Abstract
Retrograde axonal injury signalling stimulates cell body responses in lesioned peripheral neurons. The involvement of importins in retrograde transport suggests that transcription factors (TFs) might be directly involved in axonal injury signalling. Here, we show that multiple TFs are found in axons and associate with dynein in axoplasm from injured nerve. Biochemical and functional validation for one TF family establishes that axonal STAT3 is locally translated and activated upon injury, and is transported retrogradely with dynein and importin α5 to modulate survival of peripheral sensory neurons after injury. Hence, retrograde transport of TFs from axonal lesion sites provides a direct link between axon and nucleus.
Keywords: axonal transport, dynein, importin, neuronal injury, STAT
Introduction
Regeneration of lesioned peripheral nerves is dependent on transcriptional events in the neuronal cell body following an injury event in the axon (Rossi et al, 2007). Given that nerve axons may extend over tens of centimeters, specialized signalling mechanisms are required to transmit information about an injury from distant lesion sites to the cell soma (Kam et al, 2009). Studies in peripheral sensory neurons have provided compelling evidence for the importance of retrogradely transported injury signals for initiation of a regeneration response (Abe and Cavalli, 2008; Rishal and Fainzilber, 2010). Axotomy may also have major effects on neuronal viability through retrograde signalling (Keramaris et al, 2005; Welin et al, 2008). Sensory neurons of the dorsal root ganglion (DRG) extend a bifurcating axon with two branches in vivo—a peripheral branch that regenerates when injured, and a central branch that does not normally regenerate following injury. The latter failure is partially attributable to the growth inhibitory environment in the damaged central nervous system (CNS) (Schwab, 2010). However, a conditioning lesion (Smith and Skene, 1997) of the peripheral branch 1–2 weeks before a central tract lesion will enhance regeneration of centrally projecting neurites (Neumann and Woolf, 1999). Moreover, recent publications have demonstrated that regeneration responses can be re-activated in dormant neurons by carrying out new axonal lesions weeks-months after the original injury (Kadoya et al, 2009; Ylera et al, 2009). These results indicate that retrograde injury signals travel from the peripheral injury site in sensory neurons back to the cell body to increase intrinsic neurite growth capacity, helping to overcome inhibitory cues in the environment.
A large variety of retrogradely transported proteins have been identified in peripheral nerve following injury (Abe et al, 2009; Michaelevski et al, 2010a, 2010b), and one of the interesting categories of such molecules is those which might be trafficked by importin nuclear transport factors. In rodent sciatic nerve, a number of importin α's were found in axons of both control and injured nerve in constitutive association with the retrograde motor dynein, while importin β1 protein was present only after injury (Hanz et al, 2003). Interestingly, mRNA for importin β1 is found in sensory axons, and is locally translated to protein at the injury site after lesion (Hanz et al, 2003). This leads to the formation of importin α/β heterodimers bound to dynein; thereby enabling transport of signalling cargos that bind to the importins complex. Competition with the nuclear localization signal (NLS) binding site on importins (Hanz et al, 2003), or perturbation of regulation of the importins complex (Yudin et al, 2008) inhibits or delays conditioning lesion responses and regeneration. These data suggest that in parallel with local axonal synthesis of importin β1, local activation of nuclear targeted signalling proteins creates signalling cargos that access the retrograde transport pathway via importins. However, apart from the atypical example of phospho-Erks which bind vimentin complexed with importin β1 (Perlson et al, 2005), importin-dependent retrograde injury signalling cargos have not yet been identified.
Transcription factors (TFs) are adapted to take advantage of nucleocytoplasmic transport mechanisms, hence they are intriguing candidates for importin-dependent retrograde cargo molecules (Perry and Fainzilber, 2009). Indeed, a number of studies suggested that factors such as ATFs and STATs might be involved in retrograde signalling (Lee et al, 2004; Lindwall and Kanje, 2005; Qiu et al, 2005), although the mechanism of their involvement remained unclear. A recent report suggested that the TF CREB is locally translated in sensory axons in cultured neurons and retrogradely transported to the cell body in response to NGF (Cox et al, 2008). Although these observations were not replicated in sympathetic neurons (Andreassi et al, 2010), other components of the CREB or NFκB pathways were reported to translocate from synapse to nucleus in hippocampal neurons (Mikenberg et al, 2007; Dieterich et al, 2008; Lai et al, 2008; Behnisch et al, 2011). Taken together, these in-vitro studies raise the interesting possibility that injured axons may utilize TFs as retrograde signalling entities for direct communication with the nucleus. A comprehensive analysis of the links between axonal injury signalling and cell body transcriptional responses in the sciatic nerve–DRG system recently unveiled ∼400 signalling networks connecting to 39 TFs in the sensory neuron response to nerve injury (Michaelevski et al, 2010b). Here, we examine TF contribution to the injury response in more detail, and demonstrate that a number of factors are injury-regulated cargo molecules of the dynein retrograde complex. For one factor in particular, STAT3, we use biochemical and functional approaches to show that it is locally translated in axons, binds the importins retrograde complex, and is transported retrogradely in sciatic nerve in vivo to influence cell body responses after injury.
Results
Dynein-bound TFs in axons
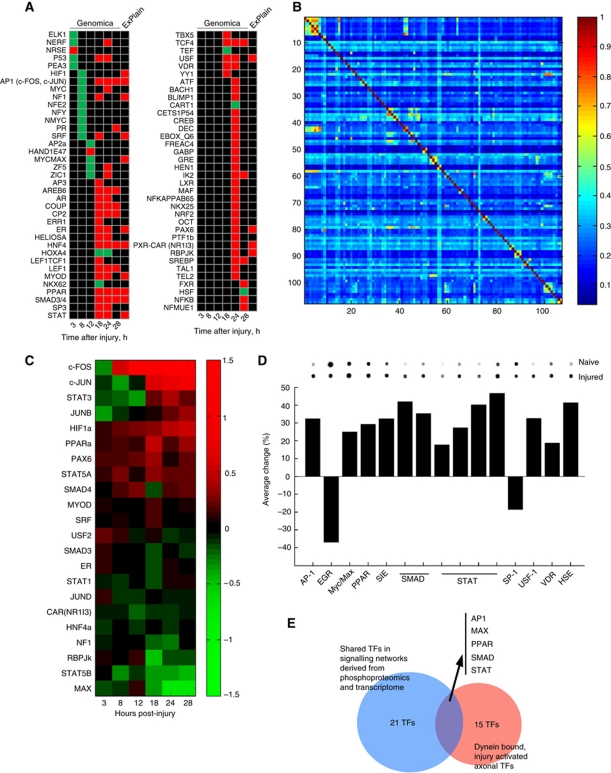
Michaelevski et al (2010b) delineated networks linking axonal signalling events to cell body transcriptional responses in the sciatic nerve–DRG system, implicating 39 TFs in this process. In order to test these TF predictions independently, we examined the relative representation of TF binding sites (TFBS) in promoter regions of 6000 regulated annotated genes from the microarray data of Michaelevski et al using the Genomica analysis tool (http://genomica.weizmann.ac.il), which cross-references an input list of all genes on the array with a matrix listing the TFBS present in a 2-kb window upstream of the coding sequence for each such gene. It then assesses whether there is overrepresentation of downregulated or upregulated genes for each TFBS set across the whole array list. This approach is complementary to that implemented in ExPlain as used by Michaelevski et al (2010b), which analyses gene sets at individual time points, and identifies TFBS overrepresented in each such set versus a matched control background set. TF's independently flagged by both methods are prioritized for follow-up analyses. Figure 1A shows a heat map for TF groupings with binding site matrices found to be enriched at different time points in the data set using this approach, and Supplementary Table S1 provides the lists of genes from the microarray data associated with each matrix. At the earlier time points (3–12 h post injury), most of the candidate TFs correlate with downregulated gene sets, while TF's highlighted from 18 h onwards are mostly correlated with upregulation of gene expression. We then examined the degree of overlap in regulated genes between TF's by comparing the numbers of overlaps between pairs of matrices. The degree of co-occurrence observed in this gene set for most matrix pairs is <30% (Figure 1B), with only 86 pairs (out of 11 342 such comparisons) showing a degree of co-regulation of 60% or more (Supplementary Table S2). Most of the latter are highly related TFBS for the same factor or a close homologue (Supplementary Table S2). This relatively low overlap contrasts strikingly with the high degree of promiscuity observed for the main hub proteins in the axonal signalling networks characterized by Michaelevski et al (2010b). Thus, although there might be some degree of functional backup between different TF's, certain aspects of the injury response may be highly dependent on individual TF's.
Figure 1.
