Abstract
EMBO J 31 7, 1654–1665 February 21 2012
A sperm-specific cation channel (CatSper) facilitates the entry of calcium necessary for rapid changes in sperm motility allowing the cell to navigate the hurdles of the female reproductive tract and successfully to locate the egg. Brenker and colleagues show that CatSper is (directly) activated by a diverse range of small organic molecules that are reported to evoke chemotaxis in human spermatozoa, suggesting that CatSper may function as a polymodal, chemosensory calcium channel. Additionally, they provide strong evidence that calcium entry into the spermatozoon does not employ specific, G protein-coupled odorant receptors or activation of trans-membrane adenylate cyclase and is not induced by cyclic nucleotides.
The human spermatozoon is a remarkable cell. It is characterised by extremely small size, absence of most organelles, a lack of transcription or translation (DNA is tightly condensed and almost crystalline), extreme polarity and rapid motility. These unusual characteristics have presented great challenges to the application of traditional methods of physiology, cell and molecular biology, such as imaging, electrophysiology, transfection and analysis of gene/protein expression patterns. Consequently, progress in understanding the function of the normal spermatozoon has been painfully slow. However, recently there has been remarkable progress in the understanding of one aspect—sperm motility—largely through developments in technology: patch clamping (Lishko et al, 2010), imaging (Publicover et al, 2007) and the rudimentary synergy of mathematics/physics with sperm biology (systems biology of sperm) (Kirkman-Brown and Smith, 2011). A primary example is the role of Ca2+ in regulating the activity of the flagellum (Darszon et al, 2011). Central to this has been the discovery of sperm-specific CatSper channels (cation channel of sperm), which facilitate the entry of Ca2+ necessary for rapid changes in sperm motility (termed as hyperactivation) (Qi et al, 2007). Two independent studies, utilising patch clamping, previously demonstrated that the induction of Ca2+ entry into human sperm by progesterone is at least partly via CatSper channels (Lishko et al, 2011; Strünker et al, 2011).
The study presented in this issue (Brenker et al, 2012) takes these developments much further, potentially unravelling several signalling ‘knots’ for sperm biologists. Brenker et al show that CatSper is (directly) activated by a diverse range of small organic molecules including odorants (e.g., cyclamal and bourgeonal) that are reported to evoke chemotaxis in human spermatozoa (Figure 1A). Thus, CatSper may function as a polymodal, chemosensory Ca2+ channel in the female tract. A significant consequence of CatSper's promiscuity is that interpretation of drug effects on sperm [Ca2+]i, particularly where high concentrations are used, must be undertaken with caution. Additionally, they provide strong evidence that odorants do not elevate sperm [Ca2+]i in a manner equivalent to that in nasal olfactory receptors (via specific, G protein-coupled odorant receptors and consequent activation of trans-membrane adenylate cyclase; tmAC). A well-established tenet in the reproductive literature is the functional role of tmAC in sperm. Brenker and colleagues show that tmAC in human sperm has no detectable effect on sperm [cAMP], soluble adenylate cyclase activated by calcium and HCO3 being critical. In fact, their data argue persuasively that the tmAC pathway does not functionally operate in human spermatozoa. Finally, they show that the increase in human sperm [Ca2+]i induced by membrane-permeant cAMP and cGMP analogues reflects an action of these compounds at the extracellular face of the CatSper channel. This surprising observation explains why more direct approaches to elevating cAMP (e.g., via example photorelease of cAMP) fail to raise [Ca2+]i in human spermatozoa. The failure of cyclic nucleotides to activate Ca2+ channels in human sperm contrasts with observations in sea urchin sperm (a key model in research on sperm physiology) and bovine sperm, providing further evidence for the existence of important physiological differences between sperm of different species.
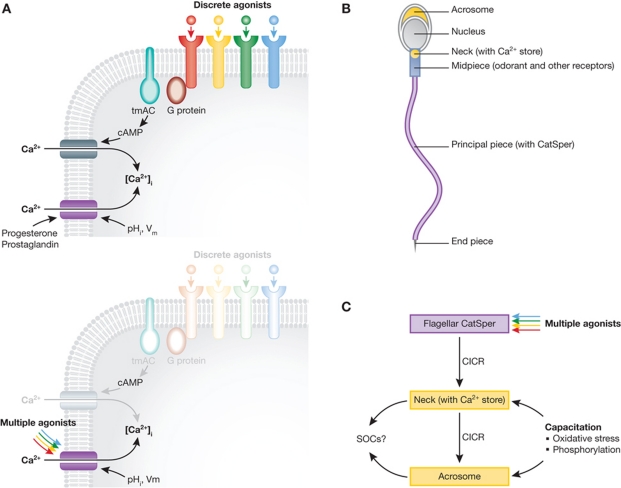
Figure 1.
