Abstract
The sperm-specific CatSper channel controls the intracellular Ca2+ concentration ([Ca2+]i) and, thereby, the swimming behaviour of sperm. In humans, CatSper is directly activated by progesterone and prostaglandins—female factors that stimulate Ca2+ influx. Other factors including neurotransmitters, chemokines, and odorants also affect sperm function by changing [Ca2+]i. Several ligands, notably odorants, have been proposed to control Ca2+ entry and motility via G protein-coupled receptors (GPCRs) and cAMP-signalling pathways. Here, we show that odorants directly activate CatSper without involving GPCRs and cAMP. Moreover, membrane-permeable analogues of cyclic nucleotides that have been frequently used to study cAMP-mediated Ca2+ signalling also activate CatSper directly via an extracellular site. Thus, CatSper or associated protein(s) harbour promiscuous binding sites that can host various ligands. These results contest current concepts of Ca2+ signalling by GPCR and cAMP in mammalian sperm: ligands thought to activate metabotropic pathways, in fact, act via a common ionotropic mechanism. We propose that the CatSper channel complex serves as a polymodal sensor for multiple chemical cues that assist sperm during their voyage across the female genital tract.
Keywords: 8-Br-cAMP, 8-Br-cGMP; Ca2+ signalling; fertilization; odorants; patch-clamp
Introduction
In mammalian sperm, the intracellular Ca2+ concentration ([Ca2+]i) is controlled by sperm-specific Ca2+ channels (CatSper, cation channel of sperm) (Quill et al, 2001; Ren et al, 2001; Kirichok et al, 2006; Lishko et al, 2010). The CatSper channel complex comprises four homologous α subunits (CatSper 1–4) (Navarro et al, 2008; Kirichok and Lishko, 2011) and at least three auxiliary subunits: CatSper β, CatSper γ, and CatSper δ (Liu et al, 2007; Wang et al, 2009; Chung et al, 2011). In human sperm, progesterone and prostaglandins—two important ingredients of the oviduct (Schuetz and Dubin, 1981)—directly activate CatSper channels without involving classical nuclear receptors or G protein-coupled receptors (GPCRs; Lishko et al, 2011; Strünker et al, 2011). The progesterone-induced Ca2+ influx has been implicated in sperm capacitation, chemotaxis, hyperactivation, and acrosomal exocytosis (Harper et al, 2003; Oren-Benaroya et al, 2008; Publicover et al, 2008). Ca2+ influx stimulated by prostaglandins evokes acrosomal exocytosis and affects motility (Aitken and Kelly, 1985; Schaefer et al, 1998) but their function in chemotaxis and sperm hyperactivation is not known.
Chemosensation in sperm appears to be complex. A plethora of chemicals including vitamin D, chemokines, small peptides, the gas NO, neurotransmitters, analogues of cyclic nucleotides, and odorants (De Jonge, 2005; Eisenbach and Giojalas, 2006; Florman et al, 2008; Kaupp et al, 2008; Suarez, 2008) affect sperm motility and/or acrosomal exocytosis in vitro. However, the underlying signalling mechanisms are ill defined. These chemicals have been proposed to activate various ionotropic and metabotropic receptors that are reportedly expressed in mammalian sperm (Meizel, 2004; Naz and Sellamuthu, 2006).
Several ligands for GPCRs have been proposed to control sperm function via cAMP-signalling pathways that regulate [Ca2+]i. A case in point concerns odorants that might serve as chemoattractants for sperm. Odorants have been proposed to activate olfactory receptors, stimulate the synthesis of cAMP by transmembrane adenylyl cyclases (tmACs), and, thereby, open unknown Ca2+ channels (Spehr et al, 2003, 2004; Neuhaus et al, 2006; Veitinger et al, 2011). This notion has received strong support by the observations that (i) membrane-permeable analogues of cyclic nucleotides evoke an increase of [Ca2+]i (Kobori et al, 2000; Ren et al, 2001; Machado-Oliveira et al, 2008) and (ii) odorant-induced Ca2+ influx is impaired by inhibitors of tmACs and antagonists of odorant receptors (Spehr et al, 2003, 2004; Veitinger et al, 2011).
Here, we show by patch-clamp recording, Ca2+ fluorimetry, and cAMP radioimmunoassay (RIA) that structurally diverse odorants directly activate the CatSper channel in human sperm by a mechanism that does not involve metabotropic receptors and cAMP signalling. Moreover, several other GPCR ligands that reportedly stimulate cAMP-signalling pathways in mouse sperm fail to elevate cAMP levels in human sperm. Finally, membrane-permeable analogues of cAMP and cGMP directly activate CatSper from the outside. These results resolve several long-standing issues concerning odorants, cyclic nucleotides, and Ca2+ signalling in human sperm and provide new insight into the function of CatSper channels as target for female factors.
Results
Bourgeonal stimulates Ca2+ entry via CatSper channels
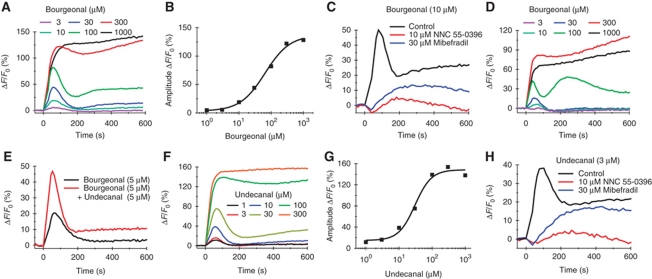
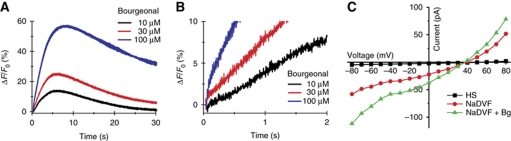
In human sperm loaded with the fluorescent Ca2+ indicator Fluo-4, the odorant bourgeonal stimulated a dose-dependent increase of [Ca2+]i (Figure 1A and B; Spehr et al, 2003, 2004; Veitinger et al, 2011). At bourgeonal concentrations ⩽100 μM, the Ca2+ signal consisted of two components: a rapid Ca2+ transient followed by a slower sustained Ca2+ increase (Figure 1A). At higher concentrations, bourgeonal evoked a rapid, sustained elevation of [Ca2+]i (Figure 1A). The Ca2+ signal was abolished at low (30 nM) extracellular [Ca2+] (Supplementary Figure S1A); thus, the Ca2+ signal rests upon Ca2+ entry (Spehr et al, 2003). The sustained component might reflect Ca2+ release from intracellular stores that is triggered by Ca2+ influx (Publicover et al, 2007; Bedu-Addo et al, 2008), or bourgeonal might also inhibit Ca2+ export by Ca2+-ATPase or Na+/Ca2+ exchange.
Figure 1.
Odorant-induced Ca2+ signals in human sperm. (A) Ca2+ signals induced by bourgeonal. ΔF/F0 (%) indicates the percent change in fluorescence (ΔF) with respect to the mean basal fluorescence (F0) before application of bourgeonal. (B) Dose–response relationship of the signal amplitudes from (A) (K1/2=65 μM). (C) Bourgeonal-induced Ca2+ signals in the presence of the CatSper channel inhibitors NNC 55-0396 and mibefradil. (D) Ca2+ signals induced by bourgeonal in non-capacitated sperm. (E) Ca2+ signals induced by bourgeonal and simultaneous application of bourgeonal and undecanal. (F) Ca2+ signals induced by undecanal. (G) Dose–response relationship of the signal amplitudes from (F) (K1/2=30 μM). (H) Undecanal-induced Ca2+ signals in the presence of the CatSper channel inhibitors NNC 55-0396 and mibefradil.
CatSper is the principle Ca2+ channel in mouse and human sperm (Kirichok et al, 2006; Lishko et al, 2010). We reasoned that Ca2+ entry stimulated by bourgeonal involves CatSper. In fact, the CatSper inhibitors NNC 55-0396 (Lishko et al, 2011; Strünker et al, 2011) and mibefradil (Strünker et al, 2011) suppressed and slowed down the bourgeonal-evoked Ca2+ signals (Figure 1C): the signal amplitude was reduced by 70±20% (10 μM NNC 55-0396, four experiments) and 65±11% (30 μM mibefradil, three experiments). We did not test higher concentrations, because at ⩾10 and ⩾30 μM, NNC 55-0396 and mibefradil, respectively, evoke Ca2+ signals themselves (Strünker et al, 2011) probably by intracellular alkalization (Supplementary Figure S2).
The activation of CatSper by progesterone is sensitized in capacitated sperm (Strünker et al, 2011). We therefore examined whether bourgeonal-induced Ca2+ signals differ in capacitated and non-capacitated sperm. Capacitation was assessed by an FITC-CD46 assay probing the acrosome reaction (Supplementary Figure S3). The amplitude and waveform of bourgeonal-induced Ca2+ signals were similar in sperm incubated under capacitating or non-capacitating conditions (Figure 1A and D). However, the constant of half-maximal activation, K1/2, was smaller in capacitated sperm (K1/2=68±32 μM, five experiments; Figure 1B) than in non-capacitated sperm (K1/2=102±44 μM, four experiments); thus, capacitated sperm are more sensitive to bourgeonal.
The CatSper channel is promiscuously activated by a variety of odorants
The odorant undecanal reportedly acts as a competitive antagonist that inhibits the bourgeonal-evoked Ca2+ influx (Spehr et al, 2003, 2004; Veitinger et al, 2011). We failed to reproduce this result: in the presence of undecanal, the Ca2+ response evoked by bourgeonal was enhanced rather than abolished (Figure 1E). Moreover, undecanal itself evoked Ca2+ signals that were similar to those evoked by bourgeonal (compare Figure 1A and F) and that were also due to Ca2+ influx (Supplementary Figure S1B). The dose–response relationship for undecanal yielded a K1/2 of 28±6 μM (four experiments) (Figure 1G). Finally, NNC 55-0396 (10 μM) and mibefradil (30 μM) suppressed the amplitude of the undecanal-evoked Ca2+ signals by 85±23% (four experiments) and 74±34% (three experiments), respectively (Figure 1H). Other odorants like cyclamal (K1/2=318±138 μM, four experiments) and helional (K1/2=98±15 μM, four experiments) also evoked Ca2+ signals that were inhibited by NNC 55-0396 and mibefradil (Supplementary Figure S4). These results suggest that diverse odorants activate CatSper channels and, thereby, stimulate Ca2+ entry.
