Abstract
Background
Uric acid is tightly linked to the metabolic syndrome (MetS) and among adults higher uric acid levels are associated with future risk for diabetes, cardiovascular disease, hypertension and renal disease.
Objective
Evaluate the sensitivity of MetS to identify adolescents with elevated uric acid levels on a race/ethnicity and gender-specific basis.
Methods
We evaluated 3,296 males and female adolescents 12-19y participating in the National Health and Nutrition Evaluation Survey ‘99-’06, comprised of 67.6% non-Hispanic whites, 15.1% non-Hispanic blacks, and 17.3% Hispanics. We used a definition of MetS modified for use in adolescents and evaluated the sensitivity of a diagnosis of MetS to identify individuals with uric acid elevations (approximately the 95th percentile of uric acid by gender among normal-weight adolescents).
Results
When used as a screening test to identify individuals with uric acid elevations MetS performed more poorly among females (18.0%) than among males (37.0%)(p<0.001). Among males, MetS exhibited a lower sensitivity among non-Hispanic blacks (17.8%) compared to Hispanics (45.9%)(p<0.01) and non-Hispanic whites (37.4%)(p<0.05). There were no race/ethnicity differences in detecting elevated uric acid levels among females (non-Hispanic-white 15.5%, non-Hispanic-black 19.4%, Hispanic 26.5%, p>0.05).
Conclusion
Current criteria to diagnose MetS exhibit racial/ethnic and gender differences in the ability to identify adolescents with elevated uric acid levels, performing poorly among non-Hispanic-black males and among females. Given emerging data regarding the ability of uric acid elevations for predicting future disease, these data may have implications regarding the use of MetS as a marker of risk among all gender and racial/ethnic groups.
Keywords: metabolic syndrome, uric acid, adolescents, insulin resistance, cardiovascular disease risk
Introduction
Uric acid is a product of purine breakdown that is linked to oxidative stress [1] and has emerged in adults as an independent risk factor for future cardiovascular disease (CVD)[2,3], type 2 diabetes mellitus (T2DM)[4,5], hypertension [6] and renal failure [7]. Among adolescents, levels of uric acid are associated with an increase in carotid artery media thickness [8] and future hypertension [9]. These associations have raised the prospect of using uric acid as a marker of future disease risk [10].
Uric acid is also tightly linked to the metabolic syndrome (MetS)[11,12], a cluster of cardiovascular risk factors including elevated waist circumference (WC), increased blood pressure (BP), high triglycerides, low HDL-cholesterol and fasting hyperglycemia. Currently-utilized criteria for diagnosing MetS such as those from the Adult Treatment Panel III (ATP-III) are based on specific cut-off values for these individual components [13]. MetS is strongly associated with insulin resistance and is a predictor of future T2DM in adolescents [14] and of future cardiovascular disease in adults [15]. Indeed, some have advocated that a diagnosis of MetS be a trigger for increased intervention among obese adolescents [16], who are at increasing risk for future CVD [17].
However, MetS exhibits racial/ethnic discrepancies that may decrease its effectiveness in predicting long-term risk for disease among all ethnicities [18-20]. Non-Hispanic-black adolescents exhibit a lower prevalence of MetS despite having a higher degree of insulin resistance [21,22] and higher rates of T2DM [23,24] and death from CVD [25]. This suggests that among non-Hispanic-black adolescents MetS may be under-diagnosed [26]. Additionally, females have an overall lower prevalence of MetS than males [19] but appear to have a tighter link between high uric acid and CVD [12].
Given the long-term associations of elevated uric acid and future disease, the association of uric acid with MetS, and racial/ethnic and gender discrepancies in MetS, our goal was to evaluate the ability of a classification of MetS to identify individuals with elevated uric acid levels on a race/ethnicity- and gender-specific basis. We applied a commonly-used set of pediatric MetS criteria (the ATP- III criteria adapted for use in adolescence [11,22,27]) to adolescent data from the National Health and Nutrition Examination Survey (NHANES), with the hypothesis that MetS would perform more poorly at detecting elevated uric acid levels among non-Hispanic black adolescents than among non-Hispanic whites and Hispanics. In such a way we aimed to further detail racial/ethnic differences in currently-used MetS criteria in assessing long-term risk.
Methods
Data were obtained from NHANES (1999-2006), a complex, multistage probability sample of the US population. These annual cross-sectional surveys are conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control (CDC), with randomly-selected subjects undergoing anthropometric and blood pressure measurements, answering questionnaires and undergoing phlebotomy (http://www.cdc.gov/nchs/nhanes.htm). The NCHS ethics review board reviewed and approved the survey and participants gave informed consent prior to participation. WC, blood pressure (BP), and laboratory measures of triglycerides, HDL-C, and glucose were obtained using standardized protocols and calibrated equipment [11]. All blood samples used for analyses were obtained following a fast ≥8 hours prior to the blood draw. Serum uric acid was measured by a colorimetric method in which uric acid is oxidized by uricase to form allantoin and H2O2. For NHANES ‘99-’02 this method was used by Hitachi model 704 analyzer, Roche Diagnostics and from ‘03-’06 this was measured by Beckman Synchron LX20, Beckman Coulter, Inc.
