Abstract
Background
Maraviroc, the first approved CCR5 antagonist, demonstrated 48-week safety and virologic efficacy in CCR5-tropic HIV-infected, treatment-experienced patients; however, critical longer-term safety and durability of responses are unknown.
Methods
Two-year follow-up of 2 prospective, randomized, blinded studies of maraviroc once daily or twice daily, or placebo in treatment-experienced patients with R5-tropic HIV-1 receiving an optimized background regimen. Unblinding occurred after the week-48 visit of the last enrolled patient. Safety and virologic parameters were assessed through week 96.
Results
One thousand forty-nine patients were randomized and received study drugs. HIV-1 RNA was <50 copies per milliliter at week 96 in 39% and 41% of patients receiving maraviroc every day or twice a day, respectively. Among patients with HIV-1 RNA <50 copies per milliliter at week 48, 81% and 87% of patients receiving maraviroc every day or twice a day, respectively, maintained this response at week 96. At week 96, median CD4+ T-cell counts increased from baseline by 89 and 113 cells per cubic millimeter with maraviroc every day and twice a day, respectively. Exposure-adjusted rates of adverse events were similar with maraviroc or placebo. No new or unexpected events were observed after week 48.
Conclusions
Maraviroc-containing antiretroviral regimens maintained durable responses in treatment-experienced patients with R5 HIV-1 through 96 weeks of treatment with a safety profile similar to placebo.
Keywords: HIV, host-based therapies, maraviroc, long-term safety, treatment experienced
INTRODUCTION
As the first approved CC chemokine receptor 5 (CCR5) antagonist, maraviroc is the prototypic agent of this new class of antiretroviral medications. Utilizing a novel mechanism of action by blocking attachment between HIV-1 gp120 and the human CCR5 receptor, maraviroc is the first antiretroviral that binds to a human protein.1
Maraviroc is approved for treatment, in combination with other antiretrovirals, of both treatment-experienced and treatment-naive patients with CCR5-tropic (R5) HIV-1 infection.2 The 48-week analysis of the MOTIVATE 1 (Canada and United States) and MOTIVATE 2 (Australia, Europe, and United States) studies demonstrated significantly greater mean decreases in HIV-1 RNA levels compared with baseline, greater proportions of study patients with HIV-1 RNA <400 copies per milliliter and <50 copies per milliliter, and greater rises in CD4+ T cells per cubic millimeter in maraviroc-treated patients compared with those receiving placebo.3,4 In addition, the adverse event profile of maraviroc was similar to that of placebo with similar incidences of both all-cause and treatment-related adverse events, AIDS-defining events (CDC category C), non–AIDS-defining malignancies and grade 3 or 4 hepatic transaminase elevations.3,5,6
As with any novel class of medication, concerns regarding maraviroc's long-term safety and durability of virologic efficacy must be addressed. Will altering the conformation of CCR5, an important part of the human chemokine system, have untoward, downstream immunologic consequences? What about adverse events seen with other agents in this class (eg, elevated liver enzymes and increased incidence of malignancies)7–10 or ones potentially resulting from maraviroc novel mechanism of action (eg, new, unusual, or reactivated infections)? These concerns warrant continuing evaluation and report of long-term clinical data with this new agent. To this end, we report the 96-week safety and efficacy analyses from the combined MOTIVATE studies.