Multiple transcription factors in the neuronal injury response. (A) Genomica analyses of transcription factor binding sites (TFBS) enriched in dorsal root ganglion microarray data sets at different time points after sciatic nerve lesion, compared with those found in the ExPlain analyses of Michaelevski et al (2010a, 2010b). Green indicates enrichment in the downregulated gene set, red indicates enrichment in the upregulated gene set, and black indicates no enrichment at that time point. For lists of genes associated with each TFBS, please see Supplementary Table S1. (B) Matrix of TFBS co-occurrence within regulated genes in the microarray data set. Genes containing the 107 enriched TFBS identified by Genomica were scanned for all possible instances of TFBS co-occurrence and the average degree of co-occurrence between all possible pairs is shown. The heat map colour code goes from no co-occurrence (0) to complete co-occurrence (1). Numerical data for the matrix are provided in Supplementary Table S2. (C) Cluster analysis of expression levels of mRNA's encoding transcription factors (TF) found in both Genomica and ExPlain analyses. Expression is shown in log2 values versus the reference time point of 1 h after injury. Colour code for TF mRNA regulation shown on the right. (D) Active (DNA binding) transcription factors co-precipitated with dynein from axoplasm of control or injured sciatic nerve (6 h post injury) quantified by Panomics Protein/DNA array analysis. Percent activity is normalized to hybridization controls on the array, and representative blots for each TF are shown above the graph. Multiple bars for STAT or Smad families represent multiple probes on the array. Complete data for all TFs represented on the array are provided in Supplementary Table S3. (E) Intersection of TFs found in the different screens highlights six families as sources for candidate retrograde injury signals.
Upon examination of the mRNA expression profile of TFs identified by both Genomica (Figure 1A) and ExPlain (Michaelevski et al, 2010b) in the original microarray data, it is interesting to note that several mRNAs encoding TFs were not themselves upregulated at time points when their transcriptional activity was already apparent (Figure 1C). This suggests that their transcriptional activity might be activated primarily by post-transcriptional mechanisms after nerve injury. Since axonal retrograde signalling mechanisms must be post-transcriptional in nature, the data raise the possibility that some of these TFs might be activated in axons. We therefore used a protein-DNA array approach (Jiang et al, 2004) to examine the interaction of activated TFs with the retrograde transport machinery in sciatic nerve axoplasm. Axoplasm from control or injured nerve (6 h post lesion) was immunoprecipitated using an anti-dynein antibody. The immunoprecipitates were then mixed with biotin-labelled DNA binding probes to form DNA/protein complexes, free probes were separated from bound, and the bound probes were eluted and hybridized to a membrane array of 56 consensus TFBS. Eleven of the TF classes pulled down with dynein in this assay differed significantly in preparations from injured versus naive nerve (Figure 1D; Supplementary Table S3). Cross-referencing these data with the ExPlain and Genomica data revealed five TF classes common to all the screens (Figure 1E), and hence likely candidates for participation in retrograde injury signalling.
STAT3 is an injury-activated locally translated axonal TF
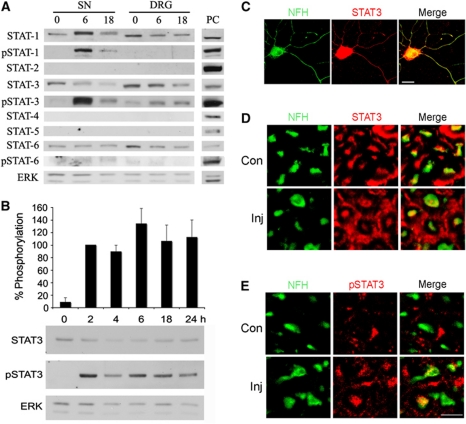
The prominent representation of STATs in the final group of candidates led us to focus on this family of TFs for further validation. We first checked occurrence of individual STAT family members in axons of sciatic nerve and in the DRG, and their activation after nerve injury. Western blot analysis of sciatic nerve axoplasm and DRG extracts revealed the presence of STAT-1, -3, and -6, but not STAT-2, -4, -5a or -5b (Figure 2A; Supplementary Figure S1). Among the proteins present in both sciatic nerve and DRG, only STAT1 and STAT3 were activated after injury, as indicated by tyrosine phosphorylation, and only STAT3 was phosphorylated in both axons and DRG. Moreover, STAT3 exhibited prolonged phosphorylation in sciatic nerve axoplasm after crush lesion (Figure 2B; Supplementary Figure S2), and is found within processes of DRG neurons both in culture (Figure 2C) and in vivo in the sciatic nerve (Figure 2D and E). We therefore focused our further efforts on STAT3.
Figure 2.
Activation of STAT3 in sciatic nerve axons after injury. (A) Western blot analyses of STAT family members in sciatic nerve (SN) axoplasm or DRG extract from control or injured rat SN (6 or 18 h after injury). Only STAT3 is phosphorylated in both SN and DRG after injury. ERK was used as a loading control. PC denotes positive control (HeLa extract for STATs-1-4 and 6; K562 cells extract for STAT-5; HeLa cells were treated with IFNα for pSTAT-1 and -3; or with IL-4 for pSTAT-6). The experiment was repeated four times with similar results. (B) Western blot analysis of 20 μg aliquots of rat sciatic nerve axoplasm from 0 to 24 h post lesion reveals prolonged phosphorylation of STAT3 after injury. ERK was used as a loading control. Quantification is in percentage of pSTAT3 levels at 2 h post lesion (average±s.e.m.; n=5). (C) Immunostaining for STAT3 and the axonal marker NFH on adult rat DRG neurons in culture shows that STAT3 is found in NFH-positive processes. Scale bar 20 μm. (D) Immunostaining for STAT3 and (E) for pSTAT3 on cross-sections of control or injured (6 h post lesion) rat sciatic nerve reveals the presence of STAT3 in NFH-positive axons in vivo and its phosphorylation after injury. Scale bar 10 μm. Figure source data can be found in Supplementary data.
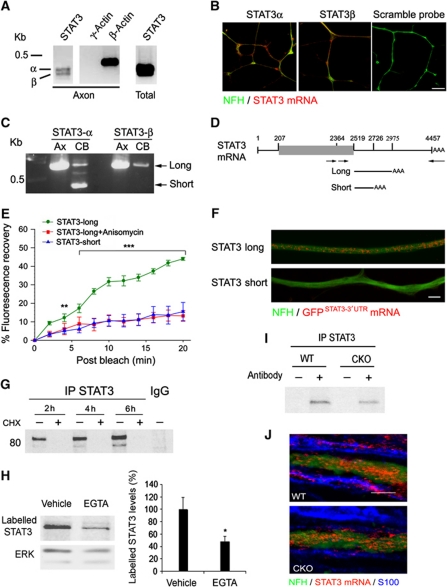
Our previous studies have shown that key components of the retrograde injury signalling complex, such as importin β1, vimentin, and RanBP1, are locally synthesized in axons in response to injury. These findings prompted us to check whether STAT3 message is also found and locally translated in axons. RT–PCR on isolated DRG axons from filter compartmentalized cultures (Zheng et al, 2001) revealed the presence of mRNAs for two alternatively spliced STAT3 isoforms, STAT3α and STAT3β (Figure 3A). In-situ hybridization with specific probes for the two splice variants further confirmed their presence in the processes of cultured DRG neurons (Figure 3B). Targeting of specific transcripts into axons is usually via untranslated (UTR) sequences that interact with RNA transport proteins (Donnelly et al, 2010). We therefore carried out 3′RACE RT–PCR on RNA extracted from sensory neuron cell bodies or axons to identify and clone STAT3 3′UTR sequence variants. A single and longer UTR variant was identified from axons, with identical sequence for STAT3α and STAT3β, while two variants were obtained for STAT3α from cell bodies (Figure 3C and D). We then tested the capacity of both UTR variants to induce axonal localization of a myristoylated destabilized GFP reporter. Importantly, fusion of the myristoylation sequence to GFP limits diffusion of newly synthesized protein, allowing its use as a reliable reporter of localized protein synthesis after photobleaching (Aakalu et al, 2001; Yudin et al, 2008). The constructs were transfected into cultured DRG neurons for fluorescence recovery after photobleaching (FRAP) analyses. Fluorescence recovery was observed for the construct containing the axonal form of STAT3 3′UTR, but not for the cell body-specific UTR variant (Figure 3E; Supplementary Figure S3). The recovery observed for the axonal 3′UTR construct was blocked upon incubation with the translation inhibitor anisomycin, consistent with the new fluorescence signal arising from local translation in the axon (Figure 3E; Supplementary Figure S3). Furthermore, in-situ hybridizations with a GFP riboprobe on sensory neurons transfected with the reporter constructs revealed axonal GFP transcripts in neurons transfected with constructs containing the long form of STAT3 3′UTR, but not the short form (Figure 3F; Supplementary Figure S4). These results indicate that the long form of STAT3 3′UTR contains a targeting sequence for localization and translation within axons of peripheral sensory neurons.
Figure 3.
