Abstract
Neuronal survival critically depends on the integrity and functionality of mitochondria. A hierarchical system of cellular surveillance mechanisms protects mitochondria against stress, monitors mitochondrial damage and ensures the selective removal of dysfunctional mitochondrial proteins or organelles. Mitochondrial proteases emerge as central regulators that coordinate different quality control (QC) pathways within an interconnected network of mechanisms. A failure of this system causes neuronal loss in a steadily increasing number of neurodegenerative disorders, which include Parkinson's disease, spinocerebellar ataxia, spastic paraplegia and peripheral neuropathies. Here, we will discuss the role of the mitochondrial QC network for neuronal survival and neurodegeneration.
Keywords: mitochondria, mitochondrial fusion, mitophagy, mitochondrial proteases, neurodegeneration
Introduction
Eukaryotic cells have evolved elaborate and powerful quality control (QC) systems to detect and eliminate damage in several compartments. The complex biogenesis of mitochondria as endosymbiontic organelles and their manifold biological functions render these organelles particularly vulnerable to accumulating damage during cell life. Mitochondria contain their own genome, which encodes 13 polypeptides, 22 tRNAs and 2 rRNAs; yet, the vast majority of mitochondrial proteins are encoded by the nuclear genome, synthesized in the cytoplasm and subsequently imported in the organelle (Larsson, 2010; Schmidt et al, 2010). Although nuclear-mitochondria communication systems coordinate the expression of the two genomes, a possible imbalance between nuclear-encoded and mitochondrial-encoded respiratory chain subunits and the accumulation of misfolded polypeptides is a risk that mitochondria continuously face. Mitochondria are the sites of ATP production through oxidative phosphorylation, but as a by-product they are exposed to high concentrations of reactive oxygen species (ROS), which can induce protein modifications, lipid peroxidation and DNA damage (Murphy et al, 2011). Finally, mitochondria are intimately connected to the endoplasmic reticulum and play an important role in cellular Ca2+ homeostasis (Csordas et al, 2010). Mitochondrial Ca2+ uptake and release regulates several processes, such as ATP production and hormone metabolism, while an overload of mitochondria with Ca2+ can in turn trigger apoptosis and cell death (Giorgi et al, 2008).
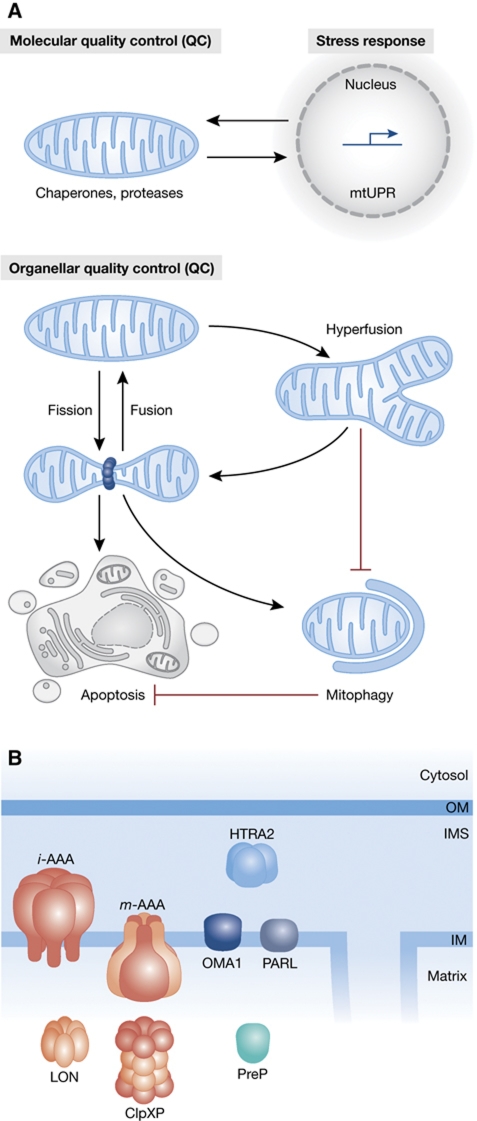
Cells have solved the challenge of maintaining the functionality of mitochondria by establishing rigorous surveillance systems that allow it to detect mitochondrial dysfunction and to eliminate the damage. Studies in recent years unravelled a hierarchical system of interdependent QC mechanisms that ensure cell survival (Figure 1A; Tatsuta and Langer, 2008). The first line of defense operates within mitochondria and consists of an ‘army’ of chaperones and proteases, which promote folding of newly imported preproteins, protect mitochondrial proteins against heat stress and degrade irreversibly damaged polypeptides (intraorganellar QC system). ATP-dependent proteases are able to recognize specifically misfolded polypeptides and degrade them to peptides, which are subsequently exported from the organelle or further degraded to amino acids by the action of oligopeptidases (Figure 1B). The i-AAA (intermembrane space-ATPase Associated with various cellular Activities) and m-AAA (matrix-ATPase Associated with various cellular Activities) proteases are located in the inner membrane and expose their catalytic sites to opposite membrane surfaces, while Lon and ClpXP proteases are active in the matrix. Many but not all proteases with a role in protein quality surveillance are ATP dependent and utilize the energy derived from ATP hydrolysis to unfold substrate proteins and processively degrade them to peptides (Sauer and Baker, 2011). They harbour an AAA ATPase domain with chaperone-like properties (Leonhard et al, 1999), which is characteristic of the AAA+ family of proteins. Oligomeric, in most cases hexameric, complexes form a proteolytic chamber allowing proteolysis to occur in a sequestered environment. The capacity of the mitochondrial QC system can be adjusted to increased demands, for example, under stress conditions, by a transcriptional programme that induces the nuclear expression of mitochondrial chaperone proteins and proteases (the so-called mitochondrial unfolded protein response, mtUPR) (Zhao et al, 2002; Benedetti et al, 2006). The signal transduction cascade that controls the mtUPR signal has been identified in C. elegans, and is beginning to be unravelled in mammalian cells. Although the nature of the signal triggering the response remains enigmatic, the identification of an ABC transporter with a role in the export of peptides from mitochondria raises the intriguing possibility that peptides generated upon proteolysis within mitochondria play a critical role in this pathway (Young et al, 2001; Haynes et al, 2010).
Figure 1.
(A) The hierarchical network of mitochondrial QC mechanisms. Intramitochondrial proteases and molecular chaperone proteins maintain mitochondrial proteostasis. Stress conditions induce a transcriptional programme (mitochondrial unfolded protein response, mtUPR) that increases protein levels of chaperones and proteases in mitochondria. Fusion allowing content mixing contributes to mitochondrial integrity and stress-induced mitochondrial hyperfusion may alleviate mild mitochondrial stress. A dysfunction of mitochondria inhibits fusion and triggers fragmentation of the mitochondrial network, which is associated with mitophagy or, under conditions of severe damage, leads to mitochondrial outer membrane permeabilization and apoptosis. (B) Submitochondrial localization of proteases with functions in mitochondrial QC. i-AAA, i-AAA protease; m-AAA, m-AAA protease; OM, outer membrane; IMS, intermembrane space; IM, inner membrane.
The second line of defense is concerned with the mitochondrial population of a cell (organellar QC system). Mitochondria form a dynamic network that is maintained by opposing fusion and fission events (Chen and Chan, 2009; Westermann, 2010). The fusion of mitochondrial membranes allows exchange of genetic material and protects against accumulation of damage (Chen et al, 2005, 2010). In stress conditions, for instance during nutrient starvation, oxidative stress, or when cytosolic protein synthesis is impaired, mitochondria respond by hyperfusing (Tondera et al, 2009). Nutrient starvation triggers the inhibition of mitochondrial fission resulting in unopposed fusion (Gomes et al, 2011; Rambold et al, 2011). Stress-induced mitochondrial hyperfusion allows mitochondria to increase ATP production, protects against the autophagic removal of mitochondria (mitophagy) and promotes cell survival. Mitochondrial dysfunction, however, causes the inhibition of mitochondrial fusion. Ongoing fission events lead to a fragmentation of the mitochondrial network, which facilitates the segregation and elimination of dysfunctional organelles through mitophagy. Finally, severe mitochondrial damage results in the release of pro-apoptotic factors like cytochrome c from the mitochondrial intermembrane space inducing the apoptotic programme.
Numerous studies have revealed in recent years that mitochondrial QC systems are highly regulated and interdependent and have established an intricate network of responses that ensures cell survival. Here, we will summarize these findings focussing on the central role of mitochondrial proteases for the regulation of mitochondrial QC at all levels.
Challenging aspects of mitochondrial QC in neurons
Neurons critically rely on mitochondrial function and oxygen supply, since most neuronal ATP is produced by oxidative phosphorylation. High ATP levels are required to sustain axonal transport of macromolecules and organelles such as mitochondria, to maintain ionic gradients and the membrane potential, to load synaptic vesicles with neurotransmitters and to release neurotransmitters into the synaptic cleft. Moreover, synaptic mitochondria are exposed to extensive Ca2+ influx and have a key role to buffer the cytosolic Ca2+ concentration.
To fully comprehend the relationship between mitochondria and neuronal function, it is important to consider that neurons are extremely polarized cells, with complex and diverse morphologies, characterized by the extension of long axons and more or less elaborate dendritic trees. These specialized areas have very different energetic demands and require local and fast adaptation of mitochondrial function (Li et al, 2004; MacAskill et al, 2010). Mitochondrial trafficking machineries, which are intimately coupled to mitochondrial dynamics (Misko et al, 2010), ensure mitochondrial delivery and localization to regions of high energy and Ca2+ buffering requirements within neurons (MacAskill and Kittler, 2010). However, the need to transport mitochondria far away from the cell body, where most of mitochondrial biogenesis is believed to occur, has other important consequences. First, mitochondria have a longer half-life in neurons than in other post-mitotic tissues (Menzies and Gold, 1971; Miwa et al, 2008; O’Toole et al, 2008) and in this regard are more likely to accumulate damage. Second, although local translation of a subset of mitochondrial proteins might occur in axons (Kaplan et al, 2009), the import of most newly synthesized proteins involves mitochondria in the cell body. Mitochondria located in the cell periphery thus might become ‘old and out of touch’ and be less efficient in coping with accumulating misfolded polypeptides or Ca2+ overload (Brown et al, 2006). Finally, removal of damaged mitochondria by mitophagy can be a demanding task in neurons. Autophagosomes form in axons, however, as they need to travel all the way back to the cell body to fuse with lysosomes (Wang et al, 2006), it is possible that this energy-dependent process could be impaired in the face of mitochondrial dysfunction.
The aforementioned processes are linked to and influenced by mitochondrial dynamics. The mitochondrial network is elongated in dendrites and close to the cell body, but tends to be more fragmented in axons, probably to enhance transport of mitochondria along great distances (Popov et al, 2005). Consistently, fission occurs in axons more frequently than fusion, and in general both events seem to happen at a lower rate than in other cell types (Berman et al, 2009). Moreover, the rate of fission to fusion events changes with the age of neurons (Arnold et al, 2011). In conclusion, though neurons possess the same mitochondrial QC systems as other cells, the challenges these systems face are more arduous given the high mitochondrial demands, increasing the risk of accumulating damage, and spatial restraints beset by the peculiar morphology of the cells. Any mutation or insult lowering the mitochondrial QC capacity is therefore predicted to affect neurons preferentially. Remarkably, this is exactly what human genetics has taught us in the last few years through the identification of genes involved in mitochondria QC as the cause of a large number of neurodegenerative diseases.
