Abstract
Glutathione has previously been identified as a reaction target for toluene diisocyanate (TDI) in vitro and in vivo, and has been suggested to contribute to toxic and allergic reactions to exposure. In this study, the reactivity of reduced glutathione (GSH) with TDI in vitro was further investigated using a mixed phase (vapor/liquid) exposure system to model the in vivo biophysics of exposure in the lower respiratory tract. HPLC/MS/MS was used to characterize the observed reaction products. Under the conditions tested, the major reaction products between TDI vapor and GSH were S-linked bis(GSH)-TDI and to a lesser extent mono(GSH)-TDI conjugates (with one N=C=O hydrolyzed). The vapor phase generated GSH-TDI conjugates were capable of transcarbamoylating human albumin in a pH-dependent manner, resulting in changes in the self-protein’s conformation/charge, based on electrophoretic mobility under native conditions. Specific sites of human albumin-TDI conjugation, mediated by GSH-TDI, were identified (Lys73, Lys159, Lys190, Lys199, Lys212, Lys351, Lys136/137, Lys413/414, Lys524/525) and overlap with those susceptible to direct conjugation by TDI. Together, the data extend proof-of-principle for GSH to act as a “shuttle” for a reactive form of TDI, which could contribute to clinical responses to exposure.
INTRODUCTION
The health hazards of TDI exposure are well recognized (1–3). Airborne concentrations >2.5 ppm are considered immediately dangerous to life or health based on animal studies, while concentrations <20 parts/per billion (ppb), below the short-term permissible exposure limits set by regulatory agencies, have been shown to trigger asthma attacks in sensitized individuals (4–6). Globally, TDI is the 2nd most abundantly produced diisocyanate, and is commonly used for making polyurethane foam (7).
The process by which TDI exposure causes disease remains unclear, in part due to TDI’s potent chemical reactivity, which has hampered mechanistic studies (8, 9). A possible role for GSH in TDI asthma pathogenesis is suggested by several lines of evidence. In vitro studies with primary human bronchial cells, and in vivo mouse studies, have shown that TDI vapors can cause marked decreases in cellular thiol levels, and induce oxidative stress (10–14). Genetic studies in humans have shown an association of TDI asthma, and TDI metabolites in urine and serum, with specific GSH S-transferase polymorphisms (15–17). Furthermore, the overall importance of GSH in human asthma caused by other occupational and environmental allergens is increasingly being recognized (18).
Generally, GSH plays a protective role against exposure to “xenobiotics” (19, 20). However, for TDI it is hypothesized that GSH plays a pathogenic role by acting as a “shuttle” that stabilizes a reactive form (of TDI) for delivery to another location, potentially extending the target range for protein modification and immune presentation (21, 22). This hypothesis is supported by in vitro studies demonstrating that GSH can form conjugates with TDI, capable of transcarbamoylating a model peptide (10). Similar findings have been demonstrated in studies with other mono-isocyanates, most notably, 2-chloroethyl-isocyanate, released by the cancer drug 1,3-bis(2-chloroethyl)-1-nitrosourea, methylisocyanate, the cause of the catastrophic industrial disaster in Bhopal India in 1984, and phenyl isocyanate, a sensitizer in animal studies (23–26). Thus, substantial evidence for reversible formation of thiocarbamate linkages between isocyanates and GSH, suggest this process might contribute to TDI’s toxicity and/or allergenicity.
To date, liquid phase reactions have been used almost exclusively for in vitro studies of TDI, and other isocyanates’, reactivity with GSH (10, 23–27). However, vapor, rather than liquid TDI, is the major phase to which GSH in the lower airway fluid is exposed in vivo (28). Vapor phase TDI may react with compounds in solution differently than liquid phase TDI, as evidenced by recent studies with human albumin (9). It remains unclear if differences between TDI vapor vs. liquid phase are due to biophysics, maximum/minimum achievable TDI concentration, or technical issues, especially (a) TDI’s insolubility and tendency to “self-aggregate” in aqueous solution, creating localized areas of high concentrations and a “self-encapsulating” physical barrier, as well as (b) TDI’s reactivity with water and conversion of its N=C=O groups to NH2, which may further co-polymerize with unhydrolyzed TDI.
In the present study, we investigated the interaction of GSH (in solution) with TDI in its vapor phase, to better model exposure biophysics in vivo in the lower respiratory tract. The mixed phase exposure approach alleviated many of the technical challenges previously noted in studying TDI / GSH reactivity in solution; namely problems with mixing, precipitation formation, and large numbers of reaction products (10). GSH-TDI conjugates formed via mixed phase exposure, and subsequent transcarbamoylation of human albumin (a major carrier protein mediating TDI allergenicity), were further studied through tandem mass-spectrometry approaches. The data support the hypothetical pathogenic role of GSH in response to TDI exposure.
EXPERIMENTAL PROCEDURES
TDI Vapor Phase Exposure of GSH
Ten mM solution of reduced or oxidized glutathione (GSH or GSSG respectively) from Sigma (St. Louis, MO) in 20 mM phosphate buffered saline (PBS, pH 7.4) from Gibco (Grand Island, NY), were exposed to room air, or TDI vapor (≤200 ppb) for 18 hrs, in 35 × 10 mm Petri dishes obtained through VWR International (Bridgeport, NJ) as depicted in Fig. 1A. All solutions and reaction products were 0.2 μm filtered (Millipore; Billerica, MA) to ensure sterility. The TDI solution used was an 80/20 mixture of 2,4/2,6-isomers, obtained from Sigma-Aldrich.
