Abstract
Liver metastasis is a major lethal complication associated with colon cancer, and post-intravasation steps of the metastasis are important for its clinical intervention. In order to identify inhibitory microRNAs (miRNAs) for these steps, we performed ‘dropout’ screens of a miRNA library in a mouse model of liver metastasis. Functional analyses showed that miR-493 and to a lesser extent miR-493* were capable of inhibiting liver metastasis. miR-493 inhibited retention of metastasized cells in liver parenchyma and induced their cell death. IGF1R was identified as a direct target of miR-493, and its inhibition partially phenocopied the anti-metastatic effects. High levels of miR-493 and miR-493*, but not pri-miR-493, in primary colon cancer were inversely related to the presence of liver metastasis, and attributed to an increase of miR-493 expression during carcinogenesis. We propose that, in a subset of colon cancer, upregulation of miR-493 during carcinogenesis prevents liver metastasis via the induction of cell death of metastasized cells.
Keywords: colon cancer, IGF-1R, metastasis, miR-493
Introduction
Colon cancer is the second leading cause of cancer-related death in the western world, and the third in the entire world (http://globocan.iarc.fr/). A major cause of the lethality of colon cancer is distant metastasis, especially metastasis to the liver, which is found in ∼60% of colon cancer patients (Hess et al, 2006). In spite of recent progress, overall curative effects of chemotherapy, radiotherapy, and liver surgery for liver metastasis are dismal, resulting in a high rate of lethality for patients (Cunningham et al, 2010).
The development of liver metastasis of colon cancer is, like many other types of cancer metastasis, the result of a sequence of events that cancer cells pass through successfully (Gupta and Massagué, 2006; Chaffer and Weinberg, 2011). First, tumour cells acquire motility through a transforming process called epithelial mesenchymal transition (EMT), and break through the basement membrane into surrounding tissues. Second, the transformed cells intravasate local blood vessels that lead to a major portal vein, and survive in the blood stream. Third, the survived cells settle in liver parenchyma. Finally, cancer cells that settle in a new environment restart proliferation to form a metastatic foci.
For successful formation of metastatic foci in the liver, the final two steps of metastasis seem challenging, because the cells must adapt to the harsh microenvironment of the liver. On the other hand, the former steps seem relatively easy to be overcome due to direct blood circulation via portal veins and the porous nature of liver sinusoids (Chambers et al, 2002). Thus, the biological mechanisms by which the metastatic cells settle and proliferate in liver parenchyma are of particular interest for clinical intervention (Shibue and Weinberg, 2011).
In spite of the potential importance of the regulation of metastasis, our knowledge of the regulatory mechanism of the settlement and proliferation of metastatic cells in the liver is still limited. It is unclear which gene or network of genes plays a regulatory role in determining whether cancer cells successfully settle and grow in the liver. This is partly due to difficulties in setting up an appropriate in vitro system in which the effects of potential regulatory genes on metastatic cells can be evaluated. Without such a system, it is difficult to systematically search for functional regulators of tumour proliferation in the liver. Systematic screening of such regulators in experimental animals in vivo is an alternative, but such an attempt has not been reported, probably due to associated technical difficulties.
Accumulating reports have firmly established microRNAs (miRNAs) as one of the central players that regulate many aspects of cancer progression, including regulation of metastasis (Lotterman et al, 2008; Dumont and Tlsty, 2009; Iorio and Croce, 2009; Nicoloso et al, 2009; Ventura and Jacks, 2009). miRNA is ∼22 nt RNA in its mature form, and produced from its precursor mRNA (pri-miRNA) after sequential processing (Winter et al, 2009). Mature miRNA, by forming an RISC complex with other associated proteins, inhibits its downstream target genes by regulating mRNA stability and translation of their products (Bartel, 2009; Winter et al, 2009).
Like other types of cancer, many miRNAs are differentially regulated during the development of colon cancer (Dong et al, 2011), and some have been shown to play regulatory roles. While most of such regulatory miRNAs are involved in cell proliferation and apoptosis of colon cancer, some are involved in the regulation of metastasis. For example, miR-21, miR-26, miR-31, miR-141, miR-145, miR-196, and miR-200 were shown to be associated with the migration/invasiveness of colon cancer, miR-107 with angiogenesis, and the miR-200 family with stem cell-like properties (de Krijger et al, 2011; Wu et al, 2011). However, the phenotypes of these regulatory miRNAs were, in general, linked to the early stages of metastasis, and no miRNA has been identified that is clearly responsible for the last stages, that is, settlement or proliferation of metastasized cells in the liver or other distant target organs.
In order to identify regulatory miRNAs that inhibit these post-intravasation steps of liver metastasis, we performed a functional screening of miRNAs that inhibit the metastasis of colon cancer cells under experimental settings that reflect the final metastatic processes. Because ‘dropout’ assays have been successfully used to isolate genes and miRNAs that inhibit cell growth and the invasion of cancer cells (Voorhoeve et al, 2006; le Sage et al, 2007; Huang et al, 2008; Schlabach et al, 2008; Izumiya et al, 2011), we utilized the genetic power of dropout assays to isolate anti-metastatic miRNAs in a mouse model of liver metastasis. The following analyses revealed that miR-493 and miR-493* were capable of inhibiting the settlement of colon cancer cells in the liver parenchyma, at least in part by inducing their cell death. Possible roles of miR-493 in regulating liver metastasis, in light of their expression profiles in clinical specimens, are discussed.
Results
Functional screening for miRNAs that inhibit liver metastasis of colon cancer cells
We aimed to functionally isolate miRNAs that inhibit liver metastasis under experimental conditions in which the settlement of metastatic cells into the liver parenchyma via portal vein is reproduced (Figure 1A). We introduced a GFP-expressing lentivirus library that expresses miRNA precursors from mini-miRNA genes (a mixture of 445 human miRNAs) into HCT116 colon cancer cells, and a pool of the library-introduced cells was injected into the spleen of immunocompromised NOG mice. Two weeks after the injection, the development of liver metastasis was observed as multiple GFP-positive foci on liver surfaces while a fraction of the cells proliferated in the inoculated spleen (Figure 1B). GFP expression in the cancer cells allowed us to visualize the process of liver metastasis as reported previously (Wang et al, 2004).
Figure 1.