Odorants enhance CatSper currents
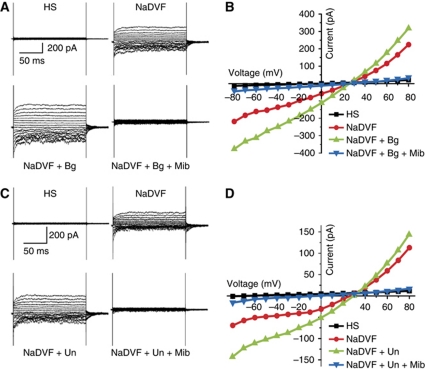
To scrutinize these findings by an independent technique, we recorded membrane currents from human sperm by whole-cell patch clamping. The pipette solution did neither include ATP nor GTP to prevent metabotropic signalling via G proteins. In standard extracellular solution containing Ca2+ and Mg2+ (HS), little or no currents were evoked by stepping the membrane voltage (Vm) from 0 to ±80 mV (Figure 2; Lishko et al, 2010, 2011; Strünker et al, 2011). However, monovalent CatSper currents could be recorded in divalent-free solution (NaDVF) (Figure 2; Lishko et al, 2010, 2011; Strünker et al, 2011). Mean inward currents at −60 mV were −96±58 pA, range −30 to −236 pA (20 experiments). Bourgeonal (80 μM) potentiated monovalent currents by 2.3±0.4-fold (−60 mV) and by 1.8±0.3-fold (+60 mV) (six experiments) (Figure 2A and B). The reversal potential (Vrev) of monovalent CatSper currents and bourgeonal-evoked currents was indistinguishable (26.5±5 mV, eight experiments each; Figure 2B). Moreover, mibefradil (30 μM) completely inhibited both CatSper and bourgeonal-evoked currents (Figure 2A and B). Undecanal (50 μM) also potentiated monovalent currents by 2.1±0.2-fold (−60 mV) and by 1.7±0.2-fold (+60 mV) (six experiments) (Figure 2C and D). Currents evoked by undecanal displayed a Vrev of 27.5±5 mV (seven experiments), similar to the Vrev of CatSper, and were completely inhibited by mibefradil (Figure 2C and D). We conclude that CatSper mediates the odorant-induced Ca2+ influx in human sperm.
Figure 2.
Electrophysiological characterization of whole-cell CatSper currents from human sperm. Currents were recorded at pHi 7.3 in the absence of intracellular divalent ions. The membrane voltage was stepped from −80 to +80 mV in steps of 10 mV from a holding potential of 0 mV. (A) Currents in extracellular solution containing Mg2+ and Ca2+ (HS) and monovalent CatSper currents in divalent-free Na+-based bath solution (NaDVF); perfusion with 80 μM bourgeonal potentiated monovalent currents (NaDVF+Bg). CatSper currents and currents evoked by bourgeonal were completely blocked by 30 μM mibefradil (NaDVF+Bg+Mib). (B) Current–voltage relationship from (A). (C) Currents in solution containing Ca2+ and Mg2+ (HS) and CatSper currents in divalent-free solution (NaDVF). Perfusion with 50 μM undecanal potentiated monovalent currents (NaDVF+Un). Currents were completely blocked by 30 μM mibefradil (NaDVF+Un+Mib). (D) Current–voltage relationship from (C).
Odorants do not activate a cAMP-signalling pathway
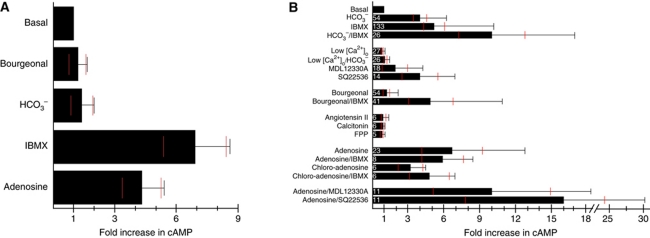
We examined whether bourgeonal stimulates the synthesis of cAMP as predicted by an olfactory signalling pathway. Bourgeonal failed to elevate cAMP levels in human sperm (Figure 3A; Veitinger et al, 2011). We scrutinized our experimental conditions and entertained several eventualities that might explain this unexpected failure. Different measures that elevate cAMP levels in mammalian sperm also enhance cAMP levels under our experimental conditions. In high (25 mM) HCO3− medium, the inhibition of cAMP hydrolysis by isobutylmethylxanthine (IBMX) and the stimulation by adenosine elevated cAMP levels by 7-fold and 4.5-fold, respectively (Figure 3A). A further increase of HCO3−, which potently (K1/2 ∼10 mM) stimulates a soluble AC (sAC; Litvin et al, 2003; Kamenetsky et al, 2006), did not elevate cAMP (Figure 3A), indicating that sAC is either fully activated or desensitized in the high HCO3− medium. In fact, sperm bathed in low (4 mM) HCO3− and stimulated with 50 mM HCO3− displayed a sizeable 4-fold cAMP increase (Figure 3B). Low extracellular [Ca2+] (<1 μM) did not affect resting cAMP levels (Figure 3B), but abolished activation of the sAC by HCO3− (Figure 3B; Carlson et al, 2007).
Figure 3.
Bourgeonal does not activate a cAMP-signalling pathway. (A) Changes in total cAMP concentration in human sperm bathed in high (25 mM) bicarbonate (HCO3−) evoked by (in mM): 50 HCO3−, 0.5 IBMX; (in μM) 50 bourgeonal, and 50 adenosine (5 measurements from 2 donors). The red lines indicate the 95% confidence intervals (CIs). Overlapping CIs indicate that there is no significant difference between conditions. (B) cAMP changes in sperm bathed in low (4 mM) bicarbonate evoked by (in mM): 50 HCO3−, 0.5 IBMX; (in μM) 50 bourgeonal,50 adenosine, 30 chloro-adenosine, 500 SQ22536, 100 MDL12330A; (in nM) 100 FPP, 10 angiotensin II, 1.5 calcitonin (5–133 measurements from 4 to 16 donors). Data comprise data from Strünker et al (2011) combined with data from additional experiments.
In kinetic experiments, we studied the time course of the HCO3−-stimulated cAMP synthesis. cAMP levels commenced to rise with a latency of >2 s that probably reflects the time required for HCO3− to enter the cell. Within 60 s cAMP rose to a peak and then slowly declined to lower levels (Supplementary Figure S5). After 25 min, cAMP levels were still 2–3-fold elevated. In sperm that were bathed for ⩾2 h in 4 mM or 25 mM HCO3−, resting cAMP levels were similar (0.082±0.042 pmol cAMP/106 sperm, 310 measurements from 16 donors, versus 0.031±0.024 pmol cAMP/106 sperm, 7 measurements from 2 donors), supporting the notion that sAC activity is downregulated via PKA by negative feedback (Nolan et al, 2004; Burton and McKnight, 2007). We were concerned that a similar negative feedback might hamper the activation of tmACs. However, bourgeonal also failed to stimulate cAMP synthesis in a low (4 mM) HCO3− medium (Figure 3B).
cAMP levels were determined >15 s after mixing sperm with bourgeonal. We wondered if a bourgeonal-evoked cAMP response is transient and cAMP levels decline to basal levels within 15 s. We tested for this caveat by inhibition of cAMP hydrolysis. In fact, simultaneous application of HCO3− and IBMX elevated cAMP levels beyond values reached by IBMX or HCO3− alone (Figure 3B; Supplementary Figure S5; Strünker et al, 2011). Importantly, the HCO3−/IBMX.-induced cAMP increase persisted on a higher level for at least 25 min (Supplementary Figure S5). In contrast, the cAMP increase was similar in sperm stimulated either by IBMX alone or by bourgeonal/IBMX (Figure 3B), arguing against a transient cAMP response stimulated by bourgeonal.
Support for an olfactory cAMP-signalling pathway rested primarily on pharmacological studies using two inhibitors of tmACs—SQ22536 and MDL12330A (Spehr et al, 2003, 2004; Veitinger et al, 2011). We examined whether these drugs affect cAMP levels. Incubation of sperm with SQ22536 or MDL12330A did not lower resting cAMP levels (Figure 3B). Surprisingly, SQ22536 rather elevated cAMP (Figure 3B). There is also evidence in mouse sperm that SQ22536 elevates cAMP. The drug accelerates the flagellar beat (Schuh et al, 2006), which is another, yet indirect, measure of a rise of cAMP.
Several other ligands for GPCRs do also not stimulate cAMP signalling
We also tested other ligands—angiotensin, calcitonin, fertilization promoting peptide (FPP), and adenosine—that reportedly activate cAMP signalling in mouse sperm via GPCRs (Fraser and Duncan, 1993; Adeoya-Osiguwa et al, 1998; Adeoya-Osiguwa and Fraser, 2003; Mededovic and Fraser, 2004; Burnett et al, 2010). In low (4 mM) HCO3− medium, angiotensin, calcitonin, and FPP did not elevate cAMP levels (Figure 3B). In contrast, adenosine and chloro-adenosine elevated cAMP levels by 6.7- and 3.2-fold, respectively (Figure 3B). Whether the cAMP increase by adenosine involves GPCRs is unclear (Burnett et al, 2010). The tmAC inhibitors SQ22536 and MDL12330A did not suppress the cAMP increase evoked by adenosine in mouse (Schuh et al, 2006) and in human sperm (Figure 3B). Moreover, the combination of IBMX and adenosine or chloro-adenosine did not elevate cAMP levels beyond the level reached by each substance alone (Figure 3B). We conclude that adenosine and chloro-adenosine inhibit PDEs rather than activate GPCRs. A similar conclusion has been reached for the action of adenosine in mouse sperm (Schuh et al, 2006, 2007). In summary, besides odorants, various other agonists thought to control cAMP signalling via GPCRs, in fact, do not elevate cAMP levels in human sperm. Angiotensin, calcitonin, and FPP also did not evoke Ca2+ signals in human sperm (Supplementary Figure S6). Most importantly, also adenosine did not elevate [Ca2+]i (Supplementary Figure S6), supporting the notion that cAMP does not stimulate Ca2+entry (Strünker et al, 2011).