MetS Classification
MetS was defined by a commonly-used pediatric/adolescent adaptation of the Adult Treatment Panel III (ATP III) criteria [11,13,22,27]. Participants had to meet ≥3 of the following 5 criteria: concentration of triglycerides ≥110 mg/dL, HDL-C ≤40 mg/dL, WC ≥90th percentile for age/sex (or ATP III limit of 102 cm for males and 88 cm for females, whichever was lower)[13,28], glucose concentration ≥100 mg/dL, and systolic or diastolic BP ≥90th percentile (age, height, and sex-specific)[29]. Similarly, hypertension was defined as systolic or diastolic BP ≥90th percentile for age, height, and sex. Elevated uric acid levels were defined as approximately the 95th percentile of uric acid levels among lean adolescents (BMI <85th percentile) on a gender-specific basis. As in prior studies [22] lean individuals were chosen to determine these cut-offs because of the strong association between uric acid and adiposity [11].
Data from non-Hispanic-white, non-Hispanic-black, or Hispanic (Mexican-American/other Hispanic) adolescents 12-19 y.o. were analyzed. Children <12y were excluded since fasting values for triglycerides and glucose were only obtained in participants ≥12 y.o. Subjects were excluded if they were pregnant or taking antihyperlipidemic or anti-diabetic medications, as these are all likely to alter lipid and insulin levels in a manner that may not reflect baseline MetS-uric acid correlations. Individuals taking anti-hypertensive medication were classified as having hypertension.
Statistical Analysis
Statistical significance was defined as a p-value<0.05. Statistical analysis was performed using SAS (version 9.2, Cary, NC) and SUDAAN (version 10; Research Triangle Institute, Research Triangle Park, NC), which accounts for the survey design when estimating standard errors to obtain population-based estimates. We combined all data sets from the 3 two-year cycles (1999-2006) for statistical analyses to increase total sample size. Prevalence rates of MetS were calculated by gender, race/ethnicity, and compared via chi-square tests. Mean uric acid levels were compared among groups using either unpaired t-tests or analysis of variance (ANOVA). The homeostasis model of insulin resistance (HOMA) was calculated as described previously [22].
Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of MetS to identify elevated uric acid (approximately the 95th percentile of uric acid levels among lean adolescents, as described above) was computed by gender and race/ethnicity. All analyses incorporated the sampling weights included in NHANES.
Results
Sample Characteristics
The sample consisted of 3,296 non-Hispanic-white, non-Hispanic-black and Hispanic adolescents age 12-19 y.o. with data for all variables tested. Numbers of subjects with and without and values for individual MetS components by race/ethnicity are shown for male and female subjects in Tables 1 and 2, respectively. The numbers of subjects with MetS are the total number in the sample and have not been adjusted to reflect oversampling of minorities in NHANES. Regarding use of anti-hypertensive medications, 12 subjects total were on anti-hypertensive medications, and of these 3 had MetS, including 1 subject on each of the following classes: beta-blocker, angiotensin-converting-enzyme inhibitor and diuretic. Non-Hispanic-black males had the lowest rate of MetS of all the groups (Table 1). Among males non-Hispanic blacks with and without MetS had lower levels of triglycerides than non-Hispanic whites (Table 1) while among females non-Hispanic blacks only had the lowest levels of triglycerides among those without MetS (Table 2). Among males, non-Hispanic blacks with and without MetS had higher systolic BP than non-Hispanic whites and Hispanics (Table 1), while among females non-Hispanic blacks only had the highest systolic BP among those without MetS (Table 2).
Table 1. Race/ethnicity comparison of MetS components and related factors for Males stratified by Metabolic Syndrome.