METHODS
Study Subjects and Design
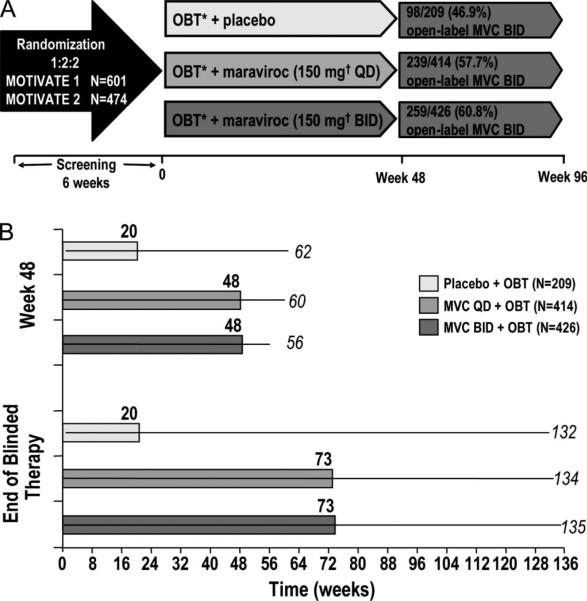
The design and enrollment criteria of the MOTIVATE 1 and 2 studies have been described in detail previously (Fig. 1A).3 Briefly, both studies enrolled patients aged 16 years or older with only R5 HIV-1 detected at screening using the original Trofile assay (Monogram Biosciences, South San Francisco, CA), plasma HIV-1 RNA >5000 copies per milliliter and experience with or resistance to at least 3 antiretroviral drug classes. Patients were randomized 2:2:1 to receive maraviroc twice a day or every day or identical placebo combined with an investigator-selected optimized background therapy (OBT) of 3–6 other antiretrovirals based on treatment history and viral resistance testing at screening (Phenosense GT, Monogram Biosciences). Of note, agents that were investigational at the start of the study, such as darunavir, etravirine, and raltegravir, were excluded from the OBT. Patients receiving potent CYP3A4 inhibitors (ritonavir-boosted protease inhibitors other than tipranavir or delavirdine) received 150 mg of maraviroc every day or twice a day, whereas others received 300 mg dosed identically. As the 2 studies had identical designs, similar enrollment demographics and baseline characteristics, pooled analysis of their data was conducted.
FIGURE 1.
A, Study design, and B, duration of blinded study treatment. A, Patients were stratified by enfuvirtide use and HIV-1 RNA < or > 100,000 copies per milliliter. Patients without treatment failure at end of blinded therapy (last patient week-48 visit) were given the option to roll over to open-label MVC twice a day. Week 96 efficacy analysis included all patients Post week 48. Safety analysis included only patients on blinded randomized therapy between individual week 48 visit and end of blinded therapy. B, Median (bars) and range (lines) of study treatment duration at indicated study time point.
Patients received their double-blinded, randomized study treatment until the last enrolled patient reached the week-48 visit. At this point, all patients’ study therapy was unblinded, and maraviroc every day and twice a day recipients who had not experienced treatment failure during the study were offered open-label maraviroc twice a day. Thus, there was a wide range of study drug exposure at the end of blinded treatment (EBT), depending on when a patient was enrolled into the study. Placebo recipients with a history of treatment failure or drug intolerance were also offered open-label maraviroc twice a day. Treatment failure was defined as 1 of 4 virologic endpoints confirmed by a second consecutive measurement within 14 days as follows: (1) increase in HIV-1 RNA to ≥3 times the baseline level at or after week 2; (2) decrease of >0.5 log10 copies per milliliter at or after week 8; (3) decrease of <1.0 log10 copies per milliliter at or after week 8, after a decrease of ≥2.0 log10 copies per milliliter; and (4) increase in HIV-1 RNA to ≥5000 copies per milliliter after a decrease to <400 copies per milliliter had been recorded on 2 consecutive visits.
The study protocol was approved by the institutional review board or independent ethics committee at each study center. Written informed consent was obtained from all participants. The studies were performed in accordance with International Conference on Harmonization Good Clinical Practice guidelines and applicable local regulatory requirements and laws.
