Abstract
Shugoshins (Sgo) are conserved proteins that act as protectors of centromeric cohesion and as sensors of tension for the machinery that eliminates improper kinetochore–microtubule attachments. Most vertebrates contain two Sgo proteins, but their specific functions are not always clear. Xenopus laevis Sgo1, XSgo1, protects centromeric cohesin from the prophase dissociation pathway. Here, we report the identification of XSgo2 and show that it does not regulate cohesion. Instead, we find that it participates in bipolar spindle assembly. Both Sgo proteins interact physically with the Chromosomal Passenger Complex (CPC) containing Aurora B, a key regulator of mitosis, but the functional consequences of such interaction are distinct. XSgo1 is required for proper localization of the CPC while XSgo2 positively contributes to its activation and the subsequent phosphorylation of at least one key substrate for bipolar spindle assembly, the microtubule depolymerizing kinesin MCAK (Mitotic Centromere-Associated Kinesin). Thus, the two Xenopus Sgo proteins have non-overlapping functions in chromosome segregation. Our results further suggest that this functional specificity could rely on the association of XSgo1 and XSgo2 with different regulatory subunits of the PP2A complex.
Keywords: Aurora B, chromosome segregation, cohesion, mitosis, phosphatase
Introduction
Accurate chromosome segregation ensures proper division of replicated genetic material to daughter cells. The establishment of sister chromatid cohesion, efficient spindle assembly and proper attachment between sister kinetochores and microtubules emanating from opposite poles of the spindle (i.e., biorientation) are key events in this process (Gadde and Heald, 2004; Kline-Smith et al, 2005; Losada and Hirano, 2005; Tanaka, 2010). Members of an evolutionary conserved family of proteins named Shugoshin (Sgo) have emerged as major regulators of chromosome segregation. The founding member, MEI-S332, was identified in Drosophila melanogaster as a protector of centromeric cohesion in meiosis (Kerrebrock et al, 1995). Functional homologues were later found in yeast (Katis et al, 2004; Kitajima et al, 2004; Marston et al, 2004; Rabitsch et al, 2004). The first vertebrate Sgo protein was identified in Xenopus laevis in a microtubule formation assay and was proposed to regulate kinetochore microtubule stability. Downregulation of the homologous protein in HeLa cells by RNA interference caused premature loss of cohesion in mitosis, suggesting that Sgo would functionally link sister centromere cohesion and microtubule–kinetochore interactions (Salic et al, 2004). Soon afterwards, it was reported that human Sgo prevents dissociation of centromeric cohesin during prophase by antagonizing its phosphorylation by Polo (McGuinness et al, 2005). Consistent with this idea, the protein phosphatase PP2A was found associated with Sgo both in human and in yeast cells (Kitajima et al, 2006; Riedel et al, 2006; Tang et al, 2006).
A single Sgo protein is present in Saccharomyces cerevisiae and Drosophila melanogaster whereas Schizosaccharomyces pombe and mammals contain two paralogues, Sgo1 and Sgo2 (Rabitsch et al, 2004; Kitajima et al, 2006; Huang et al, 2007; Llano et al, 2008). Sequence homology among the members of this protein family is restricted to a coiled-coil domain located near the N terminus and a basic sequence near the C terminus (Watanabe, 2005). In terms of functional specificity, Sgo1 has been reported to protect centromeric cohesin during mitosis both in human cells (McGuinness et al, 2005; Kitajima et al, 2006) and in the Xenopus egg cell-free system (Rivera and Losada, 2009; Shintomi and Hirano, 2009) whereas mammalian Sgo2 protects centromeric cohesin in meiosis but a similar role in mitosis is more controversial (Kitajima et al, 2006; Huang et al, 2007; Lee et al, 2008; Llano et al, 2008). In human cells, Sgo2 also serves as a sensor of tension across sister kinetochores, a function essential to correct erroneous microtubule–kinetochore attachments and thereby achieve biorientation (Gomez et al, 2007; Huang et al, 2007; Lee et al, 2008). The later mechanism has been ascribed to its role in the centromeric recruitment of the microtubule-destabilizing protein MCAK (Mitotic Centromere-Associated Kinesin) (Huang et al, 2007; Tanno et al, 2010). In addition, MCAK localization and activity are regulated by Aurora B, the kinase of the Chromosomal Passenger Complex (CPC) (Andrews et al, 2004; Lan et al, 2004; Ohi et al, 2004; Zhang et al, 2007).
Sgo proteins are also CPC targets. Although the primary signal to attract Sgo proteins to the centromere appears to be a residue in histone H2A phosphorylated by Bub1 (Kawashima et al, 2010), Aurora B is also required for this targeting in most organisms (Resnick et al, 2006; Boyarchuk et al, 2007; Huang et al, 2007; Tsukahara et al, 2010). In turn, fission yeast Sgo2 (the only Sgo protein present in mitosis) specifies the centromeric localization of the CPC (Kawashima et al, 2007; Vanoosthuyse et al, 2007) and the same is true for Xenopus Sgo1 (Rivera and Losada, 2009; Shintomi and Hirano, 2009), whereas both Sgo1 and Sgo2 contribute to this regulation in HeLa cells (Tsukahara et al, 2010). Recent reports show that an additional pathway driving CPC accumulation at centromeres is mediated by Haspin, a kinase that phosphorylates histone H3 at Threonine 3 in mitosis (Kelly et al, 2010; Wang et al, 2010; Yamagishi et al, 2010).
Phosphorylation of Sgo2 by Aurora B promotes recruitment of PP2A to centromeres in human cells (Kitajima et al, 2006; Tanno et al, 2010) whereas Sgo1 fulfills this function in Xenopus (Rivera and Losada, 2009). The phosphatase is a holoenzyme composed of a catalytic subunit (PP2A-C), a scaffolding subunit (PP2A-A) and one of four classes of the B regulatory subunit (B/PR55, B′/B56/PR61, B″/PR72 and B′″/PR93/PR110). Furthermore, each subunit exists in several isoforms (Janssens and Goris, 2001). In human cells, both Sgo1 and Sgo2 have been found associated with the same PP2A-B56α isoform although, at least in vitro, Sgo2 appears to be capable of binding other types of B subunits (Kitajima et al, 2006; Xu et al, 2009; Orth et al, 2011).
What is the main function of Sgo2 in vertebrate mitosis? Does it collaborate with Sgo1 in the regulation of cohesion? Do both Sgo proteins act as CPC adaptors? To address these questions we have turned to the Xenopus egg cell-free system. We had previously characterized the function and regulation of Xenopus Sgo1, XSgo1 (Rivera and Losada, 2009). Here, we report the identification of XSgo2 and show that it is recruited to centromeres in a Bub1- and Aurora B-dependent manner, but independently of XSgo1. XSgo2 does not cooperate with XSgo1 in the regulation of cohesion in mitosis. Instead, it contributes to chromosome alignment and, strikingly, to spindle assembly. Two signalling cascades control spindle assembly in Xenopus, the RanGTP and the CPC pathways (Sampath et al, 2004; Maresca et al, 2009). The RanGTP gradient is locally generated by chromosome-bound RCC1 and nucleates and stabilizes microtubules by releasing several spindle assembly factors (Kalab and Heald, 2008), whereas the CPC regulates two major microtubule-destabilizing proteins, MCAK and Op18/Stathmin (Sampath et al, 2004; Gadea and Ruderman, 2006). Phosphorylation of these two substrates inhibits their microtubule depolymerizing activity promoting microtubule assembly (Andrews et al, 2004; Lan et al, 2004; Ohi et al, 2004; Sampath et al, 2004; Zhang et al, 2007). Furthermore, mitotic chromosomes and microtubules activate Aurora B in a positive feedback loop restricted to the area surrounding the chromosomes to drive spindle assembly (Tseng et al, 2010). We found that XSgo2 contributes to the CPC-mediated spindle assembly pathway by promoting Aurora B activation, but not its localization. Thus, we show for the first time that an Sgo protein can modulate CPC activity. We also provide evidence that suggests that association of XSgo1 and XSgo2 with different regulatory subunits of the PP2A holoenzyme could mediate their specific functions in the chromosome segregation process.