| Metabolic Syndrome | Without Metabolic Syndrome | |||||||
|---|---|---|---|---|---|---|---|---|
| Non-Hispanic White | Hispanic | Non-Hispanic Black | p-value* | Non-Hispanic White | Hispanic | Non-Hispanic Black | p-value* | |
| n | 58 | 92 | 28 | 415 | 570 | 566 | ||
|
| ||||||||
| MetS prevalence | 12.7 | 13.6 | 4.8 | <0.01 | ||||
|
| ||||||||
| Mets components | ||||||||
| Waist Circumference | ||||||||
| Mean (95% CI), cm | 104.7 (99.8,109.6) | 103.3 (100.1,106.4) | 108.4 (101.6,115.2) | 0.30 | 78.6 (77.5,79.8) | 79.8 (78.1,81.5) | 76.8 (75.6,78.0) | <0.01 |
| Percent above 90% pctile | 73.4 | 79.6 | 82.2 | 0.65 | 7.4 | 11.5 | 9.5 | 0.17 |
| Triglycerides | ||||||||
| Mean (95% CI), m/.dL | 183.7 (161.0,206.4) | 148.1 (130.8,165.5) | 143.1 (115.6,170.6) | 0.01** | 85.5 (81.7,89.4) | 84.0 (79.3,88.6) | 66.7 (63.9,69.5) | <0.01** |
| Percent above 110 | 92.0 | 81.6 | 73.0 | 0.08 | 22.1 | 17.4 | 7.5 | <0.01 |
| HDL | ||||||||
| Mean (95%CI),mg/dL | 36.4 (34.4,38.4) | 36.7 (35.1,38.3) | 39.4 (35.9,42.9) | 0.34 | 48.2 (47.1,49.2) | 50.4 (48.9,51.8) | 55.0 (53.7,56.3) | <0.01 |
| Percent below 40 | 71.9 | 80.5 | 60.3 | 0.24 | 17.0 | 13.4 | 7.6 | <0.01 |
| SBP | ||||||||
| Mean (95%CI),mmHg | 124.2 (120.8,127.7) | 118.6 (116.1,121.2) | 128.3 (125.0,131.7) | <0.01 | 110.8 (109.7,112.0) | 110.0 (108.0,112.1) | 113.7 (112.8,114.6) | <0.01 |
| Percent above 90% pctile | 40.4 | 24.4 | 73.0 | <0.01 | 4.2 | 2.8 | 9.5 | <0.01 |
| DBP | ||||||||
| Mean (95%CI),mmHg | 63.9 (60.9,66.9) | 59.1 (54.3,63.9) | 61.2 (56.8,65.5) | 0.27 | 61.2 (60.0,62.3) | 58.9 (57.9,60.0) | 60.6 (59.4,61.8) | 0.01 |
| Percent above 90% pctile | 9.6 | 6.3 | 6.1 | 0.66 | 1.2 | 1.3 | 2.9 | 0.06 |
| Fasting Glucose | ||||||||
| Mean(95%CI),mg/dL | 100.8 (98.8,102.9) | 101.0 (98.6,103.3) | 101.5 (98.7,104.2) | 0.94 | 94.3 (93.5,95.1) | 95.1 (94.2,96.1) | 92.5 (91.8,93.2) | <0.01 |
| Percent above 100 | 57.9 | 71.6 | 58.9 | 0.22 | 16.9 | 21.4 | 12.2 | <0.01 |
Chi-square test comparing percents, ANOVA comparing means (overall difference among the groups).
Comparison of In(Triglyceride).
Table 2. Race/ethnicity comparison of MetS components and related factors for Females stratified by Metabolic Syndrome.
| Metabolic Syndrome | Without Metabolic Syndrome | |||||||
|---|---|---|---|---|---|---|---|---|
| Non-Hispanic White | Hispanic | Non-Hispanic Black | p-value* | Non-Hispanic White | Hispanic | Non-Hispanic Black | p-value* | |
| n | 25 | 58 | 19 | 409 | 606 | 450 | ||
|
| ||||||||
| MetS prevalence | 5.8 | 8.1 | 4.6 | 0.35 | ||||
|
| ||||||||
| Mets components | ||||||||
| Waist Circumference | ||||||||
| Mean (95% CI), cm | 98.0 (92.2,103.8) | 103.9 (95.6,112.3) | 106.3 (99.1,113.6) | 0.17 | 78.7 (77.1,80.2) | 79.9 (78.3,81.4) | 81.4 (80.1,82.8) | 0.02 |
| Percent above 90% pctile | 84.6 | 97.5 | 95 | 0.26 | 17.1 | 22.8 | 28.9 | <0.01 |
| Triglycerides | ||||||||
| Mean (95% CI), m/.dL | 142.1 (130.2,153.9) | 232.3 (112.7,351.9) | 142.0 (113.3,170.7) | 0.26** | 88.6 (83.5,93.7) | 87.7 (82.4,93.0) | 65.2 (62.2,68.1) | <0.01** |
| Percent above 110 | 79.3 | 90.4 | 82.3 | 0.50 | 21.9 | 18.4 | 7.0 | <0.01 |
| HDL | ||||||||
| Mean (95% CI), mg/dL | 36.1 (33.6,38.7) | 37.9 (35.8,40.1) | 41.0 (37.1,44.9) | 0.12 | 53.6 (52.4,54.8) | 52.9 (51.7,54.1) | 56.0 (54.4,57.6) | 0.02 |
