Abstract
EMBO J 31 6, 1350–1363 (2012); published online January 13 2012
In this issue of The EMBO Journal, Ben-Yaakov et al (2012) explore the contribution of transcription factors (TFs) in directly communicating information about injury between the axon and the nucleus. They show that multiple TFs bind the retrograde molecular motor dynein in injured axons. Focusing on one TF family, the authors reveal that STAT3 is locally translated and activated in injured axons, and then transported retrogradely to the nucleus to promote survival of peripheral sensory neurons.
Recovery from peripheral nerve injury can be relatively successful, but the insult can also lead to permanent neurological deficits including failure of reinnervation and neuronal loss. What determines whether a neuron survives and regenerates after injury? The existence of injury signals ascending from the axon to the cell body was proposed to account for the changes observed in sensory neurons’ cell bodies following injury (Cragg, 1970). Much progress has since been made to reveal the identities of such injury signals (Abe and Cavalli, 2008). Downstream of positive injury signals, TFs control the expression of regeneration-associated genes. The involvement of nuclear localization signals to target proteins to the axonal retrograde transport system (Schmied et al, 1993) and the role of importin nuclear transport factors in injury signalling (Yudin et al, 2008) suggests that TFs themselves might be involved in direct communication between injured axon and the nucleus, a possibility that Ben-Yaakov and colleagues directly test in the current issue of The EMBO Journal.
The authors used computational tools to analyse TF binding sites (TFBS) in a previously generated dorsal root ganglion (DRG) microarray data set (Michaelevski et al, 2010). This analysis revealed a list of TFs involved in the neuronal injury response and suggested that their activity in injured DRG neurons might be regulated at the post-transcriptional level. These findings led the authors to test the interaction between activated TFs identified in the computational analysis and the retrograde motor dynein. They found 11 TFs whose association with dynein differed between injured and naive nerves. Five TFs classes (AP, Myc/Max, PPAR, Smad, and STAT) were common across all computational screens and thus were strong candidates as injury signalling molecules.
The authors focused subsequent investigations on the STAT family of TFs. Among the STAT family members, they found that only STAT3 is phosphorylated after nerve injury in both DRG cell bodies and axons. Previous studies have shown that STAT3 activation is directly involved in the conditioning injury response of DRG neurons (Schwaiger et al, 2000; Qiu et al, 2005) and in the ability of DRG neurons to survive (Alonzi et al, 2001) and regenerate (Miao et al, 2006). Yet, it is unclear whether STAT3 exerts these effects in the axon or cell body. Indeed, while the retrograde axonal transport of locally activated STAT3 has been suggested (Lee et al, 2004) later studies proposed a model for STAT3 activation in the cell body (O’Brien and Nathanson, 2007).
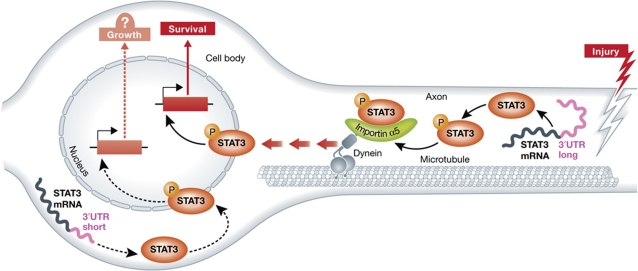
Previously, the Fainzilber laboratory revealed that key components of the retrograde injury signalling are locally translated in axons in response to injury (Yudin et al, 2008). Here, the authors demonstrate that STAT3 is synthesized locally in injured axons, revealing that STAT3 function in the neuronal injury response starts in the axon. First, they show that the mRNA for STAT3 is localized in cultured DRG axons and in sensory axons in vivo. Second, they identified axonal and cell body STAT3 3′UTR variants. Only the axonal form of STAT3 3′UTR, but not the cell body form, was able to drive the expression of a reporter in axons. This local expression was blocked by translation inhibitor, indicating that axonal STAT3 3′UTR contains a targeting sequence for axonal localization and translation in peripheral sensory neurons. In addition, metabolic labelling of sciatic nerve segments revealed that STAT3 synthesis requires calcium and occurs specifically in axons. These findings not only convincingly show that STAT3 is translated in injured axons, but also further emphasize the importance of 3′UTR sequences for axonal sorting of mRNAs and their role in injury response (Yoo et al, 2010).