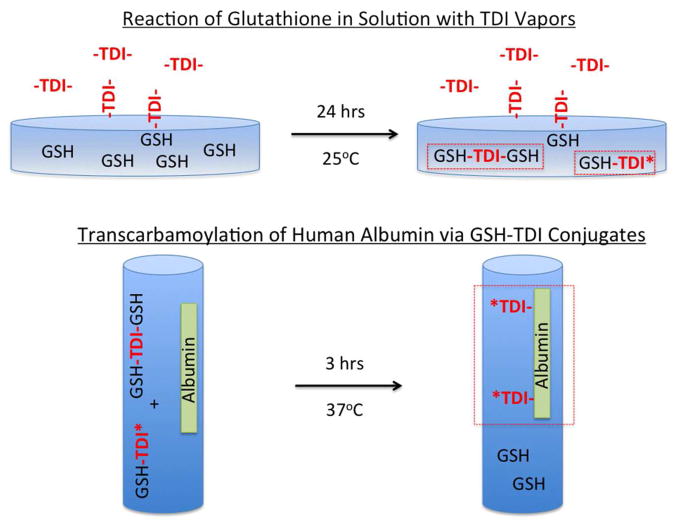
Figure 1.
Depiction of methodology used in the study. Panel A (top) depicts vapors of reactive TDI interacting with GSH in solution. Panel B (bottom) depicts the co-incubation of GSH-TDI reaction products with human albumin in solution.
Reverse phase HPLC analysis of GSH-TDI reaction products
Samples were initially fractionated by the Yale Keck Center as previously described, on a Hewlett Packard 1090 HPLC system equipped with an Isco Model 2150 Peak Separator and a 1 mm × 25 cm Vydac C-18 (5 micron particle size, 300 Å pore size) reverse phase column (29). Following equilibration with 98% buffer A (0.06% TFA) and 2% buffer B (0.052% TFA, 80% acetonitrile), TDI-GSH reaction products were eluted over the course of 1 hour, by increasing buffer B from 2 to 37%.
Mass spectrometry analysis of GSH-TDI reaction products
GSH-TDI reaction products were initially analyzed by the Yale Keck Center on a Micro Q-Tof MS instrument (Agilent Technologies; Santa Clara, CA). Twenty five microliters of each HPLC purified sample was diluted with 25 μL 0.1% formic acid, and injected onto a column (RP C4 micro column: PROTO 300 C4 5 μm 50 × 0.3 mm) for desalting. The bound analyte was eluted over a 30 minute gradient (mobile phase is acetonitrile with 0.1% TFA) directly into the Micro Q-Tof MS instrument where accurate mass was recorded. In other studies a separate aliquot (2 μL) of the HPLC purified reaction products were diluted in 60 μL of 60% acetonitrile/ 0.1% formic acid and directly infused via Triversa NanoMate into a Bruker 9.4T FT-ICR MS (Bruker Daltonics; Billerica, MA) (30).
GSH-TDI mediated transcarbamoylation of human albumin
HPLC purified TDI-GSH reaction products, or total (10 mM) GSH solutions exposed to TDI vapors, were incubated 1:2 (v/v) with a 5 mg/mL solution of human albumin at 37°C, as depicted in Fig. 1B. Initial reactions, including those analyzed by native gel (Fig. 7) and MS/MS (Table 1), were performed in isotonic saline (140 mM NaCl) buffered to pH 8.8 with 0.1 M carbonate. Subsequent experiments, exploring pH-dependence, were performed using 0.1 M buffers made with citric acid, phosphate or carbonate (Sigma) to achieve pH levels of 3, 7, and 9 respectively, or varying ratios of mono-/di-basic phosphate to achieve pH values ranging from 5.8 to 8.2.
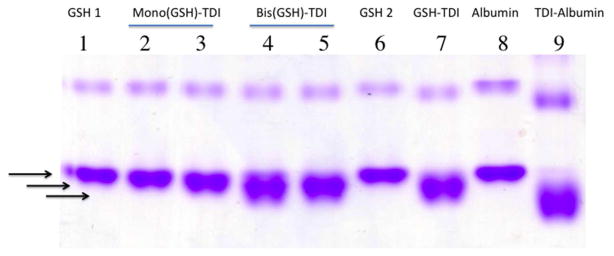
Figure 7.
Native gel analysis of human albumin transcarbamoylated by purified GSH-TDI reaction products. Human albumin co-incubated with HPLC purified GSH-TDI reaction products, or controls (labeled above the lane) was subjected to electrophoresis under native conditions and stained for protein. A downward shift in electrophoresis is consistent with TDI conjugation based on previous published data. All co-incubations used albumin in 0.1 M carbonate pH 8.8, plus the following: Lane 1- GSH from HPLC Peak ∅, Lanes 2 and 3- mono(GSH)-TDI* from HPLC peaks A and B respectively, Lanes 4 and 5- bis(GSH)-TDI from HPLC peaks C and D respectively, Lane 6- mock exposed GSH (unfractionated), Lane 7- TDI vapor exposed GSH (unfractionated), Lane 8-mock exposed albumin, Lane 9- albumin exposed directly to TDI vapors.
Table 1.
Sites of TDI conjugation to human albumin mediated via GSH-TDI.
| Observed TDI Conjugation Sites | Mono(GSH)-TDI* | Bis(GSH)-TDI | Total GSH-TDI RXN Products | ||
|---|---|---|---|---|---|
| HPLC Peak A | HPLC Peak B | HPLC Peak C | HPLC Peak D | ||
| LYS73 | X | X | |||
| LYS137 | X | X | X | X | |
| LYS159 | X | X | X | ||
| LYS190 | X | X | X | X | |
| LYS199 | X | X | X | ||
| LYS212 | X | X | |||
| LYS351 | X | X | X | X | |
| LYS525 | X | X | X | X | |
Native gel and anti-TDI Western blot
TDI conjugation to human albumin was detected in native gels based on characteristic changes in electrophoretic mobility as previously described (31, 32). For native protein analysis, samples were prepared in a glycerol buffer, run on 10% polyacrylamide gels, and stained with Imperial™ protein stain from Pierce (Rockford, IL). For Western blot analysis, samples were electrophoresed under reducing conditions on precast 4–15% gradient gels and a nitrocellulose trans-blot system from BioRad (Hercules, CA). Nitrocellulose membranes were blocked with 3% dry milk in PBS, probed with 1 μg/mL of the anti-TDI mAb 79G7 (33), followed by anti-mouse IgG1 from Pharmingen (San Diego, CA), and developed with electrochemical luminescence reagent from Thermo Fisher Scientific (Rochester, NY).