Activation of CatSper by odorants does not involve a metabotropic pathway
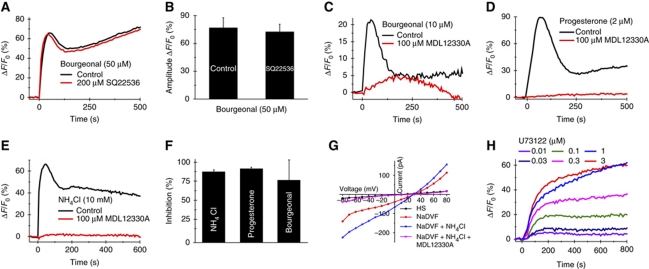
Notwithstanding the failure of bourgeonal and tmAC inhibitors to elevate and lower cAMP levels, respectively, we examined whether SQ22536 and MDL12330A impair bourgeonal-induced Ca2+ signals. In a previous report, incubation of sperm with SQ22536 (200 μM) completely inhibited bourgeonal-evoked Ca2+ signals (Veitinger et al, 2011). We failed to reproduce this result: Ca2+ signals were similar in the absence and presence of SQ22536 (Figure 4A and B); SQ22536 itself did not change [Ca2+]i at concentrations ⩽400 μM (Supplementary Figure S7A). In the presence of MDL12330A (100 μM), the amplitude of the bourgeonal-evoked Ca2+ signal was strongly attenuated (82±26%, seven experiments) (Figure 4C and F). In contrast to SQ22536, MDL12330A affected [Ca2+]i on its own (Supplementary Figure S7B). MDL12330A not only inhibits tmACs, but is also known to block Cav channels, TRP channels, and store-operated Ca2+ channels (Lee et al, 1985; Palfreyman et al, 1989; Rampe et al, 1989). The drug has been widely used as a tool to directly inhibit Ca2+ entry in somatic cells (Rampe et al, 1987; van Rossum et al, 2000; Snetkov et al, 2001; Wang, 2003). We wondered whether MDL12330A also blocks CatSper. The CatSper channel is directly activated by progesterone and intracellular alkalization (Lishko et al, 2010, 2011; Strünker et al, 2011). MDL12330A suppressed Ca2+ signals evoked both by progesterone (Figure 4D and F; 97±2% inhibition, four experiments) and by intracellular alkalization (Figure 4E and F; 93±3% inhibition, four experiments). The constant of half-maximal inhibition of alkaline-evoked Ca2+ signals was 19.2±8.2 μM (three experiments) (Supplementary Figure S7C and D). Moreover, in patch-clamp experiments, MDL12330A completely abolished CatSper currents (Figure 4G). Thus, MDL12330A and SQ22536 are not suitable to probe cAMP-mediated Ca2+ signalling in sperm: suppression of odorant-evoked Ca2+ signals by MDL12330A reflects inhibition of CatSper channels rather than inhibition of tmACs; and SQ22536 does not inhibit bourgeonal-evoked Ca2+ signals and elevates rather than lowers cAMP levels. In summary, CatSper activation by bourgeonal does not involve tmACs and cAMP.
Figure 4.
Pharmacology of inhibitors for transmembrane adenylyl cyclases (tmACs) and phospholipase C (PLC). (A) Ca2+ signals induced by bourgeonal in the presence of the tmAC inhibitor SQ22536. (B) Mean amplitude of bourgeonal-induced Ca2+ signals in the absence and presence of 200 μM SQ22536 (four experiments). (C–E) Ca2+ signals induced by bourgeonal (C), progesterone (D) and ammonium chloride (NH4Cl) (E) in the presence of the tmAC inhibitor MDL12330A. (F) Relative inhibition of the signal amplitudes by 100 μM MDL12330A (⩾4 experiments). (G) Whole-cell membrane currents in extracellular solution containing Ca2+ and Mg2+ (HS). Monovalent CatSper currents in divalent-free Na+-based bath solution before (NaDVF) and after intracellular alkalization by ammonium chloride (10 mM) (NaDVF+NH4Cl) in the absence and presence of MDL12330A (100 μM). MDL12330A completely inhibited basal and alkaline-activated monovalent CatSper currents. Currents were recorded at pHi 7.3 in the absence of intracellular divalent ions. (H) Ca2+ signals induced by the PLC inhibitor U73122.
It has been reported that olfactory receptors can activate phospholipase C (PLC; Klasen et al, 2010). Therefore, we wondered whether odorants might act via a PLC rather than a tmAC pathway. The PLC inhibitor U73122 has been used at micromolar concentrations to interfere with thermotaxis and the acrosome reaction in human sperm (Bahat and Eisenbach, 2010; Chavez et al, 2011). However, at nanomolar concentrations (K1/2=0.98±1.1 μM, three experiments), the drug itself evoked Ca2+ signals in human (Figure 4H; Supplementary Figure S7E) and in mouse sperm (Chavez et al, 2011). U73122 is a steroid derivative that might directly activate CatSper channels. Moreover, U73122 is not very specific; in somatic cells, the drug inhibits Ca2+ pumps, releases Ca2+ from intracellular stores, activates and inhibits ion channels, and binds to GPCRs (Mogami et al, 1997; Hughes et al, 2000; Macmillan and McCarron, 2010). Thus, U73122—like MDL12330A and SQ22536—is ill suited to investigate Ca2+ signalling in sperm. Consequently, we did not test whether the drug inhibits bourgeonal-induced Ca2+ signals.
We further investigated the signalling pathway for bourgeonal by kinetic Ca2+ fluorimetry and patch-clamp recordings. Cellular responses involving metabotropic pathways often display a latency. For example, olfactory neurons respond to odorants with a latency of ∼200 ms (Firestein and Werblin, 1989; Lowe and Gold, 1993; Kaupp, 2010). However, there are notable examples of ultrafast metabotropic signalling pathways: for example, phototransduction in insect photoreceptors involving a PLC occurs within <10 ms (Gonzalez-Bellido et al, 2011). Such almost instantaneous responses usually involve ionotropic chemoreceptors or mechanoreceptors (Gillespie and Muller, 2009; Kaupp, 2010). We determined the latency of bourgeonal-induced Ca2+ signals by rapid mixing of odorants and sperm in a stopped-flow apparatus (Figure 5A and B). After mixing with bourgeonal, [Ca2+]i rose with no detectable latency (time resolution of the mixing device was 36.6 ms) (Figure 5B), arguing against an olfactory pathway and indicating that bourgeonal directly activates CatSper.
Figure 5.
Activation of CatSper by odorants does not involve a metabotropic pathway. (A) Bourgeonal-induced Ca2+ signals recorded in a stopped-flow apparatus. (B) Ca2+ signals from (A) shown on an extended time scale. Intracellular Ca2+ concentration rose within time resolution of the stopped-flow apparatus (36.6 ms). (C) Whole-cell membrane currents in extracellular solution containing Ca2+ and Mg2+ (HS). Monovalent CatSper currents in divalent-free Na+-based bath solution before (NaDVF) and after perfusion with 50 μM bourgeonal (NaDVF+Bg). Currents were recorded at pHi 7.3 in the absence of intracellular divalent ions. The pipette solution contained GDPβS (250 μM) to preclude activation of G proteins.
To rule out that CatSper is activated by a GPCR pathway, we recorded monovalent CatSper currents in the presence of intracellular GDPβS (250 μM), which has been shown to almost completely abolish G protein action on ion channels and tmACs (Eckstein et al, 1979; Holz et al, 1986; Cuevas and Adams, 1997; Nörenberg et al, 1997; Wu and Barish, 1999; So and Kim, 2003; Gerevich et al, 2005; Aman et al, 2007; Drdla et al, 2009). However, GDPβS did neither change basal monovalent CatSper currents (−65±27 pA at −60 mV; range −27 to −141 pA; 30 experiments) nor prevent stimulation of CatSper currents by odorants (Figure 5C; Supplementary Figure S8). In the presence of the drug, bourgeonal and undecanal potentiated CatSper currents at −60 mV by 1.9±0.4-fold (four experiments) and 1.7±0.2-fold (eight experiments), respectively. Similarly, GDPβS did not prevent activation of CatSper by progesterone and PGE1 (Supplementary Figure S8). In summary, odorants directly activate CatSper without involving a metabotropic pathway and synthesis of second messengers.
Analogues of cyclic nucleotides activate human CatSper
cAMP enhances the frequency of the flagellar beat (Esposito et al, 2004; Schuh et al, 2006; Xie et al, 2006). It is contentious, however, whether the control of the flagellar beat by cAMP involves a rise of [Ca2+]i. Elevation of cAMP levels in mouse and human sperm by either HCO3− or adenosine, or by photorelease of cAMP from caged cAMP fails to increase [Ca2+]i (Wennemuth et al, 2003; Carlson et al, 2007; Strünker et al, 2011). In contrast, extracellular application of 8-Br-cAMP and 8-Br-cGMP stimulates an increase of [Ca2+]i (Kobori et al, 2000; Ren et al, 2001; Liu et al, 2007; Xia et al, 2007; Machado-Oliveira et al, 2008; Xia and Ren, 2009) that is abolished in CatSper−/− mice (Ren et al, 2001; Xia et al, 2007; Xia and Ren, 2009), suggesting that Ca2+ enters via CatSper and that CatSper is controlled by cyclic nucleotides. How can we reconcile the two sets of conflicting results?