| Percent below 40 | 79.9 | 73.4 | 67.8 | 0.67 | 7.5 | 8.9 | 5.9 | 0.28 |
| SBP | ||||||||
| Mean (95% CI), mmHg | 117.2 (114.5,119.9) | 118.0 (113.8,122.2) | 117.5 (112.1,122.8) | 0.94 | 105.6 (104.4,106.7) | 105.8 (104.8,106.7) | 109.1 (107.9,110.2) | <0.01 |
| Percent above 90% pctile | 20.4 | 24.4 | 37.3 | 0.56 | 2.8 | 2.1 | 5.2 | 0.04 |
| DBP | ||||||||
| Mean (95% CI), mmHg | 65.4 (60.1,70.7) | 67.6 (63.9,71.2) | 65.9 (61.1,70.7) | 0.76 | 63.5 (62.4,64.5) | 62.2 (61.1,63.3) | 63.2 (62.3,64.2) | 0.22 |
| Percent above 90% pctile | 11.6 | 4.5 | 9.2 | 0.52 | 2.7 | 2 | 3.4 | 0.60 |
| Fasting Glucose | ||||||||
| Mean (95% CI), mg/dL | 96.8 (91.7,101.8) | 97.5 (95.2,99.7) | 99.1 (89.0,109.3) | 0.91 | 90.8 (90.0,91.6) | 91.0 (90.3,91.7) | 88.9 (88.1,89.8) | <0.01 |
| Percent above 100 | 52.6 | 35.2 | 30.6 | 0.42 | 6.8 | 5.0 | 4.0 | 0.33 |
Chi-square test comparing percents, ANOVA comparing means (overall difference among the groups).
Comparison of In(Triglyceride).
Table 3 shows MetS-related characteristics by gender, race/ethnicity and MetS status. Among males with MetS, non-Hispanic blacks with had the highest BMI of the three groups and also had the highest levels of insulin of the three groups. Among females non-Hispanic blacks with and without MetS had the highest levels of BMI and insulin of the three groups. Among males with MetS there were no differences in levels of uric acid between racial/ethnic groups, while among males without MetS non-Hispanic-white males had the highest levels of uric acid. Among females with and without MetS non-Hispanic whites had the highest level of uric acid of the three groups.
Table 3. Race/ethnicity comparison of MetS related factors for Males and Females stratified by Metabolic Syndrome.
| Metabolic Syndrome | Without Metabolic Syndrome | |||||||
|---|---|---|---|---|---|---|---|---|
| Non-Hispanic White | Hispanic | Non-Hispanic Black | p-value* | Non-Hispanic White | Hispanic | Black | p-value* | |
| Males (Mean (95% CI)) | ||||||||
| BMI | 30.9 (29.2,32.5) | 31.1 (29.7, 32.4) | 34.6 (32.1, 37.1) | 0.02 | 21.9 (21.5, 22.3) | 22.3 (21.7, 22.9) | 22.8 (22.3, 23.3) | 0.01 |
| Insulin | 24.5 (19.4, 29.7) | 25.1 (20.3, 29.9) | 26.3 (22.7, 29.9) | 0.05** | 8.3 (7.7,8.9) | 9.5 (8.8, 10.3) | 9.3 (8.7, 9.9) | 0.04** |
| HOMA | 6.4 (4.6, 8.1) | 6.3 (5.0, 7.6) | 6.7 (5.7, 7.7) | 0.06** | 2.0 (1.8,2.1) | 2.3 (2.1, 2.5) | 2.1 (2.0, 2.3) | 0.03** |
| Uric acid | 7.1 (6.5, 7.6) | 6.4 (6.0, 6.8) | 6.5 (6.1, 6.8) | 0.09 | 5.7 (5.6,5.8) | 5.5 (5.3, 5.7) | 5.2 (5.1, 5.3) | <0.01 |
|
| ||||||||
| Females (Mean (95% CI)) | ||||||||
| BMI | 29.6 (27.2, 32.1) | 32.7 (29.6, 35.8) | 35.4 (32.2,38.7) | 0.02 | 22.5 (21.8,23.1) | 23.3 (22.6,23.9) | 25.3 (24.8, 25.8) | <0.01 |
| Insulin | 20.2 (12.9, 27.6) | 24.9 (21.8, 28.0) | 35.0 (28.4, 41.6) | <0.01** | 9.2 (8.5, 9.8) | 11.5 (10.4, 12.6) | 12.7 (11.9, 13.5) | <0.01** |
| HOMA | 5.0 (2.9, 7.0) | 6.0 (5.2, 6.8) | 9.1 (6.2, 11.9) | 0.01** | 2.1 (1.9, 2.2) | 2.6 (2.3, 2.9) | 2.8 (2.6, 3.0) | <0.01** |
| Uric acid | 5.2 (4.8, 5.5) | 5.5 (4.7, 6.3) | 5.5 (4.9, 6.0) | 0.47 | 4.6 (4.5, 4.6) | 4.3 (4.2, 4.5) | 4.3 (4.2, 4.4) | <0.01 |
Chi-square test comparing percents, ANOVA comparing means (overall difference among the groups).