Results
Identification of Xenopus Sgo2
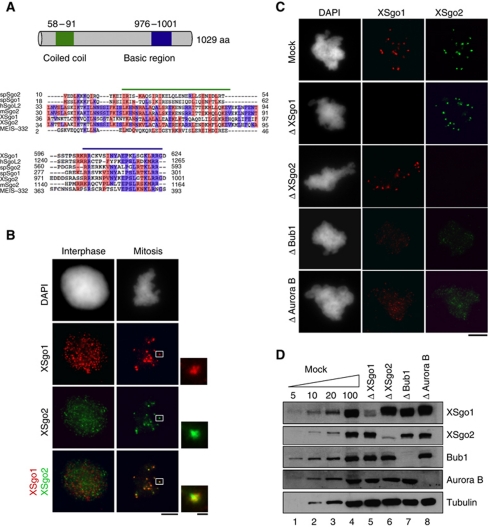
A BLAST search for Sgo2 orthologues in X. laevis identified a cDNA encoding a polypeptide of 127 amino acids with significant homology (61%) to the C-terminal region of Sgo proteins. By means of the RACE (Rapid Amplification of cDNA Ends) technique, we obtained a 3-kb long full-length cDNA that encodes a protein of 1029 amino acids showing weak but significant homology to human and mouse Sgo2 and which contains the N-terminal coiled-coil and C-terminal basic regions characteristic of the Sgo protein family (Figure 1A; Supplementary Figure S1A; Rabitsch et al, 2004; Kitajima et al, 2006). An antibody raised against this protein recognizes a main band of the expected size in the egg extracts, 130 kDa, as well as an unspecific band of 200 kDa (Supplementary Figure S1B). Only the former is immunodepleted by the antibody to <5% of the endogenous levels and addition of the mRNA encoding full-length XSgo2 to the depleted extracts restores the presence of the 130-kDa protein (Supplementary Figure S1C). By immunofluorescence, we observed that XSgo2 distributes all over the chromatin in interphase nuclei assembled in the egg extracts, same as XSgo1, although there is no colocalization between the two proteins (Figure 1B, left). In mitosis, XSgo2 accumulates at centromeres, labelled by XSgo1 (Figure 1B, right) and this accumulation depends on Bub1 and Aurora B mitotic kinases, but not on XSgo1 (Figure 1C and D). Thus, the regulation of Sgo2 targeting is conserved between human and Xenopus (Huang et al, 2007).
Figure 1.
Characterization of Xenopus Sgo2. (A) Schematic drawing of XSgo2 (top) and sequence alignment of the conserved coiled-coil and basic regions of Sgo proteins from the indicated species, including XSgo2 (bottom). Identical and similar amino acids are shown in red and blue, respectively. (B) Localization of XSgo1 (red) and XSgo2 (green) in interphase nuclei and mitotic chromosomes assembled from sperm chromatin in Xenopus egg extracts. (C) Replicated mitotic chromosomes assembled in mock-depleted extracts and extracts depleted of XSgo1 (Δ XSgo1), XSgo2 (Δ XSgo2), Bub1 (Δ Bub1) or Aurora B (Δ Aurora B) were fixed and stained with antibodies against XSgo1 (red) and XSgo2 (green). DNA was counterstained with DAPI. Scale bars, 1 μm (insets) and 10 μm. (D) To estimate the efficiency of depletion in the extracts used in (C), 1 μl aliquots were analysed by immunoblotting alongside different amounts of the mock-depleted extract (expressed as percentage of 1 μl). Tubulin was used as a loading control.
Proper sister chromatid cohesion in the absence of XSgo2
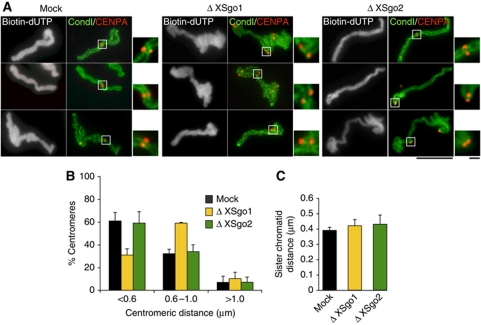
The contribution of Sgo2 to sister chromatid cohesion in mitosis is unclear (Kitajima et al, 2006; Huang et al, 2007; Llano et al, 2008). We found that, unlike XSgo1, depletion of XSgo2 has no impact on centromeric cohesion, assayed by measuring the distances between sister centromeres in replicated chromosomes assembled in the egg extracts (Figure 2A and B). Similarly, the accumulation of cohesin at centromeres observed in metaphase chromosomes is not perturbed in the absence of XSgo2 (data not shown). Thus, XSgo1 appears to be solely responsible for protecting centromere cohesion. This is consistent with our previous results showing that depletion of Bub1, which targets both XSgo1 and XSgo2 to centromeres, does not increase the extent of the centromeric cohesion defects observed when only XSgo1 is absent (Rivera and Losada, 2009). Analysis of the distances between sister chromatids along individual chromosomes revealed that arm cohesion requires neither XSgo1 nor XSgo2 (Figure 2C). Hence, the role of XSgo2 must be other than regulating sister chromatid cohesion in mitosis.
Figure 2.
XSgo2 does not regulate sister chromatid cohesion. (A) Representative images of replicated mitotic chromosomes assembled in mock-depleted, Δ XSgo1 or Δ XSgo2 extracts that were stained with antibodies against the condensin I subunit XCAP-G (green) and the centromeric histone CENP-A (red). Biotin-dUTP (grayscale images) was added to the extract to monitor DNA replication. Bars, 10 and 1 μm (insets). (B) The distance between sister centromeres was measured for >100 pairs for each condition and shown as the average from three independent experiments. Error bars, s.e.m. (C) Quantitation of the distance between sister chromatids along the length of several chromosomes (n=10 for each condition). Error bars, s.e.m.
XSgo2 promotes spindle assembly
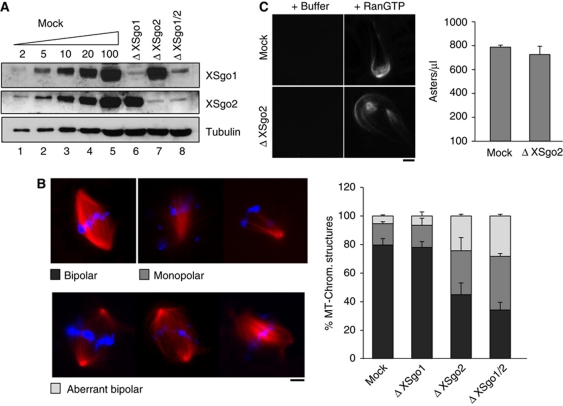
XSgo1 was originally cloned for its ability to induce microtubule polymerization (Salic et al, 2004). A microtubule pelleting assay shows that both XSgo1 and XSgo2 can associate with microtubules (Supplementary Figure S2). Thus, we decided to test whether depletion of Sgo proteins affects spindle assembly around sperm chromatin (Figure 3A). In mock-depleted or XSgo1-depleted extracts, most sperm nuclei directed the formation of robust bipolar spindles (79 and 78%, respectively; Figure 3B). In extracts lacking XSgo2, this number was reduced to 45% and instead monopolar spindles and aberrant bipolar spindles were observed (30.5 and 24.5%, respectively). Double depletion of both Sgo proteins did not lead to a significant increase of the defects compared with the single depletion of XSgo2 (Figure 3B, compare the third and fourth bars from left). Because perturbations on spindle assembly can lead to fatal chromosome segregation errors several parameters must be controlled to ensure this process, including microtubule length and density (Walczak et al, 2010). We did not observe changes in spindle length in the absence of XSgo2 (Supplementary Figure S3A) and, consistent with this result, localization of Nuclear Mitotic Apparatus (NuMA) protein, a major regulator of spindle length (Gaetz and Kapoor, 2004), was not perturbed (Supplementary Figure S3B). These data suggest that XSgo2 regulates microtubule stabilization and/or nucleation to promote the formation of proper bipolar spindles and show for the first time a role for an Sgo protein in spindle assembly.
Figure 3.
Defective spindle assembly in the absence of XSgo2. (A) Immunoblot analysis of extract depletion, as in Figure 1D. (B) Depleted extracts from (A) were cycled into interphase and back into mitosis. Metaphase spindles were assembled for 90 min after CSF addition in the presence of rhodamine-tubulin (red) and DNA was stained with DAPI (blue). The spindle structures found were classified in the categories indicated and quantified for each condition. Data represent mean values (expressed as percentage) obtained from >200 structures in each of three independent experiments. Error bars, s.e.m. (C) Microtubule asters were assembled in mock and Δ XSgo2 mitotic extracts containing rhodamine-labelled tubulin by addition of 10 μM RanQ69L (or buffer as control). Several aliquots of 1 μl were taken, mounted on a slide with fixative solution, and the number of asters in each slide counted and plotted in the bar graph on the right (bars represent mean values, and error bars s.e.m.). Scale bar on images, 10 μm.