STAT3 message and local translation in axons. (A) RT–PCR reveals the presence of STAT3 transcripts (α and β) in isolated DRG axons as well as in cell bodies. β-Actin and γ-actin transcripts are differentially represented in axons and were used as positive and negative controls, respectively. (B) STAT3 mRNA revealed by in-situ hybridization in processes of cultured DRG neurons. Scale bar 10 μm. (C) Nested PCR of STAT3 3′UTR on axonal and cell body cDNA reveals different 3′UTR variants in the cell body and axons. The major variants subsequently cloned for analysis are indicated by arrows. (D) Schematic of STAT3 transcripts, with open reading frame denoted by the grey box. The lines under the schematic delineate regions subcloned for constructs containing axonal (short) or cell body (long) 3′UTR variants, as indicated in (C). (E) Fluorescence recovery after photobleaching of adult DRG neurons transfected with the indicated constructs. Average recoveries (% of prebleach levels±s.e.m.) are shown (n=6, **P<0.01, ***P<0.005). For representative images, please see Supplementary Figure S3. (F) In-situ hybridization with a GFP riboprobe reveals mRNA for the STAT3-long reporter construct, but not for STAT3-short, in the processes of cultured DRG neurons (scale bar 5 μm). For additional images and quantification of these data, please see Supplementary Figure S4. (G–I) Metabolic labelling of sciatic nerve (SN) axoplasm reveals Ca2+-dependent local synthesis of STAT3 in axons. SN segments from rat (G, H) or from STAT3-GFAP-CKO or wild-type (WT) mice (I) were incubated for 2, 4, 6 h (G) or 6 h (H, I) in Met/Cys-deficient DMEM medium containing 40 μCi/ml of [S35]Met/Cys, with or without 10 μg/ml cycloheximide or 100 mM EGTA. EGTA (100 mM) or Vehicle (PBS) was injected to SN before incubation in the medium. Equal samples of axoplasm proteins were then subjected to immunoprecipitation with antibodies against STAT3 or control IgG, followed by gel electrophoresis and autoradiography. Quantification of labelled STAT3 intensity normalized to the loading control ERK is shown in the graph as percent of vehicle treatment (average±s.e.m., n=3, *P<0.05). (J) Fluorescent in-situ hybridization on SN longitudinal sections from wild-type or STAT3-GFAP-CKO mice reveals the presence of STAT3 transcript (red) in NFH (green) positive axons. Note the presence of STAT3 transcript in Schwann cells (blue, identified by immunostaining for S100) in the WT mouse, but not in the CKO mouse (scale bar 5 μm). Figure source data can be found in Supplementary data.
Local axonal translation of STAT3 was further examined by incubation of sciatic nerve segments with radiolabelled amino acids, followed by STAT3 immunoprecipitation and autoradiography (Figure 3G). De-novo synthesis of STAT3 in the isolated nerve segments was reduced by calcium chelation (Figure 3H), as previously shown also for importin β1 and for RanBP1 (Yudin et al, 2008). In order to verify that the synthesis thus observed originates in axons and not in Schwann cells, we conducted similar experiments with sciatic nerve segments from STAT3-GFAP-CKO mice, which lack STAT3 in glial cells (Herrmann et al, 2008). All Schwann cell precursors express GFAP (Jessen and Mirsky, 1992), thus crossing a GFAP-Cre line with a floxed line for the gene of interest creates an animal that lacks expression of the gene of interest in adult Schwann cells (Gregorian et al, 2009). As shown in Figure 3I, metabolic labelling of sciatic nerves from STAT3-GFAP-CKO mice also revealed local translation of STAT3, indicating that the local translation occurs in axons. The presence of STAT3 mRNA in axons in vivo was further confirmed by in-situ hybridization on sciatic nerve sections from wild-type and STAT3-GFAP-CKO mice (Figure 3J).
STAT3 is transported by dynein from the axonal injury site to the DRG cell body
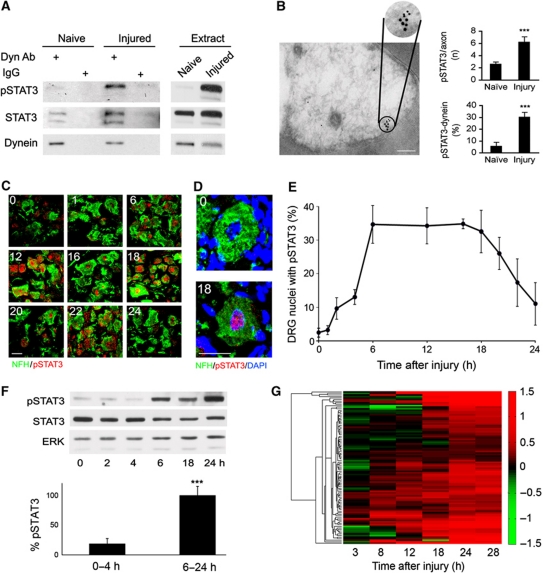
Having established the presence of STAT3 in axons, we next examined whether it interacts with the retrograde injury signalling complex. As shown in Figure 4A, STAT3 co-precipitates with dynein from axoplasm of both control and injured (6 h post lesion) rat sciatic nerve. The phosphorylated form of STAT3 (pSTAT3) is found in interaction with dynein only after injury. We further examined the interaction between dynein and pSTAT3 by electron microscopy of nerve sections labelled with immunogold, and observed a marked increase in pSTAT3 labelling in injured axons, together with an extremely significant increase in co-occurrence of dynein and pSTAT3 particles (Figure 4B). Two STAT3 immunoreactive bands were observed in the pulldowns of Figure 4A, corresponding to the two alternative spliced forms of STAT3: STAT3α and STAT3β. These isoforms are reported to have overlapping but distinct biochemical properties, with differing physiological impact in some biological systems (Dewilde et al, 2008). The main band we observe in sciatic nerve axoplasm corresponds in most cases to the longer variant STAT3α, although the relative occurrence of the two forms varies. Hence, unless otherwise indicated, we include both splice variants within the designation when referring to STAT3.
Figure 4.
Activated STAT3 interacts with dynein in axons. (A) Co-immunoprecipitation of STAT3 and pSTAT3 with dynein. Anti-dynein or non-relevant control IgGs were used to precipitate rat axoplasm (500 μg) from naive or injured (6 h post lesion) sciatic nerve (n=3). (B) Electron microscopy of immunogold labelling of dynein heavy chain 1 and pSTAT3 in sciatic nerve cross-sections. A representative micrograph from injured nerve (6 h post lesion) is shown on the left (dynein, 15 nm particles; pSTAT3, 10 nm particles; scale bar 0.2 μm). The number of pSTAT3 particles per axon and the percent of pSTAT3 particles adjacent to a dynein particle are quantified on the right (average±s.e.m., n⩾38 axons, ***P<0.001). (C–E) Accumulation of pSTAT3 in DRG after SN injury. (C) Sections of L4/L5 DRGs after sciatic nerve crush (indicated time points in hours) were immunostained for the neuronal marker NFH (green) and pSTAT3 (red). Concentration of pSTAT3 in DRG neuronal nuclei peaks at 6–18 h after injury. Scale bar 20 μm. (D) Zoom-in on representative DRG cells from the experiment described in (B) (0 and 18 h time points). DAPI staining of the nucleus is in blue. Note the concentration of pSTAT3 in DRG cell nucleus after lesion. Scale bar 20 μm. (E) Quantification of the experiment described in (B), showing the fraction of DRG neurons with pSTAT3 concentrated in the nucleus at the indicated time points (average±s.e.m., n=3). (F) Western blot analysis of DRG extracts at different time points after SN lesion. Quantification of pSTAT3/STAT3 intensity ratio in the early time points (0–4 h) versus the late time points (6–24 h) is shown in the graph as percentage of maximum (6 h) (average±s.e.m., n=3, ***P<0.005). (G) Microarray expression data for STAT3 responsive genes at different time points after sciatic nerve lesion, shown as a heat map of fold changes (colour code on the right). For a complete list of gene i.d.'s and fold changes, please see Supplementary Table S4. Figure source data can be found in Supplementary data.
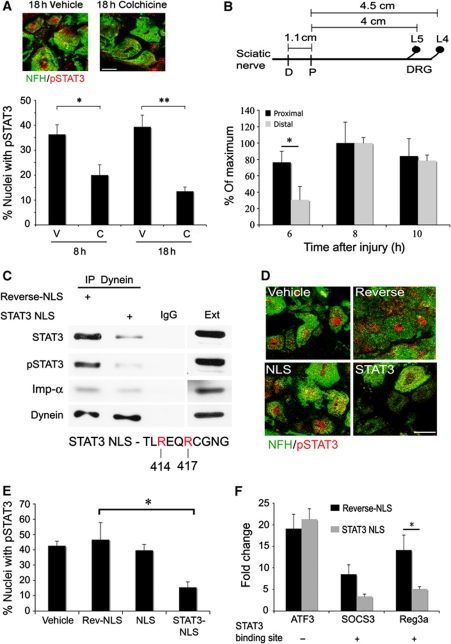
The interaction between STAT3 and the dynein motor protein suggests that STAT3 is transported retrogradely from axonal injury sites to neuronal cell bodies. We therefore checked whether the time course of accumulation of pSTAT3 in DRG nuclei after sciatic nerve injury correlates with the time of induction of putative STAT3 responsive genes. The accumulation of pSTAT3 in L4/L5 DRG peaks at 6–18 h after sciatic nerve injury, as shown by confocal imaging of immunostained neuronal nuclei (Figure 4C–E) and western blots of ganglionic extracts (Figure 4F). This time frame correlates well with the induction of STAT3 responsive genes, which starts at 8 h after nerve injury, and increases with time (Figure 4G; Supplementary Table S4). To further examine the retrograde trafficking of pSTAT3, we injected the drug colchicine to sciatic nerve to inhibit dynein transport via microtubule disruption. As shown in Figure 5A, injection of colchicine to the nerve concomitantly with a crush injury inhibited the accumulation of pSTAT3 in DRG neuronal nuclei. Furthermore, applying a sciatic nerve injury 1 cm more distant from the DRG delayed the accumulation of pSTAT3 in neuronal cell bodies (Figure 5B). At 6 h post lesion we observed 70% nuclear accumulation of pSTAT3 from the proximal injury site, while accumulation from the distal injury site reached only 30%.