Mutations in genes involved in organellar or intraorganellar levels of QC in mitochondria cause distinct neurodegenerative diseases mainly characterized by involvement of the peripheral nerves, the central motor neurons, cerebellar neurons and/or the optic nerve (Table I). Different forms of peripheral neuropathies are caused by mutations in mitofusin 2 (MFN2; Charcot-Marie-Tooth disease type 2A) (Zuchner et al, 2006), one of two dynamin-like GTPases mediating mitochondrial outer membrane (OM) fusion, and in GDAP1 (ganglioside-induced differentiation-associated protein 1), a mitochondrial OM protein whose function has been linked to the cellular glutathione metabolism and which is required for efficient fission (Niemann et al, 2005; Noack et al, 2011). Mutations in the dynamin-like GTPase OPA1 (optic atrophy 1) mediating mitochondrial inner membrane fusion and cristae organization are associated with dominant optic atrophy characterized by the progressive loss of retinal ganglion cells (Alexander et al, 2000; Delettre et al, 2000; Lenaers et al, 2009). Some familial forms of Parkinson's disease (PD) are caused by mutations in the E3 ubiquitin ligase Parkin and the PTEN-induced kinase PINK1 (Kitada et al, 1998; Valente et al, 2004), which are crucial regulators of mitophagy and mitochondrial turnover. Here, we focus on the link between mitochondrial proteases and neurodegeneration. We will first introduce neurodegenerative diseases that have been associated with specific components of intramitochondrial or organellar quality surveillance systems, before we discuss potential pathogenic mechanisms.
Table 1. Neurodegenerative disorders associated with components of the mitochondrial QC system.
| Name | Location | Process | Disease |
|---|---|---|---|
| Intramitochondrial quality control | |||
| Paraplegin/SPG7 (m-AAA protease) | IM, M | Degradation of non-native proteins; protein processing | Hereditary spastic paraplegia, AR (Casari et al, 1998) |
| AFG3L2 (m-AAA protease) | IM, M | Degradation of non-assembled and damaged proteins; protein processing | Spinocerebellar ataxia SCA28, AD (Di Bella et al, 2010) |
| Spastic ataxia neuropathy syndrome, AR (Pierson et al, 2011) | |||
| HTRA2/OMI | IMS | Degradation of damaged proteins; regulation of apoptosis | Parkinson's disease (?) |
| PARL | IM | Processing of PINK1, anti-apoptotic activity | Parkinson's disease |
| PreP | M | Degradation of presequences and oligopeptides, Aβ degradation | Alzheimer's disease (?) |
| HSP60 | M | Protein folding, protection against heat stress | Hereditary spastic paraplegia, AD (Hansen et al, 2002); Mit-CHAP60, AR (Magen et al, 2008) |
|
Organellar quality control
| |||
| Mfn2 | OM, MAM | Fusion of OM, axonal transport of mitochondria, ER-mitochondria interaction | Charcot-Marie Tooth diseases 2A, AD (Zuchner et al, 2006) |
| OPA1 | IM, IMS | Fusion of IM, cristae morphogenesis, mtDNA stability | Dominant optic atrophy, AD (Alexander et al, 2000; Delettre et al, 2000) |
| GDAP1 | IM | Mitochondrial fission | Charcot-Marie-Tooth disease 4A, AD, AR (Baxter et al, 2002) |
| Parkin | Cyt, OM | Mitophagy | Parkinson's disease (Kitada et al, 1998) |
| PINK1 | IM, OM | Mitophagy | Parkinson's disease (Valente et al, 2004) |
| AD, autosomal dominant; AR, autosomal recessive; IM, inner membrane; IMS, intermembrane space; M, matrix; MAM, mitochondria-associated membrane; OM, outer membrane. | |||
| Genes unambiguously identified in neurodegenerative diseases are referenced. | |||
Intramitochondrial QC and neurodegeneration
Mutations in the m-AAA protease cause three neurological disorders
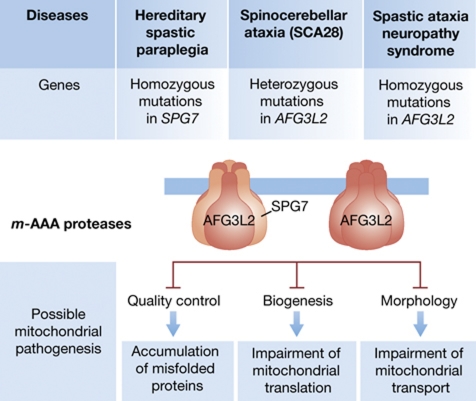
The first evidence that dysfunctional mitochondrial proteases can cause neurodegeneration came in 1998 with the identification of disease-causing mutations in SPG7, which encodes a subunit of the hexameric, ATP-dependent m-AAA protease in the mitochondrial inner membrane (Casari et al, 1998). Loss-of-function mutations in the SPG7 gene were found in patients affected by an autosomal recessive form of hereditary spastic paraplegia (HSP), a progressive disorder clinically defined by weakness, spasticity (muscle rigidity) and loss of the vibratory sense of the lower limbs. HSP is genetically heterogeneous and caused by the selective retrograde degeneration of the longest motor and sensory axons of the central nervous system, the corticospinal tracts and the fasciculus gracilis (Reid and Rugarli, 2010). Corticospinal axons can reach the remarkable length of 1 m in adults, contain >99% of the cytoplasm of the cell, and heavily rely on transport of mitochondria and other cargos to synaptic terminals for their function. SPG7 is mutated in a small subset of familiar recessive HSP cases but in up to 11% of sporadic HSP patients (Brugman et al, 2008).
Recently, one form of spinocerebellar ataxia, SCA28, was associated with heterozygous mutations in the molecular partner of paraplegin within the m-AAA protease, the homologous protein AFG3L2 (Cagnoli et al, 2010; Di Bella et al, 2010). Ataxia indicates lack of motor coordination and is associated with cerebellar dysfunction. SCA28 patients are affected by a slowly progressive gait and limb ataxia of juvenile onset, dysarthria (disturbance in articulation of speech), hyperreflexia at lower limbs (a sign of upper motor neuron dysfunction), nystagmus, ptosis and ophthalmoparesis. Different missense mutations were found in both familial and sporadic patients. Strikingly, mutations hit conserved residues in the peptidase domain. When the effect of these mutations was tested for their ability to rescue the loss of the yeast m-AAA protease they were found to be inactive variants (Di Bella et al, 2010). Therefore, while gain-of-function or dominant-negative effects cannot be formally excluded, evidence strongly suggest that these mutations lead to protein haploinsufficiency.
A homozygous mutation in AFG3L2, substituting a conserved tyrosine at the beginning of the peptidase domain to a cysteine, has been also detected in two siblings of a consanguineous marriage who were affected by a severe early-onset syndrome characterized by severe spastic paraplegia, ataxia, ptosis, oculomotor apraxia, dystonic movements and stimulus-induced myoclonus (Pierson et al, 2011). Strikingly, this phenotype combines manifestations of both HSP and SCA28 along with clinical features of other mitochondrial diseases (such as ptosis, ophthalmoparesis and myoclonic epilepsy). Again, a yeast model system was employed to assess the pathogenicity of the identified mutation. In contrast to mutations involved in SCA28, this variant was found to retain some residual activity, but its assembly into m-AAA protease complexes was strongly impaired. Reduced levels of assembled m-AAA complexes were confirmed in fibroblasts from one of the patients (Pierson et al, 2011).
One of the biggest puzzles in both neurodegenerative and mitochondrial diseases is the remarkable tissue specificity of the clinical phenotypes caused by the disruption of proteins that are often ubiquitously expressed and that perform conserved housekeeping functions. Combining biochemical, genetic and expression data, we start to decipher this conundrum for neurodegenerative diseases caused by mutations in subunits of the m-AAA protease (Figure 2). Paraplegin and AFG3L2 are highly homologous, evolutionary conserved proteins (54% protein sequence homology), containing two transmembrane domains, the AAA domain, and an M41 metallopeptidase domain (Gerdes et al, 2011). However, they differ in their ability to homo-oligomerize. While AFG3L2 forms homo-oligomeric assemblies or hetero-oligomeric complexes with paraplegin, the activity of paraplegin depends on its assembly with AFG3L2 into functional m-AAA proteases (Koppen et al, 2007). Both human isoenzymes are functionally conserved and can substitute for the orthologous yeast m-AAA protease, a hetero-oligomer of Yta10 and Yta12 subunits, which degrades misfolded inner membrane proteins and regulates mitochondrial translation (Leonhard et al, 2000; Nolden et al, 2005). The presence of different m-AAA isoenzymes, however, has important implications for understanding the pathogenesis of the associated human diseases. Mutations in SPG7 only affect the hetero-oligomeric m-AAA complex, while mutations in AFG3L2 perturb both isoforms. In view of the functional redundancy of m-AAA isoenzymes, the relative expression level of both subunits will determine the total residual amount of m-AAA proteases in a given patient, which will also be different if the patient carries a homozygous mutation (as in HSP or in the severe spastic-ataxia syndrome patients) or a heterozygous one (as in SCA28 patients). The expression levels of the two subunits indeed vary among murine tissues and within different regions of the brain. While Afg3l2 is ubiquitously expressed at high level in any neuronal cell type, Spg7 appears to be expressed at lower levels and specifically in certain neurons, such as pyramidal cells of the cerebral cortex (Martinelli et al, 2009). It is therefore plausible that different neurons might contain different amounts of homo-oligomeric versus hetero-oligomeric complexes and be differentially affected by mutations in AFG3L2 or SPG7. Moreover, it is conceivable that specific substrates might exist which can be handled preferentially by one type of complex, or that individual tissues or neuronal cell types might express specific substrates. In conclusion, dosage effects, combined with possible mishandling of critical substrates, could reconcile the different severity and pattern of neuronal degeneration observed in human patients.
Figure 2.
Possible pathogenic mechanisms of neurodegenerative disorders caused by mutations in m-AAA protease subunits. Mutations in the m-AAA protease subunit AFG3L2 impair the activity of both homo- and hetero-oligomeric m-AAA isoenzymes, while mutations in paraplegin (SPG7) affect only the hetero-oligomeric m-AAA protease. Accumulating misfolded polypeptides or deficiencies in mitochondrial biogenesis and dynamics may interfere with mitochondrial energy output and axonal trafficking of mitochondria.
The analysis of mouse models for individual m-AAA protease subunits has largely supported this scenario. Paraplegin-deficient mice show a late-onset progressive distal axonopathy of long sensory and central motor axons, optic nerves and peripheral nerves (Ferreirinha et al, 2004). Heterozygous Afg3l2+/− animals exhibit late-onset cerebellar degeneration, while double Spg7−/− Afg3l2+/− animals show a striking acceleration of the phenotype of the two individual mouse models, demonstrating functional redundancy in vivo (Maltecca et al, 2009; Martinelli et al, 2009). Two different Afg3l2 models, a spontaneous mutant carrying a missense mutation in the ATPase domain and a knockout model generated by retroviral insertion in the gene, have instead a very severe developmental phenotype, and die as early as P15 (Duchen et al, 1983; Maltecca et al, 2008). These mice still bear residual complex activity since the mouse expresses Afg3l1, a highly homologous gene to Afg3l2 that in human has become a pseudogene (Kremmidiotis et al, 2001). Interestingly, these mice do not show a reduced number of neurons or abnormalities in neuronal migration or lamination, but a defect to develop and myelinate axons (Maltecca et al, 2009). Thus, the total cellular capacity of mitochondrial QC appears to become limited when neurons elongate axons and form synaptic contacts.
PD and mitochondrial HTRA2
PD is one of the most common neurodegenerative diseases in the aging population. It is characterized by the clinical triad of rigidity, bradikinesia and tremor, and by the neuropathological loss of dopaminergic neurons (DNs) in the substantia nigra with typical intracytoplasmatic ubiquitin- and α-synuclein-positive inclusions, the Lewy bodies. A strong link between mitochondrial dysfunction and PD is supported by the findings that neurotoxins affecting respiratory complex I induce specific death of DNs, and by the discovery that a number of causative genes in familial forms of PD encode mitochondrial proteins. Remarkably, emerging pathogenic pathways in PD are related to an impaired mitochondrial QC.