XSgo2 does not contribute to RanGTP-dependent microtubule nucleation
The two major signalling cascades that promote microtubule polymerization around chromatin in Xenopus are the RanGTP pathway (Harel and Forbes, 2004) and the CPC pathway (Sampath et al, 2004). To address the contribution of XSgo2 to Ran-induced microtubule formation, we added a hydrolysis-deficient mutant Ran protein, RanQ69L, to mitotic extracts (Carazo-Salas et al, 1999). This mutant form of Ran induces aster formation by releasing microtubule binding proteins such as TPX2, NuMa, XMAP215, γTuRC and Rae from sequestration by Importins (Wilde and Zheng, 1999; Gruss et al, 2001; Nachury et al, 2001; Blower et al, 2005). RanGTP-dependent asters were formed with similar efficiency in mock-depleted and XSgo2-depleted extracts (Figure 3C). Thus, the requirement of XSgo2 in microtubule polymerization or/and stabilization is independent of the RanGTP-driven spindle assembly pathway.
XSgo2 depletion abolishes centromeric targeting of MCAK and alters the balance between active/inactive MCAK along chromosome arms
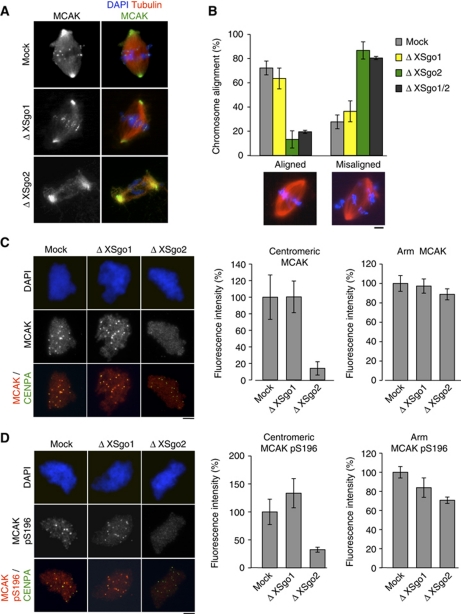
If the role of XSgo2 in spindle assembly is independent of the RanGTP pathway, it is likely to be part of the CPC pathway instead. Since the microtubule depolymerase MCAK is an important target of the CPC, we decided to examine its localization. In spindles assembled around sperm chromatin in control extracts, MCAK is present at centromeres, centrosomes and along the spindle microtubules, as previously reported (Figure 4A; Walczak et al, 1996). The same is true for spindles assembled in XSgo1-depleted extracts. In contrast, centromeric localization of MCAK was specifically abolished in the absence of XSgo2 (see also Supplementary Figure S4A). Total levels of soluble MCAK were not affected in any condition (data not shown). Given the critical role of MCAK in the correction of erroneous kinetochore–microtubule attachments, its absence from centromeres should affect chromosome alignment (Kline-Smith et al, 2005; Zhang et al, 2007) and this is indeed the case (Figure 4B). Unlike results in human cells (Salic et al, 2004; McGuinness et al, 2005), depletion of XSgo1 does not lead to chromosome misalignment, probably due to the milder effects on cohesion observed under this condition in the egg extracts.
Figure 4.
XSgo2 is required for MCAK targeting to centromeres and chromosome alignment. (A) Spindles assembled in mock, Δ XSgo1 or Δ XSgo2 cycled extracts containing rhodamine-labelled tubulin were fixed and analysed for immunofluorescence with antibodies against MCAK. DNA was stained with DAPI (blue). (B) Quantification of bipolar spindles with aligned or misaligned chromosomes assembled in the indicated extracts. The data represent the mean from three independent experiments. Error bars, s.e.m. Representative images of each condition are shown (bottom). (C, D) Chromosomes assembled in the indicated mitotic extracts in the presence of nocodazole were immunostained with antibodies against CENP-A and either anti-MCAK (C) or anti-MCAKpS196 (D). DNA was counterstained with DAPI (blue). Quantitation of the fluorescence intensity of anti-MCAK (C) or anti-MCAKpS196 (D) at centromeres and arm regions from at least 10 nuclei per condition in each of three independent experiments is shown on the right. Error bars, s.e.m. Scale bars, 10 μm.
Previous studies have shown that increased presence of active MCAK along chromosomes (e.g., in the absence of Aurora B regulation) leads to reduced microtubule polymerization around chromatin (Sampath et al, 2004; Zhang et al, 2007). We measured the fraction of chromatin-bound MCAK by quantitative immunofluorescence and found that depletion of XSgo2 removed most centromeric MCAK, as in Figure 4A, but had little impact on the amount of MCAK bound to chromosome arms (Figure 4C). However, we observed that the levels of MCAK phosphorylated by Aurora B at Serine 196, a modification that inhibits its microtubule depolymerase activity (Lan et al, 2004), were significantly reduced in the absence of XSgo2 not only at centromeres, but also on chromosome arms (Figure 4D; Supplementary Figure S4B). Thus, the balance between active and inactive MCAK along chromosome arms is changed in the absence of XSgo2 and this likely contributes to the observed defects in spindle assembly.
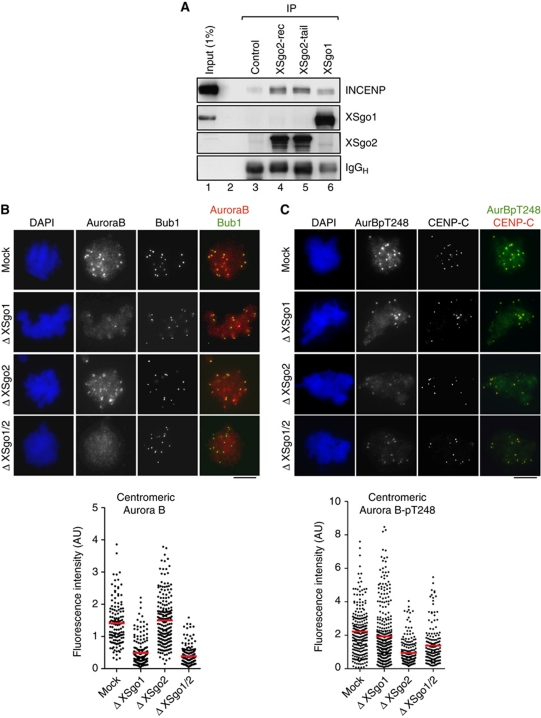
XSgo2 interacts with the CPC and modulates its activity but not its localization
How does XSgo2 modulate Aurora B-dependent phosphorylation of targets such as MCAK? Both XSgo1 and XSgo2 can interact physically with the CPC although the fraction of the CPC that coimmunoprecipitates with either Sgo protein from the soluble egg extracts is very small (Figure 5A; Supplementary Figure S5A). We previously reported that localization of the CPC is impaired in extracts lacking XSgo1 (Rivera and Losada, 2009). However, the same is not true for extracts with no XSgo2, in which Aurora B and INCENP localization is normal (Figure 5B; Supplementary Figure 5B). Formation of the CPC is not affected by depletion of XSgo1 or XSgo2 (Supplementary Figure S5C). An alternative possibility is that XSgo2 modulates Aurora B kinase activity. The binding of INCENP to Aurora B followed by phosphorylation of the TSS motif of INCENP and autophosphorylation of Aurora B on Threonine 248, a residue present in the activation loop of the kinase, are all required events for full activation of the kinase in mitosis (Kaitna et al, 2000; Adams et al, 2001; Bishop and Schumacher, 2002; Honda et al, 2003; Sessa et al, 2005). We failed to observe changes in the activity of the soluble CPC upon depletion of Sgo proteins (Supplementary Figure S5D). However, when we monitored Aurora B activation at centromeres with a phospho-specific antibody against phospho-Threonine 248 (Aurora B-pT248), we observed a significant reduction in active Aurora B in the absence of XSgo2 while total Aurora B levels remain unchanged (Figure 5B and C). This result suggests that Aurora B activation depends, at least in part, on XSgo2. In contrast, depletion of XSgo1 has little impact on the fraction of active Aurora B present at centromeres whereas it reduces the total amount of Aurora B at this location, as we showed previously (Rivera and Losada, 2009). One explanation for this striking result is that XSgo1 not only promotes the centromeric recruitment of Aurora B but also inhibits its kinase activity, an effect that would be counteracted by XSgo2. However, double depletion of both Sgo proteins does not rescue the activation of Aurora B, arguing against this possibility (Figure 5C; Supplementary Figure 4B).
Figure 5.