Figure 5.
Retrograde transport of activated STAT3 after injury. (A) Injection of colchicine (C, 100 μg) 1 cm proximal to the injury site in sciatic nerve (SN) reduced nuclear accumulation of pSTAT3 at the indicated post-injury time points in DRG neurons. Controls were injected with PBS vehicle (V). Quantification as for Figure 4E (average±s.e.m., n=3, *P<0.05, **P<0.01, scale bar 20 μm). (B) Influence of injury site location on the nerve on accumulation of pSTAT3 in DRG neuronal nuclei. The scheme shows average distances between the injury sites in sciatic nerve and the L4/L5 DRGs (D-distal injury, P-proximal injury). The fraction of DRG neurons with pSTAT3 concentrated in the nucleus after sciatic nerve proximal or distal injury is shown as percent of maximum after normalization to the 4-h time point (average±s.e.m., n=3, *P<0.05). (C) In all, 500 μg axoplasm from injured sciatic nerve (6 h) was subjected to dynein immunoprecipitation, in the presence of 50 μg reverse-NLS or STAT3-NLS peptide, followed by western blot analysis with antibodies against STAT3, pSTAT3, importin α, and dynein. Precipitation with an unrelated IgG serves as a control (n=3). For more details on the STAT3-NLS used, please see Supplementary Figure S5. (D, E) Injection of STAT3-NLS peptide to sciatic nerve inhibits accumulation of pSTAT3 in DRG neuronal nuclei after injury. In all, 250 μg of NLS, reverse-NLS, or STAT3-NLS peptide, or PBS vehicle alone, was injected to sciatic nerve, and L4/L5 DRGs were fixed 18 h later. DRG sections were stained for pSTAT3 and NFH, representative pictures are shown in (D) (scale bar 20 μm), and the fraction of DRG cells with concentrated pSTAT3 in the nucleus was quantified as shown in (E) (average±s.e.m., n=3, *P<0.05). (F) RNA was extracted from L4/L5 DRG 18 h after injection of STAT3-NLS or control reverse-NLS peptides to sciatic nerve concomitantly with a crush injury. Control RNA was extracted from the contralateral DRG. The RNA was reverse transcribed and qPCR was performed for the genes of interest. Results are normalized to β-tubulin and presented in fold changes versus control (average±s.e.m., n=3, *P<0.05). Figure source data can be found in Supplementary data.
We then sought to perturb retrograde signalling of pSTAT3 by using peptides that might compete with its interaction with the transport machinery. A number of sequence elements have been proposed to mediate STAT3 interaction with importins for nuclear translocation, and a short synthetic peptide based on a sequence that affects STAT3 interaction with importin α5 (Ma et al, 2003; Ma and Cao, 2006) reduced the amount of STAT3 and pSTAT3 that co-precipitated with dynein from axoplasm, without significant perturbation of importin α co-precipitation (Figures 5C; Supplementary Figure S5). This result suggests that an importin α, likely importin α5, mediates the interaction between STAT3 and dynein. The STAT3-NLS peptide enters injured sensory axons when injected to sciatic nerve concomitantly with crush lesion (Supplementary Figure S6), and significantly inhibits injury-induced accumulation of pSTAT3 in DRG nuclei (Figure 5D and E), further supporting injury-induced retrograde transport of pSTAT3. In contrast, injection of classical NLS peptide did not affect pSTAT3 accumulation, as might be the case for a cargo molecule that binds via a non-classical nuclear localization motif. Quantitative PCR (qPCR) analysis shown in Figure 5F then revealed that injection of STAT3 peptide to sciatic nerve also inhibits the upregulation of two STAT3 responsive genes from the microarray data, SOCS3 and Reg3a, in DRG following injury, but does not affect upregulation of ATF3, a known injury-induced factor (Tsujino et al, 2000) that lacks an STAT3 binding site. Taken together, these findings indicate that axonal pSTAT3 is transported in vivo from the injury site to the neuronal cell body after nerve injury.
Functional effects of retrogradely transported pSTAT3
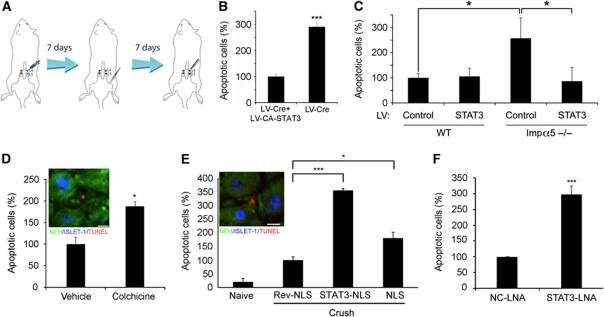
STAT3 has been implicated in both outgrowth and survival responses of neurons downstream of cytokines and growth factors (Alonzi et al, 2001; Ng et al, 2006; Smith et al, 2011) and after injury (Qiu et al, 2005; Miao et al, 2006), and has profound effects on the viability of lesioned adult neurons (Schweizer et al, 2002; Zhang et al, 2008). Hence, we examined the functional connection between axonal STAT3 and cell body injury responses. To this end, we first generated a knockdown of STAT3 in adult sensory neurons by injection of lentivirus expressing the Cre recombinase to L4/L5 DRG in adult STAT3 floxed animals, followed by a unilateral sciatic nerve crush after one week (Figure 6A). Lentivirus-Cre-treated ganglia showed much reduced levels of STAT3 mRNA (Supplementary Figure S7) and a marked increase in TUNEL-positive apoptotic neurons 7 days after nerve crush versus ganglia that received the lentivirus-Cre concomitantly with another virus expressing constitutively active STAT3 (Figure 6B). We did not observe major differences in neurite outgrowth responses in neurons from lentivirus-Cre-treated ganglia (Supplementary Figure S8A and B). Taken together, these data indicate that STAT3 functions primarily as an anti-apoptotic factor in adult sensory neurons after nerve injury.
Figure 6.
Axonal STAT3 promotes neuronal survival after nerve injury. (A) Schematic of lentivirus (LV) transduction protocol. A high titre LV of interest was injected to the L4 and L5 DRGs, and the animals were allowed 7 days for recovery and expression of the LV delivered gene product. Then sciatic nerve crush was performed, and 7 days afterwards the L4/L5 DRGs were excised, fixed, sectioned and stained for the neuronal markers NFH and ISLET-1 together with TUNEL staining for monitoring of cell death. (B) Expression of constitutively active (CA) STAT3 in DRG reduces neuronal death after SN injury in mice deprived of STAT3 in DRG. Reduction of STAT3 expression in L4/L5 DRG was achieved using the cre-lox system, by injection of LV expressing Cre recombinase to L4/L5 DRGs of floxed-STAT3 mice as described in (A). Co-infection with CA-STAT3 caused a three-fold reduction in the fraction of apoptotic cells (average±s.e.m., n=6, ***P<0.005). (C) Enhanced neuronal cell death in L4/L5 DRG of importin α5 KO mice is attenuated by LV-mediated expression of CA-STAT3 in the ganglia (average±s.e.m., n=4, *P<0.05). LV-GFP was used as a control. (D) Colchicine (100 μg) injection to rat SN concomitantly with lesion increased the fraction of TUNEL-positive neurons in the L4/L5 DRG 7 days later. Vehicle control is PBS. (E) Effects of injection of 250 μg of NLS, reverse-NLS or STAT3-NLS peptides concomitantly with SN lesion in rat on the fraction of TUNEL-positive neurons in the L4/L5 DRG 7 days later. Insets in (D) and (E) show representative images (scale bar 15 μm). (F) Effects of injection of 0.3 μg anti-STAT3 or negative control (NC) locked nucleic acid (LNA) into sciatic nerve 2 days before and then again concomitantly with lesion, followed by counts of TUNEL-positive neurons in the L4/L5 DRG 7 days afterwards. Similar results were obtained when monitoring cleaved caspase 3 instead of TUNEL (Supplementary Figure S10). Results in (D–F) are presented as percentage of the control treatments (average±s.e.m., n⩾3, *P<0.05, ***P<0.005).
STAT3 has been reported to interact with multiple importin α isoforms, including importin α5, and the STAT3-derived peptide we used to disrupt STAT3 retrograde transport (Figure 5) was designed based on sequences that affect STAT3 interaction with importin α5 (Ma and Cao, 2006). A mouse knockout for importin α5 is viable and fertile (Shmidt et al, 2007), enabling testing of whether this importin α isoform is involved in STAT3 anti-apoptotic signalling after nerve injury. Indeed sciatic nerve crush in importin α5 knockout animals caused a significant elevation in the fraction of TUNEL-positive neurons in the L4/L5 DRG compared with wild-type animals (Figure 6C), similarly to the effect observed with STAT3 knockdown in the DRG (Figure 6B). Lentiviral-mediated transduction of constitutively active STAT3 directly to the DRG rescues this neuronal death phenotype (Figure 6C), suggesting that importin α5 is essential for the delivery of axonal STAT3 to the cell body, but not for its nuclear import. We therefore tested whether survival-promoting STAT3 originates in the axon of injured sensory neurons. Perturbation of retrograde transport by colchicine injection to rat sciatic nerve increased the number of apoptotic neurons in L4/L5 DRG (Figure 6D). Furthermore, injection of the STAT3-NLS peptide to sciatic nerve concomitantly with crush lesion tripled the number of dying neurons subsequently observed in the DRG, whereas similar experiments using an SV40-derived classical NLS peptide had more modest effects (Figure 6E). STAT3-NLS peptide introduction to the sciatic nerve had very mild effects on conditioning lesion-induced neurite outgrowth, in contrast to the previously published (Hanz et al, 2003) marked effect of the classical NLS peptide (Supplementary Figure S8C–E). Since the classical NLS is a widely used nuclear import motif, it might compete with the binding of several other factors to the complex. Taken together, these results indicate that retrogradely transported axonal STAT3 has primarily anti-apoptotic effects in sensory neuron cell bodies after nerve injury.