Reduction, alkylation, and trypsin digestion of albumin samples
In preparation for MS/MS analysis of GSH-mediated TDI conjugation sites of human albumin, 50 μL aliquots of GSH-TDI / albumin co-culture reactions were treated with tributylphosphine for 30 min at room temperature, followed by alkylation with iodoacetamide for 1 hour at room temperature. Alkylation was quenched by further addition of tributylphosphine for 15 min at room temperature and samples were twice dialyzed against 3 L of 25 mM NH4HCO3 using 3500 molecular weight cutoff mini dialysis units (Slide-A-Lyzer, Thermo Scientific, Waltham, MA). Porcine trypsin in 25 mM NH4HCO3 was then added at a 40:1 (protein:trypsin) ratio. Samples were incubated overnight at 37°C with shaking (400 rpm), and finally centrifuged at 14,000 × g in a microcentrifuge (MiniSpin, Eppendorf, Hamburg, Germany) to pellet any insoluble material.
Ultra-performance liquid chromatography
Tryptic peptides of albumin were separated on a Waters (Milford, MA) nanoACQUITY ultra-performance liquid chromatography system. Aliquots (1 μL) of the digest mixture were injected and trapped/desalted on a 5 μm SymmetryC18 (180 μm × 20 mm) trapping column with 99.5/0.5 A/B (A:0.1% formic acid; B:0.1% formic acid in acetonitrile) at a flow rate of 15 μL/min for 1 minute. Separation was performed on a 1.7 μm BEH130 C18 (100 μm × 100 mm) analytical column utilizing gradient elution at a flow rate of 400 nL/min and a gradient of 99/1 to 60/40 A/B over 60 min.
Tandem mass spectrometry of transcarbamoylated albumin peptides
The eluent from the ultra-performance liquid chromatography system was directed to the nanoelectrospray source of a Waters SYNAPT MS quadrupole time-of-flight (qTOF) mass spectrometer. Positive ion nanoelectrospray was performed utilizing 10 μm PicoTip (Waters) emitters held at a potential of +3.5 kV. The cone voltage was held constant at +40 V for all experiments. Dry N2 desolvation gas was supplied to the instrument via a nitrogen generator (NitroFlowLab, Parker Hannifin Corp., Haverhill, MA). [Glu]1-Fibrinopeptide B (100 fmol/μL in 75/25 A/B) was supplied to an orthogonal reference probe and the [M+2H]2+ ion (m/z = 785.84265 u) measured as an external calibrant at 30 sec intervals. Ultra-high purity argon was used as collision gas. Spectra were acquired in an “MSe” fashion (34). Alternating one-second mass spectra were acquired. The collision energy was set to 6 eV (1 sec low energy scan) and a 15–30 eV ramp (1 sec high energy scan).
Data analysis
Data were analyzed with BioPharmaLynx v. 1.2 (Waters), a software program for analysis of peptide mass maps and identification of sites of modification on known protein sequences. Default peptide mass map analysis criteria of 30 ppm mass error in both low and high collision energy mode were specified. Trypsin was specified as the digestion enzyme, and 2 missed cleavages were allowed. The submitted protein sequence was taken from P02768, “serum albumin precursor, Homo sapiens” (www.uniprot.org/uniprot/P02768) excluding the signal and propeptides (residues 1–24). Two custom modifiers were created for TDI. The first (TDI: C9H6N2O2, m/z = 174.0429 u) represents TDI with both isocyanate moieties bound to a peptide via urea bonds. The second (TDI*: C8H8N2O, m/z = 148.0637 u) represents one isocyanate moiety bound to a peptide via a urea bond, while the second isocyanate moiety is hydrolyzed to the primary amine. Identification of an isocyanate binding site proceeded via a rigorous procedure that involved the following steps: 1) Observation of a potential peptide-isocyanate conjugation product with less than 30 ppm m/Δm mass error in the analyte peptide mass map. 2) Comparison of analyte and control peptide mass map from unmodified human serum albumin shows that observed m/z and chromatographic retention time are unique to analyte. 3) MS/MS data contains bn- and yn-type ions consistent with the assigned sequence and modifier.
RESULTS
TDI vapor phase exposure of GSH and purification of reaction products
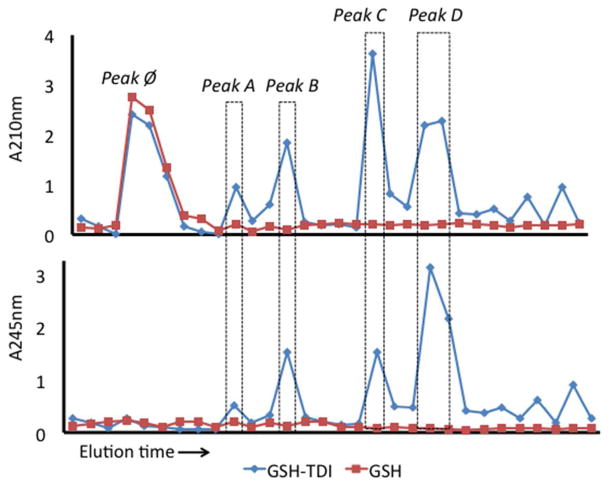
Solutions of GSH that had been exposed to TDI vapors, as depicted in Fig. 1A, were subjected to reverse phase HPLC, and the UV light absorbance of each fraction of column eluent was measured. Comparison of the HPLC chromatograms of TDI vapor vs. room air exposed GSH revealed fractions that likely contained GSH-TDI reaction products, based on increases in A210 and A250 (Fig. 2). As highlighted, the bulk of the reaction products were contained in the fractions labeled peaks A through D, which eluted after unreacted GSH (peak ∅), indicating increased hydrophobicity.
Figure 2.