XSgo2 interacts with the CPC and modulates its activity but not its localization. (A) Immunoprecipitation reactions from mitotic extracts using IgG as a control, anti-XSgo1 and two different antibodies against XSgo2, one raised against recombinant protein (XSgo2-rec) and another against a synthetic peptide (XSgo2-tail). (B, C) Mitotic chromosomes were assembled in depleted extracts (as indicated) and immunostained with antibodies against Aurora B and Bub1 (B) or Aurora B-pT248 and the centromeric protein CENP-C (C). DNA was counterstained with DAPI. The fluorescence intensity of Aurora B and Aurora B-pT248 stainings at individual centromeres is plotted. Data come from n>10 nuclei per condition from two experiments.
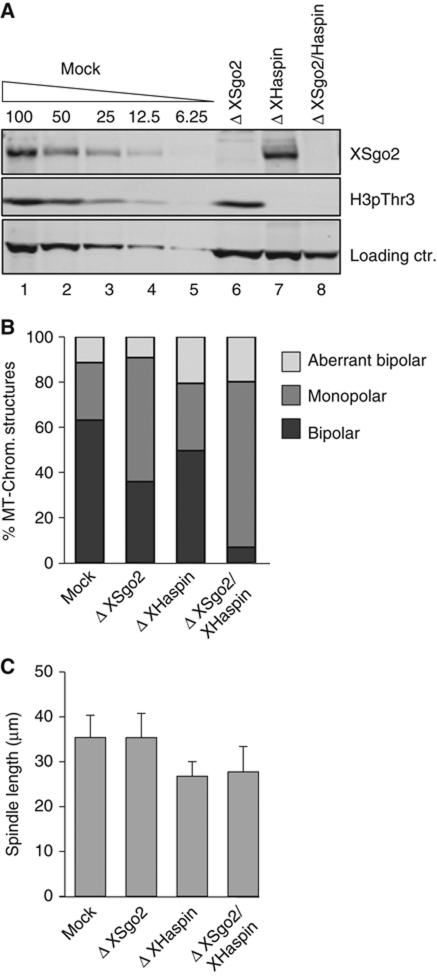
XSgo2 and Haspin function in distinct pathways to activate Aurora B
The consequences of XSgo2 depletion for spindle assembly are not as dramatic as the consequences of depleting the CPC, which completely abolishes microtubule assembly around chromatin (Kelly et al, 2007). This result suggests that additional pathways regulate the activation of the CPC to promote spindle formation. One such pathway is mediated by Haspin, whose phosphorylation of H3 at Threonine 3 (H3pThr3) promotes recruitment of the CPC to chromatin and its subsequent activation. As previously shown, the impact of Haspin depletion is limited to a modest shortening of the spindle (Kelly et al, 2010). However, codepletion of Haspin and XSgo2 caused more severe spindle defects than single depletions, resulting in formation of monoasters (Figure 6). This contrasts to the spindle phenotype of XSgo1–XSgo2 double depletion (Figure 3B), and illustrates the distinct roles of XSgo2 and Haspin in Aurora B-mediated spindle assembly.
Figure 6.
XSgo2 and Haspin contribute to proper spindle assembly through distinct pathways involving the CPC. (A) Immunoblot analysis of the XSgo2- and Haspin-depleted extracts used for spindle assembly. H3pThr3 is used to measure the efficiency of Haspin depletion whereas a robust non-specific band served as loading control. (B) Spindles assembled in the indicated extracts were classified as in Figure 3B. At least 100 spindle structures were quantified for each condition. A single, representative experiment is shown. (C) Spindle length was measured in at least 25 bipolar spindles assembled in (B). Error bars, s.d.
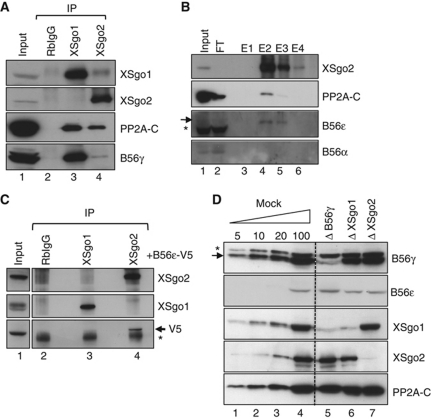
XSgo1 and XSgo2 interact with different PP2A complexes
At least some of the functions attributed to Sgo proteins, like protection of centromeric cohesion, appear to be mediated by their association with the protein phophatase PP2A. XSgo1 associates with PP2A-C in the egg extract (Rivera and Losada, 2009; Shintomi and Hirano, 2009), and the same is true for XSgo2 (Figure 7A). Analysis of an affinity-purified fraction of XSgo2 by mass spectrometry identified the catalytic subunit beta isoform (PP2A-Cβ) and the scaffolding subunit beta isoform (PP2A-Aβ; Supplementary Table 1), but none of the B56 regulatory subunits. However, immunoblot analyses of the eluted fractions allowed us to detect specifically the regulatory subunit B56ε but not B56α, a subunit previously found associated with hSgo2 (Figure 7B, lanes 4 and 5; Tanno et al, 2010). The low abundance of PP2A-B56ε in the egg extracts and the fact that its size coincides with that of the heavy chain of immunoglobulins hinders its detection in the immunoprecipitates of XSgo2. To overcome these problems, we performed the immunoprecipitation reactions in extracts that contained a tagged version of PP2A-B56ε and found that the exogenous protein coimmunoprecipitates specifically with XSgo2 and not with XSgo1 (Figure 7C). Conversely, a small amount of XSgo2 could be detected in the immunoprecipitates obtained with an antibody against the tag (data not shown). Another isoform of PP2A-B56, B56γ, interacts preferentially with XSgo1 in the egg extracts (Figure 7A, lower panel). Moreover, depletion of PP2A-B56γ codepletes specifically XSgo1 but does not affect XSgo2 levels in the extracts (Figure 7D). Thus, most if not all XSgo1 molecules stored in the oocyte are associated with a PP2A complex containing the B56γ subunit. Consistently, little XSgo1 can be found at centromeres of chromosomes assembled in these B56γ-depleted extracts whereas XSgo2 and MCAK targeting are unaffected (Supplementary Figure S6). Depletion of XSgo1 or XSgo2, in turn, do not have a major effect on the levels of PP2A-B56γ or PP2A-B56ε (Figure 7D, lanes 6 and 7). Unfortunately, efficient depletion of PP2A-B56ε could not be performed. In any case, our results indicate that XSgo1 and XSgo2 associate with distinct isoforms of the PP2A-B56 subunit. Since the PP2A-B subunits are supposed to determine the substrate specificity of the phosphatase, we speculate that these specific associations contribute to the non-overlapping functions of XSgo1 and XSgo2.
Figure 7.
XSgo1 and XSgo2 interact with different PP2A complexes. (A) Immunoprecipitates obtained from mitotic extracts with control rabbit IgG, anti-XSgo1 and anti-XSgo2 were analysed by immunoblotting. (B) Affinity purification of XSgo2 from mitotic extracts. Input, flow-through (FT) and eluted fractions (E1–E4) were analysed by immunoblotting. In the blot with anti-PP2A-B56ε, the arrow indicates the position of the phosphatase subunit and the asterisk a cross-reacting band. Fraction E2 was subjected to mass spectrometry analysis (Supplementary Table S1). (C) In-vitro translated PP2A-B56ε tagged with V5 (PP2A-B56ε-V5) was added to a mitotic extract and immunoprecipitation reactions were carried out with control IgG, XSgo1 and XSgo2 antibodies. The presence of PP2A-B56ε-V5 in the immunoprecipitates was revealed by immunoblot with anti-V5 antibodies (arrow). The asterisk marks the position of IgG heavy chains. Input, 1% of the reaction. (D) Immunoblot analyses of mitotic extracts depleted of PP2A-B56γ, XSgo1 or XSgo2. The asterisk marks a cross-reacting band.
Discussion
Shugoshin proteins have been assigned two major functions: protection of centromeric cohesin and sensing tension across sister centromeres. We have previously described the function and regulation of XSgo1 in this system and concluded that it has a role in preserving cohesin at centromeres until anaphase (Rivera and Losada, 2009). We now show that this function is not shared by XSgo2 since its depletion does not affect either cohesion or the distribution of cohesin on chromosomes assembled in the egg extracts, consistent with studies in mammalian mitotic cells (Huang et al, 2007; Llano et al, 2008; Tanno et al, 2010). Here, we revealed that XSgo2 contributes to spindle assembly. The participation of XSgo2 in microtubule dynamics seems to be particularly important in Xenopus egg extracts, probably because Aurora B plays an essential role in spindle assembly in this experimental system, while the functional interaction between Sgo2 and Aurora B is mostly required for the error correction machinery in human somatic cells (Huang et al, 2007; Tanno et al, 2010).