We then examined the possibility that active retrogradely transported STAT3 originates from local translation in axons. To this end, we generated and validated a locked nucleic acid (LNA) oligonucleotide targeted to the 5′ end of STAT3 coding sequence, and verified that it causes a reduction in STAT3 levels upon injection to injured sciatic nerve (Supplementary Figure S9A). We then tested the LNA effects in experiments where we first injected the nucleic acid reagent, and then 2 days later carried out sciatic nerve crush together with a boost LNA injection, with subsequent examination of apoptotic responses in L4/L5 DRG neurons 7 days afterwards. As shown in Figure 6F, reduction of axonal STAT3 by LNA injection caused elevation in TUNEL-positive neurons, while control reagents had no effect. Similar results were obtaining using cleaved caspase 3 immunostaining to monitor neuronal cell death (Supplementary Figure S10). Since oligonucleotides injected by this method are found only locally near the axonal injection site and do not reach neuronal cell bodies (Supplementary Figure S9B), these data indicate that local synthesis of STAT3 from axonal transcripts after injury is required for subsequent survival promoting effects in neuronal cell bodies.
Discussion
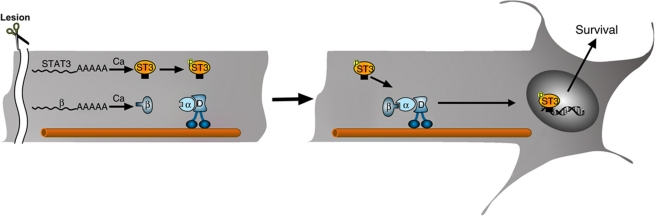
Much effort has been invested in recent years in attempts to elucidate the cell body response to peripheral nerve injury, with the goal of identifying ‘master regulators’ for functional nerve repair (Michaelevski et al, 2010b; Rishal and Fainzilber, 2010; Smith et al, 2011). Retrograde signalling from axonal lesion sites stimulates the cell body response, and can even re-evoke such a response if elicited after a significant time delay from an initial injury (Kadoya et al, 2009; Ylera et al, 2009). The prevailing notion has been that such retrograde signalling is dependent on transported complexes of classical signalling molecules, which activate TFs and other nuclear regulators upon arrival at the cell body. On the other hand, the fact that nuclear import factors are involved in axonal retrograde transport leaves open the possibility that TFs and other nuclear regulators might be involved in axonal signalling (Perry and Fainzilber, 2009). In this study, we examined the involvement of TFs in cell body response and retrograde injury signalling after peripheral nerve lesion. Computational analyses suggested that multiple TFs are involved in elicitation of the cell body response, and a number of these were found in association with dynein in nerve axoplasm. Biochemical and functional validation for one TF family establishes that STAT3 is locally translated in axons, is activated upon injury, and is transported retrogradely to modulate neuronal survival responses in the cell body (Figure 7).
Figure 7.
A model for STAT3 retrograde signalling after nerve injury. Sciatic nerve injury induces local synthesis of importin β1 (Hanz et al, 2003) and STAT3 (current manuscript) in lesioned sensory axons. The newly synthesized STAT3 is phosphorylated and then links up with the importins–dynein complex for retrograde transport to neuronal cell bodies, where it activates new transcription with survival-promoting consequences.
Multiple TFs are activated post-translationally and affect the cell body response in injured neurons
The search for ‘master regulators’ of nerve regeneration has naturally directed attention to TFs as the canonical master regulators in biological systems. Accordingly, a growing list of TFs and their associated regulators have been implicated in nerve injury responses in diverse neuronal subtypes (Raivich and Behrens, 2006; Seijffers et al, 2007; Stam et al, 2007; Wu et al, 2007; Jankowski et al, 2009; MacGillavry et al, 2009; Moore et al, 2009; Smith et al, 2009, 2011; Tedeschi and Di Giovanni, 2009; Zou et al, 2009; Michaelevski et al, 2010b). Many of these studies had focused on TFs that were themselves upregulated at transcript level by injury, such as ATF3, driven by the hope that such robustly regulated factors would prove to be key regulators of nerve injury responses. However, our data clearly show that an additional and prominent aspect of transcriptional control of the injury response is provided by factors regulated solely at translational or post-translational levels in injured neurons. These findings significantly increase the number of TFs implicated in injury responses, and support a mechanism wherein injury signalling is channeled to and through multiple TFs that coordinate the cell body response. The limited overlap in regulated gene sets in our data (Figure 1B) suggests that individual factors may control very discrete aspects of the injury response. It follows that broad regulation of neuronal regeneration most likely requires concerted action by a number of factors.
Retrograde transport of TFs in neuronal processes
Neurons utilize importins and associated proteins in long distance transport mechanisms in axons and dendrites, and a few studies have suggested that different TFs and related molecules might be transported in an NLS and importin-dependent manner in neuronal processes (Perry and Fainzilber, 2009). These observations have for the most part been restricted to in-vitro cultures. Our in-vivo data from axoplasmic dynein pulldowns show that multiple TFs are likely transported on dynein in peripheral nerve (Supplementary Table S3). A significant fraction of these show injury-related changes in dynein association (Figure 1D). The broad specificity range of most TFBS does not enable direct definition of the transported entity, but clearly members of the AP, Myc/Max, PPAR, Smad, and STAT families are likely retrogradely transported by dynein and regulated by injury in rodent sciatic nerve. A focused analysis revealed STAT3 as the most robust candidate from the STAT family (Figures 2,3,4,5,6), and different experimental approaches indeed confirmed dynein-dependent transport of STAT3 in vivo, and showed that a peptide based on an importin α5 association motif in STAT3 (Ma et al, 2003) disrupts its retrograde transport, supporting an importin-dependent transport mechanism (Figures 4,5,6). The fact that a classical NLS peptide did not inhibit retrograde transport of STAT3 indicates that both types of cargos do not compete for an identical binding site, allowing concomitant transport of multiple TFs by dynein complexes likely utilizing different importin α isoforms as cargo binding adaptors.
STAT3 activation for retrograde injury signalling involves both local translation (Figure 3) and post-translational phosphorylation (Figures 2, 4 and 5). The importance of mRNA translocation and local translation in neuronal processes has become increasingly clear, especially in the response to injury in peripheral neurons (Donnelly et al, 2010; Gumy et al, 2011). A wide range of mRNA's have been implicated in local translation upon injury (Willis et al, 2007; Taylor et al, 2009; Vogelaar et al, 2009), but technical limitations have usually restricted direct proof of axonal translation to in-vitro culture systems, with the notable exception of very recent studies on β-actin (Donnelly et al, 2011; Willis et al, 2011). Our study demonstrates axonal localization and local translation of STAT3 mRNA, taking advantage of a glia-specific deletion of STAT3 (Herrmann et al, 2008) to confirm the in-vitro culture data also in an in-vivo context in sciatic nerve (Figure 3). Previous studies have shown that scaffolding (Hanz et al, 2003; Perlson et al, 2005) and regulatory (Yudin et al, 2008) components of the retrograde injury signalling complex arise from local translation, and the current observations show that STAT3 as a signalling component can be produced in the same way. The 3′UTR axon-targeting motif in STAT3 (Figure 3) does not reveal any obvious sequence homologies with those of other components of the complex, but many other 3′UTR targeting motifs bear structural and not sequence homology. It will be interesting in the future to find out what fraction of complex components arise wholly or partly from local translation, and to determine the transport entities that shuttle these mRNAs into axons.