Functional screening for miRNAs that inhibit liver metastasis of colon cancer cells. (A) Schematic for functional screening of miRNAs that inhibit liver metastasis of colon cancer cells. (1) HCT116 cells that were infected with a human miRNA lentivirus library were injected into the spleens of NOG mice. (2, 3) Two weeks after the injection, library-introduced cells were recovered from the spleen and liver, and used to isolate genomic DNA. (4, 5) The introduced miRNA was amplified from the genomic DNA by PCR, labelled with Cy3 (spleen) or Cy5 (liver), and the Cy5/Cy3 ratio of each library-introduced miRNA was determined with the custom-made microarray (see Materials and methods). (6) miRNAs with low Cy5/Cy3 values were regarded as positive ‘dropout’ clones. (B) Bright-phase (left) and GFP (right) images of metastasized liver (2 weeks after splenic injection). (C) Bright-phase (left) and GFP (right) images of the metastasized liver at higher magnification. Arrow shows cut surface. (D) HE section of the metastasized liver (2 weeks after splenic injection). (E) Immunostaining of the metastasized liver (5 days after splenic injection). The metastasized liver was immunostained with E-cadherin (left and lower right), or stained with HE (upper right). The right panels show magnified images. Arrow indicates a metastasized HCT116 focus. (F) A list of ‘dropout’ clones with low Cy5/Cy3 ratio (>10-fold). miRNAs with low P-values (P<0.01) are shown in bold. P-values were calculated by standardizing each of the four experimental data sets by Z-score transformation and then by examining null hypothesis that the dropout value of certain miRNA is identical to the average of dropout values of all miRNAs in the library. Welch’s t-test was performed to calculate P-value.
While metastatic foci were formed on liver surfaces (Figure 1B), GFP images (Figure 1C), and HE staining (Figure 1D) of liver sections revealed that they were also formed in the liver parenchyma. HE staining of metastasized liver at an early stage (day 5 after splenic injection) indicated that the metastasized foci were generally localized in the vicinity of E-cadherin-positive areas surrounding the portal triads (Figure 1E). These data and previous reports (Bouvet et al, 2006) indicate that the generated metastasis was indeed disseminated through a portal vein after splenic injection.
In order to isolate miRNAs that inhibit liver metastasis, library-introduced cells were recovered from the spleen and liver 2 weeks after splenic injection, and DNA fragments of library miRNAs that were integrated into the genome of infected cells were amplified by PCR. Subsequently, the fragments were labelled either with Cy3 (miRNAs isolated from spleen) or with Cy5 (miRNAs isolated from liver), and used for two-colour microarray analyses in order to quantify the Cy5/Cy3 ratio of each library miRNA. This ‘dropout’ screen has been successfully employed by other groups (Voorhoeve et al, 2006; Huang et al, 2008; Schlabach et al, 2008) as well as in our previous studies (Izumiya et al, 2010; Tsuchiya et al, 2011) to isolate genes that inhibit cell proliferation. We repeated the screening four times (the dropout assays were performed with the genomic DNA isolated from the two independently injected mice in duplicate), and identified 25 miRNAs whose expression induced >10-fold reduction of the Cy5/Cy3 ratios on average (Supplementary Table S1; Figure 1F). Of the 25 miRNAs, 7 miRNAs (miR-128a, miR-668, miR-657, miR-493, miR-583, miR-659, and miR-125b) induced statistically significant reduction (P<0.01) of the Cy5/Cy3 ratios (Figure 1F).
miR-493 expression inhibits liver metastasis of colon cancer cells
In order to quantitatively determine if the candidate miRNAs identified by screening inhibit liver metastasis, HCT116 cells were individually infected with GFP-expressing lentiviruses that express the corresponding miRNA precursor, mixed with cells infected with RFP-expressing lentiviruses (HCT116/RFP) at a 1:1 ratio, and injected into the spleen of NOG mice to generate liver metastasis (Figure 2A). Two weeks after injection, the extent of inhibition of liver metastasis was examined by evaluating the ratios of the number of GFP- or RFP-positive cells from metastasized liver by GFP/RFP dual imaging of metastatic foci (Figure 2C) or by quantification of GFP- and RFP-positive cells by flow cytometry (Figure 2B). We speculated that two types of miRNAs are selected after the screening; miRNAs that inhibit attachment or proliferation of the cancer cells in the liver, and those that induce them in the spleen. This validation step should identify miRNAs that belong to the former, and eliminate the latter.
Figure 2.
miR-493 expression inhibits liver metastasis of colon cancer cells. (A) Schematic for evaluation of inhibitory effects of each miRNA on liver metastasis. HCT116/GFP cells introduced with individual miRNA were mixed with HCT116/RFP cells at a 1:1 ratio, and the mixture was used for splenic injection to generate liver metastasis. (B, C) Inhibition of liver metastasis by miRNA precursors. HCT116/GFP cells were infected with the lentiviruses that express the indicated miRNA precursors, and the mixtures of the infected HCT116/GFP cells and HCT116/RFP cells were used for the splenic injection. (B) Metastasized cells were isolated from the liver 14 days after the splenic injection, and a number of GFP-positive and RFP-positive cells were counted by flow cytometry. The inhibitory effect of each miRNA was evaluated by calculating GFP/RFP ratio. (C) Inhibition of liver metastasis by miRNAs (day 14). GFP/RFP dual fluorescence images for the indicated miRNA precursors were taken. (D) miR-493 expression in colon cancer cells and colon epithelial cells.miR-493 and miR-493* expression of the indicated cells was determined by RT–qPCR. (E) RT–qPCR analyses of miR-493 (left) and miR-493* (right) in FHC cells and HCT116 cells infected with the lentiviruses that express the miR-493 precursor. (F) Inhibition of liver metastasis by miR-493 and miR-493* mimics. GFP/RFP dual fluorescence images for the indicated miRNAs were taken as described in (C). (G) Inhibition of liver metastasis by miR-493 and miR-493* mimics. Inhibitory effect of each miRNA was evaluated as described in (B). (H) Inhibitory effect of miR-493 and miR-493* mimics in DLD-1 cells. For liver metastasis assays, the inhibitory effect was evaluated by counting the number of GFP/RFP fluorescent foci 14 days after the splenic injection. For in vitro growth assays, the same mixture of cells was grown under normal culture condition for 14 days, and the GFP/RFP ratios were calculated by flow cytometry.
GFP/RFP dual imaging showed that the formation of GFP-positive foci was markedly inhibited by introducing the viruses that expressed precursors of miR-493, or miR-125b, while the formation of RFP-positive foci was not significantly affected in either case (Figure 2C), indicating that these miRNAs inhibited formation of the metastatic foci in the liver. The other candidate miRNAs did not cause apparent inhibition of GFP foci (Figure 2B and unpublished results). In accordance with these observations, quantification of the ratios of a number of GFP- and RFP-positive metastasized cells demonstrated that miR-493 expression caused the strongest inhibition of liver metastasis while miR-125b and let-7e also showed significant inhibition (Figure 2B). Proliferation of miRNA-introduced cells in vitro under normal culture conditions revealed that the expression of miR-125b or let-7e, but not miR-493, apparently inhibited cell growth (Supplementary Figure S1A), indicating that the inhibitory effects of miR-125b and let-7e may be mainly caused by general inhibition of cell growth. Therefore, we focused on analysing miR-493 functions in the following studies.