The authors then examined whether locally translated STAT3 binds dynein, similarly to other components of the injury signal complexes (Yudin et al, 2008). If STAT3 is transported from the injury site back to the cell bodies, then the amount of time it takes for activated STAT3 to appear in the cell body depends on the distance to the axotomy site. The authors show that pSTAT3 accumulation in DRG nuclei correlates with the induction of STAT3 target genes and indeed depends on the distance between the injury site and the cell body, as has been reported for JNK injury signalling (Kenney and Kocsis, 1998). Furthermore, injection of colchicine into the sciatic nerve, which disrupts microtubules and thus retrograde transport, prevents the accumulation of pSTAT3 in DRG nuclei after nerve injury. To characterize the mechanism of interaction between STAT3 and dynein, the authors performed a competition experiment using a peptide that competes with STAT3 for its interaction with the nuclear import factor importin α5. Injection of this peptide into injured sciatic nerves reduced the amount of STAT3 and pSTAT3 that co-immunoprecipitated with dynein as well as the amount of pSTAT3 accumulation in DRG nuclei, suggesting that importin α5 is involved in the interaction between STAT3 and dynein. Together, this extensive set of experiments demonstrates that STAT3 is locally synthesized in axons following injury and then transported back to the cell body via an interaction with an importin α5–dynein complex. It will be interesting to determine whether STAT3 phosphorylation in response to injury, in addition to its transcriptional activity in the cell body, is also needed for retrograde transport, for example by increasing its affinity for the importin α5–dynein complex.
What is the functional consequence of retrogradely transported pSTAT3? Previous work suggested that STAT3 activation is required for sensory neuron survival (Alonzi et al, 2001) and regeneration (Miao et al, 2006). The authors found that viral-mediated knockdown of STAT3 in adult sensory neurons increased the level of apoptotic neurons following nerve injury, indicating that STAT3 functions to promote sensory neuron survival following peripheral nerve injury. However, this experiment did not address whether STAT3 was mediated its anti-apoptotic effects in the DRG cell body or axon. Therefore, the authors took advantage of the peptide that blocked STAT3 interaction with importin α5 and dynein to prevent STAT3 retrograde transport to the cell body. Remarkably, when injected concomitantly with the injury, the peptide increased by three-fold the number of apoptotic neurons. Accordingly, crush lesion in importin α5 knockout mice also led to an elevation of neuronal death. This apoptotic phenotype was rescued by viral expression of active STAT3 in the DRG cell bodies of importin α5 knockout mice. Finally, interfering with STAT3 coding sequence in the sciatic nerve reduced axonal STAT3 levels and increased the number of apoptotic DRG neurons after nerve injury. Together, these experiments demonstrate that retrogradely transported axonal STAT3 originates from injury-induced local axonal translation and exerts an anti-apoptotic role in sensory neuron cell bodies after sciatic nerve injury (Figure 1).
Figure 1.
Injury-induced STAT3 axonal translation and retrograde transport promotes sensory neurons survival. Following the peripheral nerve injury, Ca2+ influx activates STAT3 local protein synthesis. The newly synthesized STAT3 is phosphorylated and binds to the retrograde molecular motor dynein via the nuclear import factor importin α5. Retrograde transport of the STAT3 injury signal complex back to the DRG cell body activates the transcription of target genes to induce a survival program. Whether STAT3 expression and activation in DRG cell body plays a similar or distinct role following the injury remains to be determined.
In the current study, the authors did not observe major differences in DRG neurite outgrowth responses following the reduction of STAT3 level or following the expression of constitutively active STAT3. This is in contrast to previous reports showing that transduction of an active STAT3 increases neurite outgrowth (Miao et al, 2006) and central DRG branches (Bareyre et al, 2011) and that deletion of STAT3 leads to reduced neurite outgrowth and sensory neuron regeneration (Qiu et al, 2005; Bareyre et al, 2011). These differences may result from the type of assays used to assess axon outgrowth and regeneration. Indeed, it appears that STAT3 may regulate the initiation but not the later perpetuation of axonal growth (Bareyre et al, 2011). Nonetheless, given that STAT3 mRNA is found in both the axon and the cell body with distinct 3′UTR sequences, it will be interesting to determine whether the functional consequence of STAT3 activity depends on its translational origin in the neuron.
In summary, this elegant work demonstrates that the retrograde transport of an axonally derived STAT3-injury signalling complex promotes survival of sensory neurons. This work expands our understanding of injury signals and supports the notion proposed over 40 years ago (Cragg, 1970) that more than one signalling mechanism operates to account for the variety of responses that occur after injury.