Functional consequences of dynein transport of axonal STAT3
A number of reports have implicated STAT3 as a beneficial regulator of neurite outgrowth or survival after injury, either directly (Schwaiger et al, 2000; Alonzi et al, 2001; Liu and Snider, 2001; Schweizer et al, 2002; Qiu et al, 2005; Smith et al, 2011), or indirectly via inactivation of its regulator SOCS3 (Miao et al, 2006; Smith et al, 2009). Most previous studies have suggested that retrograde transport of STAT3 activating cytokines and their receptors in signalling endosomes link axonal injury to STAT3 effects in the cell body (Kirsch et al, 2003; O’Brien and Nathanson, 2007; Habecker et al, 2009), although one report postulated transport of STAT3 itself based on indirect evidence from immunostainings (Lee et al, 2004). Our study provides in-vivo evidence for retrograde transport of STAT3 itself from the injury site to the cell body, and functional consequences thereof. Phosphorylated STAT3 (pSTAT3) was co-immunoprecipitated with dynein from axoplasm after injury, and inhibition of microtubule-dependent transport by colchicine reduced retrograde transport of STAT3 (Figures 4 and 5). A 1-cm shift in the injury site caused a corresponding delay in retrograde transport, and injection of a competing STAT3 peptide to sciatic nerve reduced retrograde accumulation of pSTAT3 in DRG, and upregulation of STAT3 responsive genes, Reg3a and SOCS3. Moreover, a battery of complementary approaches has shown that inhibiting STAT3 translation or association with the retrograde transport machinery in axons affects cell body apoptosis after nerve injury (Figure 6). Hence, these data provide direct evidence for retrograde transport of pSTAT3 from an axonal injury site to DRG cell bodies, to mediate gene expression and neuronal survival after injury (Figure 7). Neuronal loss in certain primary sensory neuron populations can reach ∼40% following peripheral nerve injury and this may be one of the principal limitations to effective recovery of sensory functions in human patients (Hart et al, 2002, 2008). Thus, the retrograde anti-apoptotic role of STAT3 may offer new opportunities for sensory recovery in the clinical setting. Moreover, STAT3 has also been reported to promote survival of adult motor neurons (Schweizer et al, 2002) and of retinal ganglion neurons (Zhang et al, 2008) after injury. Taken together with our findings, this suggests that promotion of resistance to apoptosis following injury is both a widespread and fundamental role for STAT3 in the nervous system. The involvement of additional TFs in the retrograde injury response is also supported by the more potent effects on neurite outgrowth we observed upon injection of the NLS peptide (Supplementary Figure S8). These might include Max, PPARs, or Smads, as well as other yet unidentified cargos. Additional work will be required to delineate the full complement of retrogradely transported TFs in axons, and to determine the range of physiological phenomena impacted by this direct route from axon to nucleus.
Materials and methods
Animals, preparations, and cultures
Adult (8–12 weeks old) male Wistar rats were from Harlan. Wild-type or floxed-STAT3 (Takeda et al, 1998) or GFAP-STAT3-CKO (Herrmann et al, 2008) or importin α5 KO (Shmidt et al, 2007) mice were as described. Sciatic nerve crush, conditioning lesions, and axoplasm and DRG extract preparations were as previously described (Hanz et al, 2003; Perlson et al, 2005; Rishal et al, 2010). Neuronal process lengths were quantified using WIS-Neuromath (http://www.wisdom.weizmann.ac.il/~vision/NeuroMath/).
TFBS analyses
Normalized expression values from microarray analyses (Michaelevski et al, 2010b) were uploaded to Genomica (http://genomica.weizmann.ac.il) with a gene set of TFs generated as a binary matrix M of size n × m; n represents the number of genes and m the number of TFs. Mi,j=1 if TF j is predicted to bind the promoter of gene i, otherwise Mi,j=0. In order to determine the interaction between TFs and genes the upstream regions (2000 bp) of all rat open reading frames were downloaded and scanned using the SCANACE program (http://arep.med.harvard.edu/mrnadata/mrnasoft.html). Positional weight matrices representing the binding affinities for all vertebrate TFs were downloaded from the Transfac 7 database (http://www.gene-regulation.com/pub/databases.html). For each gene i and TF j Mi,j was set to one if at least one position in gene's i upstream sequence had an identity of >95%. The original microarrays data are available in the NCBI Geo database (accession number GSE26350).
Quantitative PCR
qPCR was performed as previously described (Nilsson et al, 2005) using Taqman primer kits for β-tubulin III or β-actin (normalization control), ATF3, Reg3a, SOCS3, and STAT3.
Protein/DNA array analysis
Sciatic nerve axoplasm was subjected to dynein immunoprecipitation and co-precipitating TFs were identified using Panomics array #MA1210, according to manufacturer's instructions.
Antibody-based methods
Western blots, immunostainings, and immunoprecipitations were carried out as previously described (Hanz et al, 2003; Perlson et al, 2005), for antibody details please see Supplementary data. For competition assays, 50 μg of reverse-NLS (CTPVKRKKKP) or STAT3 peptide (TLREQRCGNG) was added during incubation with primary antibody.
Fluorescence in-situ hybridization
Antisense oligonucleotide probes for STAT3 were designed using Oligo 6 software and checked for homology and specificity by BLAST. cRNA probes for GFP reporter mRNA in transfected neurons were as previously described (Vuppalanchi et al, 2010). Hybridization to DRG neuronal cultures was as previously described (Willis et al, 2007). Hybridization to tissue sections was performed as previously published (Muddashetty et al, 2007), with minor modifications as detailed in Supplementary data.
Electron microscopy
Rat sciatic nerves from naive or 6 h post injury animals were fixed and sectioned, followed by co-immuno-gold labelling (dynein, 15 nm particles; pSTAT3, 10 nm particles), as detailed in Supplementary data. The number of pSTAT3 particles per axon and the percent of pSTAT3 particles adjacent to a dynein particle were quantified.
Pure axonal Preps, RT–PCRs, and 3′ RACE RT–PCR
Isolation of DRG axons was carried out as previously described (Zheng et al, 2001). Two hundred nanograms of RNA from cell body and axons were used as template for reverse transcription and PCR. For details of reaction conditions and primers, please see Supplementary data.
Fluorescence recovery after photobleaching
Dissociated DRG cultures were transfected with STAT3 3′ UTR axonal and cell body variants using Amaxa nucleofection. Terminal axons were subjected to FRAP sequence at 37°C with 488 nm laser line of Leica TCS/SP2 confocal microscope as described with minor modifications (Yudin et al, 2008). Prior to bleaching, neurons were imaged every 30 s for 2 min at 15% laser power. For photobleaching, the region of interest (ROI) was exposed to 75% laser power every 1.6 s for 40 frames. Recovery was monitored every 60 s over 20 min at 15% laser power. To test for translation dependence, cultures were pretreated with 50 μM anisomycin for 30 min before the photobleaching sequence. FRAP quantification and statistical tests are detailed in Supplementary data.
Lentiviral transduction of DRG
Recombinant lentiviruses expressing Cre recombinase (LV-Cre) or constitutively active STAT3 (LV-CA-STAT3) or GFP (LV-GFP) were produced by transient transfection in HEK293T cells as described (Tiscornia et al, 2006). For in-vivo transduction of DRG, a lateral back incision was performed in floxed-STAT3 or importin α5 KO mice to expose the L4/L5 DRGs, and 0.5 μl of the appropriate LV was injected to each DRG using a microinjector. The surgery zone was then sutured, and the mice were left to recover for 7 days before future procedures.
TUNEL analysis
Rat L4/L5 DRGs were dissected 7 days after sciatic nerve injury and injection of 250 μg NLS, reverse-NLS or STAT3-NLS peptide; or 10 μg colchicine or its vehicle (PBS); or 0.3 μg anti-STAT3 or negative control LNA. L4/L5 DRGs from floxed-STAT3 mice were dissected 14 days after lentivirus injection to the DRG and 7 days after sciatic nerve crush. The DRG was then fixed in 4% paraformaldehyde, and sectioned at 20 μm thickness on a Leica cryostat. Cell death analyses in DRG were as previously described (McKay Hart et al, 2002). TUNEL staining was with the TMR red In-Situ Cell Death Detection kit (Roche) according to manufacturer's instructions. Sections were later immunostained for NFH, Islet1, and DAPI. Images were taken using an Olympus IX71 inverted fluorescence microscope. Islet1-positive cells were counted using the Cell Profiler software (http://www.cellprofiler.org) and the mean number of TUNEL-positive cells per number of Islet1-positive cells was determined.
Supplementary Material
Acknowledgments
We gratefully thank Tal Gradus and Zehava Levy for technical assistance; and Eric Martz, Vladimir Kiss, Vera Shinder and Michael Tolmasov for expert assistance with structure modelling, fluorescence microscopy, electron microscopy, and microsurgery, respectively. We also thank Prof. Shizuo Akira for floxed-STAT3 mice; Alon Chen for the LV-Cre virus; and Michael Sofroniew for GFAP-STAT3-CKO mice. This work was supported by the Israel Science Foundation (1157/09), the US-Israel Binational Science Foundation (2003208), the International Foundation for Research in Paraplegia (P86 and P116), the Dr Miriam and Sheldon G Adelson Medical Research Foundation, the German-Israeli Foundation (1010-168.1/2008), the Deutsche Forschungsgemeinschaft (KO 1950/3-1); and the NIH (R01-NS041596 and K99-NR010797). MF is the incumbent of the Chaya Professorial Chair in Molecular Neuroscience at the Weizmann Institute of Science.
Author contributions: KBY, SD, and MF designed the study; KBY, SD, YSR, DV, DEW, DY, and IR performed experiments; SD, OS, and YP carried out computational analyses; FR and MB contributed importin α5 null mice; AB contributed STAT3 lentiviruses; KBY, SD, JLT, and MF analysed the data; KBY and MF wrote the paper, and all listed authors commented and corrected on manuscript drafts.
Footnotes
The authors declare that they have no conflict of interest.