Efficacy Analysis
Patients receiving maraviroc (twice a day or every day) without a history of treatment failure or intolerance and plasma HIV-1 RNA <50 copies per milliliter at their week-48 visit were followed through week 96. Data between weeks 48 and 96 were derived from a mixture of blinded and unblinded therapy that varied due to individual differences in the duration of blinded therapy between week 48 and EBT. In this analysis, patients were categorized according to their originally randomized treatment assignment irrespective of subsequent change to open-label maraviroc twice a day. Placebo recipients who began open-label maraviroc twice a day at EBT were censored from the 96-week analysis. Those placebo recipients who had not reached a treatment failure endpoint were followed through week 96 for descriptive purposes but were not included in the efficacy analysis, as the studies became noncomparative after unblinding.
Efficacy was assessed as the proportion of patients with plasma HIV-1 RNA <50 copies per milliliter at week 48 who maintained virologic control through week 96. Virologic control was stratified as follows: (1) those who maintained <50 copies per milliliter through week 96; (2) those with plasma HIV-1 RNA between 50 and 400 copies per milliliter at week 96; and (3) those with HIV-1 RNA >400 copies per milliliter at week 96 but who had not reached the criterion for virologic failure (on study, not failed). For the week-96 analysis, this failure criterion was specified as a rise in plasma HIV-1 RNA to at least 3 times the value at baseline.
Safety Analysis
This analysis includes all blinded data until EBT for study patients according to their original randomization. It does not include any open-label treatment data. Consistent with the change in study analysis plan at EBT, patient data could contribute to the efficacy analysis both before and after EBT; however, for the safety analysis, only data collected while patients were receiving original blinded treatment were included. Hence, the safety analysis comprises a wide range of blinded exposure durations to maraviroc or placebo between week 48 and EBT. Safety data for both week 48 and EBT are presented.
Due to the considerable differences in the range of exposures to blinded study therapy between the maraviroc-treated and placebo-treated patients (Fig. 1B), the safety analysis examined both unadjusted and exposure-adjusted incidences of all-cause adverse events, AIDS-defining events (CDC category C), malignancies, and hepatic enzyme and function tests. Event counts were adjusted to 100 years of subject exposure, and if the same subject had more than 1 occurrence in the same event category, only the most severe occurrence was considered. Reported changes in hepatic transaminase and total bilirubin tests were limited to the occurrence of moderate-to-severe events (>3 × ULN for transaminases and >1.5 × ULN for total bilirubin) irrespective of baseline grading. Of note, MOTIVATE inclusion criteria allowed enrollment of otherwise eligible patients with mild-to-moderate elevations of transaminases (≤5 × ULN) or total bilirubin (≤2.5 × ULN).
Statistical Methods
Demographics and baseline characteristics were summarized using descriptive statistics. Unless otherwise noted, percentages are based on the total number of subjects initially randomized to the 3 study arms. Median change from baseline in CD4+ T-cell count at week 96 was calculated using the last available observation on each subject.
The study was designed by the sponsor, Pfizer Global Research and Development, with input from the study investigators. Data were gathered by the study investigators and the sponsor, with data summaries provided by an independent statistician (Covance CAPS, Berkshire, United Kingdom) from the Statistical Data Analysis Center to the data and safety monitoring board for periodic review. All statistical analyses were carried out by the study sponsor according to a predefined plan. One of the authors wrote the paper, with extensive input from the study team. The study team confirms the accuracy and completeness of the data and analysis as presented.
RESULTS
The combined MOTIVATE 1 and 2 studies included 1049 randomized patients who received at least 1 dose of maraviroc every day (n = 414), maraviroc twice a day (n = 426), or placebo (n = 209). Patient demographic and baseline characteristics were similar across the 3 treatment groups (Table 1). Two-thirds of enrolled patients received OBTs with 2 or fewer potentially active antiretroviral agents, assessed by the overall regimen susceptibility score.11 More than 40% of patients received enfuvirtide as part of their OBT; 59% of this group were receiving enfuvirtide for the first time. Less than 15% of subjects in MOTIVATE received tipranavir as a new protease inhibitor.
TABLE 1.