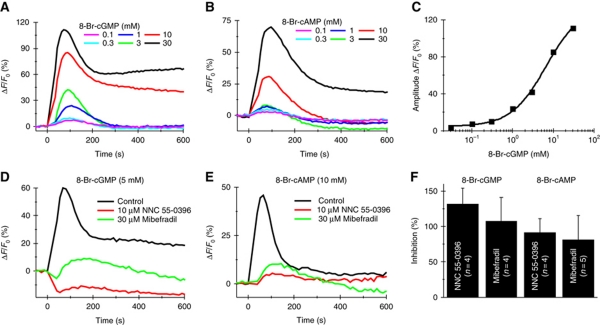
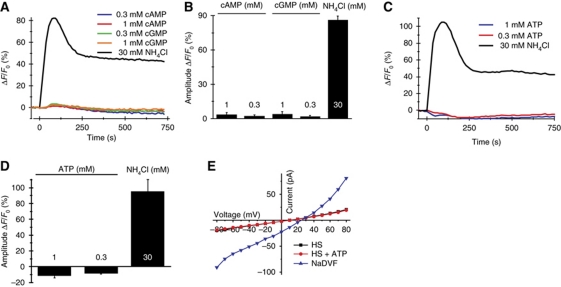
We examined whether 8-Br-cNMPs stimulate Ca2+ signals in human sperm (Figure 6A and B). 8-Br-cGMP and 8-Br-cAMP evoked a rapid and transient Ca2+ signal at concentrations ⩽3 and 10 mM, respectively; at higher concentrations, the transient was followed by a sustained elevation of [Ca2+]i. At 30 nM extracellular [Ca2+], the Ca2+ signal evoked by 8-Br-cGMP was abolished (Supplementary Figure S9). The K1/2 for 8-Br-cGMP was 7.9±1.8 mM (three experiments) (Figure 6C). 8-Br-cAMP was less potent, which prevented us from determining a complete dose-response curve. The Ca2+ signals were suppressed by NNC 55-0396 and mibefradil (Figure 6 D–F), indicating that Ca2+ enters the cell via CatSper. As a control, neither cAMP nor cGMP (⩽1 mM) stimulated Ca2+ influx (Figure 7A and B), indicating that CatSper is not activated by physiologically relevant concentrations of extracellular cAMP and cGMP. ATP (⩽1 mM) also failed to stimulate Ca2+ signals (Figure 7C and D), and in patch-clamp experiments, ATP failed to evoke membrane currents (Figure 7E). This indicates that CatSper is insensitive to ATP and that human sperm do not express functional P2X2 receptors that exist in mouse sperm (Navarro et al, 2011).
Figure 6.
Ca2+ signals evoked by 8-Bromo analogues of cyclic nucleotides. Signals induced by 8-Br-cGMP (A) or 8-Br-cAMP (B). (C) Dose–response relationship for 8-Br-cGMP based on the signal amplitudes from (A) (K1/2=6.5 mM). (D) 8-Br-cGMP-induced Ca2+ signals in the presence of the CatSper inhibitors NNC 55-0396 or mibefradil. (E) 8-Br-cAMP-induced Ca2+ signals in the presence of NNC 55-0396 or mibefradil. (F) Relative inhibition of the signal amplitudes by NNC 55-0396 and mibefradil. Inhibition of signal amplitudes >100% indicates that 8-Br-cGMP evoked a decrease in [Ca2+]i in the presence of the inhibitors.
Figure 7.
Cyclic nucleotides and ATP do not stimulate Ca2+ influx. (A) Time course of [Ca2+]i after stimulation with cAMP and cGMP. (B) Signal amplitudes evoked by cAMP, cGMP, and ammonium chloride (NH4Cl) (four experiments). (C) Time course of [Ca2+]i after stimulation with ATP. (D) Signal amplitudes induced by ATP and NH4Cl (four experiments). (E) Whole-cell membrane currents in extracellular solution containing Ca2+ and Mg2+ (HS) before and after perfusion with ATP (100 μM). ATP did not stimulate membrane currents. Monovalent CatSper currents in divalent-free Na+-based bath solution (NaDVF) served as a positive control. Currents were recorded at pHi 7.3 in the absence of intracellular divalent ions.
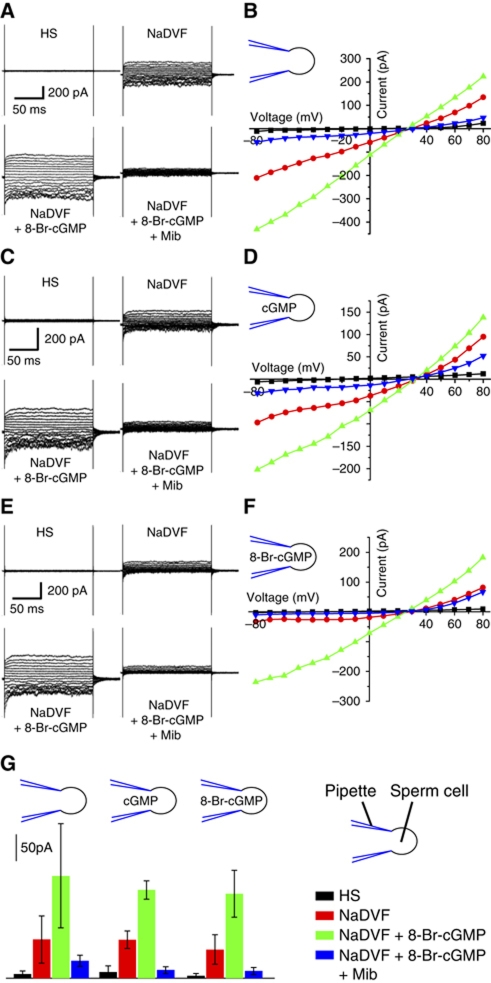
We studied the action of 8-Br-cGMP on CatSper currents (Figure 8). 8-Br-cGMP enhanced monovalent currents. The Vrev of CatSper currents (30±3 mV, nine experiments) and currents evoked by 8-Br-cGMP (28.6±3 mV, nine experiments) was similar. Mibefradil blocked the currents completely (Figure 8A and B), indicating that 8-Br-cNMPs activate CatSper. We tested whether 8-Br-cGMP acts from the inside or from the outside. Currents in standard HS and divalent-free solutions (NaDVF) recorded in the presence of 5 mM cGMP (Figure 8C and D) or 8-Br-cGMP (Figure 8E and F) in the pipette solution were similar to control currents recorded in the absence of intracellular cyclic nucleotides (Figure 8G). However, superfusion of sperm with 8-Br-cGMP enhanced monovalent CatSper currents, no matter whether cGMP or 8-Br-cGMP was included in the pipette solution (Figure 8C–F). Under all conditions tested, currents evoked by extracellular 8-Br-cGMP were completely blocked by mibefradil (Figure 8A–G). In sperm stimulated with 8-Br-cGMP, [Ca2+]i rose without detectable latency (<36.6 ms) and 8-Br-cGMP potentiated CatSper currents also in the presence of intracellular GDPβS (Supplementary Figure S9C). Thus, 8-substituted analogues of cyclic nucleotides directly activate CatSper channels through a site that is accessible from the extracellular space only. This explains why extracellular cNMP analogues but not intracellular cAMP elevate [Ca2+]i. Moreover, this result supports the notion that CatSper is promiscuously activated by diverse small molecules.
Figure 8.
Whole-cell CatSper currents are potentiated by extracellular 8-Br-cGMP. Currents were recorded at pHi 7.3 in the absence of intracellular divalent ions. Voltage was stepped from −80 to +80 mV in steps of 10 mV from a holding potential of 0 mV. (A) Currents in extracellular solution containing Ca2+ and Mg2+ (HS) and monovalent CatSper currents in divalent-free Na+-based bath solution (NaDVF). Extracellular 8-Br-cGMP (5 mM) potentiated monovalent currents (NaDVF+8-Br-cGMP). Currents were completely blocked by 30 μM mibefradil (NaDVF+8-Br-cGMP + Mib). (B) Current–voltage relationship from (A). (C) Currents recorded as described in (A), with 5 mM cGMP included in the pipette solution. (D) Current–voltage relationship from (C). (E) Currents recorded as described in (A), with 5 mM 8-Br-cGMP included in the pipette solution. (F) Current–voltage relationship from (E). (G) Mean current amplitudes at −60 mV (4–10 experiments) recorded in Ca2+ and Mg2+ containing solution (HS), in divalent-free solution (NaDVF), after perfusion with 5 mM 8-Br-cGMP (NaDVF+8-Br-cGMP), and after perfusion with 5 mM 8-Br-cGMP and 30 μM mibefradil (NaDVF+8-Br-cGMP+Mib). The currents were recorded either in the absence or in the presence of 5 mM cGMP or 8-Br-cGMP in the pipette solution. Extracellular but not intracellular application of cyclic nucleotides potentiated CatSper currents.
Even ligands for TRPM8 channels that evoke Ca2+ entry in human sperm (De Blas et al, 2009) might target CatSper. The TRPM8 agonist menthol stimulates a rapid Ca2+ signal (De Blas et al, 2009; Supplementary Figure S10A) that is inhibited by NNC 55-0396 and mibefradil (Supplementary Figure S10B and C). In support of the notion that CatSper mediates menthol-induced Ca2+ entry, icilin, a potent TRPM8 agonist (K1/2=0.36 μM; McKemy et al, 2002), did not elevate [Ca2+]i in human sperm at concentrations up to 100 μM (Supplementary Figure S10D).