HPLC separation of GSH-TDI reaction products. The absorbance at 210 nm (top) and 245 nm (bottom) was measured (Y-axis) for each 1 min eluent sample collected over time (X-axis). Samples of TDI vapor (<200 ppb) exposed GSH (10mM), shown as blue diamonds, were compared with “mock” exposed GSH, shown as red squares, to identify TDI reaction products, highlighted as peaks A-D, and unreacted GSH (peak ∅).
Identification and characterization of vapor GSH-TDI reaction products by mass spectrometry
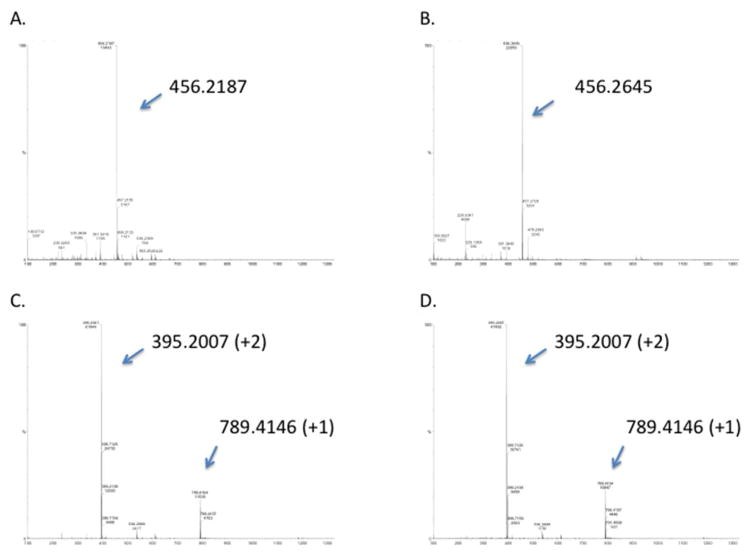
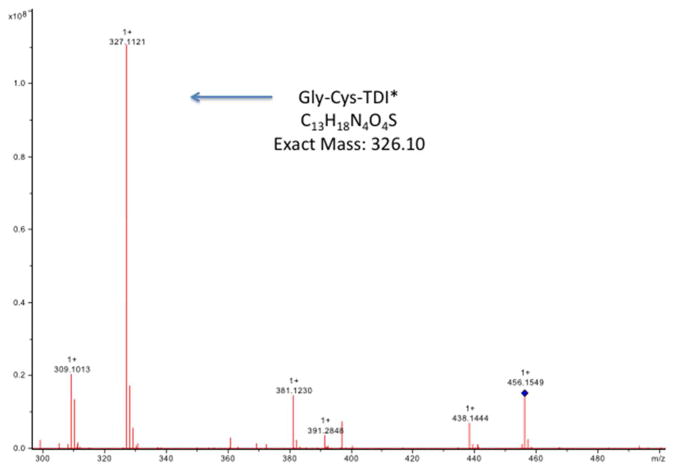
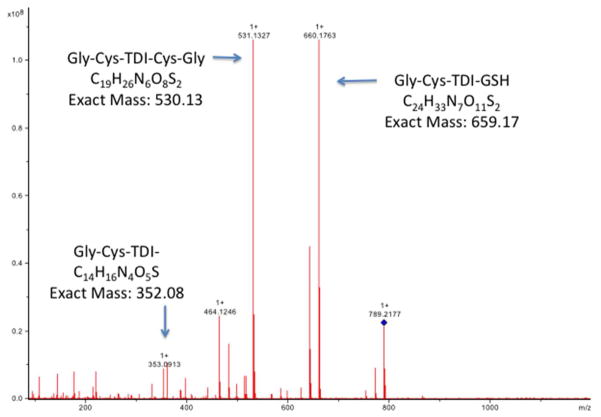
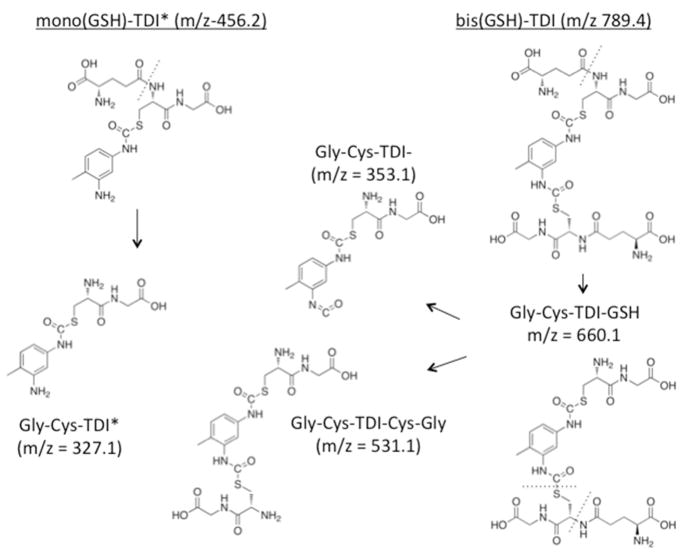
The specific HPLC fractions with elevated A210 and A250 were further characterized by MS. As shown in Fig. 3, Peaks A and B contained primarily a singly charged 456.2187 or 456.2645 m/z species, while Peaks C and D contained predominantly the doubly charged 395.2007 m/z, and its singly charged species (m/z = 789.4146). Fragmentation of these species yielded the tandem mass spectra shown in Figs. 4 and 5, which are consistent with the chemical structures shown in Fig. 6. Together, the data indicate HPLC fractions A and B contain mono(GSH)-TDI conjugate, with one N=C=O group hydrolyzed to a free amine (indicated as mono(GSH)-TDI* here forward), while fractions C and D contain bis(GSH)-TDI. As shown in Fig 6, the fragmentation data (along with functional data, see below) are consistent with TDI conjugation via the thiol group of cysteine, rather than the α-amine of the glutamate residue. The reason that GSH-TDI conjugates with identical masses elute from the HPLC in 2 distinct peaks remains unclear, however, the 2 distinct peaks may represent GSH conjugates with different isomers of TDI (2,4 vs. 2,6) present in the starting vapors.
Figure 3.