In (insulin), In(HOMA).
Sensitivity of MetS for detecting high uric acid
A classification of MetS exhibited a lower sensitivity among females (18.0%, CI 12.1-29.1) than males (37.0%, CI 29.1-45.8) at identifying individuals with elevated uric acid levels (approximately the 95th percentile among lean individuals, 7.0 mg/dL for males and 5.5 mg/dL for females)(p<0.05). There was no gender-specific difference in specificity of MetS to detect an elevated level of uric acid (males 92.3%, CI 89.1-94.7; females 95.7%, CI 94-96.9).
On a race/ethnicity specific basis (Figure 1a), a classification of MetS performed more poorly at detecting elevated levels of uric acid among non-Hispanic black males compared to Hispanics (p<0.01) and non-Hispanic whites (p<0.05). There was a high degree of specificity in the ability of MetS to detect elevations in uric acid, with each racial/ethnic/gender group exceeding 90% specificity (Figure 1b). This is to say that the vast majority of individuals without elevations in uric acid did not have MetS. The degree of specificity was higher among non-Hispanic black males compared to Hispanics and compared Hispanics and non-Hispanic whites combined (p<0.05). There were no differences in PPV for MetS to detect elevated uric acid, while the NPV was higher among non-Hispanic-black males compared to non-Hispanic whites (p<0.05)(Figure 1c-d). Among females there were no significant racial/ethnic differences in sensitivity, specificity, PPV or NPV for MetS to detect elevated levels of uric acid.
Figure 1. Sensitivity and specificity of MetS to detect uric acid elevations.
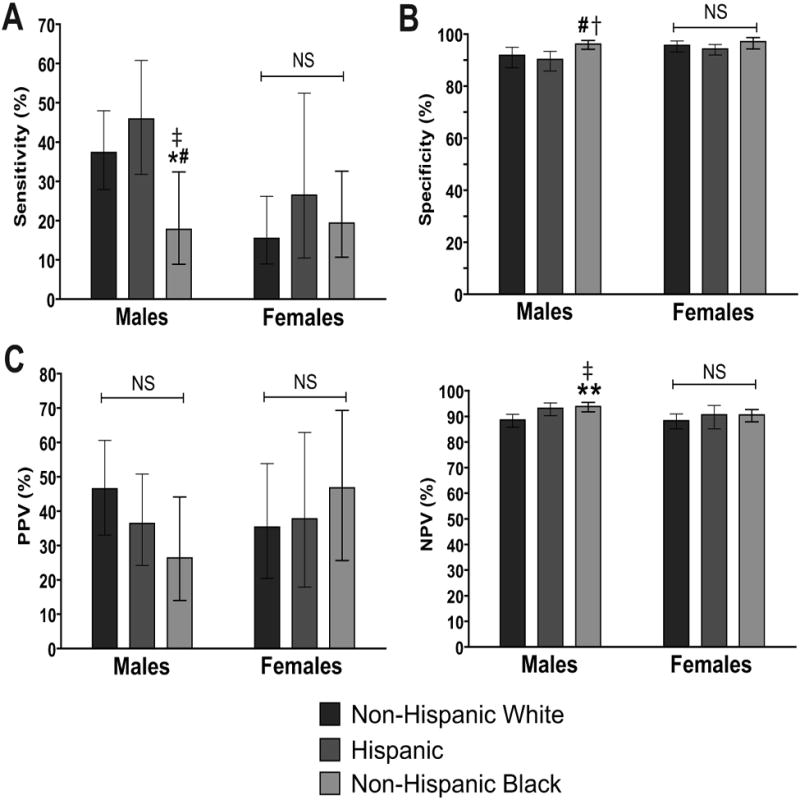
A. Sensitivity of a diagnosis of MetS to detect elevated uric acid level by gender-and race/ethnicity. A “high” uric acid level was defined as approximately the 95th percentile for non-overweight adolescents (7.0 mg/dL for males and 5.5 mg/dL for females). B. Specificity of a diagnosis of MetS for identifying individuals with high uric acid. C. Positive predictive value (PPV) of MetS for identifying uric acid elevations. D. Negative predictive value (NPV) of MetS for identifying uric acid elevations. Comparisons between racial/ethnic groups by corresponding gender and MetS status are: * p<0.05 vs.non-Hispanic whites; ** p<0.01 vs. non-Hispanic whites; # p<0.01 vs. Hispanics; † p<0.05 vs. Hispanics and non-Hispanic whites combined; ‡ p<0.01 vs. Hispanics and non-Hispanic whites combined. NS = not significant (p≥0.05).