From the miR-493 precursor introduced by the lentiviruses, a minor starform of miR-493 (miR-493*) as well as authentic miR-493 can be produced. In fact, examination of miRNA expression revealed that both forms of miRNAs were expressed in the infected HCT116 (Figure 2E). Examination of the levels of endogenous miR-493 and miR-493* by RT–qPCR indicated that neither form of miRNA was expressed in any colon cancer cells examined, while they were expressed in FHC, normal colon epithelial cells (Figure 2D). The levels of miR-493 and miR-493* in FHC cells approximately correspond to 25 and 10% of those of the infected HCT116 cells (Figure 2E).
In order to determine which miRNA, miR-493 or miR-493*, was responsible for the inhibitory effect on liver metastasis, we transfected miRNA mimics of each form into GFP-expressing HCT116 cells (HCT116/GFP), and their inhibitory effects on liver metastasis were examined in liver metastasis assays. Interestingly, both forms of miRNA were capable of inhibiting liver metastasis (Figure 2F and G), and miR-493 and miR-493* caused ∼20-fold and ∼5-fold reduction of the GFP/RFP ratio, respectively (Figure 2G). In contrast, miR-493 and miR-493* caused only an ∼2.6-fold and ∼2.1-fold reduction of the ratio in cells that proliferated in spleen (Supplementary Figure S1B). Likewise, growth of cells transfected with miRNA mimics in vitro under normal culture conditions showed that introduction of miR-493 or miR-493* caused only an ∼2.5-fold reduction of GFP/RFP ratios (Supplementary Figure S1C). These results suggest that the inhibitory function of the miRNAs cannot be attributed to mere inhibition of cell growth and are at least in part associated with liver metastasis.
We also examined the inhibitory effects of miR-493 on DLD-1, another colon cancer cell line. DLD-1 cells were transfected with the miRNA mimics and used for the splenic injection. Because metastasized DLD-1 cells grow slowly and it is difficult to obtain a sufficient number of cells to quantify GFP/RFP ratio by flow cytometry (unpublished results), the number of foci was counted from the GFP/RFP dual image to evaluate their effects on liver metastasis. Introduction of miR-493 or miR-493* caused an ∼4-fold or ∼2.5-fold inhibition of liver metastasis, whereas both caused an ∼2-fold inhibition of the GFP/RFP ratios under normal culture conditions (Figure 2H). Thus, miR-493 and to a lesser extent miR-493* were capable of inhibiting liver metastasis in at least two different colon cancer cell lines.
miR-493 expression induces cell death of colon cancer cells in metastasized liver
Next, we attempted to determine the mechanisms by which miR-493 or miR-493* inhibits liver metastasis. In order to determine whether the inhibitory effects of miR-493 can be observed during early phases of the experiments, we examined GFP/RFP dual images 2 and 4 days after splenic injection. Evaluation of GFP/RFP ratios indicated that miR-493 expression did not significantly affect liver metastasis on day 2, but caused marked inhibition on day 4 (Figure 3A and B). HE staining of metastasized liver indicated that the transfected HCT116/GFP cells were found in liver parenchyma 2 days after the splenic injection (Supplementary Figure S2A). Combined with the data presented in Figure 3A and B, these results indicate that miR-493 induced reduction of metastatic cells that already entered liver parenchyma.
Figure 3.
miR-493 expression induces cell death of colon cancer cells in metastasized liver. (A) GFP/RFP dual fluorescence images (higher magnification) of metastasized liver at day 2 or day 4 after splenic injection. HCT116/GFP cells were transfected with the indicated miRNA, and used for splenic injection as described in Figure 2C. (B) A number of GFP-positive and RFP-positive foci in (A) were counted and the relative ratio of GFP/RFP foci was calculated in each experiment. (C) Annexin V assays of metastasized cells transfected with miR-493. HCT116/RFP cells transfected with the indicated miRNA mimics were injected into the spleen. Three days after splenic injection, Alexa 488-Annexin V was injected via a tail vein, and the fraction of liver-metastasized HCT116/RFP cells that were positive for Annexin V staining was quantified under fluorescent microscope. Upper pictures show representative staining of HCT116/RFP cells with Alexa 488-Annexin V.
In order to determine whether the marked reduction of GFP-positive foci by miR-493 on day 4 was caused by accelerated cell death in the liver parenchyma, Alexa 488-labelled Annexin V was injected via the tail vein on day 3 after the splenic injection of miRNA-transfected HCT116/RFP. Evaluation of a fraction of Annexin V-positive cells indicated that miR-493 markedly stimulates the induction of cell death of metastatic cells (Figure 3C). Thus, it is likely that the induction of cell death contributes to the inhibition of liver metastasis by miR-493.
Unexpectedly, transfection of miR-493* caused moderate induction of cell death (Figure 3C), although the GFP/RFP ratio did not significantly change between day 2 and day 4 (Figure 3B). Given that the number of RFP-positive foci was somewhat reduced when co-injected with miR-493*-transfected HCT116/GFP cells (Figure 2F), miR-493* may induce cell death not only in miR-493*-expressing cells but also in neighbouring metastatic cells in a cell non-autonomous manner.
In contrast to the marked increase of cell death in metastasized liver (Figure 3C), introduction of either miR-493 or miR-493* did not cause a significant increase of a fraction of Annexin V-positive cells in vitro under normal culture conditions (Supplementary Figure S2B). The lack of induction of cell death by miR-493 in in vitro condition was further supported by analyses of cells with a sub-G1 DNA content (data not shown). Thus, our data indicate that the induction of cell death by miR-493 is associated with liver metastasis.
IGF1R is a direct target of miR-493 and partially mediates the inhibition of liver metastasis by miR-493
In order to clarify the molecular mechanisms of miR-493-mediated inhibition of liver metastasis, we looked for its direct targets. We narrowed down the target genes by combining two criteria: (1) a combination of predicted targets from three in-silico programs (Targetscan, PITA, miRanda), (2) genes reduced by >2-fold after miRNA expression. Our functional analyses presented in Figure 2 suggest that miR-493* is less effective than miR-493 in inhibiting metastasis. In addition, predicted targets of miR-493* were not reported in two (Targetscan and PITA) out of the three programs. Therefore, we focused on identifying targets of miR-493 in the following studies.
