawa H. Structure and organization of rRNA operons in the region of the replication origin of the Bacillus subtilis chromosome. Nucleic Acids Res. 1983 Sep 24;11(18):6301–6318. doi: 10.1093/nar/11.18.6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkubo S., Muto A., Kawauchi Y., Yamao F., Osawa S. The ribosomal protein gene cluster of Mycoplasma capricolum. Mol Gen Genet. 1987 Dec;210(2):314–322. doi: 10.1007/BF00325700. [DOI] [PubMed] [Google Scholar]
- Rogers M. J., Simmons J., Walker R. T., Weisburg W. G., Woese C. R., Tanner R. S., Robinson I. M., Stahl D. A., Olsen G., Leach R. H. Construction of the mycoplasma evolutionary tree from 5S rRNA sequence data. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1160–1164. doi: 10.1073/pnas.82.4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers M. J., Steinmetz A. A., Walker R. T. The nucleotide sequence of a tRNA gene cluster from Spiroplasma meliferum. Nucleic Acids Res. 1986 Apr 11;14(7):3145–3145. doi: 10.1093/nar/14.7.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson T., Elias P., Lustig F., Guindy Y. S. Cloning and nucleotide sequence analysis of transfer RNA genes from Mycoplasma mycoides. Biochem J. 1985 Nov 15;232(1):223–228. doi: 10.1042/bj2320223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson T., Guindy Y. S., Lustig F., Borén T., Lagerkvist U. Apparent lack of discrimination in the reading of certain codons in Mycoplasma mycoides. Proc Natl Acad Sci U S A. 1987 May;84(10):3166–3170. doi: 10.1073/pnas.84.10.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada M., Muto A., Iwami M., Yamao F., Osawa S. Organization of ribosomal RNA genes in Mycoplasma capricolum. Mol Gen Genet. 1984;196(2):311–316. doi: 10.1007/BF00328064. [DOI] [PubMed] [Google Scholar]
- Sawada M., Osawa S., Kobayashi H., Hori H., Muto A. The number of ribosomal RNA genes in Mycoplasma capricolum. Mol Gen Genet. 1981;182(3):502–504. doi: 10.1007/BF00293942. [DOI] [PubMed] [Google Scholar]
- Triglia T., Peterson M. G., Kemp D. J. A procedure for in vitro amplification of DNA segments that lie outside the boundaries of known sequences. Nucleic Acids Res. 1988 Aug 25;16(16):8186–8186. doi: 10.1093/nar/16.16.8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Vold B. S. Structure and organization of genes for transfer ribonucleic acid in Bacillus subtilis. Microbiol Rev. 1985 Mar;49(1):71–80. doi: 10.1128/mr.49.1.71-80.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. T., RajBhandary U. L. The nucleotide sequence of formylmethionine tRNA from Mycoplasma mycoides sp. capri. Nucleic Acids Res. 1978 Jan;5(1):57–70. doi: 10.1093/nar/5.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawrousek E. F., Hansen J. N. Structure and organization of a cluster of sic tRNA genes in the space between tandem ribosomal RNA gene sets in Bacillus subtilis. J Biol Chem. 1983 Jan 10;258(1):291–298. [PubMed] [Google Scholar]
- Wawrousek E. F., Narasimhan N., Hansen J. N. Two large clusters with thirty-seven transfer RNA genes adjacent to ribosomal RNA gene sets in Bacillus subtilis. Sequence and organization of trrnD and trrnE gene clusters. J Biol Chem. 1984 Mar 25;259(6):3694–3702. [PubMed] [Google Scholar]
- Yamada Y., Ohki M., Ishikura H. The nucleotide sequence of Bacillus subtilis tRNA genes. Nucleic Acids Res. 1983 May 25;11(10):3037–3045. doi: 10.1093/nar/11.10.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamao F., Iwagami S., Azumi Y., Muto A., Osawa S., Fujita N., Ishihama A. Evolutionary dynamics of tryptophan tRNAs in Mycoplasma capricolum. Mol Gen Genet. 1988 May;212(2):364–369. doi: 10.1007/BF00334708. [DOI] [PubMed] [Google Scholar]
- Yamao F., Muto A., Kawauchi Y., Iwami M., Iwagami S., Azumi Y., Osawa S. UGA is read as tryptophan in Mycoplasma capricolum. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2306–2309. doi: 10.1073/pnas.82.8.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]