Discussion
Chemicals as diverse as steroids, prostaglandins, odorants, menthol, and analogous of cyclic nucleotides activate the human CatSper channel without involving metabotropic signalling pathways. Activation happens through promiscuous, extracellularly accessible site(s) either on the channel itself or on associated proteins. Our results resolve several controversial issues and have important bearings on future studies of Ca2+ signalling in sperm.
Are there tmACs in sperm?
For more than two decades, ligands of GPCRs including odorants have been proposed to control sperm via cAMP-signalling pathways. However, a growing body of evidence now suggests that mouse and human sperm, in fact, are lacking functional tmACs. First, several tmAC−/− mice (AC1−/−, AC5−/−, AC6−/−, and AC8−/−) display no obvious fertility phenotype. Although tmAC3−/− mice are subfertile (Livera et al, 2005), subfertility might reflect faulty sperm development (Livera et al, 2005) and mating behaviour (Wang et al, 2006). Second, forskolin and its congeners fail to elevate cAMP levels (Aitken et al, 1986; Rojas and Bruzzone, 1992; Rojas et al, 1993; Jaiswal and Conti, 2003; Hess et al, 2005; Strünker et al, 2011); see however Baxendale and Fraser (2003) and Livera et al (2005). In countless studies of many cell types from worm to humans, forskolin has proven its worth as a powerful and reliable stimulator of most if not all tmAC isoforms (Kamenetsky et al, 2006). Therefore, the failure needs to be taken seriously. Third, in sperm lacking the sAC, no cAMP synthesis can be detected, cAMP levels are below detection threshold (Xie et al, 2006), and inhibition of cAMP hydrolysis by IBMX fails to increase flagellar beat frequency (Schuh et al, 2006). Moreover, in tmAC3−/− and wild-type sperm, basal cAMP levels are similar (Livera et al, 2005). Finally, several ligands for GPCRs that reportedly activate tmACs in mouse sperm (Fraser and Duncan, 1993; Adeoya-Osiguwa et al, 1998; Adeoya-Osiguwa and Fraser, 2003; Mededovic and Fraser, 2004), in fact, fail to raise cAMP levels in human sperm. Altogether, these observations are hard to reconcile with the presence of functional tmACs in sperm. Alternatively, tmACs together with PDE, PKA, and its substrates might be organized in sparse signalling complexes. Such local cAMP signalling might escape detection by techniques that measure total cAMP content. For the time being, until local cAMP signalling in sperm has been unequivocally demonstrated with spatio-temporal resolution, we argue that cAMP levels in both mouse and human sperm are controlled by sAC (Schuh et al, 2006; Xie et al, 2006).
Does cAMP stimulate Ca2+ entry?
Another issue concerns the relationship between cAMP and Ca2+ entry. Membrane-permeable analogues of cyclic nucleotides stimulate Ca2+ entry in mouse and human sperm (Kobori et al, 2000; Ren et al, 2001; Liu et al, 2007; Xia et al, 2007; Machado-Oliveira et al, 2008; Xia and Ren, 2009). In contrast, several measures that increase intracellular cAMP levels—HCO3−, IBMX, photorelease from caged cAMP, and adenosine—fail to stimulate a Ca2+ influx (Wennemuth et al, 2003; Carlson et al, 2007; Strünker et al, 2011; Supplementary Figure S6). Our finding that extracellular 8-Br-cGMP and 8-Br-cAMP, but not intracellular cAMP and cGMP, directly activate human CatSper provides an explanation for these seemingly contradictory findings. However, there might be differences among mammalian species. In mouse sperm, no direct action of 8-Br-cNMPs on CatSper was observed (Kirichok et al, 2006). Different experimental conditions and drug concentrations might account for this difference. Alternatively, mouse CatSper might not be directly activated by cyclic nucleotide analogues. Such a species difference has been shown for progesterone, which activates human but not mouse CatSper (Lishko et al, 2011). Moreover, in bovine sperm, intracellular photorelease of cNMP derivatives in the flagellum evokes a rapid and robust Ca2+ influx (Wiesner et al, 1998), and in sperm of marine invertebrates, a well-established cGMP-signalling pathway controls Ca2+ entry (Kaupp et al, 2003; Matsumoto et al, 2003; Strünker et al, 2006; Darszon et al, 2008; Bönigk et al, 2009). In summary, there is no evidence that a rise of cyclic nucleotides elevates [Ca2+]i at least in mouse and human sperm.
How many different Ca2+-permeable channels exist in sperm?
Another issue is concerned with the type of Ca2+ channels that mediate Ca2+ entry. Four different channel types—CatSper, CNG channels, various voltage-activated Cav channels, and TRP channels—have been proposed to pass Ca2+ into sperm (Publicover et al, 2007; Darszon et al, 2011). However, only CatSper has been shown to be required for fertilization: Mice deficient in either one of the pore-forming subunits (CatSper 1–4) or the auxiliary CatSper δ subunit are infertile due to impaired Ca2+ signalling and motility (Qi et al, 2007; Chung et al, 2011). In humans, mutations in CatSper genes have been correlated with male infertility (Hildebrand et al, 2010). Finally, CatSper is the only voltage-activated Ca2+ channel that can be detected by patch-clamp recordings from ejaculated human and epididymal mouse sperm (Kirichok et al, 2006; Navarro et al, 2008; Xia and Ren, 2009; Lishko et al, 2010, 2011; Kirichok and Lishko, 2011; Strünker et al, 2011). Currents carried by other Cav channels have only been recorded from precursor cells or testicular sperm (Martinez-Lopez et al, 2009; Xia and Ren, 2009). The only other Ca2+-permeable ion channel that has been functionally characterized is a purinergic P2X2 receptor present in mouse sperm (Navarro et al, 2011). However, this purinergic channel is functionally absent in human sperm.
Pharmacology of sperm
The study of signalling pathways in sperm has heavily rested upon pharmacological tools. Our results regarding three inhibitors and a GPCR agonist are alarming. Adenosine, which activates adenosine receptors in somatic cells, probably inhibits PDE activity and thereby elevates cAMP. SQ22536, a tmAC inhibitor, enhances rather than lowers cAMP levels probably also by inhibiting PDE activity. MDL12330A, another tmAC inhibitor, potently blocks CatSper channels. U73122, a PLC inhibitor used at micromolar concentrations for motility assays, provokes on its own sizeable Ca2+ signals at nanomolar concentrations. In Ca2+ fluorimetry, but not patch clamping, also the pharmacology of CatSper inhibitors is complex, because at high concentrations, these drugs evoke Ca2+ signals on their own. These examples caution against rash interpretations of results obtained by pharmacological intervention, even more so, if drug concentrations are used that are orders of magnitude larger than those required to specifically inhibit the function of the respective target. The problem is particularly severe in sperm: because of the miniscule flagellar volume, even minute changes in the activity of a few CatSper channels or Ca2+ transport systems can evoke sizeable Ca2+ signals.
CatSper might be a polymodal sensor for chemical cues
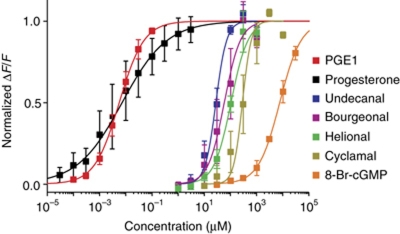
We show that the CatSper channel complex or associated protein(s) can accommodate diverse chemicals. Therefore, we suspect that ligands thought to act via GPCRs or ligand-gated ion channels, in fact, activate CatSper. The potency of structurally diverse CatSper agonists (Supplementary Figure S11) differs by orders of magnitude, ranging from nanomolar to millimolar concentrations (Figure 9). Notably, the physiological factors progesterone and prostaglandins represent by far the most potent CatSper agonists. However, we note that CatSper activation by menthol, cyclamal, and helional needs to be confirmed by patch-clamp recordings. Although the activation of CatSper by odorants, 8-Br-cNMPs, or menthol is physiologically irrelevant, it points to a potential source of artifacts that needs to be considered in future studies when testing sperm for native factors from the oviduct.
Figure 9.
Normalized dose–response relationships for various compounds that activate CatSper. Each data point represents the mean of n⩾3.
Promiscuous binding sites for small ligands exist in other ion channels. For example, the cardiac human ether-a-go-go-related-gene K+ channel (HERG) is inhibited by structurally diverse molecules that bind to a few amino-acid residues in transmembrane segment S6 (Zou et al, 2010; Windisch et al, 2011). Crystal structures of acetylcholine receptors reveal a common binding site for general anaesthetics. The site hosts functionally and structurally diverse compounds at different depths along the binding cavity (Nury et al, 2011). Finally, the TRPV1 channel is modulated by different chemical and physical stimuli (Vriens et al, 2009; Baez-Nieto et al, 2011). The channel is directly activated by lipophilic compounds such as capsaicin and other vanilloids. Various lipids, such as conjugates of biogenic amines and oxygenated eicosatetraenoic acids, derived from the fatty-acid pool, also activate TRPV1. Moreover, TRPV1 is activated by acidic extracellular pH, heat, and membrane depolarization. In neurons of dorsal root ganglia, the polymodal TRPV1 channel integrates various noxious stimuli (Stucky et al, 2009).
We envisage a similar principle operating in CatSper channels: The complex regulation of CatSper by pH, voltage, and ligands might be functionally important. On their voyage across the oviduct, sperm are exposed to a series of chemical milieus that differ in viscosity, temperature, pH, HCO3−, progesterone, and possibly several other factors. These factors enable sperm to locate and fertilize the egg. We propose that CatSper is a polymodal ‘stimulus integrator’ that translates the chemical and physical code of each microenvironment into spatio-temporal Ca2+ patterns and, therefore, is key for navigation of sperm over long distances to reach the site of fertilization. It needs to be studied whether female factors act synergistically or even antagonistically on CatSper. Moreover, it needs to be studied whether agonists evoke stereotypic or unique motility responses.