A combination of the two criteria revealed that there are eight predicted targets whose expression was reduced by >2-fold after miR-493 expression (Figure 4A; Supplementary Table S2). Because miR-493 induces the cell death of metastasized cells (Figure 3C), IGF1R is of particular interest due to its known roles in cell survival and promotion of metastasis of various types of cancer, including colon cancer (Ewing and Goff, 2010). In fact, the introduction of miR-493 mimic or the infection with miR-493-expressing lentiviruses inhibited the expression of IGF1R, whereas other miRNAs, except miR-125b, did not suppress its expression (Figure 4B). The predicted target site for miR-493 is located near the 3′ end of the 3′ UTR of the IGF1R gene (Figure 4C). Functional luciferase assays indicated that the 3′ UTR of IGF1R was repressed by miR-493, and mutation of the predicted target site completely abolished the repression (Figure 4D), indicating that miR-493 directly targets IGF1R for suppression via its 3′ UTR. In contrast, other miRNAs (miR-493*, let-7e, miR-125b, miR-34a, miR-668) did not inhibit the same 3′ UTR (Supplementary Figure S3A). Thus, inhibition of the 3′ UTR of IGF1R by miR493 is specific among miRNAs. Inhibition of IGF1R by miR-493 was also observed in DLD-1 (Figure 4E; Supplementary Figure S3B), indicating that miR-493 is capable of inhibiting IGF1R expression in at least two colon cancer cells.
Figure 4.
IGF1R is a direct target of miR-493 and partially mediates the inhibition of liver metastasis by miR-493. (A) Two-cycle Venn diagram shows the overlap of predicted target genes of miR-493 (40 genes) and genes inhibited by miR-493 expression (421 genes). (B) HCT116 cells were transfected with the indicated miRNA mimics (left panels), or infected with the control or the miR-493-expressing lentiviruses (right panels), and used for western blot analyses with the indicated antibodies. (C) Sequence comparison of IGF1R 3′UTR (positions 1311–1317), the mutated IGF1R 3′UTR, and human miR-493. (D) Transient luciferase assays. The indicated reporter plasmid was transfected into HCT116 cells together with control miRNA or miR-493 mimic, and luciferase activity was measured 2 days after transfection. (E) Western blot analyses in DLD-1 cells as shown in (B). (F) Western blot analyses in HCT116/GFP cells after transfection of control or the designated IGF1R siRNA. (G) Inhibition of liver metastasis by IGF1R siRNAs. The inhibitory effect of each siRNA was evaluated as described in Figure 2B. (H) Annexin V assays of metastasized HCT116 cells that were transfected with control or IGF1R siRNA. A fraction of Annexin V-positive cells after transfection of the indicated siRNA is shown as presented in Figure 3C.
In order to examine whether the repression of IGF1R mediates the anti-metastatic effects of miR-493, IGF1R expression was inhibited by transfection of corresponding siRNAs in HCT116/GFP (Figure 4F), and the transfected cells were used for functional analyses of liver metastasis as performed in Figure 2F. Inhibition of IGF1R caused 30–40% reduction of liver metastasis (Figure 4G). Annexin V staining of the inhibited cells revealed that inhibition of IGF1R caused ∼3-fold increase of cell death (Figure 4H). In order to determine whether IGF1R inhibition is required for miR-493-mediated suppression of metastasis, IGF1R-expressing lentiviruses were used to infect HCT116/GFP cells (Supplementary Figure S3C). IGF1R overexpression partially alleviated miR-493-mediated inhibition of liver metastasis (Supplementary Figure S3D). These data collectively indicated that IGF1R inhibition partly mediates the effects of miR-493 on the suppression of liver metastasis.
A high level of miR-493 expression is associated with the absence of liver metastasis of colon cancer
Given the observed roles of miR-493 in suppressing liver metastasis, we were interested in examining whether high levels of miR-493 expression in human colon cancer are associated with the absence of liver metastasis. Therefore, we measured the levels of miRNAs in surgical specimens from primary colon cancers by RT–qPCR assays. The 44 cases that were examined were classified into 3 groups; cases without liver metastasis (19 cases), with metachronous metastasis (12 cases), and with synchronous metastasis (13 cases). Among these groups, there was no significant difference (P>0.05 by one-way ANOVA) in terms of age, gender, location, or size of tumours (Supplementary Table S3).
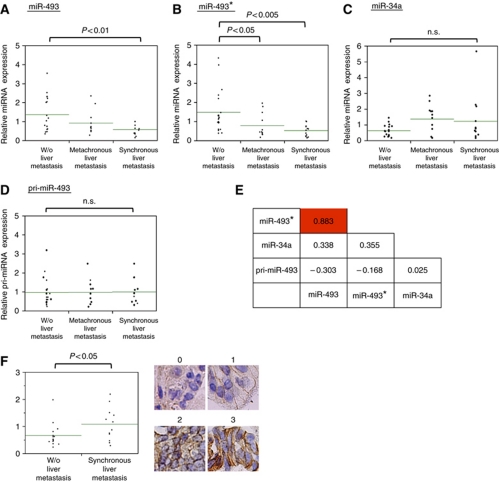
Average levels of miR-493 and miR-493* from primary cancer specimens without liver metastasis were ∼2-fold and ∼3-fold higher than those with synchronous metastasis, respectively, whereas their levels in specimens with metachronous metastasis were between those from the other two specimens (Figure 5A and B). Of note, none of the primary cancers with synchronous liver metastasis showed high levels of miR-493/miR-493* expression. It is unlikely that low levels of these miRNAs in primary tumours with liver metastasis were caused by alteration of the genomic loci because chromosomal loss of 14q32.2, the genomic region encompassing miR-493, was not commonly observed with these tumours by allelic copy number analyses (Izumiya et al, unpublished data). In contrast to miR-493 and miR-493*, levels of miR-34a, another tumour-suppressive miRNA (Hermeking, 2007; Tazawa et al, 2007), did not show an inverse relationship with the formation of liver metastasis (Figure 5C).
Figure 5.
A high level of miR-493 expression is associated with the absence of liver metastasis of colon cancer. (A–D) RT–qPCR analyses of (A) miR-493, (B) miR-493*, (C) miR-34a, and (D) pri-miR-493 from the designated groups of primary colon tumours. Green lines indicate average values of each group. The relative miRNA expression level in each sample was normalized by the average of total samples. (E) Pearson's correlation coefficient between the designated variables. (F) Levels of IGF1R immunostaining of primary tumour without metastasis or with synchronous metastasis. Right panels indicate representative immunostaining from grade 0 to 3. Each immunostaining was quantified as described in Materials and methods.
Using the same set of the tumour specimen, we also examined the expression of the six other miRNAs whose expression caused statistically significant inhibition of liver metastasis in the initial screening (Figure 1F). Because expression of miR-583 was not detectable in most of the samples, we analysed the remaining five miRNAs (miR-128a, miR-668, miR-657, miR-659, and miR-125b). Remarkably, none of these miRNAs showed the statistically significant differences among the three groups (Supplementary Figure S4A–E). These results indicated that the expression of miR-493/miR-493* was specifically elevated in a subset of primary tumours without liver metastasis, which is in agreement with their inhibitory roles in liver metastasis.
In contrast to liver metastasis, we did not observe significant reduction of levels of miR-493 or miR-493* in tumours with lung metastasis (Supplementary Figure S4F and G). Thus, the formation of liver metastasis is specifically associated with the reduced levels of these miRNAs in primary tumours.