(A) The upper panel shows the ‘classical’ model for agonist-induced Ca2+ influx in human sperm incorporating action of odorants and other agonists via trans-membrane adenyl cyclase (tmAC). Various receptors, represented by different colours, are each activated by discrete agonists. Generation of cAMP leads to activation of Ca2+-permeable channels (grey). CatSper (purple) is probably restricted to the flagellum (see B) and is regulated by progesterone, prostaglandins, pHi and (weakly) Vm. Other ionotropic and metabotropic receptors have also been described in sperm but are not shown. The lower panel shows a model based upon the conclusions of Brenker et al in which the tmAC and the regulation of Ca2+-permeable channels by cAMP are ‘greyed out’. CatSper acts as a ‘polymodal sensor for chemical cues’ as well as responding to pHi and Vm. (B) Restriction of CatSper (purple) to the membrane of the flagellar principal piece (as occurs in mouse sperm) will result in all CatSper-mediated [Ca2+]i signals originating here. Though rapid diffusion will occur there is apparently limited scope for flexibility of responses elicited by the various stimuli that converge on CatSper. This could be provided by Ca2+ storage organelles in the head and neck (yellow). (C) Propagation of Ca2+ signals into the sperm neck and head by Ca2+-induced release of stored Ca2+ (CICR) and activation of store operated channels (SOCs) may be regulated by cues from the female tract and/or by processes occurring during sperm capacitation such as oxidative stress and activation of kinases and phosphatases (lower panel), such that the spatio-temporal characteristics of the signal generated by activation of CatSper depends on the ‘readiness’ of the cell.
Thought provoking and challenging work always generates new questions. For instance, within the female tract the sperm is likely to be subject to a ‘barrage’ of signals from cell interactions and mobile factors, which provide cues and information to the sperm. If many of these stimuli activate CatSper, causing Ca2+ flux into the flagellum (Figure 1B) rather than acting through their ‘own’ receptor, how does the cell generate appropriate responses? Additionally, since humans do not synchronise ovulation with mating, the sperm cell may have to reside in the female tract for days to increase the chances of fertilising an oocyte—probably requiring a period of quiescence. How does the cell ‘ignore’ the ability of small molecules directly to activate CatSper-mediated Ca2+ influx? One possibility is that the downstream recruitment of stored Ca2+ propagates and amplifies the signal and that regulation of this process adds flexibility to CatSper-mediated signalling (Figure 1C).
Understanding sperm function and regulation is scientifically intriguing, but progress such as that reported here is also of medical importance. Our limited understanding of the working of the spermatozoon has resulted in the exclusive use of Assisted Reproductive Technology (ART; e.g. in-vitro fertilisation) therapy for sperm dysfunction (Barratt et al, 2011). A clear understanding of the function of the normal (and dysfunctional) spermatozoon will undoubtedly accelerate the long overdue development of drug-based (non-ART) therapies for sperm dysfunction.
Footnotes
The authors declare that they have no conflict of interest.
References
- Barratt CL, Mansell S, Beaton C, Tardif S, Oxenham SK (2011) Diagnostic tools in male infertility-the question of sperm dysfunction. Asian J Androl 13: 53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenker C, Goodwin N, Weyand I, Kashikar ND, Naruse M, Krähling M, Müller A, Kaupp UB, Strünker T (2012) The CatSper channel: a polymodal chemosensor in human sperm. EMBO J 31: 1654–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darszon A, Nishigaki T, Beltran C, Treviño CL (2011) Calcium channels in the development, maturation, and function of spermatozoa. Physiol Rev 91: 1305–1355 [DOI] [PubMed] [Google Scholar]
- Kirkman-Brown JC, Smith DJ (2011) Sperm motility: is viscosity fundamental to progress? Mol Hum Reprod 17: 539–544 [DOI] [PubMed] [Google Scholar]
- Lishko PV, Botchkina IL, Fedorenko A, Kirichok Y (2010) Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell 140: 327–337 [DOI] [PubMed] [Google Scholar]
- Lishko PV, Botchkina IL, Kirichok Y (2011) Progesterone activates the principal Ca2+ channel of human sperm. Nature 471: 387–391 [DOI] [PubMed] [Google Scholar]
- Publicover S, Harper CV, Barratt C (2007) [Ca2+]i signalling in sperm—making the most of what you’ve got. Nat Cell Biol 9: 235–242 [DOI] [PubMed] [Google Scholar]
- Qi H, Moran MM, Navarro B, Chong JA, Krapivinsky G, Krapivinsky L, Kirichok Y, Ramsey IS, Quill TA, Clapham DE (2007) All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc Natl Acad Sci USA 104: 1219–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strünker T, Goodwin N, Brenker C, Kashikar ND, Weyand I, Seifert R, Kaupp UB (2011) The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature 471: 382–386 [DOI] [PubMed] [Google Scholar]