Materials and methods
Materials and reagents
NNC 55-0396, mibefradil, U73122, SQ22536, and MDL12330A were purchased from Tocris (MN, USA). Bourgeonal was from Enzo Life Sciences (NY, USA). 8-Br-cAMP and 8-Br-cGMP were obtained from Biolog (Bremen, Germany). All other reagents were obtained from Sigma-Aldrich (MO, USA).
Sperm preparation
Human semen samples were obtained from healthy volunteers with their prior consent. Sperm were handled and purified as described (Strünker et al, 2011). In brief, sperm were purified by a ‘swim-up’ procedure in human tubular fluid (HTF+) containing (in mM): 97.8 NaCl, 4.69 KCl, 0.2 MgSO4, 0.37 KH2PO4, 2.04 CaCl2, 0.33 Na-pyruvate, 21.4 lactic acid, 2.78 glucose, 21 HEPES, and 25 NaHCO3 adjusted to pH 7.3–7.4 with NaOH. After washing, 3 mg/ml human serum albumin (HSA) (Irvine Scientific, CA, USA) was added to the HTF+ (making it HTF++). Before experiments, sperm were incubated for at least 2 h in HTF++ at 37°C. Under these conditions, sperm become capacitated. All Ca2+ measurements and patch-clamp recordings were done with capacitated sperm unless stated otherwise. To obtain non-capacitated sperm, samples were purified and incubated in HTF lacking HCO3− and HSA.
Measurement of changes in [Ca2+]i
Changes in [Ca2+]i were measured in sperm loaded with Fluo-4 (Invitrogen, OR, USA). Measurements were performed either in 384 multi-well plates (Greiner Bio-One, Frickenhausen, Germany) in a fluorescence plate reader (Fluostar Omega; BMG Labtech, Offenburg, Germany) at 29°C or in a rapid-mixing device in the stopped-flow mode (SFM400; Bio-Logic, Claix, France) at 37°C (Kilic et al, 2009; Strünker et al, 2011). After loading, sperm were washed and resuspended in HTF+. Ligands or inhibitors were dissolved in HTF+. Sperm were incubated with NNC 55-0396, mibefradil, SQ22536, and MDL12330A for ∼5 min prior to application of agonists.
Patch-clamp recordings
We recorded from sperm in the whole-cell configuration as described (Strünker et al, 2011). Seals between pipette and sperm were formed either at the cytoplasmic droplet or in the neck region in standard extracellular solution (HS) containing (in mM): 135 NaCl, 5 KCl, 1 MgSO4, 1 CaCl2, 5 glucose, 1 Na-pyruvate, 10 lactic acid, 20 HEPES adjusted to pH 7.4 with NaOH. Monovalent currents were recorded in a sodium-based divalent-free solution (NaDVF) containing (in mM): 140 NaCl, 40 HEPES, and 1 EGTA adjusted to pH 7.4 with NaOH; the pipette (10–15 MΩ) solution contained (in mM): 130 Cs-aspartate, 50 HEPES, 5 EGTA, 5 CsCl adjusted to pH 7.3 with CsOH. The Vrev of CatSper currents was ∼8 mV less positive as reported previously (Strünker et al, 2011), because we modified the composition of the intracellular solution (e.g., 130 mM Cs-aspartate instead of 121 mM Cs-methanesulphonate). Data were not corrected for liquid junction potentials.
Determination of cAMP content
Sperm in HTF++ containing 25 or 4 mM NaHCO3 were mixed 1:1 (v/v) with substances dissolved in the same buffer. The cAMP content was determined by RIAs (125I-labelled cAMP; IBL, Germany) (Strünker et al, 2011).
Data analysis
Statistical analysis and fitting of the data were performed using Prism 5 (GraphPad Software, Inc.), OriginPro 8.1G SR3 (OriginLab Corporation), or Clampfit 10.2 (Molecular Devices). All data are given as mean±standard deviation (number of experiments).
Supplementary Material
Acknowledgments
This work was supported by the German Research Foundation (SFB645), the International Helmholtz Research School on Biophysics and Soft Matter (BIOSOFT), and the Japanese Society for the Promotion of Science (JSPS). We thank S Stark for technical assistance, H Krause for preparing the manuscript, and Dr C Bernsdorff for preparing the figures.
Author contributions: CB, NG, IW, NK, MN, MK, AM, and TS designed and performed experiments. TS and UBK conceived the project. TS and UBK wrote the manuscript. All authors revised and edited the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Adeoya-Osiguwa SA, Dudley RK, Hosseini R, Fraser LR (1998) FPP modulates mammalian sperm function via TCP-11 and the adenylyl cyclase/cAMP pathway. Mol Reprod Dev 51: 468–476 [DOI] [PubMed] [Google Scholar]
- Adeoya-Osiguwa SA, Fraser LR (2003) Calcitonin acts as a first messenger to regulate adenylyl cyclase/cAMP and mammalian sperm function. Mol Reprod Dev 65: 228–236 [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Kelly RW (1985) Analysis of the direct effects of prostaglandins on human sperm function. J Reprod Fertil 73: 139–146 [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Mattei A, Irvine S (1986) Paradoxical stimulation of human sperm motility by 2-deoxyadenosine. J Reprod Fertil 78: 515–527 [DOI] [PubMed] [Google Scholar]
- Aman TK, Shen RY, Haj-Dahmane S (2007) D2-like dopamine receptors depolarize dorsal raphe serotonin neurons through the activation of nonselective cationic conductance. J Pharmacol Exp Ther 320: 376–385 [DOI] [PubMed] [Google Scholar]
- Baez-Nieto D, Castillo JP, Dragicevic C, Alvarez O, Latorre R (2011) Thermo-TRP channels: biophysics of polymodal receptors. Adv Exp Med Biol 704: 469–490 [DOI] [PubMed] [Google Scholar]
- Bahat A, Eisenbach M (2010) Human sperm thermotaxis is mediated by phospholipase C and inositol trisphosphate receptor Ca2+ channel. Biol Reprod 82: 606–616 [DOI] [PubMed] [Google Scholar]
- Baxendale RW, Fraser LR (2003) Evidence for multiple distinctly localized adenylyl cyclase isoforms in mammalian spermatozoa. Mol Reprod Dev 66: 181–189 [DOI] [PubMed] [Google Scholar]
- Bedu-Addo K, Costello S, Harper C, Hado-Oliveira G, Lefievre L, Ford C, Barratt C, Publicover S (2008) Mobilisation of stored calcium in the neck region of human sperm--a mechanism for regulation of flagellar activity. Int J Dev Biol 52: 615–626 [DOI] [PubMed] [Google Scholar]
- Bönigk W, Loogen A, Seifert R, Kashikar N, Klemm C, Krause E, Hagen V, Kremmer E, Strünker T, Kaupp UB (2009) An atypical CNG channel activated by a single cGMP molecule controls sperm chemotaxis. Sci Signal 2: Ra68. [DOI] [PubMed] [Google Scholar]
- Burnett LA, Blais EM, Unadkat JD, Hille B, Tilley SL, Babcock DF (2010) Testicular expression of Adora3i2 in Adora3 knockout mice reveals a role of mouse A3Ri2 and human A3Ri3 adenosine receptors in sperm. J Biol Chem 285: 33662–33670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton KA, Mcknight GS (2007) Pka, germ cells, and fertility. Physiology (Bethesda) 22: 40–46 [DOI] [PubMed] [Google Scholar]
- Carlson AE, Hille B, Babcock DF (2007) External Ca2+ acts upstream of adenylyl cyclase SACY in the bicarbonate signaled activation of sperm motility. Dev Biol 312: 183–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez JC, De Blas GA, De La Vega-Beltran JL, Nishigaki T, Chirinos M, Gonzalez-Gonzalez ME, Larrea F, Solis A, Darszon A, Trevino CL (2011) The opening of maitotoxin-sensitive calcium channels induces the acrosome reaction in human spermatozoa: differences from the zona pellucida. Asian J Androl 13: 159–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JJ, Navarro B, Krapivinsky G, Krapivinsky L, Clapham DE (2011) A novel gene required for male fertility and functional CATSPER channel formation in spermatozoa. Nat Commun 2: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas J, Adams DJ (1997) M4 muscarinic receptor activation modulates calcium channel currents in rat intracardiac neurons. J Neurophysiol 78: 1903–1912 [DOI] [PubMed] [Google Scholar]
- Darszon A, Guerrero A, Galindo BE, Nishigaki T, Wood CD (2008) Sperm-activating peptides in the regulation of ion fluxes, signal transduction and motility. Int J Dev Biol 52: 595–606 [DOI] [PubMed] [Google Scholar]
- Darszon A, Nishigaki T, Beltran C, Trevino CL (2011) Calcium channels in the development, maturation, and function of spermatozoa. Physiol Rev 91: 1305–1355 [DOI] [PubMed] [Google Scholar]
- De Blas GA, Darszon A, Ocampo AY, Serrano CJ, Castellano LE, Hernandez-Gonzalez EO, Chirinos M, Larrea F, Beltran C, Trevino CL (2009) Trpm8, a versatile channel in human sperm. PLoS One 4: E6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jonge C (2005) Biological basis for human capacitation. Hum Reprod Update 11: 205–214 [DOI] [PubMed] [Google Scholar]
- Drdla R, Gassner M, Gingl E, Sandkuhler J (2009) Induction of synaptic long-term potentiation after opioid withdrawal. Science 325: 207–210 [DOI] [PubMed] [Google Scholar]
- Eckstein F, Cassel D, Levkovitz H, Lowe M, Selinger Z (1979) Guanosine 5'-O-(2-Thiodiphosphate). An inhibitor of adenylate cyclase stimulation by guanine nucleotides and fluoride ions. J Biol Chem 254: 9829–9834 [PubMed] [Google Scholar]
- Eisenbach M, Giojalas LC (2006) Sperm guidance in mammals - an unpaved road to the egg. Nat Rev Mol Cell Biol 7: 276–285 [DOI] [PubMed] [Google Scholar]