In contrast to miR-493 and miR-493*, levels of their precursor (pri-miR-493) were not significantly altered among these specimens (Figure 5D). In fact, while levels of miR-493 and miR-493* were highly correlated (Pearson's correlation: r=0.883), neither was positively correlated with those of pri-miR-493 (r=−0.303 and r=−0.168, respectively) (Figure 5E). These results suggest that high levels of miR-493/miR-493* in a subset of cancer cells without liver metastasis were not caused by an increase of their precursor, but by enhanced processing of the precursor into mature miRNAs.
Because IGF1R was inhibited by miR-493 (Figure 4), we examined whether expression of IGF1R is increased in primary cancers with liver metastasis. Immunostaining of IGF1R of the same primary tumour demonstrated that IGF1R was expressed at higher levels in tumours with synchronous metastasis than those without metastasis (Figure 5F), although inverse correlations between levels of IGF1R immunostaining and those of miR-493 or miR-493* were rather moderate (r=−0.353 and r=−0.404, respectively). Thus, the high levels of miR-493 may partly contribute to the reduced levels of IGF1R in tumours without liver metastasis.
miR-493 expression is induced during carcinogenesis in a subset of colon cancers
Finally, we examined whether miR-493/miR-493* expression is regulated during colon carcinogenesis using the same sets of tumours (without liver metastasis and synchronous metastasis) and corresponding non-tumour specimens. As demonstrated in Figure 5A and B, levels of miR-493 and miR-493* expressions were high in a subset of tumours without liver metastasis, and comparison of tumour and non-tumour specimens revealed that the high levels were attributed to their elevation during carcinogenesis (Figure 6A and C). On average, miR-493 and miR-493* were increased by ∼2-fold and ∼4-fold, respectively, during carcinogenesis of colon tumours without liver metastasis, whereas the induction of these miRNAs was significantly less in tumours with synchronous metastasis (Figure 6B and D). Thus, high levels of miR-493/miR-493* in a subset of tumours without liver metastasis are attributed to their induction during colon carcinogenesis.
Figure 6.
miR-493 expression is induced during carcinogenesis in a subset of colon cancers. (A, C) RT–qPCR analyses of (A) miR-493 and (C) miR-493* in 27 primary tumours (14 cases without liver metastasis and 13 cases with synchronous liver metastasis) and the corresponding non-cancerous specimens. (B, D) Ratios of (B) miR-493 and (D) miR-493* levels between tumour and non-cancerous specimens analysed in (A) and (C), respectively. (E) Model for the role of miR-493 in suppressing liver metastasis in a subset of liver tumours.
In contrast to miR-493/miR-493*, the significant elevation of miR-34a or miR-125b was not observed during carcinogenesis of colon tumours without liver metastasis (∼1.3-fold for miR-34a and ∼1.2-fold for miR-125b; Supplementary Figure S5A–D). These data indicate that the increase of miR-493 during carcinogenesis in a subset of tumours with liver metastasis is unique among miRNAs.
Interestingly, miR-125b expression is apparently reduced during carcinogenesis of colon tumours with synchronous metastasis (∼0.36-fold; Supplementary Figure S5C and D). Thus, given the inhibitory role of liver metastasis of miR-125b (Figure 2B), the reduction of miR-125b in colon tumour may also contribute to the generation of liver metastasis.
Discussion
A functional ‘dropout’ screen has been applied to identify genes and miRNAs that play inhibitory roles in cell proliferation or invasion of human cancer cells (Voorhoeve and Agami, 2007; Izumiya et al, 2011). Here, we extended its application for more complicated biological process, that is, regulation of liver metastasis. This was made possible by the use of highly immunodeficient NOG mice that show high efficiency for the metastasis of human cells (Hamada et al, 2008), in combination with relatively small complexity of the library (445 miRNAs). In addition, use of RFP-positive cells as internal controls and measurement of GFP/RFP ratios as readouts for the inhibitory effect allowed us to quantitatively evaluate the effects of the identified miRNAs on liver metastasis. Presumably, a similar screening strategy will be applied to identify miRNAs, other types of non-coding RNAs, or shRNAs for a subset of genes, that can inhibit various types of distant metastasis in the future.
Through dropout screening using a mouse model of liver metastasis, we demonstrated that miR-493 induces the cell death of metastasized cells and inhibits liver metastasis of colon cancer cells. In addition, miR-493*, a starform miRNA that is derived from the same precursor, was also capable of inhibiting of liver metastasis. Expressions of two metastasis inhibitory miRNAs from the same precursor may co-operate to strengthen their inhibitory effects on liver metastasis.
An inverse correlation between miR-493 expression and the tendency to develop liver metastasis, considering its inhibitory roles against metastatic cells, strongly suggests that high levels of miR-493 prevent liver metastasis in human colon cancer. Remarkably, high levels of miR-493 expression in a subset of colon cancer were attributed to their induction during carcinogenesis. Hence, in some tumours, the induction of miR-493 during carcinogenesis may protect them from developing liver metastasis, while in others a lack of the induction may facilitate the formation of metastasis (Figure 6E).
The anti-metastatic functions of miR-493 are somewhat reminiscent of that of some known tumour suppressors, such as p53 (Vousden and Prives, 2009), which are activated in response to a variety of carcinogenic signals: carcinogenesis may trigger the induction of miR-493 in some colon cancers as one of cellular responses to prevent liver metastasis, although it is uncertain whether the induction functions to inhibit early stages of cancer development as well.
The reduced miR-493 expression in primary tumours with liver metastasis, but not with lung metastasis (Supplementary Figure S4F and G) suggests that the anti-metastatic effects of miR-493/miR-493* are specific to liver metastasis. The ‘seeds and soil’ theory of metastasis proposes that efficient formation of metastasis depends on the interaction between metastatic cells and the targeted organs in a tumour microenvironment (Nguyen et al, 2009; Psaila and Lyden, 2009; Langley and Fidler, 2011). In this context, clarification of miR-493 function may aid future understanding of why colon cancer cells preferentially metastasize in the liver.
While the expression of miR-493 and miR-493* is highly correlated in primary colon tumours, expression of pre-miR-493, their precursor, is not related to either. These observations suggest that the processing of pre-miR-493 may play a regulatory role in the induction of miR-493/miR-493* during carcinogenesis. Recently, it has been shown that some of the crucial regulators against carcinogenesis, such as p53, ATM, or TGF-β, are involved in the processing of miRNA precursors (Suzuki and Miyazono, 2011; Zhang et al, 2011).
miR-493 expression induced cell death of the metastasized cells concomitantly with their disappearance from the liver (Figure 3). Therefore, it is likely that induction of cell death is the mechanism by which miR-493 inhibits liver metastasis. Interestingly, both miR-493 and miR-493* were capable of inhibiting phosphorylation of Akt (Figure 4B), and the inhibitory function of these miRNAs on Akt may contribute to the reduced survival of cells that were metastasized in liver. On the other hand, we found that miR-493 expression decreases invasion activity in standard Matrigel assays (Supplementary Figure S2C). Considering that HCT116 is EMT (−) cells that express high levels of E-cadherin (Park et al, 2008), it is not clear whether a further decrease of invasiveness of HCT116 cells is involved in the inhibition of liver metastasis in our assays.