Our results suggest that XSgo2 participates in spindle assembly by promoting Aurora B activation and the subsequent phosphorylation of key substrates such as MCAK. Aurora B phosphorylates MCAK at multiple sites and thereby regulates its association with chromatin, its accumulation at centromeres and its microtubule depolymerizing activity (Andrews et al, 2004; Lan et al, 2004; Ohi et al, 2004; Huang et al, 2007). Recent results in human cells suggest that phosphorylation of Sgo2 by Aurora B recruits MCAK to centromeres (Tanno et al, 2010). A similar pathway could operate in Xenopus, although the residues implicated are not conserved. In fact, there are 22 consensus sites for Aurora B phosphorylation in XSgo2 and 29 in hSgo2 and only one of them coincides (Supplementary Figure S1A). We have also been unable to detect a direct interaction between XSgo2 and MCAK in the soluble egg extracts (data not shown) although this result does not rule out their possible interaction on chromatin. In any case, recruitment of MCAK to centromeres cannot be the sole function of XSgo2 since loss of MCAK from this location does not phenocopy the consequences of depleting XSgo2. The absence of centromeric MCAK causes chromosome misalignment as in the absence of XSgo2, but only in the latter case spindle assembly defects are also observed (Walczak et al, 2002). Our results suggest that impaired activation of Aurora B in extracts lacking XSgo2 alters the balance between active and inactive MCAK also along chromosome arms and this has a negative effect on the formation of proper bipolar spindles. Consistent with previously reported relevance of MCAK phosphorylation by Aurora B in the conversion of monopolar to bipolar spindles, increased number of monopolar spindles are found in XSgo2-depleted extracts (Figure 3B; Ohi et al, 2004).
The effect of XSgo2 depletion on spindle assembly is not as strong as the effect of depleting the CPC. XSgo2 collaborates with Haspin, which phosphorylates H3 on Thr3 to recruit the CPC and activates Aurora B (Kelly et al, 2010; Tanno et al, 2010; Wang et al, 2010; Yamagishi et al, 2010), to support spindle assembly (Figure 6). Double depletion of XSgo2 and H3pThr3 severely inhibited bipolar spindle formation and generated asters. This mimics the phenotype caused by Dasra A depletion, which inhibits chromatin recruitment of the CPC (Sampath et al, 2004; Kelly et al, 2007). Generation of residual microtubules in these conditions is most likely due to the presence of CPC-microtubule interaction, which also supports spindle microtubule assembly (Tseng et al, 2010).
As previously shown for human Sgo2, we found that targeting of XSgo2 to centromeres depends both on Bub1 and on Aurora B (Huang et al, 2007). In Xenopus, depletion of Aurora B completely abolishes centromeric accumulation of Bub1 (Vigneron et al, 2004) and thus directing Bub1 to centromeres could be the main function of Aurora B in the targeting of XSgo1 and XSgo2. Sgo proteins have been recently proposed to act as CPC adaptors so that their interaction is important for co-targeting to centromeres, a role apparently shared by Sgo1 and Sgo2 in human cells (Tsukahara et al, 2010). In contrast, XSgo1 and XSgo2 affect distinct aspects of CPC regulation and function. Depletion of XSgo1 alters CPC distribution, whereas depletion of XSgo2 impairs CPC activation. Because the spatial regulation of the CPC is thought to be essential for the coordination of many mitotic events (Honda et al, 2003; Vader et al, 2006; Ruchaud et al, 2007; Carmena et al, 2009), one would expect that its anomalous distribution in the absence of XSgo1 would also affect CPC function. However, we have found that depletion of XSgo1 diminishes significantly the amount of total Aurora B present at centromeres, but it barely changes the amount of active Aurora B (Figure 5). Consistently, the lack of XSgo1 does not affect MCAK distribution or spindle assembly. Downregulation of Sgo1 in human cells causes delocalization of Aurora B from centromeres but also in this case centromeric MCAK is unaffected (Wang et al, 2010). Thus, an excess of the CPC apparently accumulates at the centromeric region. It is likely that different sub-populations of the complex exist that can be modulated by proteins other than Sgo2 such as Sds22/PP1 (Posch et al, 2010) or TD60 (Rosasco-Nitcher et al, 2008).
How do XSgo1 and XSgo2 carry out their specific functions in chromosome segregation? Our results showing preferential interaction of the two proteins with distinct PP2A-B56 subunits—which could dictate the substrate specificity of the enzyme—illuminate one possible answer to this question. This is consistent with the proposal that the association of Sgo proteins with PP2A serves to specify the substrate of the phosphatase by the recruitment of different PP2A complexes to centromeres (Xu et al, 2009). We have shown that depletion of B56 gamma removes XSgo1 from the extract and, consequently, from centromeres, but does not affect XSgo2 levels, localization or function. The role of the phosphatase associated with Sgo1 in protecting cohesin from the prophase dissociation pathway is clear, that is, to counteract cohesin SA phosphorylation by Polo (McGuinness et al, 2005; Kitajima et al, 2006; Rivera and Losada, 2009). It also contributes to reverse phosphorylation of Sgo1 itself by Polo until anaphase (Tang et al, 2006; TR and AL, unpublished results). By keeping cohesin at centromeres, Sgo1 could indirectly promote the accumulation of Haspin, H3pThr3 and the CPC (Kelly et al, 2010; Tanno et al, 2010; Wang et al, 2010; Yamagishi et al, 2010). What could be the target of Sgo2-PP2A that affects activation of Aurora B? The phosphatase could stimulate the activator of Aurora B (e.g., TD-60) or suppress the inhibitor (e.g., PP1). In either case, this step is essential for the subsequent regulation of the localization and activity of its substrate MCAK, which controls microtubule dynamics. Whether the role of XSgo2 in the spindle assembly relies on a direct modulation of MCAK remains to be addressed. The clear division of labour between XSgo1 and XSgo2 renders Xenopus egg extracts a suitable system to look for the target(s) of the PP2A fraction associated specifically with XSgo2.
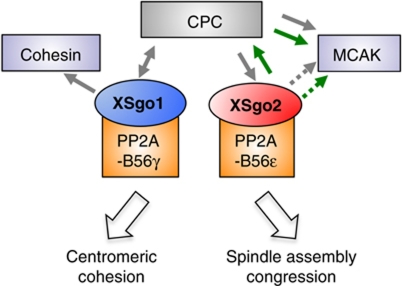
In conclusion, the identification of Xenopus Sgo2 leads us to propose a model in which Sgo proteins are essential factors that ensure proper chromosome segregation by complementary, non-overlapping functions (Figure 8). XSgo1 regulates centromeric sister chromatid cohesion while XSgo2 mediates spindle assembly and chromosome congression. All these events must be tightly regulated to prevent the generation of chromosomal instability associated with malignant cell transformation.
Figure 8.
Sgo proteins have non-overlapping functions in chromosome segregation. XSgo1 associates with PP2A-B56γ complex to counteract phosphorylation of cohesin and thereby prevent its dissociation from centromeres until anaphase. XSgo2, in association with PP2A-B56ε complex, ensures proper spindle assembly and chromosome congression by promoting proper localization and activity of MCAK. This effect appears to be mediated by the CPC, at least in part. While XSgo1 is required for centromeric accumulation of the CPC, XSgo2 contributes to its activation. In turn, the CPC is required for proper localization of both XSgo1 and XSgo2, and so is Bub1 (not depicted). Green arrows indicate activation; grey arrows indicate regulation of localization. Sgo2 could regulate MCAK not only through the CPC but also in a more direct way (dotted lines).
Materials and methods
Cloning and protein expression
For XSgo2 cloning, total RNAs were extracted from cytostatic factor (CSF)-arrested extracts using RNeasy Midi kit (Qiagen). Full cDNA sequence was obtained using the SMATer RACE cDNA Amplification kit (Clontech # 634923) according to manufacturer's instructions. The primer used for 5′ cDNA amplification was designed from the sequence of a partial X. laevis cDNA (EST name BJ623343, Clone Id: XL204e21): 5′-CACACTGTAGCTCACTATGTTGTTCT-3′. The amplified cDNA was cloned into pGEM vector (Promega) and sequenced. Complete XSgo2 cDNA was amplified using the primers: 5′-ATGGCTTTACAAACAAGTGC-3′ and 5′-TCATTTCTCCGACTTC-3′. To produce GFP-XSgo2, this cDNA was inserted between ClaI and XhoI sites of the pAFS210 vector (Sampath et al, 2004). A cDNA encoding X. laevis PP2A-B56ε was amplified from IMAGE clone 6318521 and cloned in pcDNA3.2-V5-DEST using the Gateway Cloning System (Invitrogen). PP2A-B56ε was in-vitro translated using the TNT Quick coupled transcription/translation system (Promega) according to manufacturer's instructions and diluted five-fold in the egg extracts (Figure 7C).