The mitochondrial peptidase HTRA2/OMI, which is localized to the mitochondrial intermembrane space and homologous to the bacterial HtrA stress responsive genes, DegP and DegS (Vande Walle et al, 2008; Clausen et al, 2011), plays a critical role in protecting neurons against degeneration and has been associated with PD. Both a spontaneous mutation and a targeted deletion in the murine Htra2 gene were shown to cause a progressive neurodegenerative phenotype, characterized by abnormal gait, ataxia, repetitive movements and akinesia, owing to loss of neurons in the striatum (Jones et al, 2003; Martins et al, 2004). While its role in neuronal survival is well established, the implication of HTRA2 in the pathogenesis of PD remains controversial. HTRA2 has been found to be a component of α-synuclein-containing inclusions in brains of individuals with PD, dementia with Lewy bodies and multiple-system atrophy (Strauss et al, 2005; Kawamoto et al, 2008). Furthermore, some groups have reported the association of polymorphisms and mutations in HTRA2 with sporadic PD cases (Strauss et al, 2005; Bogaerts et al, 2008). However, these results have not been reproduced in large-scale studies on PD patients (Ross et al, 2008; Simon-Sanchez and Singleton, 2008; Kruger et al, 2011). Finally, the function of HTRA2 has been linked to PINK1. HTRA2 was found to be phosphorylated by the p38 pathway in a PINK1-dependent manner, and phosphorylated HTRA2 to be increased in the brain of sporadic PD patients and decreased in PINK1 mutated patients (Plun-Favreau et al, 2007). However, studies in D. melanogaster have come to different and conflicting conclusions (Whitworth et al, 2008; Yun et al, 2008; Tain et al, 2009). Taken together, the role of HTRA2 for the pathogenesis of PD has to be taken with caution and some findings need to be revisited critically. Nevertheless, the central role of PINK1 and parkin, both associated with juvenile forms of PD (Kitada et al, 1998; Valente et al, 2004), in mitophagy suggests that impaired mitochondrial QC is of pathogenic relevance in PD as will be discussed in more detail below.
Alzheimer's disease and the Aβ-degrading mitochondrial oligopeptidase PreP
Oligopeptidases form another class of mitochondrial proteases, whose functions have been linked to neurodegeneration. The proteolytic breakdown of misfolded proteins by various ATP-dependent proteases or the maturation of mitochondrial preproteins upon import into mitochondria results in the generation of peptides, which are further degraded to amino acids by oligopeptidases. The matrix-localized oligopeptidase PreP was originally identified in plant mitochondria by its ability to degrade mitochondrial presequences (Stahl et al, 2002). The crystal structure of this conserved peptidase with homologues identified in yeast and human mitochondria revealed a large proteolytic cavity whose accessibility is regulated by substrate binding (Johnson et al, 2006). The substrate specificity appears to be degenerate (Moberg et al, 2003; Kambacheld et al, 2005). Unexpectedly, studies on human brain mitochondria have demonstrated that PreP is the main peptidase degrading the amyloid β (Aβ) peptide associated with Alzheimer's disease (AD) (Falkevall et al, 2006).
AD is a progressive neurodegenerative disorder characterized by the accumulation of intracellular neurofibrillary tangles and extracellular plaques of Aβ peptides in the brain. A dysfunction of mitochondria is among the earliest effects observed in AD brains but its causative role for disease pathogenesis is presently unclear, mainly as AD-causing mutations in mitochondrial proteins have not been identified. Interestingly, Aβ peptides were detected in post-mortem brain mitochondria of AD patients (Lustbader et al, 2004), raising the possibility of a role of mitochondrial Aβ for disease progression. Current models for the toxicity of Aβ propose that Aβ binds to specific mitochondrial proteins, such as Aβ-binding alcohol dehydrogenase (Lustbader et al, 2004) and cyclophilin D (Du et al, 2008), causes oxidative stress and increased levels of ROS, resulting in diminished complex IV activities and finally in apoptosis and neuronal death (Glaser and Alikhani, 2010). PreP can counteract this fatal cascade by clearing brain mitochondria from toxic Aβ. Accordingly, decreased proteolytic activity of PreP, as observed in the temporal lobe of AD brains (Alikhani et al, 2011), may contribute to disease progression. However, genetic variations in PITRM1 encoding PreP did not correlate with the risk of AD in >600 AD patients (Pinho et al, 2010). Therefore, despite compelling evidence for the degradation of mitochondrial Aβ by PreP, further studies are required to establish the pathogenic relevance of PreP to AD.
Mitochondrial chaperone HSP60 and neurodegeneration
The maintenance of mitochondrial proteostasis critically depends on molecular chaperone proteins within the organelle (Voos, 2009; Baker et al, 2011), and therefore it does not surprise that mutations in molecular chaperones are associated with neurodegeneration. Chaperone proteins drive the membrane translocation of nuclear-encoded mitochondrial preproteins and mediate their folding into their native state. Moreover, molecular chaperones protect against heat and other stress conditions, preventing the aggregation of misfolded mitochondrial proteins and allowing their refolding or their degradation by ATP-dependent proteases.
Shortly after the discovery that mutations in SPG7 caused autosomal recessive HSP, Hansen et al (2002) identified heterozygous missense mutations in the HSPD1 gene in two French families with an autosomal dominant pure HSP. A few years later, another family was reported in which a different missense mutation caused the disease with low penetrance (Hansen et al, 2007). HSPD1 encodes for the mitochondrial chaperonin HSP60 (Heat shock protein 60) that mediates the folding of mitochondrial matrix proteins and protects against heat stress. The implication of HSPD1 in HSP pathogenesis predicates that decreasing the QC capacity of mitochondria can lead to degeneration of specific neuronal populations. More recently, an autosomal recessive form of Pelizaeus–Merzbacher-like disease (PMLD) has been linked to a homozygous mutation in HSPD1, and therefore renamed mit-CHAP-60 (Magen et al, 2008). Affected individuals suffer from a severe phenotype characterised by hypotonia, nystagmus, severe psychomotor developmental delay, and prominent spasticity, and display severe hypomyelination as per MRI, implicating the function of the mitochondrial chaperone HSP60 not only in maintenance of motor axons but also in their myelination.
HSP patient cell lines harbouring the HSP60 mutation did not show defects in mitochondrial function, cell viability or oxidative stress sensitivity. Therefore, the pathogenicity of the mutations in HSPD1 was studied by taking advantage of the ability of HSP60 and its co-chaperonin HSP10 to rescue the growth of E. coli cells in which the homologous chaperonin genes have been deleted. HSP60 carrying the missense mutation (V98I) linked to HSP exerted a dominant-negative effect when co-expressed with a wild-type variant (Hansen et al, 2002). Furthermore, purified HSP60 bearing the V98I mutation displayed a decreased ATPase activity and a strongly reduced capacity to promote folding of denatured malate dehydrogenase in vitro (Bross et al, 2008). In contrast, HSP60 carrying the mutation identified in the severe mit-CHAP-60 syndrome induced only a very mild growth defect in the E. coli complementation assay, explaining why the disease manifests only in homozygous individuals.
Mitochondrial proteases and organellar QC
The dynamic behaviour of the mitochondrial network maintained by balanced fusion and fission events is intimately connected to mitochondrial QC and the maintenance of mitochondrial activities. Fusion is a pro-survival mechanism protecting against apoptosis and neurodegeneration. Fission, on the other hand, does not only ensure the distribution of mitochondria in a cell, but occurs early during mitophagy and apoptosis. A dysfunction of mitochondria that manifests by low matrix ATP levels or a low ΔΨm results in the inhibition of fusion, while ongoing fission events lead to the fragmentation of the mitochondrial network under these conditions. In this way, dysfunctional mitochondria are segregated from the network and selectively eliminated by mitophagy (Twig et al, 2008). Mitochondrial proteases and proteolytic events on both the inner and outer mitochondrial membrane regulate these surveillance mechanisms, which turn out to be crucial for neuronal survival.
Constitutive and stress-induced OPA1 processing
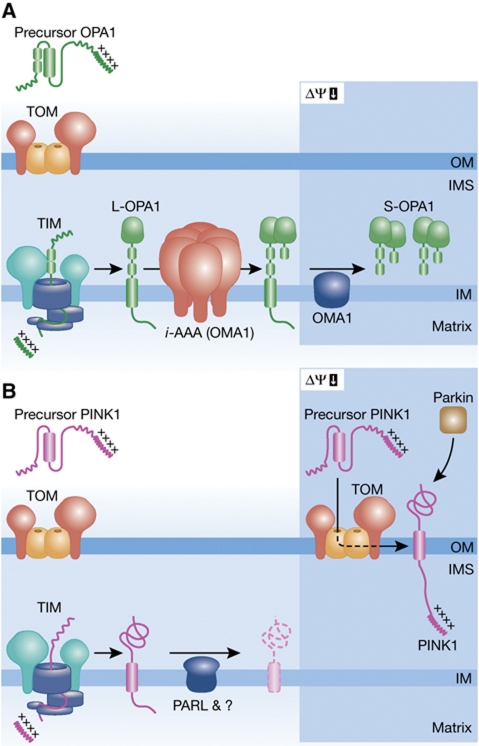
The dynamin-like GTPase OPA1 mediates mitochondrial fusion, ensures cristae morphogenesis and the maintenance of mtDNA, and protects against apoptosis (Landes et al, 2010). Eight different splice variants exist that are expressed in a tissue-specific manner (Akepati et al, 2008). Proteolytic processing converts long (L-OPA1) into short isoforms (S-OPA1) and, as mitochondrial fusion depends on the presence of both (Song et al, 2007), represents a central regulatory step determining mitochondrial morphology (Figure 3A). OPA1 processing at two proteolytic sites, S1 and S2, removes transmembrane segments and results in the activation of the GTPase activity of the dynamin (DeVay et al, 2009). Only about half of L-OPA1 is cleaved leading to the accumulation of both isoforms under normal conditions (constitutive cleavage) and the activation of fusion. The mechanisms limiting constitutive OPA1 processing and preventing the complete conversion of L- into S-OPA1 are presently not understood. As OPA1 appears to be rather unstable (Song et al, 2007), the accumulation of equimolar concentrations of L- and S-OPA1 may reflect the fine-tuned balance between proteolytic processing of newly imported molecules and complete degradation.
Figure 3.
Mitochondrial proteases regulate mitochondrial morphology and turnover. (A) Constitutive and stress-induced OPA1 cleavage. Newly synthesized OPA1 precursor molecules are matured and converted into L-OPA1 upon import into mitochondria, likely by the mitochondrial processing peptidase. A fraction of L-OPA1 molecules is subsequently cleaved at position S2 by YME1L and likely at position S1 by OMA1. Under stress conditions (e.g., low ΔΨm, low matrix ATP), OMA1 degrades L-OPA1 molecules completely into S-OPA1. (B) Accumulation of PINK1 at mitochondrial surface. PINK1 precursor proteins are imported into mitochondria in ΔΨm-dependent manner, accompanied by maturation, likely by the mitochondrial processing peptidase. Newly imported PINK1 is degraded in the inner membrane by PARL and an MG132-sensitive, yet to be identified peptidase. A dysfunction of mitochondria (low ΔΨm) impairs import but not mitochondrial targeting of PINK1 resulting in its accumulation at the mitochondrial surface. OM, outer membrane; IMS, intermembrane space; IM, inner membrane.
A number of mitochondrial peptidases have been linked to the constitutive processing of OPA1, including the rhomboid protease PARL, m- and i-AAA proteases, HTRA2 and OMA1 (Cipolat et al, 2006; Ishihara et al, 2006; Duvezin-Caubet et al, 2007; Griparic et al, 2007; Song et al, 2007; Ehses et al, 2009; Head et al, 2009). However, the analysis of cell lines depleted of individual proteases supported a direct role for OPA1 cleavage only for two of these peptidases in the inner membrane: the i-AAA protease YME1L and the metallopeptidase OMA1. While loss of OMA1 interferes with processing at S1, the i-AAA protease YME1L is required for S2 cleavage (Griparic et al, 2007; Song et al, 2007; Ehses et al, 2009; Head et al, 2009). Although OPA1 processing is not impaired in HtrA2−/− or Parl−/− MEFs, excluding an essential role of either peptidase in OPA1 cleavage, PARL affects the release of short OPA1 isoforms from mitochondria during apoptosis (Cipolat et al, 2006).