It is important to note that because of the nature of the computation for these analyses (i.e. using 2×2 tables with MetS as the predictor and high uric acid as the outcome), these graphs can be rearranged to assess the performance of high uric acid as the predictor and MetS as the outcome. Interpreting the analysis in this manner, Figure 1C reflects the sensitivity of uric acid (using our gender-specific cut-offs) to detect MetS and Figure 1D reflects the specificity for this approach. There was a nonsignificant trend (p=0.07) toward a lower sensitivity for elevated uric acid to detect MetS among non-Hispanic-black vs. non-Hispanic-white adolescent males, while there was a corresponding higher specificity for high uric acid to detect MetS in non-Hispanic black males. These data would support the concept that while elevated uric acid levels exhibit similar ability to detect the presence of MetS across races/ethnicities, there was a higher proportion of individuals without MetS who also were without uric acid elevations among non-Hispanic-black males than among non-Hispanic whites.
Discussion
We found racial/ethnic and gender differences in the ability for a diagnosis of MetS to identify adolescents with elevated levels of uric acid. While on a population level MetS exhibits a reasonable sensitivity to identify elevations in uric acid [30], in an ethnicity-specific analysis MetS performed more poorly in the ability to do so among females compared to males and among non-Hispanic-black males compared to Hispanics and non-Hispanic whites. These data may have implications regarding the use of current MetS criteria as a marker of risk, particularly among non-Hispanic black males.
Uric acid continues to gain attention as an independent risk factor for future disease. In multiple prospective studies in adults, higher levels of uric acid have linked to future risk for CVD [2,3], hypertension [6], renal disease [7] and T2DM [4,5]. Among adolescents higher levels of uric acid are associated with current carotid intima media thickness [8] and future hypertension [9]. Given the advent of the pediatric obesity epidemic and its effects on future adult disease, markers of risk such as uric acid are needed to identify adolescents at highest need for intervention [10,26].
MetS is perhaps a better known marker of risk in adolescents. While data regarding long-term risk related to elevated uric acid in adolescents remains scarce, the presence of MetS in youth has been linked to an increase risk for future T2DM [14]. Because of this some have advocated using MetS as one screening tool that could trigger increased intervention among adolescents [16].
However, MetS may not function equally well as a marker of risk among all ethnicities. Non-Hispanic blacks have a lower prevalence of MetS throughout the lifespan [18,19] despite having higher rates of T2DM [23,24] and death from CVD [25]. Many of these differences appear to result from lower baseline levels of triglycerides among non-Hispanic blacks—making them less likely to develop elevations in triglycerides that contribute to MetS diagnosis using current MetS criteria [20,26]. Despite their lower prevalence of MetS overall, non-Hispanic-black adolescents diagnosed with MetS have higher levels of C-reactive protein (CRP, another marker of cardiovascular risk)[27] and insulin [22]. This suggests that by the time non-Hispanic blacks are diagnosed with MetS they have reached a more progressed degree of risk compared to other ethnicities—potentially suggesting that additional non-Hispanic blacks are not diagnosed with MetS despite having an elevated level of risk.
Data from the current study support this concept. We essentially evaluated MetS as a screening test for its ability to identify adolescents with another marker of risk, elevated uric acid levels. While MetS performed reasonably well at detecting uric acid elevations in adolescent males overall (with sensitivity rates of 37-46% among non-Hispanic whites and Hispanics), it performed poorly (sensitivity 18%) at detecting uric acid elevations in non-Hispanic black males. This lower sensitivity among non-Hispanic blacks did not appear to be due to a weaker association between MetS and uric acid, as levels of uric acid were not significantly different between groups with MetS. The low sensitivity also did not appear to be related to higher levels of uric acid among non-Hispanic blacks without MetS, as non-MetS levels of uric acid were higher among non-Hispanic whites. Finally, the poor sensitivity did not appear to be due to a lower degree of insulin resistance among non-Hispanic-black males, as fasting insulin levels were highest among non-Hispanic black males with MetS. Instead, this finding appeared to be truly due to a poor ability of MetS itself to identify non-Hispanic-black males with higher levels of uric acid. This is particularly important given that the ability of elevated uric acid to identify risk for future CVD appears to be particularly strong among non-Hispanic blacks as compared to non-Hispanic whites.