Antibodies
Rabbit polyclonal sera against XSgo2 were obtained by using a synthetic peptide as immunogen (CKEKKRPRKIKVKSEK) and affinity purified. A second antibody raised against a C-terminal fragment of Sgo2 was also obtained and used in some experiments with indistinguishable results. Other antibodies used in this study were Haspin (Kelly et al, 2010), INCENP and Aurora B (MacCallum et al, 2002); Dasra A (Sampath et al, 2004); Survivin (Losada et al, 2002); CENP-A, Bub1 and XSgo1 (Rivera and Losada, 2009); MCAK (Walczak et al, 1996) and MCAK pS196 (Lan et al, 2004) (both obtained from T Stukenberg); PP2A-B56γ and PP2A-B56ε (Mochida et al, 2009); PP2A-B56α (07-334; Millipore); PP2A-C (05-421; Millipore); α-tubulin (DM1A; Sigma); V5 (R960-25; Invitrogen); Aurora B-pT248 (600-401-677; Rockland); XKid (Funabiki and Murray, 2000); Asf1 (Bernad et al, 2011); XCAP-G (Hirano et al, 1997); NuMA (Merdes et al, 1996) (a gift from A Merdes). An antibody recognizing an N-terminal fragment of Xenopus CENP-C was labelled with Dylight 549 Antibody Labeling kit (Thermo Scientific) and used as centromere marker in some experiments.
Preparation of Xenopus egg extracts, immunodepletion, reconstitution and immunoprecipitation experiments
CSF-arrested egg extracts were prepared in XBE2 buffer (10 mM K-Hepes (pH 7.7), 0.1 M KCl, 2 mM MgCl2, 0.1 mM CaCl2, 5 mM EGTA and 50 mM sucrose) as described (Losada et al, 1998). Interphase extracts were generated by addition of 100 mg/ml cycloheximide and 0.4 mM CaCl2 to CSF-arrested extracts. For depletions, antibodies described above were bound to 25 μl of Dynabeads protein A (Dynal) to deplete 50 μl of extract as follows: for XSgo1 depletion, 8 μg of rabbit polyclonal anti-XSgo1 (two rounds of 40 min), for XSgo2 depletion, 8 μg of rabbit polyclonal anti-XSgo2 (50 min); for double depletion of XSgo1 and XSgo2, two rounds of 40 min with XSgo1-coated beads were followed by one round of 40 min of XSgo2-coated beads; for Aurora B depletion, 3.5 μg of rabbit polyclonal anti-INCENP plus 3.5 μg of rabbit polyclonal anti-Aurora B (two rounds of 40 min); for Bub1 depletion, 8 μg of rabbit polyclonal anti-Bub1 (50 min); for PP2a-B56γ depletion, 50 μl of rabbit polyclonal serum (three rounds of 40 min). In all cases, mock depletions were performed in parallel using beads coated with non-immune rabbit IgG.
For XSgo2 reconstitution, mRNA encoding XSgo2 was made using the T7 mMessage Machine RNA transcription kit (Ambion) and added to 0.2 mg/ml extract.
For immunoprecipitation, 4 μg of affinity-purified rabbit anti-XSgo1, anti-XSgo2 or anti-Aurora B was bound to 15 μl of protein A agarose beads (Invitrogen); 4 μg of anti-V5 antibody was bound to 15 μl of protein G sepharose beads (Invitrogen). The antibody beads were incubated with 100 μl of CSF extract for 2 h at 4°C. The beads were washed with XBE2 buffer six times and bound proteins were analysed by immunoblotting.
For affinity purification of XSgo2, 100 μg of affinity-purified anti-XSgo2 antibody crosslinked to 100 μl of protein A agarose beads was incubated with 1 ml of egg extract for 1 h. After extensive washing, bound proteins were eluted with 100 μl of 0.5 mg/ml XSgo2 peptide in XBE2 buffer for 1 h at 4°C.
Morphological analysis of chromosomes assembled in vitro
Sperm nuclei (500–1000 nuclei/μl) were incubated with freshly depleted interphase extract at 22°C for 90 min. When required, 4 μM biotin-16-dUTP was added to the extract (e.g., Figure 2). The extracts were driven into mitosis by addition of an equal volume of CSF-arrested extract and incubated for another 90 min (Figures 1, 2 and 3, 4A, B and 5). For Figure 4C and D, sperm nuclei (500 nuclei/μl) were incubated in CSF-arrested extract plus 10 μg/ml nocodazole for 60 min. The assembly mixtures were fixed with 10 volumes of 2% paraformaldehyde in XBE2 containing 0.5% Triton X-100 for 10 min and centrifuged onto coverslips. Immunofluorescence was carried out as described previously (Losada et al, 1998). Primary antibodies were used at 1–2 μg/ml whereas Cy3 or FITC-conjugated donkey anti-rabbit or anti-mouse secondary antibodies (Jackson ImmunoResearch) were used at 1:200. To assess replication, incorporation of biotin-16-dUTP into sperm DNA was detected with Cy5-conjugated avidin (Jackson ImmunoResearch). DNA was counterstained with 1 μg/ml DAPI. A Leica DM6000 microscope was used to obtain grayscale images, which were later pseudo-coloured and merged using Adobe Photoshop, and analysed in Image J (http://rsb.info.nih.gov/ij). Immunostaining signals at centromeres were calculated as the integrated pixel density measured within a region defined by the diameter circle of the centromeric protein staining (CENP-A, CENP-C or Bub1). A minimum number of eight centromeres per nuclei were quantified in each condition. Quantification of immunostaining signals along chromosome arms was done by calculating the mean pixel intensity within several areas out of centromeric regions per nuclei.
Spindle assembly and Ran aster assembly in egg extracts
Extracts containing 500 nuclei/μl and 50 μg/ml rhodamine-tubulin (Cytoskeleton) were cycled into interphase at 20°C for 90 min by addition of 0.4 mM CaCl2. Metaphase spindles were formed by adding two volumes of CSF-arrested extracts and incubating for 60 min at 20°C. Spindles were processed for immunofluorescence as described (Desai et al, 1999). To assemble Ran asters, 10 μM GTPase-defective RanQ69L (a gift of I Vernos) was added to CSF-depleted extracts containing rhodamine-tubulin for 20 min at 20°C. Reactions were fixed and analysed.
In-vitro kinase assays
CSF-depleted extract (100 μl diluted 1:2 in XBE2 buffer) was incubated with 2 μl of [γ-32P] ATP (6000 Ci/mmol) and 80 mM β-glycerophosphate at 22°C for 1 h. The CPC was isolated from the extract on 10 μl protein A-Dynabeads coated with 3 μg anti-Aurora B after incubating on ice for 2 h. The soluble fraction was removed, beads were washed and bound proteins were analysed by SDS–PAGE followed by Coomassie Blue staining and autoradiography. Non-immune rabbit IgG was used in mock immunoprecipitation reactions. Phosphorylated INCENP was quantified using a Phosphorimager and total INCEP was measured from the CBB-stained gel image with Image J.
Accession codes
The GeneBank Accession number for Xenopus Shugoshin2 is JQ412129.
Supplementary Material
Acknowledgments
We are grateful to T Stukenberg (University of Virginia, Charlottesville, USA), I Vernos (Centre for Genomic Regulation, Barcelona, Spain) and A Merdes (CNRS_Pierre Fabres, Toulouse, France) for contributing reagents. We thank O Domínguez (Genomics Unit, CNIO) for advice on the RACE technique and members of our laboratories for advice and helpful discussions. In addition, we thank Dave Wynne (Funabiki laboratory) for some experimental work. This research has been supported by the Spanish Ministry of Science and Innovation (grants BFU2007-66627 and CSD-Inesgen to AL, FPI to TR), NIH (GM075249 to HF), Japanese MEXT (Grant-in-Aid for challenging Exploratory Research and Global COE Program (Cell Fate Regulation Research and Education Unit) to SM), and EMBO-short term fellowship for TR to visit the laboratory of HF.
Author contributions: TR and AL conceived and designed the experiments, which were carried out by TR with help from MR-C, except data shown in Figure 6 (CG). HF identified cDNA sequences encoding XSgo2. SM provided B56 antibodies and conditions for depletion. TR and AL wrote the paper, which was edited by CG and HF.
The authors declare that they have no conflict of interest.
03/21/2012
Since Advance Online Publication, the second author name has been corrected.