Mass spectrometry analysis of HPLC fractions containing GSH-TDI reaction products. Each panel A through B corresponds to samples from peaks A through D in Figure 2. Tandem MS/MS data (X-axis = m/z) and deduced structures for the singly charged m/z 456.2, the doubly and singly charged m/z 395.2 and 789.4 are shown in Figures 4 through 6.
Figure 4.
Tandem mass spectrometry analysis of peak A from HPLC purified GSH-TDI reaction products. A sample of peak A from the HPLC fractionation of GSH exposed to TDI vapor was analyzed by MS/MS (X-axis = m/z), focusing on the major m/z 456.2 product. The MS/MS spectrum shows a major fragment with an m/z consistent with the Gly-Cys-TDI* structure shown in Figure 6, suggesting linkage of TDI via the thiol of Cys, rather than the glutamate amino acid of GSH. Identical results were obtained with peak B from the HPLC fractionation shown in Fig. 2.
Figure 5.
Tandem mass spectrometry analysis of peak C from HPLC purified GSH-TDI reaction products. A sample of peak C from the HPLC fractionation of GSH exposed to TDI vapor was analyzed by MS/MS (X-axis = m/z), focusing on the major m/z 789.4 product. The MS/MS spectrum shows a major fragment with an m/z consistent with the Gly-Cys-TDI-GSH, Gly-Cys-TDI-Cys-Gly, and Gly-Cys-TDI structures shown in Figure 6, suggesting linkage of TDI via the thiol of Cys, rather than the glutamate amino acid of GDH. Identical results were obtained with peak D from the HPLC fractionation shown in Fig. 2.
Figure 6.
Chemical structures of the major reaction products between TDI vapors and GSH in solution. The structure of the major GSH-TDI reaction products and their predicted ionization fragments, including the m/z of the singly charged species, consistent with the observed MS/MS data shown in Figures 4 and 5.
Transcarbamoylation of human albumin by vapor GSH-TDI conjugates
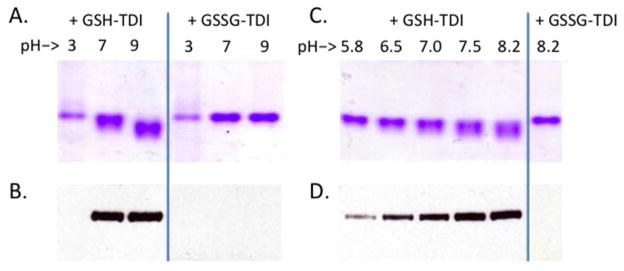
To test the functional ability of GSH-TDI conjugates generated by TDI vapor exposure to transcarbamoylate another protein, human albumin was co-incubated with GSH-TDI conjugates (Fig 1B), and subsequently analyzed by native gel electrophoresis, for conformational/charge changes indicative of TDI conjugation. HPLC purified bis(GSH)-TDI, total (unpurified) GSH-TDI, and to a lesser extent, mono(GSH)-TDI, all caused electrophoretic changes in albumin consistent with TDI transcarbamoylation (Fig. 7). As further shown in Fig. 8, through native gel analysis as well as anti-TDI Western blot, GSH-TDI mediated transcarbamoylation of human albumin was strongly pH dependent regardless of buffer ion (phosphate, citrate, carbonate), and across a wide range (pH 3–9), with lower activity at acidic pH and higher activity at basic pH levels. Further support of the thiocarbamate-dependent mechanism of TDI transcarbamoylation via GSH is provided by the fact that oxidized GSH (GSSG) did not mediate TDI transcarbamoylation, when vapor exposed and co-cultured with albumin under identical conditions.
Figure 8.
Dependence of human albumin transcarbamoylation by GSH-TDI on pH and free thiol of cysteine. Results of transcarbamoylation reactions of human albumin with total unfractionated GSH-TDI or GSSG-TDI reaction products. Co-incubations were performed at different pH levels (as labeled) and analyzed by gel electrophoresis (Panels A and C), or anti-TDI Western blot (Panels B and D). *Note- A/B, and C/D are paired gel / Western blots. Protein-stained gels were run under native conditions to highlight shift in migration due to conformational/charge differences, while Western blots were run under reducing conditions to maximize anti-TDI mAb binding.
Identification of human albumin’s specific sites of TDI conjugation via GSH-TDI
It was hypothesized that specific amino acid side chains of human albumin would be most susceptible to TDI conjugation via GSH-TDI conjugates, and the identify of these putative targets was evaluated through tandem mass spectrometry analysis. Under alkaline conditions, which yielded maximal transcarbamoylation, 8 different lysine residues were consistently identified as targets of TDI conjugation by mono(GSH)-TDI* or bis(GSH)-TDI in replicate experiments (Table 1). In each case, the 2nd (unbound) N=C=O group of the transferred TDI molecule had been hydrolyzed to the free amine. Overall, the pattern of albumin conjugation via GSH-TDI is qualitatively distinct from that recently reported via direct exposure to low dose TDI in liquid phase titration studies (35). However, two “GSH-mediated” TDI conjugation sites (Lys199, Lys525) are also targeted by direct “low dose” TDI exposure, while the other six identified “GSH-mediated” TDI conjugation sites (Lys73, Lys159, Lys190, Lys212, Lys351, Lys137) can become directly conjugated with high enough TDI exposure concentrations (35).
DISCUSSION
The reactivity of TDI with GSH was studied under mixed (vapor/liquid) phase conditions, as exists in the human airways, an important site of occupational exposure. GSH-TDI reaction products formed readily, and were further capable of transferring TDI to albumin, altering the self-protein’s native conformation/charge. Specific lysine residues of human albumin were identified as the predominant targets for TDI conjugation, via GSH-TDI conjugates, and overlap with those susceptible to direct conjugation with TDI, a process previously shown to induce antigenic changes (9). Together, these findings advance our understanding of the complex biochemistry that may connect TDI vapor inhalation with toxicity and/or allergy/asthma, and further suggest an important role for GSH in response to occupational TDI exposure.