- Esposito G, Jaiswal BS, Xie F, Krajnc-Franken MA, Robben TJ, Strik AM, Kuil C, Philipsen RLA, Van Duin M, Conti M, Gossen JA (2004) Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proc Natl Acad Sci USA 101: 2993–2998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein S, Werblin F (1989) Odor-induced membrane currents in vertebrate-olfactory receptor neurons. Science 244: 79–82 [DOI] [PubMed] [Google Scholar]
- Florman HM, Jungnickel MK, Sutton KA (2008) Regulating the acrosome reaction. Int J Dev Biol 52: 503–510 [DOI] [PubMed] [Google Scholar]
- Fraser LR, Duncan AE (1993) Adenosine analogues with specificity for A2 receptors bind to mouse spermatozoa and stimulate adenylate cyclase activity in uncapacitated suspensions. J Reprod Fertil 98: 187–194 [DOI] [PubMed] [Google Scholar]
- Gerevich Z, Müller C, Illes P (2005) Metabotropic P2Y1 receptors inhibit P2X3 receptor-channels in rat dorsal root ganglion neurons. Eur J Pharmacol 521: 34–38 [DOI] [PubMed] [Google Scholar]
- Gillespie PG, Muller U (2009) Mechanotransduction by hair cells: models, molecules, and mechanisms. Cell 139: 33–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Bellido PT, Wardill TJ, Juusola M (2011) Compound eyes and retinal information processing in miniature dipteran species match their specific ecological demands. Proc Natl Acad Sci USA 108: 4224–4229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper CV, Kirkman-Brown JC, Barratt CLR, Publicover SJ (2003) Encoding of progesterone stimulus intensity by intracellular [Ca2+] ([Ca2+]I) in human spermatozoa. Biochem J 373: 407–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess KC, Jones BH, Marquez B, Chen Y, Ord TS, Kamenetsky M, Miyamoto C, Zippin JH, Kopf GS, Suarez SS, Levin LR, Williams CJ, Buck J, Moss SB (2005) The ‘Soluble’ adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev Cell 9: 249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand MS, Avenarius MR, Fellous M, Zhang Y, Meyer NC, Auer J, Serres C, Kahrizi K, Najmabadi H, Beckmann JS, Smith RJ (2010) Genetic male infertility and mutation of CATSPER ion channels. Eur J Hum Genet 18: 1178–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Rane SG, Dunlap K (1986) GTP-binding proteins mediate transmitter inhibition of voltage-dependent calcium channels. Nature 319: 670–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SA, Gibson WJ, Young JM (2000) The interaction of U-73122 with the histamine H1 receptor: implications for the use of U-73122 in defining H1 receptor-coupled signalling pathways. Naunyn Schmiedebergs Arch Pharmacol 362: 555–558 [DOI] [PubMed] [Google Scholar]
- Jaiswal BS, Conti M (2003) Calcium regulation of the soluble adenylyl cyclase expressed in mammalian spermatozoa. Proc Natl Acad Sci USA 100: 10676–10681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamenetsky M, Middelhaufe S, Bank EM, Levin LR, Buck J, Steegborn C (2006) Molecular details of camp generation in mammalian cells: a tale of two systems. J Mol Biol 362: 623–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupp UB (2010) Olfactory signalling in vertebrates and insects: differences and commonalities. Nat Rev Neurosci 11: 188–200 [DOI] [PubMed] [Google Scholar]
- Kaupp UB, Kashikar ND, Weyand I (2008) Mechanisms of sperm chemotaxis. Annu Rev Physiol 70: 93–117 [DOI] [PubMed] [Google Scholar]
- Kaupp UB, Solzin J, Hildebrand E, Brown JE, Helbig A, Hagen V, Beyermann M, Pampaloni F, Weyand I (2003) The signal flow and motor response controlling chemotaxis of sea urchin sperm. Nat Cell Biol 5: 109–117 [DOI] [PubMed] [Google Scholar]
- Kilic F, Kashikar ND, Schmidt R, Alvarez L, Dai L, Weyand I, Wiesner B, Goodwin N, Hagen V, Kaupp UB (2009) Caged progesterone: a new tool for studying rapid nongenomic actions of progesterone. J Am Chem Soc 131: 4027–4030 [DOI] [PubMed] [Google Scholar]
- Kirichok Y, Lishko PV (2011) Rediscovering sperm ion channels with the patch-clamp technique. Mol Hum Reprod 17: 478–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirichok Y, Navarro B, Clapham DE (2006) Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature 439: 737–740 [DOI] [PubMed] [Google Scholar]
- Klasen K, Corey EA, Kuck F, Wetzel CH, Hatt H, Ache BW (2010) Odorant-stimulated phosphoinositide signaling in mammalian olfactory receptor neurons. Cell Signal 22: 150–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobori H, Miyazaki S, Kuwabara Y (2000) Characterization of intracellular Ca2+ increase in response to progesterone and cyclic nucleotides in mouse spermatozoa. Biol Reprod 63: 113–130 [DOI] [PubMed] [Google Scholar]
- Lee HR, Jaros JA, Roeske WR, Wiech NL, Ursillo R, Yamamura HI (1985) Potent enhancement of [3H]nitrendipine binding in rat cerebral cortical and cardiac homogenates: a putative mechanism for the action of MDL 12,330A. J Pharmacol Exp Ther 233: 611–616 [PubMed] [Google Scholar]
- Lishko PV, Botchkina IL, Fedorenko A, Kirichok Y (2010) Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell 140: 327–337 [DOI] [PubMed] [Google Scholar]
- Lishko PV, Botchkina IL, Kirichok Y (2011) Progesterone activates the principal Ca2+ channel of human sperm. Nature 471: 387–391 [DOI] [PubMed] [Google Scholar]
- Litvin TN, Kamenetsky M, Zarifyan A, Buck J, Levin LR (2003) Kinetic properties of ‘Soluble’ adenylyl cyclase. J Biol Chem 278: 15922–15926 [DOI] [PubMed] [Google Scholar]
- Liu J, Xia J, Cho KH, Clapham DE, Ren D (2007) CatSperbeta, a novel transmembrane protein in the CatSper channel complex. J Biol Chem 282: 18945–18952 [DOI] [PubMed] [Google Scholar]
- Livera G, Xie F, Garcia MA, Jaiswal B, Chen J, Law E, Storm DR, Conti M (2005) Inactivation of the mouse adenylyl cyclase 3 gene disrupts male fertility and spermatozoon function. Mol Endocrinol 19: 1277–1290 [DOI] [PubMed] [Google Scholar]
- Lowe G, Gold GH (1993) Nonlinear amplification by calcium-dependent chloride channels in olfactory receptor cells. Nature 366: 283–286 [DOI] [PubMed] [Google Scholar]
- Machado-Oliveira G, Lefievre L, Ford C, Herrero MB, Barratt C, Connolly TJ, Nash K, Morales-Garcia A, Kirkman-Brown J, Publicover S (2008) Mobilisation of Ca2+ stores and flagellar regulation in human sperm by S-nitrosylation: a role for NO synthesised in the female reproductive tract. Development 135: 3677–3686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan D, Mccarron JG (2010) The phospholipase C inhibitor U-73122 inhibits Ca2+ release from the intracellular sarcoplasmic reticulum Ca2+ store by inhibiting Ca2+ pumps in smooth muscle. Br J Pharmacol 160: 1295–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Lopez P, Santi CM, Trevino CL, Ocampo-Gutierrez AY, Acevedo JJ, Alisio A, Salkoff LB, Darszon A (2009) Mouse sperm K+ currents stimulated by pH and cAMP possibly coded by Slo3 channels. Biochem Biophys Res Commun 381: 204–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Solzin J, Helbig A, Hagen V, Ueno S-I, Kawase O, Maruyama Y, Ogiso M, Godde M, Minakata H, Kaupp UB, Hoshi M, Weyand I (2003) A sperm-activating peptide controls a cGMP-signaling pathway in starfish sperm. Dev Biol 260: 314–324 [DOI] [PubMed] [Google Scholar]
- Mckemy DD, Neuhausser WM, Julius D (2002) Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416: 52–58 [DOI] [PubMed] [Google Scholar]
- Mededovic S, Fraser LR (2004) Angiotensin II stimulates cAMP production and protein tyrosine phosphorylation in mouse spermatozoa. Reproduction 127: 601–612 [DOI] [PubMed] [Google Scholar]
- Meizel S (2004) The sperm, a neuron with a tail: ‘neuronal’ receptors in mammalian sperm. Biol Rev Camb Philos Soc 79: 713–732 [DOI] [PubMed] [Google Scholar]
- Mogami H, Lloyd MC, Gallacher DV (1997) Phospholipase C inhibitor, U73122, releases intracellular Ca2+, potentiates Ins(1,4,5)P3-mediated Ca2+ release and directly activates Ion channels in mouse pancreatic acinar cells. Biochem J 324 (Pt 2): 645–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro B, Kirichok Y, Chung JJ, Clapham DE (2008) Ion channels that control fertility in mammalian spermatozoa. Int J Dev Biol 52: 607–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro B, Miki K, Clapham DE (2011) ATP-activated P2X2 current in mouse spermatozoa. Proc Natl Acad Sci USA 108: 14342–14347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naz RK, Sellamuthu R (2006) Receptors in spermatozoa: are they real? J Androl 27: 627–636 [DOI] [PubMed] [Google Scholar]
- Neuhaus EM, Mashukova A, Barbour J, Wolters D, Hatt H (2006) Novel function of beta-arrestin2 in the nucleus of mature spermatozoa. J Cell Sci 119: 3047–3056 [DOI] [PubMed] [Google Scholar]