References
- Aakalu G, Smith WB, Nguyen N, Jiang C, Schuman EM (2001) Dynamic visualization of local protein synthesis in hippocampal neurons. Neuron 30: 489–502 [DOI] [PubMed] [Google Scholar]
- Abe N, Almenar-Queralt A, Lillo C, Shen Z, Lozach J, Briggs SP, Williams DS, Goldstein LS, Cavalli V (2009) Sunday driver interacts with two distinct classes of axonal organelles. J Biol Chem 284: 34628–34639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe N, Cavalli V (2008) Nerve injury signalling. Curr Opin Neurobiol 18: 276–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonzi T, Middleton G, Wyatt S, Buchman V, Betz UA, Muller W, Musiani P, Poli V, Davies AM (2001) Role of STAT3 and PI 3-kinase/Akt in mediating the survival actions of cytokines on sensory neurons. Mol Cell Neurosci 18: 270–282 [DOI] [PubMed] [Google Scholar]
- Andreassi C, Zimmermann C, Mitter R, Fusco S, De Vita S, Saiardi A, Riccio A (2010) An NGF-responsive element targets myo-inositol monophosphatase-1 mRNA to sympathetic neuron axons. Nat Neurosci 13: 291–301 [DOI] [PubMed] [Google Scholar]
- Behnisch T, Yuanxiang P, Bethge P, Parvez S, Chen Y, Yu J, Karpova A, Frey JU, Mikhaylova M, Kreutz MR (2011) Nuclear translocation of jacob in hippocampal neurons after stimuli inducing long-term potentiation but not long-term depression. PLoS One 6: e17276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LJ, Hengst U, Gurskaya NG, Lukyanov KA, Jaffrey SR (2008) Intra-axonal translation and retrograde trafficking of CREB promotes neuronal survival. Nat Cell Biol 10: 149–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewilde S, Vercelli A, Chiarle R, Poli V (2008) Of alphas and betas: distinct and overlapping functions of STAT3 isoforms. Front Biosci 13: 6501–6514 [DOI] [PubMed] [Google Scholar]
- Dieterich DC, Karpova A, Mikhaylova M, Zdobnova I, Konig I, Landwehr M, Kreutz M, Smalla KH, Richter K, Landgraf P, Reissner C, Boeckers TM, Zuschratter W, Spilker C, Seidenbecher CI, Garner CC, Gundelfinger ED, Kreutz MR (2008) Caldendrin-Jacob: a protein liaison that couples NMDA receptor signalling to the nucleus. PLoS Biol 6: e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly CJ, Fainzilber M, Twiss JL (2010) Subcellular communication through RNA transport and localized protein synthesis. Traffic 11: 1498–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly CJ, Willis DE, Xu M, Tep C, Jiang C, Yoo S, Schanen NC, Kirn-Safran CB, van Minnen J, English A, Yoon SO, Bassell GJ, Twiss JL (2011) Limited availability of ZBP1 restricts axonal mRNA localization and nerve regeneration capacity. EMBO J 30: 4665–4677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorian C, Nakashima J, Dry SM, Nghiemphu PL, Smith KB, Ao Y, Dang J, Lawson G, Mellinghoff IK, Mischel PS, Phelps M, Parada LF, Liu X, Sofroniew MV, Eilber FC, Wu H (2009) PTEN dosage is essential for neurofibroma development and malignant transformation. Proc Natl Acad Sci USA 106: 19479–19484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumy LF, Yeo GS, Tung YC, Zivraj KH, Willis D, Coppola G, Lam BY, Twiss JL, Holt CE, Fawcett JW (2011) Transcriptome analysis of embryonic and adult sensory axons reveals changes in mRNA repertoire localization. RNA 17: 85–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habecker BA, Sachs HH, Rohrer H, Zigmond RE (2009) The dependence on gp130 cytokines of axotomy induced neuropeptide expression in adult sympathetic neurons. Dev Neurobiol 69: 392–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanz S, Perlson E, Willis D, Zheng JQ, Massarwa R, Huerta JJ, Koltzenburg M, Kohler M, van-Minnen J, Twiss JL, Fainzilber M (2003) Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron 40: 1095–1104 [DOI] [PubMed] [Google Scholar]
- Hart AM, Terenghi G, Wiberg M (2008) Neuronal death after peripheral nerve injury and experimental strategies for neuroprotection. Neurol Res 30: 999–1011 [DOI] [PubMed] [Google Scholar]
- Hart AM, Wiberg M, Youle M, Terenghi G (2002) Systemic acetyl-L-carnitine eliminates sensory neuronal loss after peripheral axotomy: a new clinical approach in the management of peripheral nerve trauma. Exp Brain Res 145: 182–189 [DOI] [PubMed] [Google Scholar]
- Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, Korsak RA, Takeda K, Akira S, Sofroniew MV (2008) STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci 28: 7231–7243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MP, McIlwrath SL, Jing X, Cornuet PK, Salerno KM, Koerber HR, Albers KM (2009) Sox11 transcription factor modulates peripheral nerve regeneration in adult mice. Brain Res 1256: 43–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R (1992) Schwann cells: early lineage, regulation of proliferation and control of myelin formation. Curr Opin Neurobiol 2: 575–581 [DOI] [PubMed] [Google Scholar]
- Jiang X, Norman M, Roth L, Li X (2004) Protein-DNA array-based identification of transcription factor activities regulated by interaction with the glucocorticoid receptor. J Biol Chem 279: 38480–38485 [DOI] [PubMed] [Google Scholar]
- Kadoya K, Tsukada S, Lu P, Coppola G, Geschwind D, Filbin MT, Blesch A, Tuszynski MH (2009) Combined intrinsic and extrinsic neuronal mechanisms facilitate bridging axonal regeneration one year after spinal cord injury. Neuron 64: 165–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam N, Pilpel Y, Fainzilber M (2009) Can molecular motors drive distance measurements in injured neurons? PLoS Comput Biol 5: e1000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keramaris E, Vanderluit JL, Bahadori M, Mousavi K, Davis RJ, Flavell R, Slack RS, Park DS (2005) c-Jun N-terminal kinase 3 deficiency protects neurons from axotomy-induced death in vivo through mechanisms independent of c-Jun phosphorylation. J Biol Chem 280: 1132–1141 [DOI] [PubMed] [Google Scholar]
- Kirsch M, Terheggen U, Hofmann HD (2003) Ciliary neurotrophic factor is an early lesion-induced retrograde signal for axotomized facial motoneurons. Mol Cell Neurosci 24: 130–138 [DOI] [PubMed] [Google Scholar]
- Lai KO, Zhao Y, Ch’ng TH, Martin KC (2008) Importin-mediated retrograde transport of CREB2 from distal processes to the nucleus in neurons. Proc Natl Acad Sci USA 105: 17175–17180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N, Neitzel KL, Devlin BK, MacLennan AJ (2004) STAT3 phosphorylation in injured axons before sensory and motor neuron nuclei: potential role for STAT3 as a retrograde signaling transcription factor. J Comp Neurol 474: 535–545 [DOI] [PubMed] [Google Scholar]
- Lindwall C, Kanje M (2005) Retrograde axonal transport of JNK signaling molecules influence injury induced nuclear changes in p-c-Jun and ATF3 in adult rat sensory neurons. Mol Cell Neurosci 29: 269–282 [DOI] [PubMed] [Google Scholar]
- Liu RY, Snider WD (2001) Different signaling pathways mediate regenerative versus developmental sensory axon growth. J Neurosci 21: RC164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Cao X (2006) Regulation of Stat3 nuclear import by importin alpha5 and importin alpha7 via two different functional sequence elements. Cell Signal 18: 1117–1126 [DOI] [PubMed] [Google Scholar]
- Ma J, Zhang T, Novotny-Diermayr V, Tan AL, Cao X (2003) A novel sequence in the coiled-coil domain of Stat3 essential for its nuclear translocation. J Biol Chem 278: 29252–29260 [DOI] [PubMed] [Google Scholar]
- MacGillavry HD, Stam FJ, Sassen MM, Kegel L, Hendriks WT, Verhaagen J, Smit AB, van Kesteren RE (2009) NFIL3 and cAMP response element-binding protein form a transcriptional feedforward loop that controls neuronal regeneration-associated gene expression. J Neurosci 29: 15542–15550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay Hart A, Brannstrom T, Wiberg M, Terenghi G (2002) Primary sensory neurons and satellite cells after peripheral axotomy in the adult rat: timecourse of cell death and elimination. Exp Brain Res 142: 308–318 [DOI] [PubMed] [Google Scholar]
- Miao T, Wu D, Zhang Y, Bo X, Subang MC, Wang P, Richardson PM (2006) Suppressor of cytokine signaling-3 suppresses the ability of activated signal transducer and activator of transcription-3 to stimulate neurite growth in rat primary sensory neurons. J Neurosci 26: 9512–9519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelevski I, Medzihradszky KF, Lynn A, Burlingame AL, Fainzilber M (2010a) Axonal transport proteomics reveals mobilization of translation machinery to the lesion site in injured sciatic nerve. Mol Cell Proteomics 9: 976–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelevski I, Segal-Ruder Y, Rozenbaum M, Medzihradszky KF, Shalem O, Coppola G, Horn-Saban S, Ben-Yaakov K, Dagan SY, Rishal I, Geschwind DH, Pilpel Y, Burlingame AL, Fainzilber M (2010b) Signaling to transcription networks in the neuronal retrograde injury response. Sci Signal 3: ra53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikenberg I, Widera D, Kaus A, Kaltschmidt B, Kaltschmidt C (2007) Transcription factor NF-kappaB is transported to the nucleus via cytoplasmic dynein/dynactin motor complex in hippocampal neurons. PLoS ONE 2: e589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DL, Blackmore MG, Hu Y, Kaestner KH, Bixby JL, Lemmon VP, Goldberg JL (2009) KLF family members regulate intrinsic axon regeneration ability. Science 326: 298–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muddashetty RS, Kelic S, Gross C, Xu M, Bassell GJ (2007) Dysregulated metabotropic glutamate receptor-dependent translation of AMPA receptor and postsynaptic density-95 mRNAs at synapses in a mouse model of fragile X syndrome. J Neurosci 27: 5338–5348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S, Woolf CJ (1999) Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron 23: 83–91 [DOI] [PubMed] [Google Scholar]