If current MetS criteria were used to detect risk of exhibiting elevated uric acid (and whatever further risk this elevated uric acid level entails), a significantly greater percentage of non-Hispanic-black males would miss detection. Given the emerging evidence linking uric acid to future disease, these data provide further support that current MetS criteria are a poorer detector of risk among non-Hispanic-black males than among other ethnicities. Indeed, some have advocated for the formulation of race/ethnicity-specific MetS criteria for better overall risk assessment [20,26]. The race/ethnicity-related findings among males in the current study are particularly interesting given what appear to be stronger associations between elevated uric acid levels and future CVD among non-Hispanic blacks as compared to non-Hispanic whites [2].
The sensitivity of MetS to detect elevated uric acid was low among most ethnic/gender groups, and this was particularly true among adolescent females, who had a lower sensitivity (18%) than males (37%). While the ethnic/gender groups with the lowest sensitivities of MetS to detect elevated uric acid were all in groups a lower prevalence of MetS (i.e., non-Hispanic black-males and each of the female groups), the low sensitivity across all groups suggests a high prevalence of uric acid elevations that are not related to MetS itself. It is uncertain what other causes of these non-MetS uric acid elevations these may be, particularly given that as a group adolescents represent a population with far less disease comorbidities than are seen in adults. One potential cause of non-MetS-related elevations in uric acid is the high rate of obesity in the population [11].
Regarding the inter-gender differences, females have lower levels of uric acid than males, which is at least in part because estrogen is uricosuric [12]. This resulted in a lower cut-off to define uric acid elevations in females (approximately the 95th percentile, 5.5 mg/dL in females vs. 7.0 in males). There was a lower sensitivity (but similar specificity) of MetS for detecting uric acid elevations in females suggesting a greater proportion of uric acid elevations that are not related to MetS itself among females than is seen among males. Also, females did not exhibit the racial/ethnic differences in MetS sensitivity as had been noted in males. Overall, given the emerging utility of uric acid as a risk marker, these gender differences present the need for gender-specific comparisons of MetS as a risk factor for future disease.
This study was limited by its use of cross-sectional data from NHANES. It should be noted that assessments of the associations between MetS, uric acid and future disease among adolescents will require long-term cohort studies.
In conclusion, we noted a poorer ability for current MetS criteria to identify uric acid elevations in non-Hispanic-black adolescent males in comparison to Hispanics and non-Hispanic whites. Given emerging data on uric acid as an important risk factor for hypertension, kidney disease, T2DM and CVD, these data suggest that MetS may function more poorly as a marker of risk among non-Hispanic blacks than among other ethnicities. More data is needed regarding prospective analysis of disease risk in these populations.
Acknowledgments
This work was supported by NIH grants 5K08HD060739-03 (MDD) and 1R21DK085363 (MDD and MJG)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Gagliardi AC, Miname MH, Santos RD. Uric acid: A marker of increased cardiovascular risk. Atherosclerosis. 2009;202:11–17. doi: 10.1016/j.atherosclerosis.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 2.Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971-1992. National Health and Nutrition Examination Survey. JaAMA. 2000;283:2404–2410. doi: 10.1001/jama.283.18.2404. [DOI] [PubMed] [Google Scholar]
- 3.Rodrigues TC, Maahs DM, Johnson RJ, et al. Serum uric acid predicts progression of subclinical coronary atherosclerosis in individuals without renal disease. Diabetes Care. 2010;33:2471–2473. doi: 10.2337/dc10-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kodama S, Saito K, Yachi Y, et al. Association between serum uric acid and development of type 2 diabetes. Diabetes Care. 2009;32:1737–1742. doi: 10.2337/dc09-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viazzi F, Leoncini G, Vercelli M, et al. Serum uric acid levels predict new-onset type 2 diabetes in hospitalized patients with primary hypertension: the MAGIC study. Diabetes Care. 2011;34:126–128. doi: 10.2337/dc10-0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mellen PB, Bleyer AJ, Erlinger TP, et al. Serum uric acid predicts incident hypertension in a biethnic cohort: the atherosclerosis risk in communities study. Hypertension. 2006;48:1037–1042. doi: 10.1161/01.HYP.0000249768.26560.66. [DOI] [PubMed] [Google Scholar]