Footnotes
The authors declare that they have no conflict of interest.
References
- Abe N, Cavalli V (2008) Nerve injury signaling. Curr Opin Neurobiol 18: 276–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonzi T, Middleton G, Wyatt S, Buchman V, Betz UA, Muller W, Musiani P, Poli V, Davies AM (2001) Role of STAT3 and PI 3-kinase/Akt in mediating the survival actions of cytokines on sensory neurons. Mol Cell Neurosci 18: 270–282 [DOI] [PubMed] [Google Scholar]
- Bareyre FM, Garzorz N, Lang C, Misgeld T, Buning H, Kerschensteiner M (2011) In vivo imaging reveals a phase-specific role of STAT3 during central and peripheral nervous system axon regeneration. Proc Natl Acad Sci USA 108: 6282–6287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Yaakov K, Dagan SY, Segal-Ruder Y, Shalem O, Vuppalanchi D, Willis DE, Yudin D, Rishal I, Rother F, Bader M, Blesch A, Pilpel Y, Twiss JL, Fainzilber M (2012) Axonal transcription factors signal retrogradely in lesioned peripheral nerve. EMBO J 31: 1350–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg BG (1970) What is the signal for chromatolysis? Brain Res 23: 1–21 [DOI] [PubMed] [Google Scholar]
- Kenney AM, Kocsis JD (1998) Peripheral axotomy induces long-term c-Jun amino-terminal kinase-1 activation and activator protein-1 binding activity by c-Jun and junD in adult rat dorsal root ganglia in vivo. J Neurosci 18: 1318–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N, Neitzel KL, Devlin BK, MacLennan AJ (2004) STAT3 phosphorylation in injured axons before sensory and motor neuron nuclei: potential role for STAT3 as a retrograde signaling transcription factor. J Comp Neurol 474: 535–545 [DOI] [PubMed] [Google Scholar]
- Miao T, Wu D, Zhang Y, Bo X, Subang MC, Wang P, Richardson PM (2006) Suppressor of cytokine signaling-3 suppresses the ability of activated signal transducer and activator of transcription-3 to stimulate neurite growth in rat primary sensory neurons. J Neurosci 26: 9512–9519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelevski I, Segal-Ruder Y, Rozenbaum M, Medzihradszky KF, Shalem O, Coppola G, Horn-Saban S, Ben-Yaakov K, Dagan SY, Rishal I, Geschwind DH, Pilpel Y, Burlingame AL, Fainzilber M (2010) Signaling to transcription networks in the neuronal retrograde injury response. Sci Signal 3: ra53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien JJ, Nathanson NM (2007) Retrograde activation of STAT3 by leukemia inhibitory factor in sympathetic neurons. J Neurochem 103: 288–302 [DOI] [PubMed] [Google Scholar]
- Qiu J, Cafferty WB, McMahon SB, Thompson SW (2005) Conditioning injury-induced spinal axon regeneration requires signal transducer and activator of transcription 3 activation. J Neurosci 25: 1645–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmied R, Huang CC, Zhang XP, Ambron DA, Ambron RT (1993) Endogenous axoplasmic proteins and proteins containing nuclear localization signal sequences use the retrograde axonal transport/nuclear import pathway in Aplysia neurons. J Neurosci 13: 4064–4071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaiger FW, Hager G, Schmitt AB, Horvat A, Hager G, Streif R, Spitzer C, Gamal S, Breuer S, Brook GA, Nacimiento W, Kreutzberg GW (2000) Peripheral but not central axotomy induces changes in Janus kinases (JAK) and signal transducers and activators of transcription (STAT). Eur J Neurosci 12: 1165–1176 [DOI] [PubMed] [Google Scholar]
- Yoo S, van Niekerk EA, Merianda TT, Twiss JL (2010) Dynamics of axonal mRNA transport and implications for peripheral nerve regeneration. Exp Neurol 223: 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudin D, Hanz S, Yoo S, Iavnilovitch E, Willis D, Gradus T, Vuppalanchi D, Segal-Ruder Y, Ben-Yaakov K, Hieda M, Yoneda Y, Twiss JL, Fainzilber M (2008) Localized regulation of axonal RanGTPase controls retrograde injury signaling in peripheral nerve. Neuron 59: 241–252 [DOI] [PMC free article] [PubMed] [Google Scholar]