Demographics and Baseline Characteristics
| Treated, n = 1049 | Placebo + OBT, n = 209 | MVC Every Day + OBT, n = 414 | MVC Twice a Day + OBT, n = 426 |
|---|---|---|---|
| Mean age, yrs (range) | 46 (29–72) | 46 (17–75) | 46 (21–73) |
| Female, n (%) | 24 (12) | 51 (12) | 44 (10) |
| White, n (%) | 178 (85) | 336 (81) | 363 (85) |
| Mean CD4+ cell count, (cells/mm3)a | 171 | 171 | 167 |
| Mean HIV-1 RNA, (log10 copies/mL)b | 4.86 | 4.86 | 4.85 |
| ≤2 active drugs in OBT, %c | 66.0 | 66.0 | 69.7 |
| Enfuvirtide in OBT, % | 43.5 | 40.6 | 42.7 |
| Enfuvirtide sensitive and first use, % | 28.0 | 22.3 | 26.0 |
| Tipranavir in OBT, % | 13.9 | 15.9 | 14.8 |
MVC, maraviroc.
calculated for each patient as the mean of up to two pre-dose assessments (screening and baseline).
calculated for each patient as the mean of up to three pre-dose assessments (screening, randomization, and baseline).
according to overall susceptibility score at screening. Includes all patients who received at least one dose of study medication.
The duration of study treatment exposure was substantially greater for the maraviroc-treated patients (median 73 weeks, range up to 135 weeks) compared with those receiving placebo (median 20 weeks, range up to 132 weeks) at EBT (Fig. 1B). As expected, 47% of placebo patients had experienced treatment failure or drug intolerance and rolled over to open-label maraviroc twice a day at EBT. In contrast, 58% of maraviroc every day patients and 61% of maraviroc twice a day patients had not experienced treatment failure or intolerance at EBT and rolled over to open-label maraviroc twice a day.
Efficacy Analysis
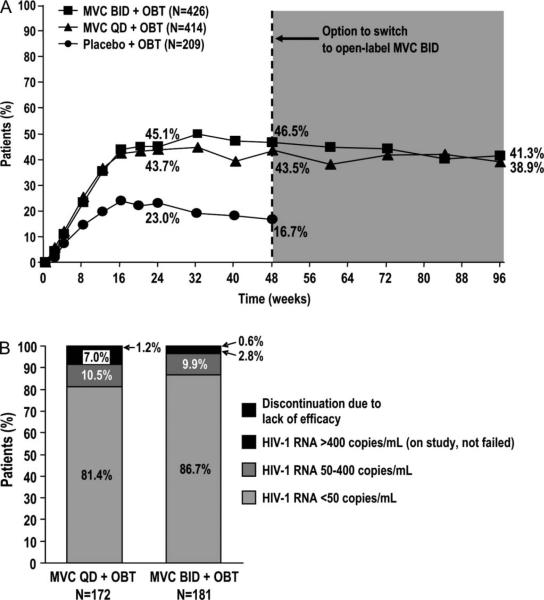
For study subjects assigned to maraviroc twice a day, whose treatment remained the same preweek and postweek 48, 41% had HIV-1 RNA <50 copies per milliliter at week 96, compared with 46% at week 48 (Fig. 2A). For those assigned to maraviroc every day, for whom postweek 48 data included a mixture of blinded every day and open-label twice a day treatment, 38.9% had HIV-1 RNA <50 copies per milliliter at week 96 compared with 43.5% at week 48.
FIGURE 2.
A, Percentage of patients with HIV-1 RNA <50 copies/mL at week 96, and B, virological outcome at week 96 of patients with HIV-1 RNA <50 copies/mL at week 48. A, Placebo + OBT data excludes patients who switched to open-label MVC BID at end of blinded therapy between weeks 48 and 96. MVC BID + OBT and MVC QD + BID data include those who switched to open-label MVC BID. B, Maraviroc groups include patients on blinded therapy and open-label maraviroc twice a day. Lack of efficacy = HIV-1 RNA levels >3 times the baseline HIV-1 RNA level. Does not include patients who discontinued for lack of efficacy reasons: adverse events (n = 4); withdrew/lost to follow-up (n = 8); other reasons (n = 4); no discontinuation and no data at 96 weeks (n = 4).