Proteolytic processing of OPA1 combined with the requirement of both isoforms for fusion offers an intriguing possibility for regulation. A dysfunction of mitochondria causes the complete conversion of L- into S-OPA1 (stress-induced cleavage) and the inhibition of fusion (Figure 3B; Duvezin-Caubet et al, 2006; Ishihara et al, 2006). Similarly, L-OPA1 isoforms are destabilized under apoptotic conditions. Stress-induced processing occurs at S1 and is mediated by the metallopeptidase OMA1 (Ehses et al, 2009; Head et al, 2009), which therefore plays a central role in mitochondrial QC: it can degrade misfolded inner membrane proteins (Kaser et al, 2003; Bestwick et al, 2010) and, by processing of OPA1, regulate mitochondrial morphology and downstream quality surveillance systems. Knockdown of OMA1 in MEFs does not inhibit fusion, likely due to the generation of S-OPA1 by YME1L-mediated cleavage at S2, but protects against apoptosis (Ehses et al, 2009; Head et al, 2009). How a dysfunction of mitochondria is sensed remains an important, not yet answered question. As activation occurs within minutes and can be induced in isolated organelles, regulation occurs likely at a post-translational level. Low ATP levels, an altered ΔΨm, or membrane damage may activate OMA1 directly or modulate the accessibility of OPA1 for the peptidase. It is noteworthy that the stability of L-OPA1 depends on prohibitin complexes and the m-AAA protease that assembles with prohibitins in the inner membrane (Steglich et al, 1999; Merkwirth et al, 2008; Ehses et al, 2009). Prohibitin complexes are putative membrane scaffolds that modulate the distribution of membrane proteins and lipids (Osman et al, 2009), suggesting that the membrane environment has major impact on the stability of L-OPA1.
PINK1 stability controls mitophagy
While occurring early during mitophagy, membrane fission and the fragmentation of the mitochondrial network are not sufficient to ensure the selective removal of damaged mitochondria. Rather, mitophagy requires the accumulation of specific receptor proteins at the mitochondrial surface recruiting the autophagic machinery to mitochondria (Youle and Narendra, 2011). The selective elimination of dysfunctional mitochondria depends on PINK1 and parkin, pointing to a crucial role of mitochondrial damage in PD, and is under strict control by mitochondrial proteases (Figure 3B).
First evidence linking PINK1 and parkin to mitochondrial dysfunction came from studies in animal model systems. Loss of either protein in Drosophila causes the accumulation of dysmorphic mitochondria with less efficient oxidative phosphorylation, resulting in male sterility and dopaminergic neuronal and muscle degeneration (Greene et al, 2003; Clark et al, 2006; Park et al, 2006; Yang et al, 2006). Although Pink1−/− and parkin−/− mice show moderate phenotypes and appear to maintain normal levels of DNs in the substantia nigra, a dysfunction of mitochondria with age was apparent in the brain, especially in the striatum (Palacino et al, 2004; Kitada et al, 2007; Gautier et al, 2008). Moreover, neurodegeneration and progressive loss of DN has recently been reported upon deletion of parkin in the adult brain of mice (Shin et al, 2011). An epistasis analysis in D. melanogaster revealed that both PINK1 and parkin act along a common genetic pathway, with PINK1 functioning upstream of parkin (Clark et al, 2006; Park et al, 2006; Yang et al, 2006). The molecular basis for the striking genetic interaction of PINK1 and parkin in D. melanogaster was discovered when analysing the subcellular localization of parkin under stress conditions in mammalian cells. Parkin, a normally cytosolic protein, relocalizes in a PINK1-dependent manner to the mitochondrial OM upon dissipation of ΔΨm (Narendra et al, 2008, 2010b; Vives-Bauza et al, 2010). Parkin recruitment to mitochondria can be induced by drugs affecting the voltage component of ΔΨm, such as carbonyl cyanide m-chlorophenylhydrazone (CCCP) or valinomycin, but not by nigericin that facilitates K+/H+ exchange (Narendra et al, 2009).
Recent studies have shed insights into the subcellular localization and site of function of PINK1. PINK1 is a nuclear-encoded mitochondrial kinase. A typical mitochondrial presequence target newly synthesized PINK1 to mitochondria and is cleaved off, likely by the matrix-localized mitochondrial processing peptidase, upon ΔΨm-dependent mitochondrial import. Strikingly, PINK1 is an unstable protein and constitutively degraded in the inner membrane (Matsuda et al, 2010; Narendra et al, 2010b). The rhomboid protease PARL cleaves PINK1 within its transmembrane segments and generates a 52-kDa fragment, which is subsequently degraded by an MG132-sensitive, yet to be identified peptidase (Jin et al, 2010; Deas et al, 2011). A dysfunction of mitochondria, as mimicked by CCCP-induced dissipation of ΔΨm in cultured cell systems, impairs mitochondrial import but does not interfere with targeting of PINK1 to mitochondria. As a consequence, non-processed PINK1 accumulates at the mitochondrial surface and promotes recruitment of parkin to mitochondria, resulting in the elimination of dysfunctional mitochondria (Narendra et al, 2010b).
Despite compelling evidence in support of this model, a number of questions are still to be addressed. Most importantly, it remains to be demonstrated whether subtle and progressive decreases in the ΔΨm of mitochondria as observed in the context of mitochondrial diseases are sufficient to stabilize PINK1 and recruit parkin to the surface of dysfunctional mitochondria in vivo or whether the PINK1/parkin pathway selectively eliminates only completely depolarized mitochondria (as in the presence of CCCP). Indeed, evidence has been provided that the unique metabolic properties of neurons interfere with parkin translocation to mitochondria in these cells (Van Laar et al, 2011), which may explain the parkin-independent neurodegeneration of DN in a respiratory-deficient mouse model (Sterky et al, 2011). Moreover, it is only poorly understood how PINK1 activates parkin. As both proteins were found to interact, parkin may translocate to the mitochondrial surface by directly binding to PINK1.
Parkin-mediated ubiquitylation and turnover of OM proteins
Some pathogenic mutations affecting the ubiquitin ligase activity of parkin inhibit mitophagy, suggesting that ubiquitylation of mitochondrial OM proteins is required (Lee et al, 2010). VDAC was the first protein that was found to be polyubiquitylated upon stress-induced recruitment of parkin to mitochondria (Geisler et al, 2010). As ubiquitylation occurs via K63 linkages and did not affect the stability of VDAC, it was proposed to serve a signalling function promoting mitophagy. At least in MEFs, however, VDAC does not appear to be essential for parkin-induced mitophagy (Narendra et al, 2010a). Mitofusins represent other mitochondrial substrates of parkin. Parkin mediates K48-linked polyubiquitylation of mitofusins in depolarized mitochondria in both D. melanogaster and mammalian cells, triggering their turnover by 26S proteasomes (Gegg et al, 2010; Poole et al, 2010; Ziviani et al, 2010; Glauser et al, 2011). As a consequence, fusion is inhibited and axonal trafficking of mitochondria impaired, as MFN2 binds directly to the Miro/Milton adaptor complex linking mitochondria to kinesin motor proteins and microtubules (Misko et al, 2010). Moreover, PINK1 and parkin were recently found to bind Miro upon mitochondrial depolarization, triggering its phosphorylation and proteasomal degradation (Wang et al, 2011).
Proteomic studies revealed that the translocation of parkin is associated with a broad activation of the ubiquitin-proteasome system (UPS), resulting in the K48-linked ubiquitylation and degradation of many OM proteins (Chan et al, 2011). This is accompanied by the recruitment of 26S proteasomes and the AAA-type ATPase p97 (Cdc48 in yeast) to the mitochondrial surface. Similarly, p97 is required for the degradation of the anti-apoptotic Bcl2-family member Mcl1 during apoptosis, after its ubiquitylation by the HECT-domain containing E3 ubiquitin ligase Mule/ARF-BP1 (Zhong et al, 2005). p97 binds to ubiquitin chains and mediates the dislocation of misfolded proteins from the ER to the cytosol during ER-associated protein degradation. It may therefore have a similar role during the degradation of mitochondrial OM proteins (Karbowski and Youle, 2011), but the mechanisms how proteins are extracted from the OM remain unclear and additional components are likely to be involved (Heo et al, 2010). Different views have been reported on the role of the parkin-induced turnover of OM proteins. It may ensure the degradation of inhibitory factor(s) of mitophagy at the mitochondrial surface or prevent the aggregation of depolarized mitochondria that accumulate in a parkin-dependent manner in the perinuclear region, and thus be essential for the elimination of damaged mitochondria (Chan et al, 2011). Alternatively, the parkin-mediated, proteasomal degradation of OM proteins may serve a protective QC function that is independent of and not required for mitophagy (Ziviani et al, 2010; Yoshii et al, 2011). Further experiments are required to resolve these issues and examine the role of other cytosolic substrates of parkin like PARIS, a repressor of PGC1α, which was found to be required for the loss of DN in a parkin-deficient mouse model (Shin et al, 2011). Regardless, numerous studies have now established the existence of intricate quality surveillance mechanisms acting on the OM and unravelled a central role of PINK1 and parkin in these processes.
Pathogenesis of neurodegenerative disorders associated with an impaired mitochondrial QC
Compelling evidence supports the notion that a failure of organellar and intramitochondrial QC leads to neurodegeneration, but the underlying pathogenic mechanisms remain largely enigmatic. Reduced respiratory chain activities and abnormalities of the mitochondrial network were observed in models for diseases associated with an impaired mitochondrial QC, suggesting that defective mitochondrial energy output and abnormalities of mitochondrial dynamics ultimately result in neuronal loss. As the machineries mediating axonal transport of mitochondria and mitochondrial fusion are closely interconnected (Misko et al, 2010), alterations in the morphology as well as decreased ATP levels per se interfere with axonal trafficking of mitochondria and result in depletion of functional mitochondria from the synapses. This will affect synaptic function, lead to axonal swelling, and ultimately dying-back axonopathy. Diseases associated with an impaired mitochondrial QC thus share common pathogenic steps with general mitochondrial disorders, which are caused by mutations in mtDNA or respiratory chain subunits and often presented clinically as neurological syndromes, and with other axonopathies due to impairment of trafficking mechanisms in axons (Blackstone et al, 2011). However, we are only beginning to understand how mutations in mitochondrial proteases and a reduced capacity of the mitochondrial QC system lead to a dysfunction of the mitochondrial respiratory chain and to the fragmentation of the mitochondrial network. Different scenarios that are not mutually exclusive can be envisioned:
Accumulation of protein damage
Substrates accumulating in protease-deficient mitochondria like peptides or oxidatively damaged proteins may be deleterious for mitochondrial activities. Non-native polypeptides could jam protein translocases, impair mitochondrial gene expression, or interfere with the assembly of multisubunit respiratory chain complexes or ΔΨm with devastating effects on mitochondrial biogenesis. Although direct evidence for an accumulation of damaged proteins or protein aggregates is scarce, beneficial effects of an increased mtQC capacity have been observed in filamentous fungi. Overexpression of matrix-localized Lon protease extends significantly the lifespan of the filamentous fungus Podospora anserina, suggesting deleterious effects of non-native polypeptides on mitochondrial function during aging (Luce and Osiewacz, 2009). Loss of HTRA2 in the mouse has been shown to result in the accumulation of unfolded proteins in the mitochondria, associated with defective respiration and enhanced ROS production (Moisoi et al, 2009). Similarly, given the QC function of m-AAA proteases, misfolded and damaged proteins may accumulate progressively within m-AAA protease-deficient mitochondria, interfere with the integrity of the inner membrane, and decrease the respiratory capacity of mitochondria (Figure 2). Respiratory deficiencies could conceivably increase mitochondrial ROS production and oxidative damage to levels that are incompatible with life. Similarly, the failure to remove dysfunctional mitochondria by mitophagy may result in continuous ROS production and overwhelm the capacity of mitochondrial QC systems. An impaired assembly of respiratory complexes I and III and the accumulation of carbonylated proteins have been observed in cerebellar mitochondria deficient of AFG3L2, while the stability of complexes I, II and IV was affected in mitochondria from Spg7−/− Afg3l2+/− mice (Maltecca et al, 2008, 2009; Martinelli et al, 2009). However, these studies could not distinguish whether oxidatively damaged proteins are merely a consequence of or are the primary cause of the reduced respiratory capacity of the affected neurons. Therefore, other scenarios are conceivable to explain how mutations in AFG3L2 or SPG7 may impact mitochondrial activities.