- Ng YP, Cheung ZH, Ip NY (2006) STAT3 as a downstream mediator of Trk signaling and functions. J Biol Chem 281: 15636–15644 [DOI] [PubMed] [Google Scholar]
- Nilsson A, Moller K, Dahlin L, Lundborg G, Kanje M (2005) Early changes in gene expression in the dorsal root ganglia after transection of the sciatic nerve; effects of amphiregulin and PAI-1 on regeneration. Brain Res Mol Brain Res 136: 65–74 [DOI] [PubMed] [Google Scholar]
- O’Brien JJ, Nathanson NM (2007) Retrograde activation of STAT3 by leukemia inhibitory factor in sympathetic neurons. J Neurochem 103: 288–302 [DOI] [PubMed] [Google Scholar]
- Perlson E, Hanz S, Ben-Yaakov K, Segal-Ruder Y, Seger R, Fainzilber M (2005) Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron 45: 715–726 [DOI] [PubMed] [Google Scholar]
- Perry RB, Fainzilber M (2009) Nuclear transport factors in neuronal function. Semin Cell Dev Biol 20: 600–606 [DOI] [PubMed] [Google Scholar]
- Qiu J, Cafferty WB, McMahon SB, Thompson SW (2005) Conditioning injury-induced spinal axon regeneration requires signal transducer and activator of transcription 3 activation. J Neurosci 25: 1645–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raivich G, Behrens A (2006) Role of the AP-1 transcription factor c-Jun in developing, adult and injured brain. Prog Neurobiol 78: 347–363 [DOI] [PubMed] [Google Scholar]
- Rishal I, Fainzilber M (2010) Retrograde signaling in axonal regeneration. Exp Neurol 223: 5–10 [DOI] [PubMed] [Google Scholar]
- Rishal I, Michaelevski I, Rozenbaum M, Shinder V, Medzihradszky KF, Burlingame AL, Fainzilber M (2010) Axoplasm isolation from peripheral nerve. Dev Neurobiol 70: 126–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi F, Gianola S, Corvetti L (2007) Regulation of intrinsic neuronal properties for axon growth and regeneration. Prog Neurobiol 81: 1–28 [DOI] [PubMed] [Google Scholar]
- Schwab ME (2010) Functions of Nogo proteins and their receptors in the nervous system. Nat Rev Neurosci 11: 799–811 [DOI] [PubMed] [Google Scholar]
- Schwaiger FW, Hager G, Schmitt AB, Horvat A, Streif R, Spitzer C, Gamal S, Breuer S, Brook GA, Nacimiento W, Kreutzberg GW (2000) Peripheral but not central axotomy induces changes in Janus kinases (JAK) and signal transducers and activators of transcription (STAT). Eur J Neurosci 12: 1165–1176 [DOI] [PubMed] [Google Scholar]
- Schweizer U, Gunnersen J, Karch C, Wiese S, Holtmann B, Takeda K, Akira S, Sendtner M (2002) Conditional gene ablation of Stat3 reveals differential signaling requirements for survival of motoneurons during development and after nerve injury in the adult. J Cell Biol 156: 287–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seijffers R, Mills CD, Woolf CJ (2007) ATF3 increases the intrinsic growth state of DRG neurons to enhance peripheral nerve regeneration. J Neurosci 27: 7911–7920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmidt T, Hampich F, Ridders M, Schultrich S, Hans VH, Tenner K, Vilianovich L, Qadri F, Alenina N, Hartmann E, Kohler M, Bader M (2007) Normal brain development in importin-alpha5 deficient-mice. Nat Cell Biol 9: 1337–1338; author reply 1339 [DOI] [PubMed] [Google Scholar]
- Smith DS, Skene JH (1997) A transcription-dependent switch controls competence of adult neurons for distinct modes of axon growth. J Neurosci 17: 646–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PD, Sun F, Park KK, Cai B, Wang C, Kuwako K, Martinez-Carrasco I, Connolly L, He Z (2009) SOCS3 deletion promotes optic nerve regeneration in vivo. Neuron 64: 617–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RP, Lerch-Haner JK, Pardinas JR, Buchser WJ, Bixby JL, Lemmon VP (2011) Transcriptional profiling of intrinsic PNS factors in the postnatal mouse. Mol Cell Neurosci 46: 32–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam FJ, MacGillavry HD, Armstrong NJ, de Gunst MC, Zhang Y, van Kesteren RE, Smit AB, Verhaagen J (2007) Identification of candidate transcriptional modulators involved in successful regeneration after nerve injury. Eur J Neurosci 25: 3629–3637 [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Yoshida N, Takeda J, Kishimoto T, Akira S (1998) Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J Immunol 161: 4652–4660 [PubMed] [Google Scholar]
- Taylor AM, Berchtold NC, Perreau VM, Tu CH, Li Jeon N, Cotman CW (2009) Axonal mRNA in uninjured and regenerating cortical mammalian axons. J Neurosci 29: 4697–4707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschi A, Di Giovanni S (2009) The non-apoptotic role of p53 in neuronal biology: enlightening the dark side of the moon. EMBO Rep 10: 576–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiscornia G, Singer O, Verma IM (2006) Production and purification of lentiviral vectors. Nat Protoc 1: 241–245 [DOI] [PubMed] [Google Scholar]
- Tsujino H, Kondo E, Fukuoka T, Dai Y, Tokunaga A, Miki K, Yonenobu K, Ochi T, Noguchi K (2000) Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: A novel neuronal marker of nerve injury. Mol Cell Neurosci 15: 170–182 [DOI] [PubMed] [Google Scholar]
- Vogelaar CF, Gervasi NM, Gumy LF, Story DJ, Raha-Chowdhury R, Leung KM, Holt CE, Fawcett JW (2009) Axonal mRNAs: characterisation and role in the growth and regeneration of dorsal root ganglion axons and growth cones. Mol Cell Neurosci 42: 102–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuppalanchi D, Coleman J, Yoo S, Merianda TT, Yadhati AG, Hossain J, Blesch A, Willis DE, Twiss JL (2010) Conserved 3′-untranslated region sequences direct subcellular localization of chaperone protein mRNAs in neurons. J Biol Chem 285: 18025–18038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welin D, Novikova LN, Wiberg M, Kellerth JO, Novikov LN (2008) Survival and regeneration of cutaneous and muscular afferent neurons after peripheral nerve injury in adult rats. Exp Brain Res 186: 315–323 [DOI] [PubMed] [Google Scholar]
- Willis DE, van Niekerk EA, Sasaki Y, Mesngon M, Merianda TT, Williams GG, Kendall M, Smith DS, Bassell GJ, Twiss JL (2007) Extracellular stimuli specifically regulate localized levels of individual neuronal mRNAs. J Cell Biol 178: 965–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis DE, Xu M, Donnelly CJ, Tep C, Kendall M, Erenstheyn M, English AW, Schanen NC, Kirn-Safran CB, Yoon SO, Bassell GJ, Twiss JL (2011) Axonal localization of transgene mRNA in mature PNS and CNS neurons. J Neurosci 31: 14481–14487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Zhang Y, Bo X, Huang W, Xiao F, Zhang X, Miao T, Magoulas C, Subang MC, Richardson PM (2007) Actions of neuropoietic cytokines and cyclic AMP in regenerative conditioning of rat primary sensory neurons. Exp Neurol 204: 66–76 [DOI] [PubMed] [Google Scholar]
- Ylera B, Erturk A, Hellal F, Nadrigny F, Hurtado A, Tahirovic S, Oudega M, Kirchhoff F, Bradke F (2009) Chronically CNS-injured adult sensory neurons gain regenerative competence upon a lesion of their peripheral axon. Curr Biol 19: 930–936 [DOI] [PubMed] [Google Scholar]
- Yudin D, Hanz S, Yoo S, Iavnilovitch E, Willis D, Gradus T, Vuppalanchi D, Segal-Ruder Y, Ben-Yaakov K, Hieda M, Yoneda Y, Twiss JL, Fainzilber M (2008) Localized regulation of axonal RanGTPase controls retrograde injury signaling in peripheral nerve. Neuron 59: 241–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Li H, Liu MG, Kawasaki A, Fu XY, Barnstable CJ, Shao-Min Zhang S (2008) STAT3 activation protects retinal ganglion cell layer neurons in response to stress. Exp Eye Res 86: 991–997 [DOI] [PubMed] [Google Scholar]
- Zheng JQ, Kelly TK, Chang B, Ryazantsev S, Rajasekaran AK, Martin KC, Twiss JL (2001) A functional role for intra-axonal protein synthesis during axonal regeneration from adult sensory neurons. J Neurosci 21: 9291–9303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H, Ho C, Wong K, Tessier-Lavigne M (2009) Axotomy-induced Smad1 activation promotes axonal growth in adult sensory neurons. J Neurosci 29: 7116–7123 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.