References
- Adams RR, Eckley DM, Vagnarelli P, Wheatley SP, Gerloff DL, Mackay AM, Svingen PA, Kaufmann SH, Earnshaw WC (2001) Human INCENP colocalizes with the Aurora-B/AIRK2 kinase on chromosomes and is overexpressed in tumour cells. Chromosoma 110: 65–74 [DOI] [PubMed] [Google Scholar]
- Andrews PD, Ovechkina Y, Morrice N, Wagenbach M, Duncan K, Wordeman L, Swedlow JR (2004) Aurora B regulates MCAK at the mitotic centromere. Dev Cell 6: 253–268 [DOI] [PubMed] [Google Scholar]
- Bernad R, Sanchez P, Rivera T, Rodriguez-Corsino M, Boyarchuk E, Vassias I, Ray-Gallet D, Arnaoutov A, Dasso M, Almouzni G, Losada A (2011) Xenopus HJURP and condensin II are required for CENP-A assembly. J Cell Biol 192: 569–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop JD, Schumacher JM (2002) Phosphorylation of the carboxyl terminus of inner centromere protein (INCENP) by the Aurora B Kinase stimulates Aurora B kinase activity. J Biol Chem 277: 27577–27580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blower MD, Nachury M, Heald R, Weis K (2005) A Rae1-containing ribonucleoprotein complex is required for mitotic spindle assembly. Cell 121: 223–234 [DOI] [PubMed] [Google Scholar]
- Boyarchuk Y, Salic A, Dasso M, Arnaoutov A (2007) Bub1 is essential for assembly of the functional inner centromere. J Cell Biol 176: 919–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carazo-Salas RE, Guarguaglini G, Gruss OJ, Segref A, Karsenti E, Mattaj IW (1999) Generation of GTP-bound Ran by RCC1 is required for chromatin-induced mitotic spindle formation. Nature 400: 178–181 [DOI] [PubMed] [Google Scholar]
- Carmena M, Ruchaud S, Earnshaw WC (2009) Making the Auroras glow: regulation of Aurora A and B kinase function by interacting proteins. Curr Opin Cell Biol 21: 796–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai A, Murray A, Mitchison TJ, Walczak CE (1999) The use of Xenopus egg extracts to study mitotic spindle assembly and function in vitro. Methods Cell Biol 61: 385–412 [DOI] [PubMed] [Google Scholar]
- Funabiki H, Murray AW (2000) The Xenopus chromokinesin Xkid is essential for metaphase chromosome alignment and must be degraded to allow anaphase chromosome movement. Cell 102: 411–424 [DOI] [PubMed] [Google Scholar]
- Gadde S, Heald R (2004) Mechanisms and molecules of the mitotic spindle. Curr Biol 14: R797–R805 [DOI] [PubMed] [Google Scholar]
- Gadea BB, Ruderman JV (2006) Aurora B is required for mitotic chromatin-induced phosphorylation of Op18/Stathmin. Proc Natl Acad Sci USA 103: 4493–4498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz J, Kapoor TM (2004) Dynein/dynactin regulate metaphase spindle length by targeting depolymerizing activities to spindle poles. J Cell Biol 166: 465–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez R, Valdeolmillos A, Parra MT, Viera A, Carreiro C, Roncal F, Rufas JS, Barbero JL, Suja JA (2007) Mammalian SGO2 appears at the inner centromere domain and redistributes depending on tension across centromeres during meiosis II and mitosis. EMBO Rep 8: 173–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss OJ, Carazo-Salas RE, Schatz CA, Guarguaglini G, Kast J, Wilm M, Le Bot N, Vernos I, Karsenti E, Mattaj IW (2001) Ran induces spindle assembly by reversing the inhibitory effect of importin alpha on TPX2 activity. Cell 104: 83–93 [DOI] [PubMed] [Google Scholar]
- Harel A, Forbes DJ (2004) Importin beta: conducting a much larger cellular symphony. Mol Cell 16: 319–330 [DOI] [PubMed] [Google Scholar]
- Hirano T, Kobayashi R, Hirano M (1997) Condensins, chromosome condensation protein complexes containing XCAP-C, XCAP-E and a Xenopus homolog of the Drosophila Barren protein. Cell 89: 511–521 [DOI] [PubMed] [Google Scholar]
- Honda R, Korner R, Nigg EA (2003) Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis. Mol Biol Cell 14: 3325–3341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Feng J, Famulski J, Rattner JB, Liu ST, Kao GD, Muschel R, Chan GK, Yen TJ (2007) Tripin/hSgo2 recruits MCAK to the inner centromere to correct defective kinetochore attachments. J Cell Biol 177: 413–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens V, Goris J (2001) Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J 353: 417–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaitna S, Mendoza M, Jantsch-Plunger V, Glotzer M (2000) Incenp and an aurora-like kinase form a complex essential for chromosome segregation and efficient completion of cytokinesis. Curr Biol 10: 1172–1181 [DOI] [PubMed] [Google Scholar]
- Kalab P, Heald R (2008) The RanGTP gradient—a GPS for the mitotic spindle. J Cell Sci 121: 1577–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katis VL, Galova M, Rabitsch KP, Gregan J, Nasmyth K (2004) Maintenance of cohesin at centromeres after meiosis I in budding yeast requires a kinetochore-associated protein related to MEI-S332. Curr Biol 14: 560–572 [DOI] [PubMed] [Google Scholar]
- Kawashima SA, Tsukahara T, Langegger M, Hauf S, Kitajima TS, Watanabe Y (2007) Shugoshin enables tension-generating attachment of kinetochores by loading Aurora to centromeres. Genes Dev 21: 420–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima SA, Yamagishi Y, Honda T, Ishiguro K, Watanabe Y (2010) Phosphorylation of H2A by Bub1 prevents chromosomal instability through localizing shugoshin. Science 327: 172–177 [DOI] [PubMed] [Google Scholar]
- Kelly AE, Ghenoiu C, Xue JZ, Zierhut C, Kimura H, Funabiki H (2010) Survivin reads phosphorylated histone H3 threonine 3 to activate the mitotic kinase Aurora B. Science 330: 235–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AE, Sampath SC, Maniar TA, Woo EM, Chait BT, Funabiki H (2007) Chromosomal enrichment and activation of the aurora B pathway are coupled to spatially regulate spindle assembly. Dev Cell 12: 31–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrebrock AW, Moore DP, Wu JS, Orr-Weaver TL (1995) Mei-S332, a Drosophila protein required for sister-chromatid cohesion, can localize to meiotic centromere regions. Cell 83: 247–256 [DOI] [PubMed] [Google Scholar]
- Kitajima TS, Kawashima SA, Watanabe Y (2004) The conserved kinetochore protein shugoshin protects centromeric cohesion during meiosis. Nature 427: 510–517 [DOI] [PubMed] [Google Scholar]
- Kitajima TS, Sakuno T, Ishiguro K, Iemura S, Natsume T, Kawashima SA, Watanabe Y (2006) Shugoshin collaborates with protein phosphatase 2A to protect cohesin. Nature 441: 46–52 [DOI] [PubMed] [Google Scholar]
- Kline-Smith SL, Sandall S, Desai A (2005) Kinetochore-spindle microtubule interactions during mitosis. Curr Opin Cell Biol 17: 35–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan W, Zhang X, Kline-Smith SL, Rosasco SE, Barrett-Wilt GA, Shabanowitz J, Hunt DF, Walczak CE, Stukenberg PT (2004) Aurora B phosphorylates centromeric MCAK and regulates its localization and microtubule depolymerization activity. Curr Biol 14: 273–286 [DOI] [PubMed] [Google Scholar]
- Lee J, Kitajima TS, Tanno Y, Yoshida K, Morita T, Miyano T, Miyake M, Watanabe Y (2008) Unified mode of centromeric protection by shugoshin in mammalian oocytes and somatic cells. Nat Cell Biol 10: 42–52 [DOI] [PubMed] [Google Scholar]
- Llano E, Gomez R, Gutierrez-Caballero C, Herran Y, Sanchez-Martin M, Vazquez-Quinones L, Hernandez T, de Alava E, Cuadrado A, Barbero JL, Suja JA, Pendas AM (2008) Shugoshin-2 is essential for the completion of meiosis but not for mitotic cell division in mice. Genes Dev 22: 2400–2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losada A, Hirano M, Hirano T (1998) Identification of Xenopus SMC protein complexes required for sister chromatid cohesion. Genes Dev 12: 1986–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losada A, Hirano M, Hirano T (2002) Cohesin release is required for sister chromatid resolution, but not for condensin-mediated compaction, at the onset of mitosis. Genes Dev 16: 3004–3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losada A, Hirano T (2005) Dynamic molecular linkers of the genome: the first decade of SMC proteins. Genes Dev 19: 1269–1287 [DOI] [PubMed] [Google Scholar]