Our data in Figure 4 firmly established IGF1R as a novel target of miR-493. IGF1R promotes the survival and metastasis of several types of cancer, including colorectal cancer (Ewing and Goff, 2010), and is regarded as a potent therapeutic target against them (Chitnis et al, 2008; Ewing and Goff, 2010). In fact, blockade of its ligand using neutralizing antibodies reduces liver metastasis of colorectal cancer cells (Miyamoto et al, 2005). In accordance, our data indicate that IGF1R enhances liver metastasis (Figure 4G), and promotes cell survival in the liver (Figure 4H). Thus, it is likely that enhanced survival of IGF1R is partly responsible for the function of miR-493 to inhibit metastasis, although the suppression of IGF1R alone cannot explain the inhibitory function of miR-493 (Supplementary Figure S3D), and there are possibly other target genes that co-operate with IGF1R. Future investigation will clarify the full picture of miR-493 targets and their functions on liver metastasis.
Given the metastasis-inhibitory function of miR-493, increasing levels of miR-493 may be effective in preventing liver metastasis, especially for patients who suffer from primary colon cancer with low levels of miR-493. Given the potential of miRNAs for therapeutic applications (Trang et al, 2008; Iguchi et al, 2010; Petrocca and Lieberman, 2011), this may be done by administrating miR-493 or its agonist analogues. Alternatively, stimulation of the processing of its precursor may induce levels of endogenous miR-493. In either case, elevation of miR-493 levels may become an effective adjuvant therapy against liver metastasis in the future.
Materials and methods
Cell lines and plasmids
All colon cancer cells and their derivatives were cultivated in Dulbecco's MEM supplemented with 10% FCS. miRNA precursor-expressing lentivirus plasmids were purchased from System Biosciences (CA, USA). In order to create a lentivirus plasmid that expresses IGF1R, an IGF1R cDNA fragment was amplified from FHC-derived cDNA and ligated into pLenti6-DEST (Invitrogen). A luciferase reporter for 3′UTR of IGF1R (psicheck-IGF1R-UTR) was created by inserting a 420-bp 3′UTR DNA fragment spanning the predicted miR-493 target site into the 3′ end of Renilla luciferase of psicheck2, a dual-luciferase reporter plasmid (Promega). A luciferase reporter with the mutated 3′UTR (psicheck-IGF1R-UTR-mut) was generated by substituting five nucleotides at the miR-493 target site with In Vitro Mutagenesis kit (Stratagene).
Lentivirus production and infection
Lentivirus plasmids were co-transfected with pLP1, pLP2, and pLP/VSVG (Invitrogen) into 293FT cells (Invitrogen), and virus-containing supernatants were prepared according to manufacturer's instructions. For lentivirus infection, cells were incubated with virus-containing supernatants in the presence of 6 μg/ml polybrene. For infection of the lentiviruses that express IGF1R or their control viruses, infected cells were selected in the presence of 8 μg/ml blasticidin. For infection of GFP-expressing viruses for miRNA expression, flow cytometry analyses (FacsCalibur, Becton Dickinson) were performed to confirm that >90% of cells were infected.
Functional screening of miRNAs that inhibit liver metastasis
HCT116 cells were infected with a human miRNA precursor expression lentivirus library that expresses GFP (System Biosciences) with 6 μg/ml polybrene. Judging from a fraction of GFP-positive cells, 80–90% of cells were infected with the virus. A pool of library-infected cells (1 × 106 cells) was suspended in medium containing 50% Matrigel (Becton Dickinson), and injected into the spleens of 8-week-old NOG (NOD/Shi-scid IL2rgnull) mice (Central Institute for Experimental Animals, Tokyo, Japan).
Two weeks after the splenic injection, dissected livers and spleens were minced, treated with 1 μg/μl DNase I (Roche Applied Science) and 1 mg/ml collagenase I (Roche Applied Science) for 30 min at 37°C, and filtered with a 100-μm cell strainer (BD Falcon). Subsequently, the library-introduced cells were isolated through centrifugal purification in phosphate-buffered saline containing Histodenz (Sigma, MO, USA). Isolation of genomic DNA, amplification and Cy5/Cy3 labelling of DNA fragments spanning library-introduced miRNA, and analyses of Cy5/Cy3 ratio of each miRNA using the custom-made microarrays were performed as previously described (Izumiya et al, 2010).
Liver metastasis assays
HCT116 cells were infected with lentiviruses that express miRNA and GFP (System Biosciences) or with control lentiviruses in the presence of 6 μg/ml polybrene. Alternatively, HCT116/GFP or DLD-1/GFP (2 × 105 cells) cells were transfected with 4 pmoles of siRNAs (Invitrogen) or 6 pmoles of miRNA mimics (pre-miR miRNA precursors; Ambion) in the presence of 20 μl Hiperfect (Qiagen) for 2 days. Subsequently, the GFP-positive cells were mixed with HCT116/RFP or DLD-1/RFP at a 1:1 ratio, suspended in normal growth medium containing 50% Matrigel, and injected into spleen of NOG mice (2–4 × 105 cells). A fraction of the cell mixture before the injection was used for flow cytometry analyses to accurately measure original ratios of GFP/RFP-positive cells.
In all, 1–14 days after splenic injection, the inhibitory effects of siRNA or miRNA on liver metastasis were evaluated by GFP/RFP imaging (OV110; Olympus), or by measuring the ratios of GFP/RFP-positive cells by flow cytometry after recovering metastasized cells from livers as described above. The inhibitory effect of each oligonucleotide was calculated by comparing the GFP/RFP ratios before and after liver metastasis.
Cell death assays of metastasized cells
HCT116/RFP cells were transfected with miRNA mimics as described above, and 4 × 105 of the transfected cells were injected into the spleens of NOG mice. Three days after the splenic injection, Alexa 488-labelled Annexin V (Molecular Probe, OR, USA) was injected into the tail vein for 1 h, and the number of Alexa 488-positive HCT116/RFP cells in the dissected liver was counted by fluorescence microscopy.
Western blot analyses
Cells were lysed in RIPA buffer and used for western blot analyses as previously described (Okamoto et al, 2005), with anti-IGF1R (Cell Signaling), anti-Actin (Sigma), anti-Akt (Cell Signaling), or anti-phospho-Akt (PS473) (Cell Signaling).