The identification of bis(GSH)-TDI conjugates as a predominant reaction product via mixed phase exposure implies that reactivity of TDI with the thiol group of GSH, rather than reactivity with water, is a primary step though which the chemical vapor crosses the human airway’s fluid phase boundary. The reversible thiocarbamates that TDI forms, via S-linked conjugation with GSH, might shelter TDI from hydrolysis thereby allowing further penetration into the body in a reactive form, increasing toxicity or allergenicity, and creating the potential for systemic effects. Thus, GSH and/or other thiols at major sites of occupational TDI exposure (respiratory tract, as well as mucous membranes and skin) could play an important role in toxic and/or allergic responses. Airway fluid pH, which normally ranges from 6.5–7.5, and is acidified in some human conditions (e.g. asthma, pneumonia), may further modulate TDI exposure outcomes via its influence of transcarbamoylation reactions involving TDI-GSH conjugates, as demonstrated here (36).
The present findings agree with the previously published studies of liquid phase TDI reactivity with GSH by Day et al, which also described predominately bis(GSH)-TDI reaction products, when using 10-fold higher GSH concentrations (100 mM), ~2% v/v TDI and alkaline pH-7.7 (10). The present study extends these observations, by demonstrating the formation of GSH-TDI under more physiologic reaction conditions, as well as the ability of GSH-TDI to transfer TDI to human albumin, an important carrier protein for allergic (IgE) responses to TDI, and potential biomarker of exposure (9, 37, 38). GSH-mediated transfer of TDI was shown to occur preferentially on specific lysine residues, some of which are also susceptible to direct conjugation by TDI, and have been associated with antigenic changes induced by other diisocyanates (HDI, MDI) (31, 32, 35). Thus, the present findings raise new questions, which may be highly relevant to TDI asthma pathogenesis; e.g. the potential competition for isocyanate reactivity in vivo between thiols, such as GSH, and amines, such as those on albumin, as well as potential similarities and/or differences between TDI-albumin resulting from direct reaction with TDI vs. transcarbamoylation via GSH.
The present data differ from previous findings that GSH protects human albumin from vapors of another diisocyanate, HDI (39). Such dual effects could be due to differences in the reaction and hydrolysis/disassociation rates of GSH with (aromatic) TDI vs. (aliphatic) HDI, as might be expected based on studies of their corresponding thiocarbamates prepared with cysteine methyl esters (kd for TDI roughly 100X that of HDI). Differences in the effect of GSH might also relate to exposure conditions, which differed in the study on HDI, particularly the pH/temperature of the transcarbamoylation reaction, and the timing of albumin exposure relative to GSH (e.g. competitive vs. sequential). Studies reevaluating the interactions of GSH, albumin, and HDI vapors, under physiologic conditions (pH 7, temp. 37°C) more favorable to thiol-amine “isocyanate exchange”, such as those used in the present investigation, are underway in our laboratory.
The major strength of the present study is the application of a mixed (vapor/liquid) phase system to investigate the interaction of GSH with TDI vapors. The study design targeted a fixed GSH concentration and pH as starting points for investigation; however, the system should readily permit evaluation of a more dynamic range of physiologic factors (e.g. glutathione/ion concentration, pH, temperature) and other volatile occupational/environmental co-exposures (amine catalysts, solvents) that may influence TDI reactivity. The vapor/liquid exposure system should be similarly useful for studying other volatile diisocyanates (HDI, MDI), and differences that have been reported in their reactivity/stability with GSH and related thiols (21, 39, 40). The basic system could be further developed with underlying human epithelial cell layers, and overlying surfactant, to more completely model the airway microenvironment. However, animal models or clinical investigations will likely be needed to ultimately determine potential systemic effects of GSH-TDI interactions in vivo.
In summary, it was demonstrated that GSH can mediate TDI vapor uptake across a fluid phase boundary, and subsequently transfer TDI onto human albumin, a protein recognized as a major carrier for TDI in vivo. GSH-mediated transfer of TDI onto human albumin occurred on specific lysine residues and altered the self-protein’s native conformation/charge, an effect previously associated with TDI’s antigenicity. Together, the data extend proof-of-principle for the hypothesis that GSH, and potentially other thiols in exposed tissue, act as a primary reaction route for entry of TDI into the body and could play an important role in pathogenic responses to exposure.
Acknowledgments
FUNDING SOURCE
The studies were supported by grants R42-ES016728 and R41-ES018021 from the National Institute of Environmental Health Safety. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the National Institute for Occupational Safety and Health.
The authors would like to acknowledge Drs. Stone, Lam, Ted Voss, Tom Abbott, and Mary LoPresti, from the Yale Keck Center for their work on the HPLC, MS, and MS/MS, as well as Jian Liu for her work with the vapor exposures.