Impaired regulation of mitochondrial biogenesis
A dysfunction of neuronal mitochondria may be the direct consequence of a disturbed mitochondrial biogenesis, as many mitochondrial proteases have dual functions as protein surveillance factors and regulatory enzymes. For instance, Lon protease, a QC enzyme in the matrix (Wagner et al, 1994; Bota and Davies, 2002; Bender et al, 2011) regulates mtDNA copy number by selectively degrading the mitochondrial transcription factor A (TFAM; Matsushima et al, 2010). Moreover, it mediates the turnover of δ-aminolevulinic acid synthase in the absence of haeme, a short-lived, rate-controlling enzyme of the mitochondrial haeme biosynthesis pathway in the matrix space (Tian et al, 2011) or of the steroidogenic acute regulatory (StAR) protein, promoting cholesterol uptake by steroidogenic mitochondria (Granot et al, 2007). Similarly, the i-AAA protease, a homologue of the m-AAA protease acting in the intermembrane space, determines the half-time of UPS/PRELI-like proteins that regulate the accumulation of specific phospholipids in mitochondrial membranes (Potting et al, 2010). Although likely to exist, short-lived regulatory proteins that are degraded by the m-AAA protease have not been identified. However, the m-AAA protease was found to regulate mitochondrial protein synthesis mediating the maturation of the nuclear-encoded ribosomal subunit MrpL32 upon import into yeast mitochondria (Nolden et al, 2005). MrpL32 does not harbour a sequence-specific recognition site for the m-AAA protease, which has degenerate cleavage specificity. Rather, it is the folding of a C-terminal domain of MrpL32 that limits degradation by the m-AAA protease thereby ensuring specific processing (Bonn et al, 2011). MrpL32 processing by the m-AAA protease is conserved from yeast to man but it is presently unclear whether an impaired maturation of MrpL32 and mitochondrial protein synthesis contributes to the pathogenesis of neurodegenerative disorders associated with mutations in subunits of the m-AAA protease (Figure 2). Regardless, the ability of the m-AAA protease to act as a processing peptidase with regulatory functions raises the possibility that neuron-specific substrates of the m-AAA protease exist, which could be specific for different isoenzymes and which may explain the selective vulnerability of specific neurons upon mutations of m-AAA protease subunits.
Inhibition of mitochondrial fusion and axonal transport
Neuronal survival depends on the mitochondrial fusion machinery (Chen et al, 2007), which is tightly coupled to the axonal transport of mitochondria. The expression of MFN2 mutants that are associated with the peripheral neuropathy Charcot-Marie-Tooth type 2A caused fragmentation of mitochondria in neurons and impaired axonal transport (Baloh et al, 2007). In addition, lack of MFN2 resulted in clustering of swollen mitochondria at dendritic junctions, with a depletion of mitochondria from distal branches in Purkinje cells in vitro (Chen et al, 2007). MFN2 plays a crucial role in tethering mitochondria to the ER, and a disruption of ER–mitochondria interaction affecting Ca2+ homeostasis was observed in MFN2-deficient cells (de Brito and Scorrano, 2008). Therefore, Ca2+ overload or inability to buffer cytosolic Ca2+ could contribute to the death cascade in these neuropathies.
Interestingly, acute depletion of m-AAA protease subunits in mouse embryonic fibroblasts does not significantly impair respiration but inhibits fusion by destabilizing OPA1 (Ehses et al, 2009). It is therefore conceivable that the fragmentation of mitochondria due to inactivation of OPA1 is the primary defect occurring in the absence of the m-AAA protease, resulting in axonal transport defects in neurons and the depletion of functional mitochondria from synapses (Figure 2). However, it is currently unclear whether impaired fusion is a very early pathogenic event in m-AAA protease-deficient neurons in vivo. An alteration of the mitochondrial network associated with abnormally swollen mitochondria with altered and disrupted cristae represents the first phenotype observed in mice lacking the m-AAA protease subunits paraplegin (SPG7) (Ferreirinha et al, 2004). These mitochondrial anomalies were first confined to synaptic terminals and then to distal regions of axons, while mitochondria in the cell bodies were normal. This reinforces the notion that the burden of mitochondrial damage might be very different in axons versus those in cell bodies and that an impaired QC capacity might be first revealed in distal regions of a neuron. Moreover, accumulation of mitochondria in distal region is highly suggestive of an impaired retrograde transport of damaged organelles. Indeed, retrograde transport was affected in old paraplegin-deficient mice (Ferreirinha et al, 2004).
Mitochondrial damage triggers a fatal cascade culminating in neuronal loss
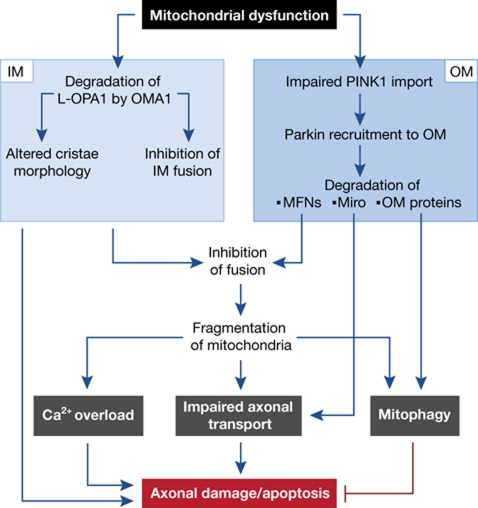
After intensive research in recent years a more detailed picture is now emerging on cellular mechanisms ensuring mitochondrial integrity and their relevance for disease. A hierarchical network of different mechanisms exists, which act on molecular or organellar levels and are highly interconnected and coordinately regulated. This intricate balance ensures high selectivity and allows the rapid adaptation to mitochondrial stress and damage. Mitochondrial proteases prevent the accumulation of damaged proteins within mitochondria, but at the same time can act as regulatory enzymes and control mitochondrial biogenesis and the fusion of mitochondrial membranes. Accordingly, pathogenic mutations associated with neurodegenerative disorders may lead to the accumulation of damaged polypeptides or organelles and/or directly impair mitochondrial activities, both with potential deleterious effects on matrix ATP levels and ΔΨm, which is emerging as central sensor to monitor mitochondrial functionality (Figure 4). Mitochondrial depolarization causes the degradation of dynamin-like GTPases, OPA1 and mitofusins, in the inner and outer membrane, respectively. This may ensure the complete inhibition of mitochondrial fusion, although fusion machineries in both membranes are only loosely coupled (Song et al, 2009). Impaired fusion has severe consequences for neuronal survival: First, fragmentation of the mitochondrial network allows the specific segregation and elimination of damaged mitochondria by mitophagy, supporting neuronal survival. Second, an impaired fusion, more specifically the turnover of MFN2, interferes directly with the axonal transport of mitochondria. Third, alterations in mitochondrial morphology affect functional interactions with the ER and trigger an altered mitochondrial Ca2+ homeostasis. Severe damage or the permanent failure of mitochondrial surveillance mechanisms as it occurs in diverse neurodegenerative disorders may ultimately result in a collapse of the mitochondrial QC network and the apoptotic loss of neurons. Clearly, much remains to be learned about this emerging fatal cascade of events. However, the increasing number of neurodegenerative disorders that are linked to an impaired mitochondrial QC illustrates the relevance of these surveillance mechanisms for neuronal survival and makes them interesting targets of therapeutic interventions.
Figure 4.
Mitochondrial dysfunction triggers a fatal cascade leading to axonal damage and apoptosis. Low ΔΨm induce the degradation of dynamin-like GTPases in the inner and outer membrane, proteolytic remodelling of the mitochondrial proteome in the outer membrane and the turnover of Miro inhibiting fusion and axonal transport of mitochondria. Mitophagy of damaged mitochondrial fragments may protect against neurodegeneration, but severe damage impairs axonal trafficking of mitochondria and Ca2+ homeostasis, ultimately leading to axonal swelling and apoptosis. IM, inner membrane; OM, outer membrane; MFNs, mitofusins.
Acknowledgments
We thank members of our laboratories for discussions, T Wai for critically reading the manuscript and M Baker for help with the figures. Work in the authors’ laboratories is supported by grants from the Deutsche Forschungsgemeinschaft (RU1653/1-1 to EIR; FOR885, RP8 and SFB635, C4 to TL) and from the European Research Council (ERC-Ad-233078) to TL.
Footnotes
The authors declare that they have no conflict of interest.