- 7.Sonoda H, Takase H, Dohi Y, et al. Uric acid levels predict future development of chronic kidney disease. Am J Nephrol. 2011;33:352–357. doi: 10.1159/000326848. [DOI] [PubMed] [Google Scholar]
- 8.Meyer AA, Kundt G, Steiner M, et al. Impaired flow-mediated vasodilation, carotid artery intima-media thickening, and elevated endothelial plasma markers in obese children: the impact of cardiovascular risk factors. Pediatrics. 2006;117:1560–1567. doi: 10.1542/peds.2005-2140. [DOI] [PubMed] [Google Scholar]
- 9.Alper AB, Jr, Chen W, Yau L, et al. Childhood uric acid predicts adult blood pressure: the Bogalusa Heart Study. Hypertension. 2005;45:34–38. doi: 10.1161/01.HYP.0000150783.79172.bb. [DOI] [PubMed] [Google Scholar]
- 10.Krishnan E, Sokolove J. Uric acid in heart disease: a new C-reactive protein? Curr Opin Rheumatol. 2011;23:174–177. doi: 10.1097/BOR.0b013e3283432dd3. [DOI] [PubMed] [Google Scholar]
- 11.Ford ES, Li C, Cook S, et al. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation. 2007;115:2526–2532. doi: 10.1161/CIRCULATIONAHA.106.657627. [DOI] [PubMed] [Google Scholar]
- 12.Borges RL, Ribeiro AB, Zanella MT, et al. Uric acid as a factor in the metabolic syndrome. Curr Hypertens Rep. 2010;12:113–119. doi: 10.1007/s11906-010-0098-2. [DOI] [PubMed] [Google Scholar]
- 13.Grundy SM, Brewer HB, Jr, Cleeman JI, et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 14.Morrison JA, Friedman LA, Wang P, et al. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J Pediatr. 2008;152:201–206. doi: 10.1016/j.jpeds.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Dekker JM, Girman C, Rhodes T, et al. Metabolic syndrome and 10-year cardiovascular disease risk in the Hoorn Study. Circulation. 2005;112:666–673. doi: 10.1161/CIRCULATIONAHA.104.516948. [DOI] [PubMed] [Google Scholar]
- 16.Zimmet P, Alberti G, Kaufman F, et al. The metabolic syndrome in children and adolescents. Lancet. 2007;369:2059–2061. doi: 10.1016/S0140-6736(07)60958-1. [DOI] [PubMed] [Google Scholar]
- 17.Bibbins-Domingo K, Coxson P, Pletcher MJ, et al. Adolescent overweight and future adult coronary heart disease. N Engl J Med. 2007;357:2371–2379. doi: 10.1056/NEJMsa073166. [DOI] [PubMed] [Google Scholar]
- 18.Park YW, Zhu S, Palaniappan L, et al. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2003;163:427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker SE, Gurka MJ, Oliver MN, et al. Racial/ethnic discrepancies in the metabolic syndrome begin in childhood and persist after adjustment for environmental factors. Nutr Metab Cardiovasc Dis. 2010 doi: 10.1016/j.numecd.2010.05.006. e-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sumner AE, Cowie CC. Ethnic differences in the ability of triglyceride levels to identify insulin resistance. Atherosclerosis. 2008;196:696–703. doi: 10.1016/j.atherosclerosis.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 21.Arslanian S, Suprasongsin C. Differences in the in vivo insulin secretion and sensitivity of healthy black versus white adolescents. J Pediatr. 1996;129:440–443. doi: 10.1016/s0022-3476(96)70078-1. [DOI] [PubMed] [Google Scholar]
- 22.DeBoer MD, Dong L, Gurka MJ. Racial/ethnic and gender differences in the ability of metabolic syndrome criteria to predict elevations in fasting insulin in adolescents: An Analyses of NHANES 1999-2008. J of Pediatr. 2011 doi: 10.1016/j.jpeds.2011.05.023. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dabelea D, Bell RA, D'Agostino RB, Jr, et al. Incidence of diabetes in youth in the United States. JAMA. 2007;297:2716–2724. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- 24.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988-2006. Diabetes Care. 2010;33:562–568. doi: 10.2337/dc09-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mensah GA, Mokdad AH, Ford ES, et al. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 26.DeBoer MD. Ethnicity, obesity and the metabolic syndrome: implications on assessing risk and targeting intervention. Expert Rev Endocrinol Metab. 2011;6:279–289. doi: 10.1586/eem.11.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeBoer MD, Gurka MJ, Sumner AE. Diagnosis of the Metabolic Syndrome Is Associated With Disproportionately High Levels of High-Sensitivity C-Reactive Protein in Non-Hispanic Black Adolescents: An analysis of NHANES 1999-2008. Diabetes Care. 2011;34:734–740. doi: 10.2337/dc10-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandez JR, Redden DT, Pietrobelli A, et al. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr. 2004;145:439–444. doi: 10.1016/j.jpeds.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 29.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 30.DeBoer MD, Gurka MJ. Ability among adolescents for the metabolic syndrome to predict elevations in factors associated with type 2 diabetes and cardiovascular disease: data from the national health and nutrition examination survey 1999-2006. Metab Syndr Relat Disord. 2010;8:343–353. doi: 10.1089/met.2010.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]