ABBREVIATIONS
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- HDI
hexamethylene diisocyanate
- MDI
methylene diphenyl diisocyanate
- ppb
parts per billion
- TDI
toluene diisocyanate
References
- 1.Baur X, Marek W, Ammon J, Czuppon AB, Marczynski B, Raulf-Heimsoth M, Roemmelt H, Fruhmann G. Respiratory and other hazards of isocyanates. Int Arch Occup Environ Health. 1994;66:141–152. doi: 10.1007/BF00380772. [DOI] [PubMed] [Google Scholar]
- 2.Collins MA. Toxicology of toluene diisocyanate. Appl Occup Environ Hyg. 2002;17:846–855. doi: 10.1080/10473220290107048. [DOI] [PubMed] [Google Scholar]
- 3.Lindberg HK, Korpi A, Santonen T, Sakkinen K, Jarvela M, Tornaeus J, Ahonen N, Jarventaus H, Pasanen AL, Rosenberg C, Norppa H. Micronuclei, hemoglobin adducts and respiratory tract irritation in mice after inhalation of toluene diisocyanate (TDI) and 4,4′-methylenediphenyl diisocyanate (MDI) Mutat Res. 2011 doi: 10.1016/j.mrgentox.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 4.NIOSH. DHHS (NIOSH) Publication Number 96–111. Cincinnati, OH: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health; 1996. Preventing Asthma and Death from Diisocyanate Exposure. [Google Scholar]
- 5.NIOSH. DHHS (NIOSH) Publication No 90–101. Cincinnati, OH: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health; 1989. Current Intelligence Bulletin 53: toluene diisocyanate (TDI) and toluenediamine (TDA); evidence of carcinogenicity. [Google Scholar]
- 6.Duncan B, Scheel LD, Fairchild EJ, Killens R, Graham S. Toluene Diisocyanate Inhalation Toxicity: Pathology and Mortality. American Industrial Hygiene Association journal. 1962;23:447– 456. [Google Scholar]
- 7.Allport DC, Gilbert DS, Outterside SM. MDI and TDI : safety, health and the environment : a source book and practical guide. Wiley; Chichester: 2003. [Google Scholar]
- 8.Wisnewski AV, Jones M. Pro/Con debate: Is occupational asthma induced by isocyanates an immunoglobulin E-mediated disease? Clin Exp Allergy. 2010;40:1155–1162. doi: 10.1111/j.1365-2222.2010.03550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ye YM, Kim CW, Kim HR, Kim HM, Suh CH, Nahm DH, Park HS, Redlich CA, Wisnewski AV. Biophysical determinants of toluene diisocyanate antigenicity associated with exposure and asthma. J Allergy Clin Immunol. 2006;118:885–891. doi: 10.1016/j.jaci.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 10.Day BW, Jin R, Basalyga DM, Kramarik JA, Karol MH. Formation, solvolysis, and transcarbamoylation reactions of bis(S-glutathionyl) adducts of 2,4- and 2,6-diisocyanatotoluene. Chem Res Toxicol. 1997;10:424–431. doi: 10.1021/tx960201+. [DOI] [PubMed] [Google Scholar]
- 11.Lange RW, Day BW, Lemus R, Tyurin VA, Kagan VE, Karol MH. Intracellular S-glutathionyl adducts in murine lung and human bronchoepithelial cells after exposure to diisocyanatotoluene. Chem Res Toxicol. 1999;12:931–936. doi: 10.1021/tx990045h. [DOI] [PubMed] [Google Scholar]
- 12.Lantz RC, Lemus R, Lange RW, Karol MH. Rapid reduction of intracellular glutathione in human bronchial epithelial cells exposed to occupational levels of toluene diisocyanate. Toxicol Sci. 2001;60:348–355. doi: 10.1093/toxsci/60.2.348. [DOI] [PubMed] [Google Scholar]
- 13.Haenen S, Vanoirbeek JA, De Vooght V, Maes E, Schoofs L, Nemery B, Hoet PH, Clynen E. Proteome analysis of multiple compartments in a mouse model of chemical-induced asthma. J Proteome Res. 2010;9:5868–5876. doi: 10.1021/pr100638m. [DOI] [PubMed] [Google Scholar]
- 14.Kim SH, Choi GS, Ye YM, Jou I, Park HS, Park SM. Toluene diisocyanate (TDI) regulates haem oxygenase-1/ferritin expression: implications for toluene diisocyanate-induced asthma. Clin Exp Immunol. 2010;160:489–497. doi: 10.1111/j.1365-2249.2010.04118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broberg KE, Warholm M, Tinnerberg H, Axmon A, Jonsson BA, Sennbro CJ, Littorin M, Rannug A. The GSTP1 Ile105 Val polymorphism modifies the metabolism of toluene di-isocyanate. Pharmacogenet Genomics. 2010;20:104–111. doi: 10.1097/FPC.0b013e328334fb84. [DOI] [PubMed] [Google Scholar]
- 16.Mapp CE, Fryer AA, De Marzo N, Pozzato V, Padoan M, Boschetto P, Strange RC, Hemmingsen A, Spiteri MA. Glutathione S-transferase GSTP1 is a susceptibility gene for occupational asthma induced by isocyanates. J Allergy Clin Immunol. 2002;109:867–872. doi: 10.1067/mai.2002.123234. [DOI] [PubMed] [Google Scholar]
- 17.Piirila P, Wikman H, Luukkonen R, Kaaria K, Rosenberg C, Nordman H, Norppa H, Vainio H, Hirvonen A. Glutathione S-transferase genotypes and allergic responses to diisocyanate exposure. Pharmacogenetics. 2001;11:437–445. doi: 10.1097/00008571-200107000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Reynaert NL. Glutathione biochemistry in asthma. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbagen.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Boyd MR, Stiko A, Statham CN, Jones RB. Protective role of endogenous pulmonary glutathione and other sulfhydryl compounds against lung damage by alkylating agents. Investigations with 4-ipomeanol in the rat. Biochem Pharmacol. 1982;31:1579–1583. doi: 10.1016/0006-2952(82)90383-5. [DOI] [PubMed] [Google Scholar]
- 20.Patterson CE, Rhoades RA. Protective role of sulfhydryl reagents in oxidant lung injury. Exp Lung Res. 1988;14(Suppl):1005–1019. doi: 10.3109/01902148809064189. [DOI] [PubMed] [Google Scholar]