- Nolan MA, Babcock DF, Wennemuth G, Brown W, Burton KA, Mcknight GS (2004) Sperm-specific protein kinase A catalytic subunit Cα2 orchestrates cAMP signaling for male fertility. Proc Natl Acad Sci USA 101: 13483–13488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nörenberg W, Wirkner K, Illes P (1997) Effect of adenosine and some of its structural analogues on the conductance of NMDA receptor channels in a subset of rat neostriatal neurones. Br J Pharmacol 122: 71–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nury H, Van RC, Weng Y, Tran A, Baaden M, Dufresne V, Changeux JP, Sonner JM, Delarue M, Corringer PJ (2011) X-ray structures of general anaesthetics bound to a pentameric ligand-gated ion channel. Nature 469: 428–431 [DOI] [PubMed] [Google Scholar]
- Oren-Benaroya R, Orvieto R, Gakamsky A, Pinchasov M, Eisenbach M (2008) The sperm chemoattractant secreted from human cumulus cells is progesterone. Hum Reprod 23: 2339–2345 [DOI] [PubMed] [Google Scholar]
- Palfreyman MG, Dudley MW, Cheng HC, Mir AK, Yamada S, Roeske WR, Obata T, Yamamura HI (1989) Lactamimides: a novel chemical class of calcium antagonists with diltiazem-like properties. Biochem Pharmacol 38: 2459–2465 [DOI] [PubMed] [Google Scholar]
- Publicover S, Harper CV, Barratt C (2007) [Ca2+]i signalling in sperm - making the most of what you’ve got. Nat Cell Biol 9: 235–242 [DOI] [PubMed] [Google Scholar]
- Publicover SJ, Giojalas LC, Teves ME, De Oliveira GS, Garcia AA, Barratt CL, Harper CV (2008) Ca2+ signalling in the control of motility and guidance in mammalian sperm. Front Biosci 13: 5623–5637 [DOI] [PubMed] [Google Scholar]
- Qi H, Moran MM, Navarro B, Chong JA, Krapivinsky G, Krapivinsky L, Kirichok Y, Ramsey IS, Quill TA, Clapham DE (2007) All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc Natl Acad Sci USA 104: 1219–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quill TA, Ren D, Clapham D, Garbers DL (2001) A voltage-gated ion channel expressed specifically in spermatozoa. Proc Natl Acad Sci USA 98: 12527–12531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampe D, Poder T, Zhao ZY, Schilling WP (1989) Calcium channel agonist and antagonist binding in a highly enriched sarcolemma preparation obtained from canine ventricle. J Cardiovasc Pharmacol 13: 547–556 [PubMed] [Google Scholar]
- Rampe D, Triggle DJ, Brown AM (1987) Electrophysiologic and biochemical studies on the putative Ca++ channel blocker MDL 12,330A in an endocrine cell. J Pharmacol Exp Ther 243: 402–407 [PubMed] [Google Scholar]
- Ren D, Navarro B, Perez G, Jackson AC, Hsu S, Shi Q, Tilly JL, Clapham DE (2001) A sperm ion channel required for sperm motility and male fertility. Nature 413: 603–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas FJ, Bruzzone ME (1992) Regulation of cyclic adenosine monophosphate synthesis in human ejaculated spermatozoa. I. Experimental conditions to quantitate membrane-bound adenylyl cyclase activity. Hum Reprod 7: 1126–1130 [DOI] [PubMed] [Google Scholar]
- Rojas FJ, Patrizio P, Do J, Silber S, Asch RH, Moretti-Rojas I (1993) Evidence for a novel adenylyl cyclase in human epididymal sperm. Endocrinology 133: 3030–3033 [DOI] [PubMed] [Google Scholar]
- Schaefer M, Hofmann T, Schultz G, Gudermann T (1998) A new prostaglandin E receptor mediates calcium influx and acrosome reaction in human spermatozoa. Proc Natl Acad Sci USA 95: 3008–3013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetz AW, Dubin NH (1981) Progesterone and prostaglandin secretion by ovulated rat cumulus cell-oocyte complexes. Endocrinology 108: 457–463 [DOI] [PubMed] [Google Scholar]
- Schuh SM, Carlson AE, Mcknight GS, Conti M, Hille B, Babcock DF (2006) Signaling pathways for modulation of mouse sperm motility by adenosine and catecholamine agonists. Biol Reprod 74: 492–500 [DOI] [PubMed] [Google Scholar]
- Schuh SM, Hille B, Babcock DF (2007) Adenosine and catecholamine agonists speed the flagellar beat of mammalian sperm by a non-receptor-mediated mechanism. Biol Reprod 77: 960–969 [DOI] [PubMed] [Google Scholar]
- Snetkov VA, Hapgood KJ, Mcvicker CG, Lee TH, Ward JP (2001) Mechanisms of leukotriene D4-induced constriction in human small bronchioles. Br J Pharmacol 133: 243–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- So I, Kim KW (2003) Nonselective cation channels activated by the stimulation of muscarinic receptors in mammalian gastric smooth muscle. J Smooth Muscle Res 39: 231–247 [DOI] [PubMed] [Google Scholar]
- Spehr M, Gisselmann G, Poplawski A, Riffell JA, Wetzel CH, Zimmer RK, Hatt H (2003) Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science 299: 2054–2058 [DOI] [PubMed] [Google Scholar]
- Spehr M, Schwane K, Riffell JA, Barbour J, Zimmer RK, Neuhaus EM, Hatt H (2004) Particulate adenylate cyclase plays a key role in human sperm olfactory receptor-mediated chemotaxis. J Biol Chem 279: 40194–40203 [DOI] [PubMed] [Google Scholar]
- Strünker T, Goodwin N, Brenker C, Kashikar ND, Weyand I, Seifert R, Kaupp UB (2011) The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature 471: 382–386 [DOI] [PubMed] [Google Scholar]
- Strünker T, Weyand I, Bönigk W, Van Q, Loogen A, Brown JE, Kashikar N, Hagen V, Krause E, Kaupp UB (2006) A K+-selective cGMP-gated ion channel controls chemosensation of sperm. Nat Cell Biol 8: 1149–1154 [DOI] [PubMed] [Google Scholar]
- Stucky CL, Dubin AE, Jeske NA, Malin SA, Mckemy DD, Story GM (2009) Roles of transient receptor potential channels in pain. Brain Res Rev 60: 2–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez SS (2008) Control of hyperactivation in sperm. Hum Reprod Update 14: 647–657 [DOI] [PubMed] [Google Scholar]
- van Rossum DB, Patterson RL, Ma HT, Gill DL (2000) Ca2+ entry mediated by store depletion, S-nitrosylation, and TRP3 channels. Comparison of coupling and function. J Biol Chem 275: 28562–28568 [DOI] [PubMed] [Google Scholar]
- Veitinger T, Riffell JR, Veitinger S, Nascimento JM, Triller A, Chandsawangbhuwana C, Schwane K, Geerts A, Wunder F, Berns MW, Neuhaus EM, Zimmer RK, Spehr M, Hatt H (2011) Chemosensory Ca2+ dynamics correlate with diverse behavioral phenotypes in human sperm. J Biol Chem 286: 17311–17325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriens J, Appendino G, Nilius B (2009) Pharmacology of vanilloid transient receptor potential cation channels. Mol Pharmacol 75: 1262–1279 [DOI] [PubMed] [Google Scholar]
- Wang H, Liu J, Cho KH, Ren D (2009) A novel, single, transmembrane protein CATSPERG is associated with CATSPER1 channel protein. Biol Reprod 81: 539–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JP (2003) Characterization of maleimide-activated Ca2+ entry in neutrophils. Biochem Pharmacol 65: 1923–1929 [DOI] [PubMed] [Google Scholar]
- Wang Z, Balet SC, Li V, Nudelman A, Chan GC, Storm DR (2006) Pheromone detection in male mice depends on signaling through the type 3 adenylyl cyclase in the main olfactory epithelium. J Neurosci 26: 7375–7379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennemuth G, Carlson AE, Harper AJ, Babcock DF (2003) Bicarbonate actions on flagellar and Ca2+-channel responses: initial events in sperm activation. Development 130: 1317–1326 [DOI] [PubMed] [Google Scholar]
- Wiesner B, Weiner J, Middendorff R, Hagen V, Kaupp UB, Weyand I (1998) Cyclic nucleotide-gated channels on the flagellum control Ca2+ entry into sperm. J Cell Biol 142: 473–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windisch A, Timin E, Schwarz T, Stork-Riedler D, Erker T, Ecker G, Hering S (2011) Trapping and dissociation of propafenone derivatives in HERG channels. Br J Pharmacol 162: 1542–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu RL, Barish ME (1999) Modulation of a slowly inactivating potassium current, I(D), by metabotropic glutamate receptor activation in cultured hippocampal pyramidal neurons. J Neurosci 19: 6825–6837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Reigada D, Mitchell CH, Ren D (2007) CATSPER channel-mediated Ca2+ entry into mouse sperm triggers a tail-to-head propagation. Biol Reprod 77: 551–559 [DOI] [PubMed] [Google Scholar]
- Xia J, Ren D (2009) Egg coat proteins activate calcium entry into mouse sperm via CATSPER channels. Biol Reprod 80: 1092–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie F, Garcia MA, Carlson AE, Schuh SM, Babcock DF, Jaiswal BS, Gossen JA, Esposito G, Van Duin M, Conti M (2006) Soluble adenylyl cyclase (sAC) is indispensable for sperm function and fertilization. Dev Biol 296: 353–362 [DOI] [PubMed] [Google Scholar]
- Zou B, Yu H, Babcock JJ, Chanda P, Bader JS, Mcmanus OB, Li M (2010) Profiling diverse compounds by flux- and electrophysiology-based primary screens for inhibition of human Ether-a-go-go related gene potassium channels. Assay Drug Dev Technol 8: 743–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.