References
- Akepati VR, Muller EC, Otto A, Strauss HM, Portwich M, Alexander C (2008) Characterization of OPA1 isoforms isolated from mouse tissues. J Neurochem 106: 372–383 [DOI] [PubMed] [Google Scholar]
- Alexander C, Votruba M, Pesch UE, Thiselton DL, Mayer S, Moore A, Rodriguez M, Kellner U, Leo-Kottler B, Auburger G, Bhattacharya SS, Wissinger B (2000) OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet 26: 211–215 [DOI] [PubMed] [Google Scholar]
- Alikhani N, Guo L, Yan S, Du H, Pinho CM, Chen JX, Glaser E, Yan SS (2011) Decreased proteolytic activity of the mitochondrial amyloid-beta degrading enzyme, prep peptidasome, in Alzheimer's disease brain mitochondria. J Alzheimers Dis 27: 75–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold B, Cassady SJ, VanLaar VS, Berman SB (2011) Integrating multiple aspects of mitochondrial dynamics in neurons: age-related differences and dynamic changes in a chronic rotenone model. Neurobiol Dis 41: 189–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker MJ, Tatsuta T, Langer T (2011) Quality control of mitochondrial proteostasis. Cold Spring Harb Perspect Biol 3: pii: a007559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baloh RH, Schmidt RE, Pestronk A, Milbrandt J (2007) Altered axonal mitochondrial transport in the pathogenesis of Charcot-Marie-Tooth disease from mitofusin 2 mutations. J Neurosci 27: 422–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter RV, Ben Othmane K, Rochelle JM, Stajich JE, Hulette C, Dew-Knight S, Hentati F, Ben Hamida M, Bel S, Stenger JE, Gilbert JR, Pericak-Vance MA, Vance JM (2002) Ganglioside-induced differentiation-associated protein-1 is mutant in Charcot-Marie-Tooth disease type 4A/8q21. Nat Genet 30: 21–22 [DOI] [PubMed] [Google Scholar]
- Bender T, Lewrenz I, Franken S, Baitzel C, Voos W (2011) Mitochondrial enzymes are protected from stress-induced aggregation by mitochondrial chaperones and the Pim1/LON protease. Mol Biol Cell 22: 541–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti C, Haynes CM, Yang Y, Harding HP, Ron D (2006) Ubiquitin-like protein 5 positively regulates chaperone gene expression in the mitochondrial unfolded protein response. Genetics 174: 229–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman SB, Chen YB, Qi B, McCaffery JM, Rucker EB III, Goebbels S, Nave KA, Arnold BA, Jonas EA, Pineda FJ, Hardwick JM (2009) Bcl-x L increases mitochondrial fission, fusion, and biomass in neurons. J Cell Biol 184: 707–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestwick M, Khalimonchuk O, Pierrel F, Winge DR (2010) The role of Coa2 in hemylation of yeast Cox1 revealed by its genetic interaction with Cox10. Mol Cell Biol 30: 172–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstone C, O′Kane CJ, Reid E (2011) Hereditary spastic paraplegias: membrane traffic and the motor pathway. Nat Rev Neurosci 12: 31–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogaerts V, Nuytemans K, Reumers J, Pals P, Engelborghs S, Pickut B, Corsmit E, Peeters K, Schymkowitz J, De Deyn PP, Cras P, Rousseau F, Theuns J, Van Broeckhoven C (2008) Genetic variability in the mitochondrial serine protease HTRA2 contributes to risk for Parkinson disease. Hum Mutat 29: 832–840 [DOI] [PubMed] [Google Scholar]
- Bonn F, Tatsuta T, Petrungaro C, Riemer J, Langer T (2011) Presequence-dependent folding ensures MrpL32 processing by the m-AAA protease in mitochondria. EMBO J 30: 2545–2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bota DA, Davies KJ (2002) Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat Cell Biol 4: 674–680 [DOI] [PubMed] [Google Scholar]
- Bross P, Naundrup S, Hansen J, Nielsen MN, Christensen JH, Kruhoffer M, Palmfeldt J, Corydon TJ, Gregersen N, Ang D, Georgopoulos C, Nielsen KL (2008) The Hsp60-(p.V98I) mutation associated with hereditary spastic paraplegia SPG13 compromises chaperonin function both in vitro and in vivo. J Biol Chem 283: 15694–15700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MR, Sullivan PG, Geddes JW (2006) Synaptic mitochondria are more susceptible to Ca2+ overload than nonsynaptic mitochondria. J Biol Chem 281: 11658–11668 [DOI] [PubMed] [Google Scholar]
- Brugman F, Scheffer H, Wokke JH, Nillesen WM, de Visser M, Aronica E, Veldink JH, van den Berg LH (2008) Paraplegin mutations in sporadic adult-onset upper motor neuron syndromes. Neurology 71: 1500–1505 [DOI] [PubMed] [Google Scholar]
- Cagnoli C, Stevanin G, Brussino A, Barberis M, Mancini C, Margolis RL, Holmes SE, Nobili M, Forlani S, Padovan S, Pappi P, Zaros C, Leber I, Ribai P, Pugliese L, Assalto C, Brice A, Migone N, Durr A, Brusco A (2010) Missense mutations in the AFG3L2 proteolytic domain account for approximately 1.5% of European autosomal dominant cerebellar ataxias. Hum Mutat 31: 1117–1124 [DOI] [PubMed] [Google Scholar]
- Casari G, De Fusco M, Ciarmatori S, Zeviani M, Mora M, Fernandez P, De Michele G, Filla A, Cocozza S, Marconi R, Dürr A, Fontaine B, Ballabio A (1998) Spastic paraplegia and OXPHOS impairment caused by mutations in paraplegin, a nuclear-encoded mitochondrial metalloprotease. Cell 93: 973–983 [DOI] [PubMed] [Google Scholar]
- Chan NC, Salazar AM, Pham AH, Sweredoski MJ, Kolawa NJ, Graham RL, Hess S, Chan DC (2011) Broad activation of the ubiquitin-proteasome system by Parkin is critical for mitophagy. Hum Mol Genet 20: 1726–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Chan DC (2009) Mitochondrial dynamics—fusion, fission, movement, and mitophagy—in neurodegenerative diseases. Hum Mol Genet 18: R169–R176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Chomyn A, Chan DC (2005) Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem 280: 26185–26192 [DOI] [PubMed] [Google Scholar]
- Chen H, McCaffery JM, Chan DC (2007) Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell 130: 548–562 [DOI] [PubMed] [Google Scholar]
- Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, Chan DC (2010) Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell 141: 280–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolat S, Rudka T, Hartmann D, Costa V, Serneels L, Craessaerts K, Metzger K, Frezza C, Annaert W, D′Adamio L, Derks C, Dejaegere T, Pellegrini L, D′Hooge R, Scorrano L, De Strooper B (2006) Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell 126: 163–175 [DOI] [PubMed] [Google Scholar]
- Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, Yoo SJ, Hay BA, Guo M (2006) Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature 441: 1162–1166 [DOI] [PubMed] [Google Scholar]
- Clausen T, Kaiser M, Huber R, Ehrmann M (2011) HTRA proteases: regulated proteolysis in protein quality control. Nat Rev Mol Cell Biol 12: 152–162 [DOI] [PubMed] [Google Scholar]
- Csordas G, Varnai P, Golenar T, Roy S, Purkins G, Schneider TG, Balla T, Hajnoczky G (2010) Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol Cell 39: 121–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L (2008) Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456: 605–610 [DOI] [PubMed] [Google Scholar]
- Deas E, Plun-Favreau H, Gandhi S, Desmond H, Kjaer S, Loh SH, Renton AE, Harvey RJ, Whitworth AJ, Martins LM, Abramov AY, Wood NW (2011) PINK1 cleavage at position A103 by the mitochondrial protease PARL. Hum Mol Genet 20: 867–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delettre C, Lenaers G, Griffoin JM, Gigarel N, Lorenzo C, Belenguer P, Pelloquin L, Grosgeorge J, Turc-Carel C, Perret E, Astarie-Dequeker C, Lasquellec L, Arnaud B, Ducommun B, Kaplan J, Hamel CP (2000) Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet 26: 207–210 [DOI] [PubMed] [Google Scholar]
- DeVay RM, Dominguez-Ramirez L, Lackner LL, Hoppins S, Stahlberg H, Nunnari J (2009) Coassembly of Mgm1 isoforms requires cardiolipin and mediates mitochondrial inner membrane fusion. J Cell Biol 186: 793–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Bella D, Lazzaro F, Brusco A, Plumari M, Battaglia G, Pastore A, Finardi A, Cagnoli C, Tempia F, Frontali M, Veneziano L, Sacco T, Boda E, Brussino A, Bonn F, Castellotti B, Baratta S, Mariotti C, Gellera C, Fracasso V et al. (2010) Mutations in the mitochondrial protease gene AFG3L2 cause dominant hereditary ataxia SCA28. Nat Genet 42: 313–321 [DOI] [PubMed] [Google Scholar]
- Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, Gunn-Moore FJ, Vonsattel JP, Arancio O, Chen JX, Yan SD (2008) Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer′s disease. Nat Med 14: 1097–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen LW, Gomez S, Guénet JL, Love S (1983) Paralysé: a new neurological mutant mouse with progressive atrophy and loss of motor nerve terminals. J Physiol 345: 166P [Google Scholar]
- Duvezin-Caubet S, Jagasia R, Wagener J, Hofmann S, Trifunovic A, Hansson A, Chomyn A, Bauer MF, Attardi G, Larsson NG, Neupert W, Reichert AS (2006) Proteolytic processing of OPA1 links mitochondrial dysfunction to alterations in mitochondrial morphology. J Biol Chem 281: 37972–37979 [DOI] [PubMed] [Google Scholar]
- Duvezin-Caubet S, Koppen M, Wagener J, Zick M, Israel L, Bernacchia A, Jagasia R, Rugarli EI, Imhof A, Neupert W, Langer T, Reichert AS (2007) OPA1 processing reconstituted in yeast depends on the subunit composition of the m-AAA protease in mitochondria. Mol Biol Cell 18: 3582–3590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehses S, Raschke I, Mancuso G, Bernacchia A, Geimer S, Tondera D, Martinou JC, Westermann B, Rugarli EI, Langer T (2009) Regulation of OPA1 processing and mitochondrial fusion by m-AAA protease isoenzymes and OMA1. J Cell Biol 187: 1023–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkevall A, Alikhani N, Bhushan S, Pavlov PF, Busch K, Johnson KA, Eneqvist T, Tjernberg L, Ankarcrona M, Glaser E (2006) Degradation of the amyloid beta-protein by the novel mitochondrial peptidasome, PreP. J Biol Chem 281: 29096–29104 [DOI] [PubMed] [Google Scholar]
- Ferreirinha F, Quattrini A, Pirozzi M, Valsecchi V, Dina G, Broccoli V, Auricchio A, Piemonte F, Tozzi G, Gaeta L, Casari G, Ballabio A, Rugarli EI (2004) Axonal degeneration in paraplegin-deficient mice is associated with abnormal mitochondria and impairment of axonal transport. J Clin Invest 113: 231–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier CA, Kitada T, Shen J (2008) Loss of PINK1 causes mitochondrial functional defects and increased sensitivity to oxidative stress. Proc Natl Acad Sci USA 105: 11364–11369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegg ME, Cooper JM, Chau KY, Rojo M, Schapira AH, Taanman JW (2010) Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy. Hum Mol Genet 19: 4861–4870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Holmstrom KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, Springer W (2010) PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol 12: 119–131 [DOI] [PubMed] [Google Scholar]
- Gerdes F, Tatsuta T, Langer T (2011) Mitochondrial AAA proteases—towards a molecular understanding of membrane-bound proteolytic machines. Biochim Biophys Acta 1823: 49–55 [DOI] [PubMed] [Google Scholar]
- Giorgi C, Romagnoli A, Pinton P, Rizzuto R (2008) Ca2+ signaling, mitochondria and cell death. Curr Mol Med 8: 119–130 [DOI] [PubMed] [Google Scholar]
- Glaser E, Alikhani N (2010) The organellar peptidasome, PreP: a journey from Arabidopsis to Alzheimer′s disease. Biochim Biophys Acta 1797: 1076–1080 [DOI] [PubMed] [Google Scholar]
- Glauser L, Sonnay S, Stafa K, Moore DJ (2011) Parkin promotes the ubiquitination and degradation of the mitochondrial fusion factor mitofusin 1. J Neurochem 118: 636–645 [DOI] [PubMed] [Google Scholar]
- Gomes LC, Di Benedetto G, Scorrano L (2011) During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol 13: 589–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granot Z, Kobiler O, Melamed-Book N, Eimerl S, Bahat A, Lu B, Braun S, Maurizi MR, Suzuki CK, Oppenheim AB, Orly J (2007) Turnover of mitochondrial steroidogenic acute regulatory (StAR) protein by Lon protease: the unexpected effect of proteasome inhibitors. Mol Endocrinol 21: 2164–2177 [DOI] [PubMed] [Google Scholar]