- MacCallum DE, Losada A, Kobayashi R, Hirano T (2002) ISWI remodeling complexes in Xenopus egg extracts: identification as major chromosomal components that are regulated by INCENP-aurora B. Mol Biol Cell 13: 25–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresca TJ, Groen AC, Gatlin JC, Ohi R, Mitchison TJ, Salmon ED (2009) Spindle assembly in the absence of a RanGTP gradient requires localized CPC activity. Curr Biol 19: 1210–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston AL, Tham WH, Shah H, Amon A (2004) A genome-wide screen identifies genes required for centromeric cohesion. Science 303: 1367–1370 [DOI] [PubMed] [Google Scholar]
- McGuinness BE, Hirota T, Kudo NR, Peters JM, Nasmyth K (2005) Shugoshin prevents dissociation of cohesin from centromeres during mitosis in vertebrate cells. PLoS Biol 3: e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdes A, Ramyar K, Vechio JD, Cleveland DW (1996) A complex of NuMA and cytoplasmic dynein is essential for mitotic spindle assembly. Cell 87: 447–458 [DOI] [PubMed] [Google Scholar]
- Mochida S, Ikeo S, Gannon J, Hunt T (2009) Regulated activity of PP2A-B55 delta is crucial for controlling entry into and exit from mitosis in Xenopus egg extracts. EMBO J 28: 2777–2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury MV, Maresca TJ, Salmon WC, Waterman-Storer CM, Heald R, Weis K (2001) Importin beta is a mitotic target of the small GTPase Ran in spindle assembly. Cell 104: 95–106 [DOI] [PubMed] [Google Scholar]
- Ohi R, Sapra T, Howard J, Mitchison TJ (2004) Differentiation of cytoplasmic and meiotic spindle assembly MCAK functions by Aurora B-dependent phosphorylation. Mol Biol Cell 15: 2895–2906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth M, Mayer B, Rehm K, Rothweiler U, Heidmann D, Holak TA, Stemmann O (2011) Shugoshin is a Mad1/Cdc20-like interactor of Mad2. EMBO J 30: 2868–2880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posch M, Khoudoli GA, Swift S, King EM, Deluca JG, Swedlow JR (2010) Sds22 regulates aurora B activity and microtubule-kinetochore interactions at mitosis. J Cell Biol 191: 61–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabitsch KP, Gregan J, Schleiffer A, Javerzat JP, Eisenhaber F, Nasmyth K (2004) Two fission yeast homologs of Drosophila Mei-S332 are required for chromosome segregation during meiosis I and II. Curr Biol 14: 287–301 [DOI] [PubMed] [Google Scholar]
- Resnick TD, Satinover DL, MacIsaac F, Stukenberg PT, Earnshaw WC, Orr-Weaver TL, Carmena M (2006) INCENP and Aurora B promote meiotic sister chromatid cohesion through localization of the Shugoshin MEI-S332 in Drosophila. Dev Cell 11: 57–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel CG, Katis VL, Katou Y, Mori S, Itoh T, Helmhart W, Galova M, Petronczki M, Gregan J, Cetin B, Mudrak I, Ogris E, Mechtler K, Pelletier L, Buchholz F, Shirahige K, Nasmyth K (2006) Protein phosphatase 2A protects centromeric sister chromatid cohesion during meiosis I. Nature 441: 53–61 [DOI] [PubMed] [Google Scholar]
- Rivera T, Losada A (2009) Shugoshin regulates cohesion by driving relocalization of PP2A in Xenopus extracts. Chromosoma 118: 223–233 [DOI] [PubMed] [Google Scholar]
- Rosasco-Nitcher SE, Lan W, Khorasanizadeh S, Stukenberg PT (2008) Centromeric Aurora-B activation requires TD-60, microtubules, and substrate priming phosphorylation. Science 319: 469–472 [DOI] [PubMed] [Google Scholar]
- Ruchaud S, Carmena M, Earnshaw WC (2007) Chromosomal passengers: conducting cell division. Nat Rev Mol Cell Biol 8: 798–812 [DOI] [PubMed] [Google Scholar]
- Salic A, Waters JC, Mitchison TJ (2004) Vertebrate shugoshin links sister centromere cohesion and kinetochore microtubule stability in mitosis. Cell 118: 567–578 [DOI] [PubMed] [Google Scholar]
- Sampath SC, Ohi R, Leismann O, Salic A, Pozniakovski A, Funabiki H (2004) The chromosomal passenger complex is required for chromatin-induced microtubule stabilization and spindle assembly. Cell 118: 187–202 [DOI] [PubMed] [Google Scholar]
- Sessa F, Mapelli M, Ciferri C, Tarricone C, Areces LB, Schneider TR, Stukenberg PT, Musacchio A (2005) Mechanism of Aurora B activation by INCENP and inhibition by hesperadin. Mol Cell 18: 379–391 [DOI] [PubMed] [Google Scholar]
- Shintomi K, Hirano T (2009) Releasing cohesin from chromosome arms in early mitosis: opposing actions of Wapl-Pds5 and Sgo1. Genes Dev 23: 2224–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka TU (2010) Kinetochore-microtubule interactions: steps towards bi-orientation. EMBO J 29: 4070–4082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z, Shu H, Qi W, Mahmood NA, Mumby MC, Yu H (2006) PP2A is required for centromeric localization of Sgo1 and proper chromosome segregation. Dev Cell 10: 575–585 [DOI] [PubMed] [Google Scholar]
- Tanno Y, Kitajima TS, Honda T, Ando Y, Ishiguro K, Watanabe Y (2010) Phosphorylation of mammalian Sgo2 by Aurora B recruits PP2A and MCAK to centromeres. Genes Dev 24: 2169–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng BS, Tan L, Kapoor TM, Funabiki H (2010) Dual detection of chromosomes and microtubules by the chromosomal passenger complex drives spindle assembly. Dev Cell 18: 903–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara T, Tanno Y, Watanabe Y (2010) Phosphorylation of the CPC by Cdk1 promotes chromosome bi-orientation. Nature 467: 719–723 [DOI] [PubMed] [Google Scholar]
- Vader G, Kauw JJ, Medema RH, Lens SM (2006) Survivin mediates targeting of the chromosomal passenger complex to the centromere and midbody. EMBO Rep 7: 85–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanoosthuyse V, Prykhozhij S, Hardwick KG (2007) Shugoshin 2 regulates localization of the chromosomal passenger proteins in fission yeast mitosis. Mol Biol Cell 18: 1657–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneron S, Prieto S, Bernis C, Labbe JC, Castro A, Lorca T (2004) Kinetochore localization of spindle checkpoint proteins: who controls whom? Mol Biol Cell 15: 4584–4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak CE, Cai S, Khodjakov A (2010) Mechanisms of chromosome behaviour during mitosis. Nat Rev Mol Cell Biol 11: 91–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak CE, Gan EC, Desai A, Mitchison TJ, Kline-Smith SL (2002) The microtubule-destabilizing kinesin XKCM1 is required for chromosome positioning during spindle assembly. Curr Biol 12: 1885–1889 [DOI] [PubMed] [Google Scholar]
- Walczak CE, Mitchison TJ, Desai A (1996) XKCM1: a Xenopus kinesin-related protein that regulates microtubule dynamics during mitotic spindle assembly. Cell 84: 37–47 [DOI] [PubMed] [Google Scholar]
- Wang F, Dai J, Daum JR, Niedzialkowska E, Banerjee B, Stukenberg PT, Gorbsky GJ, Higgins JM (2010) Histone H3 Thr-3 phosphorylation by Haspin positions Aurora B at centromeres in mitosis. Science 330: 231–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y (2005) Shugoshin: guardian spirit at the centromere. Curr Opin Cell Biol 17: 590–595 [DOI] [PubMed] [Google Scholar]
- Wilde A, Zheng Y (1999) Stimulation of microtubule aster formation and spindle assembly by the small GTPase Ran. Science 284: 1359–1362 [DOI] [PubMed] [Google Scholar]
- Xu Z, Cetin B, Anger M, Cho US, Helmhart W, Nasmyth K, Xu W (2009) Structure and function of the PP2A-shugoshin interaction. Mol Cell 35: 426–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi Y, Honda T, Tanno Y, Watanabe Y (2010) Two histone marks establish the inner centromere and chromosome bi-orientation. Science 330: 239–243 [DOI] [PubMed] [Google Scholar]
- Zhang X, Lan W, Ems-McClung SC, Stukenberg PT, Walczak CE (2007) Aurora B phosphorylates multiple sites on mitotic centromere-associated kinesin to spatially and temporally regulate its function. Mol Biol Cell 18: 3264–3276 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.