RNA preparation and RT–qPCR analysis
RNA was isolated from cells with miRNeasy Mini kit (Qiagen), and levels of miRNAs or genes were measured by performing Taqman microRNA assays or Taqman gene expression assays (Applied Biosystems) according to manufacturer's instructions. RNU48 or Actin expression was used to calculate delta Ct values for miRNA or genes, respectively.
Luciferase reporter assays
A dual-luciferase reporter construct with or without 3′UTR of IGF1R was transfected into HCT116/GFP cells in the presence of a miR-493 mimic or its control, and Firefly and Renilla luciferase activities were measured by the Dual-Luciferase Reporter System (Promega) 2 days after transfection.
Matrigel invasion assays
Ten thousand transfected HCT116 cells were serum starved with medium with 0.1% serum overnight, and seeded onto 24-well Matrigel-coated inserts (8 μm pore; BD Falcon). After 24 h, cells attached to the lower surfaces of the insert filter were counted after staining with the Diff-Quick staining kit (Sysmex, Tokyo).
Immunohistochemical analysis
Metastasized mouse liver was fixed in formalin, embedded in paraffin, sectioned, and stained with HE or with standard immunohistochemistry methods. For immunostaining with E-cadherin, deparaffinized sections were incubated with an anti-E-cadherin antibody (Santa-Cruz), and biotinylated anti-mouse secondary antibody followed by ABC reagent and DAB (Vector Laboratories). The slides were counterstained with haematoxylin. For IGF1R immunostaining of human primary cancer, freshly frozen samples of human primary colon tumours were sectioned, fixed in acetone, and immunostained with anti-IGF1R antibody (Cell Signaling). Staining with the secondary antibody and the detection steps were performed as described above. The extent of the staining was visually evaluated on a scale of 0 (no staining) to 3 (strong staining). Approximately 800 cells were evaluated for each sample by two observers, and the mean value for each staining was calculated.
Clinical specimens
Tumour and non-tumour tissues were resected from patients with informed consent at the Teikyo University Hospital, and all procedures were performed under the protocol approved by the Ethics Committee of Teikyo University Hospital. Each case without metastasis at the time of surgery was subjected to a 2-year follow-up for classification of the metastatic status. RNAs were purified from dissected samples, and a part of each tumour was freshly frozen for immunostaining.
Microarray analyses
Total RNA was extracted from the transfected HCT116 cells using miRNeasy mini kit (Qiagen). Purified RNA was labelled with Cy3 dye, and hybridized to Agilent Whole Human Genome 4 × 44K Oligo Microarrays according to manufacturer's instructions (Agilent Technologies). Microarray data are analysed using Genespring software (Agilent Technologies), and accessible through the Gene Expression Omnibus (GEO) database (GEO Series accession number GSE31751).
Statistical analysis
Statistical analyses were performed by JMP (6.0) software (SAS Institute, NC, USA). Data included in Figure 5A–D and Supplementary Figure S4 were subjected to one-way ANOVA. The other data were analysed by the two-tailed Student's t-test. All data are presented as the mean±s.d.
Supplementary Material
Acknowledgments
We thank Yoshinori Ikarashi for fluorescent imaging; Shigeki Sekine for immunohistochemical analyses; Masako Ochiai for statistical analyses; and Ibuki Kobayashi, Kazuhiro Kanemoto, and Yuki Tabe for technical assistance. NOG mice were provided by the Central Institute for Experimental Animal (Tokyo, Japan). This research was supported by a Grant-in-Aid for the Third-Term Comprehensive 10-Year Strategy for Cancer Control from the Ministry of Health, Labor and Welfare; the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (NiBio). TI is a recipient of a Research Resident Fellowship from the Foundation for Promotion of Cancer Research (FPCR, Japan). None of the authors have a financial interest related to this work.
Author contributions: KO designed and performed experiments, analysed data and wrote the manuscript. YM performed experiments and analysed data. MI and NT designed and performed experiments. TI, HO, AS, and HS performed experiments. HN designed experiments and wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136: 215–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvet M, Tsuji K, Yang M, Jiang P, Moossa AR, Hoffman RM (2006) In vivo color-coded imaging of the interaction of colon cancer cells and splenocytes in the formation of liver metastases. Cancer Res 66: 11293–11297 [DOI] [PubMed] [Google Scholar]
- Chaffer CL, Weinberg RA (2011) A perspective on cancer cell metastasis. Science 331: 1559–1564 [DOI] [PubMed] [Google Scholar]
- Chambers AF, Groom AC, MacDonald IC (2002) Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2: 563–572 [DOI] [PubMed] [Google Scholar]
- Chitnis MM, Yuen JS, Protheroe AS, Pollak M, Macaulay VM (2008) The type 1 insulin-like growth factor receptor pathway. Clin Cancer Res 14: 6364–6370 [DOI] [PubMed] [Google Scholar]
- Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, Starling N (2010) Colorectal cancer. Lancet 375: 1030–1047 [DOI] [PubMed] [Google Scholar]
- de Krijger I, Mekenkamp LJ, Punt CJ, Nagtegaal ID (2011) MicroRNAs in colorectal cancer metastasis. J Pathol 224: 438–447 [DOI] [PubMed] [Google Scholar]
- Dong Y, Wu WK, Wu CW, Sung JJ, Yu J, Ng SS (2011) MicroRNA dysregulation in colorectal cancer: a clinical perspective. Br J Cancer 104: 893–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont N, Tlsty TD (2009) Reflections on miR-ing effects in metastasis. Cancer Cell 16: 3–4 [DOI] [PubMed] [Google Scholar]
- Ewing GP, Goff LW (2010) The insulin-like growth factor signaling pathway as a target for treatment of colorectal carcinoma. Clin Colorectal Cancer 9: 219–223 [DOI] [PubMed] [Google Scholar]
- Gupta GP, Massagué J (2006) Cancer metastasis: building a framework. Cell 127: 679–695 [DOI] [PubMed] [Google Scholar]
- Hamada K, Monnai M, Kawai K, Nishime C, Kito C, Miyazaki N, Ohnishi Y, Nakamura M, Suemizu H (2008) Liver metastasis models of colon cancer for evaluation of drug efficacy using NOD/Shi-scid IL2Rgammanull (NOG) mice. Int J Oncol 32: 153–159 [PubMed] [Google Scholar]
- Hermeking H (2007) p53 enters the microRNA world. Cancer Cell 12: 414–418 [DOI] [PubMed] [Google Scholar]