- Greene JC, Whitworth AJ, Kuo I, Andrews LA, Feany MB, Pallanck LJ (2003) Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc Natl Acad Sci USA 100: 4078–4083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griparic L, Kanazawa T, van der Bliek AM (2007) Regulation of the mitochondrial dynamin-like protein Opa1 by proteolytic cleavage. J Cell Biol 178: 757–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J, Svenstrup K, Ang D, Nielsen MN, Christensen JH, Gregersen N, Nielsen JE, Georgopoulos C, Bross P (2007) A novel mutation in the HSPD1 gene in a patient with hereditary spastic paraplegia. J Neurol 254: 897–900 [DOI] [PubMed] [Google Scholar]
- Hansen JJ, Durr A, Cournu-Rebeix I, Georgopoulos C, Ang D, Nielsen MN, Davoine CS, Brice A, Fontaine B, Gregersen N, Bross P (2002) Hereditary spastic paraplegia SPG13 is associated with a mutation in the gene encoding the mitochondrial chaperonin Hsp60. Am J Hum Genet 70: 1328–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes CM, Yang Y, Blais SP, Neubert TA, Ron D (2010) The matrix peptide exporter HAF-1 signals a mitochondrial UPR by activating the transcription factor ZC376.7 in C. elegans. Mol Cell 37: 529–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head B, Griparic L, Amiri M, Gandre-Babbe S, van der Bliek AM (2009) Inducible proteolytic inactivation of OPA1 mediated by the OMA1 protease in mammalian cells. J Cell Biol 187: 959–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo JM, Livnat-Levanon N, Taylor EB, Jones KT, Dephoure N, Ring J, Xie J, Brodsky JL, Madeo F, Gygi SP, Ashrafi K, Glickman MH, Rutter J (2010) A stress-responsive system for mitochondrial protein degradation. Mol Cell 40: 465–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara N, Fujita Y, Oka T, Mihara K (2006) Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. EMBO J 25: 2966–2977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SM, Lazarou M, Wang C, Kane LA, Narendra DP, Youle RJ (2010) Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biol 191: 933–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Bhushan S, Stahl A, Hallberg BM, Frohn A, Glaser E, Eneqvist T (2006) The closed structure of presequence protease PreP forms a unique 10,000 Angstroms3 chamber for proteolysis. EMBO J 25: 1977–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JM, Datta P, Srinivasula SM, Ji W, Gupta S, Zhang Z, Davies E, Hajnoczky G, Saunders TL, Van Keuren ML, Fernandes-Alnemri T, Meisler MH, Alnemri ES (2003) Loss of Omi mitochondrial protease activity causes the neuromuscular disorder of mnd2 mutant mice. Nature 425: 721–727 [DOI] [PubMed] [Google Scholar]
- Kambacheld M, Augustin S, Tatsuta T, Muller S, Langer T (2005) Role of the novel metallopeptidase Mop112 and saccharolysin for the complete degradation of proteins residing in different subcompartments of mitochondria. J Biol Chem 280: 20132–20139 [DOI] [PubMed] [Google Scholar]
- Kaplan BB, Gioio AE, Hillefors M, Aschrafi A (2009) Axonal protein synthesis and the regulation of local mitochondrial function. Results Probl Cell Differ 48: 225–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M, Youle RJ (2011) Regulating mitochondrial outer membrane proteins by ubiquitination and proteasomal degradation. Curr Opin Cell Biol 23: 476–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaser M, Kambacheld M, Kisters-Woike B, Langer T (2003) Oma1, a novel membrane-bound metallopeptidase in mitochondria with activities overlapping with the m-AAA protease. J Biol Chem 278: 46414–46423 [DOI] [PubMed] [Google Scholar]
- Kawamoto Y, Kobayashi Y, Suzuki Y, Inoue H, Tomimoto H, Akiguchi I, Budka H, Martins LM, Downward J, Takahashi R (2008) Accumulation of HtrA2/Omi in neuronal and glial inclusions in brains with alpha-synucleinopathies. J Neuropathol Exp Neurol 67: 984–993 [DOI] [PubMed] [Google Scholar]
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N (1998) Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392: 605–608 [DOI] [PubMed] [Google Scholar]
- Kitada T, Pisani A, Porter DR, Yamaguchi H, Tscherter A, Martella G, Bonsi P, Zhang C, Pothos EN, Shen J (2007) Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc Natl Acad Sci USA 104: 11441–11446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppen M, Metodiev MD, Casari G, Rugarli EI, Langer T (2007) Variable and tissue-specific subunit composition of mitochondrial m-AAA protease complexes linked to hereditary spastic paraplegia. Mol Cell Biol 27: 758–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremmidiotis G, Gardner AE, Settasatian C, Savoia A, Sutherland GR, Callen DF (2001) Molecular and functional analyses of the human and mouse genes encoding AFG3L1, a mitochondrial metalloprotease homologous to the human spastic paraplegia protein. Genomics 76: 58–65 [DOI] [PubMed] [Google Scholar]
- Kruger R, Sharma M, Riess O, Gasser T, Van Broeckhoven C, Theuns J, Aasly J, Annesi G, Bentivoglio AR, Brice A, Djarmati A, Elbaz A, Farrer M, Ferrarese C, Gibson JM, Hadjigeorgiou GM, Hattori N, Ioannidis JP, Jasinska-Myga B, Klein C et al. (2011) A large-scale genetic association study to evaluate the contribution of Omi/HtrA2 (PARK13) to Parkinson′s disease. Neurobiol Aging 32: 548 e9–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landes T, Leroy I, Bertholet A, Diot A, Khosrobakhsh F, Daloyau M, Davezac N, Miquel MC, Courilleau D, Guillou E, Olichon A, Lenaers G, Arnaune-Pelloquin L, Emorine LJ, Belenguer P (2010) OPA1 (dys)functions. Semin Cell Dev Biol 21: 593–598 [DOI] [PubMed] [Google Scholar]
- Larsson NG (2010) Somatic mitochondrial DNA mutations in mammalian aging. Annu Rev Biochem 79: 683–706 [DOI] [PubMed] [Google Scholar]
- Lee JY, Nagano Y, Taylor JP, Lim KL, Yao TP (2010) Disease-causing mutations in parkin impair mitochondrial ubiquitination, aggregation, and HDAC6-dependent mitophagy. J Cell Biol 189: 671–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenaers G, Reynier P, Elachouri G, Soukkarieh C, Olichon A, Belenguer P, Baricault L, Ducommun B, Hamel C, Delettre C (2009) OPA1 functions in mitochondria and dysfunctions in optic nerve. Int J Biochem Cell Biol 41: 1866–1874 [DOI] [PubMed] [Google Scholar]
- Leonhard K, Guiard B, Pellecchia G, Tzagoloff A, Neupert W, Langer T (2000) Membrane protein degradation by AAA proteases in mitochondria: extraction of substrates from either membrane surface. Mol Cell 5: 629–638 [DOI] [PubMed] [Google Scholar]
- Leonhard K, Stiegler A, Neupert W, Langer T (1999) Chaperone-like activity of the AAA domain of the yeast Yme1 AAA protease. Nature 398: 348–351 [DOI] [PubMed] [Google Scholar]
- Li Z, Okamoto K, Hayashi Y, Sheng M (2004) The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell 119: 873–887 [DOI] [PubMed] [Google Scholar]
- Luce K, Osiewacz HD (2009) Increasing organismal healthspan by enhancing mitochondrial protein quality control. Nat Cell Biol 11: 852–858 [DOI] [PubMed] [Google Scholar]
- Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, Trinchese F, Liu S, Gunn-Moore F, Lue LF, Walker DG, Kuppusamy P, Zewier ZL, Arancio O, Stern D, Yan SS et al. (2004) ABAD directly links Abeta to mitochondrial toxicity in Alzheimer′s disease. Science 304: 448–452 [DOI] [PubMed] [Google Scholar]
- MacAskill AF, Atkin TA, Kittler JT (2010) Mitochondrial trafficking and the provision of energy and calcium buffering at excitatory synapses. Eur J Neurosci 32: 231–240 [DOI] [PubMed] [Google Scholar]
- MacAskill AF, Kittler JT (2010) Control of mitochondrial transport and localization in neurons. Trends Cell Biol 20: 102–112 [DOI] [PubMed] [Google Scholar]
- Magen D, Georgopoulos C, Bross P, Ang D, Segev Y, Goldsher D, Nemirovski A, Shahar E, Ravid S, Luder A, Heno B, Gershoni-Baruch R, Skorecki K, Mandel H (2008) Mitochondrial hsp60 chaperonopathy causes an autosomal-recessive neurodegenerative disorder linked to brain hypomyelination and leukodystrophy. Am J Hum Genet 83: 30–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltecca F, Aghaie A, Schroeder DG, Cassina L, Taylor BA, Phillips SJ, Malaguti M, Previtali S, Guenet JL, Quattrini A, Cox GA, Casari G (2008) The mitochondrial protease AFG3L2 is essential for axonal development. J Neurosci 28: 2827–2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltecca F, Magnoni R, Cerri F, Cox GA, Quattrini A, Casari G (2009) Haploinsufficiency of AFG3L2, the gene responsible for spinocerebellar ataxia type 28, causes mitochondria-mediated Purkinje cell dark degeneration. J Neurosci 29: 9244–9254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli P, La Mattina V, Bernacchia A, Magnoni R, Cerri F, Cox G, Quattrini A, Casari G, Rugarli EI (2009) Genetic interaction between the m-AAA protease isoenzymes reveals novel roles in cerebellar degeneration. Hum Mol Genet 18: 2001–2013 [DOI] [PubMed] [Google Scholar]
- Martins LM, Morrison A, Klupsch K, Fedele V, Moisoi N, Teismann P, Abuin A, Grau E, Geppert M, Livi GP, Creasy CL, Martin A, Hargreaves I, Heales SJ, Okada H, Brandner S, Schulz JB, Mak T, Downward J (2004) Neuroprotective role of the Reaper-related serine protease HtrA2/Omi revealed by targeted deletion in mice. Mol Cell Biol 24: 9848–9862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, Sou YS, Saiki S, Kawajiri S, Sato F, Kimura M, Komatsu M, Hattori N, Tanaka K (2010) PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol 189: 211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima Y, Goto Y, Kaguni LS (2010) Mitochondrial Lon protease regulates mitochondrial DNA copy number and transcription by selective degradation of mitochondrial transcription factor A (TFAM). Proc Natl Acad Sci USA 107: 18410–18415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies RA, Gold PH (1971) The turnover of mitochondria in a variety of tissues of young adult and aged rats. J Biol Chem 246: 2425–2429 [PubMed] [Google Scholar]
- Merkwirth C, Dargazanli S, Tatsuta T, Geimer S, Lower B, Wunderlich FT, von Kleist-Retzow JC, Waisman A, Westermann B, Langer T (2008) Prohibitins control cell proliferation and apoptosis by regulating OPA1-dependent cristae morphogenesis in mitochondria. Genes Dev 22: 476–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misko A, Jiang S, Wegorzewska I, Milbrandt J, Baloh RH (2010) Mitofusin 2 is necessary for transport of axonal mitochondria and interacts with the Miro/Milton complex. J Neurosci 30: 4232–4240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa S, Lawless C, von Zglinicki T (2008) Mitochondrial turnover in liver is fast in vivo and is accelerated by dietary restriction: application of a simple dynamic model. Aging Cell 7: 920–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberg P, Stahl A, Bhushan S, Wright SJ, Eriksson A, Bruce BD, Glaser E (2003) Characterization of a novel zinc metalloprotease involved in degrading targeting peptides in mitochondria and chloroplasts. Plant J 36: 616–628 [DOI] [PubMed] [Google Scholar]
- Moisoi N, Klupsch K, Fedele V, East P, Sharma S, Renton A, Plun-Favreau H, Edwards RE, Teismann P, Esposti MD, Morrison AD, Wood NW, Downward J, Martins LM (2009) Mitochondrial dysfunction triggered by loss of HtrA2 results in the activation of a brain-specific transcriptional stress response. Cell Death Differ 16: 449–464 [DOI] [PubMed] [Google Scholar]
- Murphy MP, Holmgren A, Larsson NG, Halliwell B, Chang CJ, Kalyanaraman B, Rhee SG, Thornalley PJ, Partridge L, Gems D, Nystrom T, Belousov V, Schumacker PT, Winterbourn CC (2011) Unraveling the biological roles of reactive oxygen species. Cell Metab 13: 361–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra D, Kane LA, Hauser DN, Fearnley IM, Youle RJ (2010a) p62/SQSTM1 is required for Parkin-induced mitochondrial clustering but not mitophagy; VDAC1 is dispensable for both. Autophagy 6: 1090–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra D, Tanaka A, Suen DF, Youle RJ (2008) Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 183: 795–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra D, Tanaka A, Suen DF, Youle RJ (2009) Parkin-induced mitophagy in the pathogenesis of Parkinson disease. Autophagy 5: 706–708 [DOI] [PubMed] [Google Scholar]
- Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ (2010b) PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol 8: e1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann A, Ruegg M, La Padula V, Schenone A, Suter U (2005) Ganglioside-induced differentiation associated protein 1 is a regulator of the mitochondrial network: new implications for Charcot-Marie-Tooth disease. J Cell Biol 170: 1067–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noack R, Frede S, Albrecht P, Henke N, Pfeiffer A, Knoll K, Dehmel T, Meyer Zu Horste G, Stettner M, Kieseier BC, Summer H, Golz S, Kochanski A, Wiedau-Pazos M, Arnold S, Lewerenz J, Methner A (2011) Charcot-Marie-Tooth disease CMT4A: GDAP1 increases cellular glutathione and the mitochondrial membrane potential. Hum Mol Genet 21: 50–62 [DOI] [PubMed] [Google Scholar]
- Nolden M, Ehses S, Koppen M, Bernacchia A, Rugarli EI, Langer T (2005) The m-AAA protease defective in hereditary spastic paraplegia controls ribosome assembly in mitochondria. Cell 123: 277–289 [DOI] [PubMed] [Google Scholar]
- O’Toole M, Latham R, Baqri RM, Miller KE (2008) Modeling mitochondrial dynamics during in vivo axonal elongation. J Theor Biol 255: 369–377 [DOI] [PubMed] [Google Scholar]
- Osman C, Merkwirth C, Langer T (2009) Prohibitins and the functional compartmentalization of mitochondrial membranes. J Cell Sci 122: 3823–3830 [DOI] [PubMed] [Google Scholar]
- Palacino JJ, Sagi D, Goldberg MS, Krauss S, Motz C, Wacker M, Klose J, Shen J (2004) Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J Biol Chem 279: 18614–18622 [DOI] [PubMed] [Google Scholar]
- Park J, Lee SB, Lee S, Kim Y, Song S, Kim S, Bae E, Kim J, Shong M, Kim JM, Chung J (2006) Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature 441: 1157–1161 [DOI] [PubMed] [Google Scholar]
- Pierson TM, Adams D, Bonn F, Martinelli P, Cherukuri PF, Teer JK, Hansen NF, Cruz P, Mullikin For The Nisc Comparative Sequencing Program JC, Blakesley RW, Golas G, Kwan J, Sandler A, Fuentes Fajardo K, Markello T, Tifft C, Blackstone C, Rugarli EI, Langer T, Gahl WA et al. (2011) Whole-exome sequencing identifies homozygous AFG3L2 mutations in a spastic ataxia-neuropathy syndrome linked to mitochondrial m-AAA proteases. PLoS Genet 7: e1002325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho CM, Bjork BF, Alikhani N, Backman HG, Eneqvist T, Fratiglioni L, Glaser E, Graff C (2010) Genetic and biochemical studies of SNPs of the mitochondrial A beta-degrading protease, hPreP. Neurosci Lett 469: 204–208 [DOI] [PubMed] [Google Scholar]
- Plun-Favreau H, Klupsch K, Moisoi N, Gandhi S, Kjaer S, Frith D, Harvey K, Deas E, Harvey RJ, McDonald N, Wood NW, Martins LM, Downward J (2007) The mitochondrial protease HtrA2 is regulated by Parkinson′s disease-associated kinase PINK1. Nat Cell Biol 9: 1243–1252 [DOI] [PubMed] [Google Scholar]
- Poole AC, Thomas RE, Yu S, Vincow ES, Pallanck L (2010) The mitochondrial fusion-promoting factor mitofusin is a substrate of the PINK1/parkin pathway. PLoS One 5: e10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov V, Medvedev NI, Davies HA, Stewart MG (2005) Mitochondria form a filamentous reticular network in hippocampal dendrites but are present as discrete bodies in axons: a three-dimensional ultrastructural study. J Comp Neurol 492: 50–65 [DOI] [PubMed] [Google Scholar]
- Potting C, Wilmes C, Engmann T, Osman C, Langer T (2010) Regulation of mitochondrial phospholipids by Ups1/PRELI-like proteins depends on proteolysis and Mdm35. EMBO J 29: 2888–2898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J (2011) Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc Natl Acad Sci USA 108: 10190–10195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid E, Rugarli EI (2010) Hereditary Spastic Paraplegia. In: Valle, Beaudet, Vogelstein, Kinzler, Antonarakis, Ballabio (eds). Scriver's Online Metabolic and Molecular Bases of Inherited Diseases. New York: Mc Graw Hill [Google Scholar]
- Ross OA, Soto AI, Vilarino-Guell C, Heckman MG, Diehl NN, Hulihan MM, Aasly JO, Sando S, Gibson JM, Lynch T, Krygowska-Wajs A, Opala G, Barcikowska M, Czyzewski K, Uitti RJ, Wszolek ZK, Farrer MJ (2008) Genetic variation of Omi/HtrA2 and Parkinson′s disease. Parkinsonism Relat Disord 14: 539–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer RT, Baker TA (2011) AAA+ proteases: ATP-fueled machines of protein destruction. Annu Rev Biochem 80: 587–612 [DOI] [PubMed] [Google Scholar]
- Schmidt O, Pfanner N, Meisinger C (2010) Mitochondrial protein import: from proteomics to functional mechanisms. Nat Rev Mol Cell Biol 11: 655–667 [DOI] [PubMed] [Google Scholar]
- Shin JH, Ko HS, Kang H, Lee Y, Lee YI, Pletinkova O, Troconso JC, Dawson VL, Dawson TM (2011) PARIS (ZNF746) repression of PGC-1alpha contributes to neurodegeneration in Parkinson′s disease. Cell 144: 689–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon-Sanchez J, Singleton AB (2008) Sequencing analysis of OMI/HTRA2 shows previously reported pathogenic mutations in neurologically normal controls. Hum Mol Genet 17: 1988–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Chen H, Fiket M, Alexander C, Chan DC (2007) OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J Cell Biol 178: 749–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Ghochani M, McCaffery JM, Frey TG, Chan DC (2009) Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol Biol Cell 20: 3525–3532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl A, Moberg P, Ytterberg J, Panfilov O, Brockenhuus Von Lowenhielm H, Nilsson F, Glaser E (2002) Isolation and identification of a novel mitochondrial metalloprotease (PreP) that degrades targeting presequences in plants. J Biol Chem 277: 41931–41939 [DOI] [PubMed] [Google Scholar]
- Steglich G, Neupert W, Langer T (1999) Prohibitins regulate membrane protein degradation by the m-AAA protease in mitochondria. Mol Cell Biol 19: 3435–3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterky FH, Lee S, Wibom R, Olson L, Larsson NG (2011) Impaired mitochondrial transport and Parkin-independent degeneration of respiratory chain-deficient dopamine neurons in vivo. Proc Natl Acad Sci USA 108: 12937–12942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss KM, Martins LM, Plun-Favreau H, Marx FP, Kautzmann S, Berg D, Gasser T, Wszolek Z, Muller T, Bornemann A, Wolburg H, Downward J, Riess O, Schulz JB, Kruger R (2005) Loss of function mutations in the gene encoding Omi/HtrA2 in Parkinson′s disease. Hum Mol Genet 14: 2099–2111 [DOI] [PubMed] [Google Scholar]
- Tain LS, Chowdhury RB, Tao RN, Plun-Favreau H, Moisoi N, Martins LM, Downward J, Whitworth AJ, Tapon N (2009) Drosophila HtrA2 is dispensable for apoptosis but acts downstream of PINK1 independently from Parkin. Cell Death Differ 16: 1118–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuta T, Langer T (2008) Quality control of mitochondria: protection against neurodegeneration and ageing. EMBO J 27: 306–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q, Li T, Hou W, Zheng J, Schrum LW, Bonkovsky HL (2011) Lon peptidase 1 (LONP1)-dependent breakdown of mitochondrial 5-aminolevulinic acid synthase protein by heme in human liver cells. J Biol Chem 286: 26424–26430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tondera D, Grandemange S, Jourdain A, Karbowski M, Mattenberger Y, Herzig S, Da Cruz S, Clerc P, Raschke I, Merkwirth C, Ehses S, Krause F, Chan DC, Alexander C, Bauer C, Youle R, Langer T, Martinou JC (2009) SLP-2 is required for stress-induced mitochondrial hyperfusion. EMBO J 28: 1589–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS (2008) Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 27: 433–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, Gonzalez-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B et al. (2004) Hereditary early-onset Parkinson′s disease caused by mutations in PINK1. Science 304: 1158–1160 [DOI] [PubMed] [Google Scholar]
- Van Laar VS, Arnold B, Cassady SJ, Chu CT, Burton EA, Berman SB (2011) Bioenergetics of neurons inhibit the translocation response of Parkin following rapid mitochondrial depolarization. Hum Mol Genet 20: 927–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vande Walle L, Lamkanfi M, Vandenabeele P (2008) The mitochondrial serine protease HtrA2/Omi: an overview. Cell Death Differ 15: 453–460 [DOI] [PubMed] [Google Scholar]
- Vives-Bauza C, Zhou C, Huang Y, Cui M, de Vries RL, Kim J, May J, Tocilescu MA, Liu W, Ko HS, Magrane J, Moore DJ, Dawson VL, Grailhe R, Dawson TM, Li C, Tieu K, Przedborski S (2010) PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci USA 107: 378–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voos W (2009) Mitochondrial protein homeostasis: the cooperative roles of chaperones and proteases. Res Microbiol 160: 718–725 [DOI] [PubMed] [Google Scholar]
- Wagner I, Arlt H, van Dyck L, Langer T, Neupert W (1994) Molecular chaperones cooperate with PIM1 protease in the degradation of misfolded proteins in mitochondria. EMBO J 13: 5135–5145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QJ, Ding Y, Kohtz DS, Mizushima N, Cristea IM, Rout MP, Chait BT, Zhong Y, Heintz N, Yue Z (2006) Induction of autophagy in axonal dystrophy and degeneration. The Journal of neuroscience: the Official Journal of the Society for Neuroscience 26: 8057–8068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Winter D, Ashrafi G, Schlehe J, Wong YL, Selkoe D, Rice S, Steen J, La Vole MJ, Schwarz TL (2011) PINK1 and parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell 147: 893–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann B (2010) Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol 11: 872–884 [DOI] [PubMed] [Google Scholar]
- Whitworth AJ, Lee JR, Ho VM, Flick R, Chowdhury R, McQuibban GA (2008) Rhomboid-7 and HtrA2/Omi act in a common pathway with the Parkinson′s disease factors Pink1 and Parkin. Dis Model Mech 1: 168–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Gehrke S, Imai Y, Huang Z, Ouyang Y, Wang JW, Yang L, Beal MF, Vogel H, Lu B (2006) Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc Natl Acad Sci USA 103: 10793–10798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii SR, Kishi C, Ishihara N, Mizushima N (2011) Parkin mediates proteasome-dependent protein degradation and rupture of the outer mitochondrial membrane. J Biol Chem 286: 19630–19640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle RJ, Narendra DP (2011) Mechanisms of mitophagy. Nat Rev Mol Cell Biol 12: 9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L, Leonhard K, Tatsuta T, Trowsdale J, Langer T (2001) Role of the ABC transporter Mdl1 in peptide export from mitochondria. Science 291: 2135–2138 [DOI] [PubMed] [Google Scholar]
- Yun J, Cao JH, Dodson MW, Clark IE, Kapahi P, Chowdhury RB, Guo M (2008) Loss-of-function analysis suggests that Omi/HtrA2 is not an essential component of the PINK1/PARKIN pathway in vivo. J Neurosci 28: 14500–14510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Wang J, Levichkin IV, Stasinopoulos S, Ryan MT, Hoogenraad NJ (2002) A mitochondrial specific stress response in mammalian cells. EMBO J 21: 4411–4419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Q, Gao W, Du F, Wang X (2005) Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell 121: 1085–1095 [DOI] [PubMed] [Google Scholar]
- Ziviani E, Tao RN, Whitworth AJ (2010) Drosophila parkin requires PINK1 for mitochondrial translocation and ubiquitinates mitofusin. Proc Natl Acad Sci USA 107: 5018–5023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuchner S, De Jonghe P, Jordanova A, Claeys KG, Guergueltcheva V, Cherninkova S, Hamilton SR, Van Stavern G, Krajewski KM, Stajich J, Tournev I, Verhoeven K, Langerhorst CT, de Visser M, Baas F, Bird T, Timmerman V, Shy M, Vance JM (2006) Axonal neuropathy with optic atrophy is caused by mutations in mitofusin 2. Ann Neurol 59: 276–281 [DOI] [PubMed] [Google Scholar]