- 21.Chipinda I, Stetson SJ, Depree GJ, Simoyi RH, Siegel PD. Kinetics and mechanistic studies of the hydrolysis of diisocyanate-derived bis-thiocarbamates of cysteine methyl ester. Chem Res Toxicol. 2006;19:341–350. doi: 10.1021/tx050311t. [DOI] [PubMed] [Google Scholar]
- 22.Karol MH, Macina OT, Cunningham A. Cell and molecular biology of chemical allergy. Ann Allergy Asthma Immunol. 2001;87:28–32. doi: 10.1016/s1081-1206(10)62337-x. [DOI] [PubMed] [Google Scholar]
- 23.Davis MR, Kassahun K, Jochheim CM, Brandt KM, Baillie TA. Glutathione and N-acetylcysteine conjugates of 2-chloroethyl isocyanate. Identification as metabolites of N,N′-bis(2-chloroethyl)-N-nitrosourea in the rat and inhibitory properties toward glutathione reductase in vitro. Chem Res Toxicol. 1993;6:376–383. doi: 10.1021/tx00033a020. [DOI] [PubMed] [Google Scholar]
- 24.Pearson PG, Slatter JG, Rashed MS, Han DH, Baillie TA. Carbamoylation of peptides and proteins in vitro by S-(N-methylcarbamoyl)glutathione and S-(N-methylcarbamoyl)cysteine, two electrophilic S-linked conjugates of methyl isocyanate. Chem Res Toxicol. 1991;4:436–444. doi: 10.1021/tx00022a007. [DOI] [PubMed] [Google Scholar]
- 25.Pearson PG, Slatter JG, Rashed MS, Han DH, Grillo MP, Baillie TA. S-(N-methylcarbamoyl)glutathione: a reactive S-linked metabolite of methyl isocyanate. Biochem Biophys Res Commun. 1990;166:245–250. doi: 10.1016/0006-291x(90)91937-n. [DOI] [PubMed] [Google Scholar]
- 26.Fleischel O, Gimenez-Arnau E, Lepoittevin JP. Nuclear magnetic resonance studies on covalent modification of amino acids thiol and amino residues by monofunctional aryl 13C-isocyanates, models of skin and respiratory sensitizers: transformation of thiocarbamates into urea adducts. Chem Res Toxicol. 2009;22:1106–1115. doi: 10.1021/tx9000539. [DOI] [PubMed] [Google Scholar]
- 27.Slatter JG, Rashed MS, Pearson PG, Han DH, Baillie TA. Biotransformation of methyl isocyanate in the rat. Evidence for glutathione conjugation as a major pathway of metabolism and implications for isocyanate-mediated toxicities. Chem Res Toxicol. 1991;4:157–161. doi: 10.1021/tx00020a006. [DOI] [PubMed] [Google Scholar]
- 28.Ebino K, Lemus R, Karol MH. The importance of the diluent for airway transport of toluene diisocyanate following intranasal dosing of mice. Inhal Toxicol. 1999;11:171–185. doi: 10.1080/089583799197131. [DOI] [PubMed] [Google Scholar]
- 29.Williams KR, Stone KL. Enzymatic cleavage and HPLC peptide mapping of proteins. Mol Biotechnol. 1997;8:155–167. doi: 10.1007/BF02752260. [DOI] [PubMed] [Google Scholar]
- 30.Stone KL, Bjornson RD, Blasko GG, Bruce C, Cofrancesco R, Carriero NJ, Colangelo CM, Crawford JK, Crawford JM, daSilva NC, Deluca JD, Elliott JI, Elliott MM, Flory PJ, Folta-Stogniew EJ, Gulcicek E, Kong Y, Lam TT, Lee JY, Lin A, LoPresti MB, Mane SM, McMurray WJ, Tikhonova IR, Westman S, Williams NA, Wu TL, Hongyu Z, Williams KR. Keck Foundation Biotechnology Resource Laboratory, Yale University. Yale J Biol Med. 2007;80:195–211. [PMC free article] [PubMed] [Google Scholar]
- 31.Wisnewski AV, Liu J, Redlich CA. Antigenic changes in human albumin caused by reactivity with the occupational allergen diphenylmethane diisocyanate. Anal Biochem. 2010;400:251–258. doi: 10.1016/j.ab.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wisnewski AV, Stowe MH, Cartier A, Liu Q, Liu J, Chen L, Redlich CA. Isocyanate vapor-induced antigenicity of human albumin. J Allergy Clin Immunol. 2004;113:1178–1184. doi: 10.1016/j.jaci.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Ruwona TB, Johnson VJ, Schmechel D, Simoyi RH, Beezhold D, Siegel PD. Monoclonal antibodies against toluene diisocyanate haptenated proteins from vapor-exposed mice. Hybridoma (Larchmt) 2010;29:221–229. doi: 10.1089/hyb.2009.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chakraborty AB, Berger SJ, Gebler JC. Use of an integrated MS--multiplexed MS/MS data acquisition strategy for high-coverage peptide mapping studies. Rapid Commun Mass Spectrom. 2007;21:730–744. doi: 10.1002/rcm.2888. [DOI] [PubMed] [Google Scholar]
- 35.Hettick JM, Siegel PD. Determination of the toluene diisocyanate binding sites on human serum albumin by tandem mass spectrometry. Anal Biochem. 2011 doi: 10.1016/j.ab.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 36.Bodem CR, Lampton LM, Miller DP, Tarka EF, Everett ED. Endobronchial pH. Relevance of aminoglycoside activity in gram-negative bacillary pneumonia. Am Rev Respir Dis. 1983;127:39–41. doi: 10.1164/arrd.1983.127.1.39. [DOI] [PubMed] [Google Scholar]
- 37.Brown WE, Burkert AL. Biomarkers of toluene diisocyanate exposure. Appl Occup Environ Hyg. 2002;17:840–845. doi: 10.1080/10473220290107039. [DOI] [PubMed] [Google Scholar]
- 38.Wass U, Belin L. Immunologic specificity of isocyanate-induced IgE antibodies in serum from 10 sensitized workers. J Allergy Clin Immunol. 1989;83:126–135. doi: 10.1016/0091-6749(89)90487-9. [DOI] [PubMed] [Google Scholar]
- 39.Wisnewski AV, Liu Q, Liu J, Redlich CA. Glutathione protects human airway proteins and epithelial cells from isocyanates. Clin Exp Allergy. 2005;35:352–357. doi: 10.1111/j.1365-2222.2005.02185.x. [DOI] [PubMed] [Google Scholar]
- 40.Reisser M, Schmidt BF, Brown WE. Synthesis, characterization, and solvolysis of mono- and bis-S-(glutathionyl) adducts of methylene-bis-(phenylisocyanate) (MDI) Chem Res Toxicol. 2002;15:1235–1241. doi: 10.1021/tx0255020. [DOI] [PubMed] [Google Scholar]