- Hess KR, Varadhachary GR, Taylor SH, Wei W, Raber MN, Lenzi R, Abbruzzese JL (2006) Metastatic patterns in adenocarcinoma. Cancer 106: 1624–1633 [DOI] [PubMed] [Google Scholar]
- Huang Q, Gumireddy K, Schrier M, le Sage C, Nagel R, Nair S, Egan DA, Li A, Huang G, Klein-Szanto AJ, Gimotty PA, Katsaros D, Coukos G, Zhang L, Pure E, Agami R (2008) The microRNAs miR-373 and miR-520c promote tumour invasion and metastasis. Nat Cell Biol 10: 202–210 [DOI] [PubMed] [Google Scholar]
- Iguchi H, Kosaka N, Ochiya T (2010) Versatile applications of microRNA in anti-cancer drug discovery: from therapeutics to biomarkers. Curr Drug Discov Technol 7: 95–105 [DOI] [PubMed] [Google Scholar]
- Iorio MV, Croce CM (2009) MicroRNAs in cancer: small molecules with a huge impact. J Clin Oncol 27: 5848–5856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumiya M, Okamoto K, Tsuchiya N, Nakagama H (2010) Functional screening using a microRNA virus library and microarrays: a new high-throughput assay to identify tumor-suppressive microRNAs. Carcinogenesis 31: 1354–1359 [DOI] [PubMed] [Google Scholar]
- Izumiya M, Tsuchiya N, Okamoto K, Nakagama H (2011) Systematic exploration of cancer-associated microRNAs through functional screening assays. Cancer Sci 102: 1615–1621 [DOI] [PubMed] [Google Scholar]
- Langley RR, Fidler IJ (2011) The seed and soil hypothesis revisited--the role of tumor-stroma interactions in metastasis to different organs. Int J Cancer 128: 2527–2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- le Sage C, Nagel R, Egan DA, Schrier M, Mesman E, Mangiola A, Anile C, Maira G, Mercatelli N, Ciafre SA, Farace MG, Agami R (2007) Regulation of the p27 (Kip1) tumor suppressor by miR-221 and miR-222 promotes cancer cell proliferation. EMBO J 26: 3699–3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotterman CD, Kent OA, Mendell JT (2008) Functional integration of microRNAs into oncogenic and tumor suppressor pathways. Cell Cycle 7: 2493–2499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S, Nakamura M, Shitara K, Nakamura K, Ohki Y, Ishii G, Goya M, Kodama K, Sangai T, Maeda H, Shi-Chuang Z, Chiba T, Ochiai A (2005) Blockade of paracrine supply of insulin-like growth factors using neutralizing antibodies suppresses the liver metastasis of human colorectal cancers. Clin Cancer Res 11: 3494–3502 [DOI] [PubMed] [Google Scholar]
- Nguyen DX, Bos PD, Massague J (2009) Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer 9: 274–284 [DOI] [PubMed] [Google Scholar]
- Nicoloso MS, Spizzo R, Shimizu M, Rossi S, Calin GA (2009) MicroRNAs—the micro steering wheel of tumour metastases. Nat Rev Cancer 9: 293–302 [DOI] [PubMed] [Google Scholar]
- Okamoto K, Kashima K, Pereg Y, Ishida M, Yamazaki S, Nota A, Teunisse A, Migliorini D, Kitabayashi I, Marine JC, Prives C, Shiloh Y, Jochemsen AG, Taya Y (2005) DNA damage-induced phosphorylation of MdmX at serine 367 activates p53 by targeting MdmX for Mdm2-dependent degradation. Mol Cell Biol 25: 9608–9620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SM, Gaur AB, Lengyel E, Peter ME (2008) The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev 22: 894–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrocca F, Lieberman J (2011) Promise and challenge of RNA interference-based therapy for cancer. J Clin Oncol 29: 747–754 [DOI] [PubMed] [Google Scholar]
- Psaila B, Lyden D (2009) The metastatic niche: adapting the foreign soil. Nat Rev Cancer 9: 285–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlabach MR, Luo J, Solimini NL, Hu G, Xu Q, Li MZ, Zhao Z, Smogorzewska A, Sowa ME, Ang XL, Westbrook TF, Liang AC, Chang K, Hackett JA, Harper JW, Hannon GJ, Elledge SJ (2008) Cancer proliferation gene discovery through functional genomics. Science 319: 620–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibue T, Weinberg RA (2011) Metastatic colonization: settlement, adaptation and propagation of tumor cells in a foreign tissue environment. Semin Cancer Biol 21: 99–106 [DOI] [PubMed] [Google Scholar]
- Suzuki HI, Miyazono K (2011) Emerging complexity of microRNA generation cascades. J Biochem 149: 15–25 [DOI] [PubMed] [Google Scholar]
- Tazawa H, Tsuchiya N, Izumiya M, Nakagama H (2007) Tumor-suppressive miR-34a induces senescence-like growth arrest through modulation of the E2F pathway in human colon cancer cells. Proc Natl Acad Sci USA 104: 15472–15477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trang P, Weidhaas JB, Slack FJ (2008) MicroRNAs as potential cancer therapeutics. Oncogene 27 (Suppl 2): S52–S57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya N, Izumiya M, Ogata-Kawata H, Okamoto K, Fujiwara Y, Nakai M, Okabe A, Schetter AJ, Bowman ED, Midorikawa Y, Sugiyama Y, Aburatani H, Harris CC, Nakagama H (2011) Tumor suppressor miR-22 determines p53-dependent cellular fate through post-transcriptional regulation of p21. Cancer Res 71: 4628–4639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura A, Jacks T (2009) MicroRNAs and cancer: short RNAs go a long way. Cell 136: 586–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorhoeve PM, Agami R (2007) Classifying microRNAs in cancer: the good, the bad and the ugly. Biochim Biophys Acta 1775: 274–282 [DOI] [PubMed] [Google Scholar]
- Voorhoeve PM, le Sage C, Schrier M, Gillis AJ, Stoop H, Nagel R, Liu YP, van Duijse J, Drost J, Griekspoor A, Zlotorynski E, Yabuta N, De Vita G, Nojima H, Looijenga LH, Agami R (2006) A genetic screen implicates miRNA-372 and miRNA-373 as oncogenes in testicular germ cell tumors. Cell 124: 1169–1181 [DOI] [PubMed] [Google Scholar]
- Vousden KH, Prives C (2009) Blinded by the light: the growing complexity of p53. Cell 137: 413–431 [DOI] [PubMed] [Google Scholar]
- Wang J, Yang M, Hoffman RM (2004) Visualizing portal vein metastatic trafficking to the liver with green fluorescent protein-expressing tumor cells. Anticancer Res 24: 3699–3702 [PubMed] [Google Scholar]
- Winter J, Jung S, Keller S, Gregory RI, Diederichs S (2009) Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol 11: 228–234 [DOI] [PubMed] [Google Scholar]
- Wu WK, Law PT, Lee CW, Cho CH, Fan D, Wu K, Yu J, Sung JJ (2011) MicroRNA in colorectal cancer: from benchtop to bedside. Carcinogenesis 32: 247–253 [DOI] [PubMed] [Google Scholar]
- Zhang X, Wan G, Berger FG, He X, Lu X (2011) The ATM kinase induces microRNA biogenesis in the DNA damage response. Mol Cell 41: 371–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.