A total of 373 patients receiving maraviroc (194 of 426 twice a day and 179 of 414 every day) had HIV-1 RNA <50 copies per milliliter at week 48. Sixteen of these patients [12 twice a day (6.2% of total) and 4 every day (2.2%)] were censored from the week-96 efficacy analysis for nonefficacy-related study discontinuations after week 48 [adverse events (n = 4); withdrew/lost to follow up (n = 8); other reasons (n = 4; including 3 deaths and 1 serious adverse event-cerebral vascular accident]. Four additional patients, including 1 twice a day (0.5% of total) and 3 every day (1.7% of total) were excluded for missing HIV-1 RNA data at week 96. The efficacy analysis population therefore comprised 353 patients (181 twice a day, 172 every day) with HIV-1 RNA <50 copies per milliliter at week 48 and with adequate virologic data available at week 96 (Fig. 2B).
Overall, 86.7% of maraviroc twice a day and 81.4% of maraviroc every day recipients with HIV-1 RNA <50 copies per milliliter at week 48 had this level of virologic suppression at week 96. An additional 9.9% of the twice a day group and 10.5% of the every day group had HIV-1 RNA >50 but <400 copies per milliliter. Of those with postweek-96 data available, 13 of 18 (72%) in the twice a day group and 10 of 18 (56%) in the every day group resuppressed to <50 copies per milliliter after the week 96 visit (data not shown). Only 0.6% and 1.2% of maraviroc twice a day and every day patients, respectively, were discontinued for lack of efficacy between weeks 48 and 96.
At week 96, the median change from baseline in CD4+ T-cell count was +89 and +113 cells per cubic millimeter in patients assigned to maraviroc every day and twice a day, respectively. These results were similar to those observed at week 48 (+92, +103 cells/mm3 for the every day and twice a day arms, respectively).
Safety Analysis
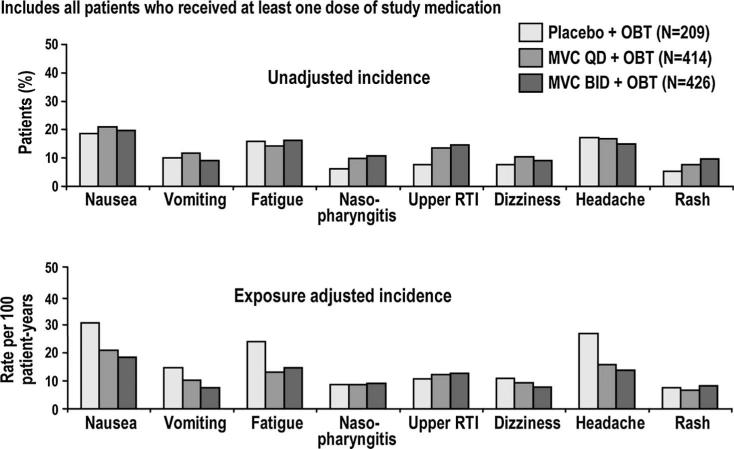
The distribution of all-cause adverse events occurring in at least 5% of patients at EBT is shown in Figure 3. The unadjusted analysis showed that the occurrence of nasopharyngitis, upper respiratory infections, rash, and dizziness seemed to be more frequent in maraviroc than placebo recipients. The frequencies of these adverse events were similar in both treatment groups in the exposure-adjusted analysis.
FIGURE 3.
Adverse events occurring in ≥5% of patients at end of blinded therapy. Includes all patients who received at least 1 dose of study medication. If the same patient in a given treatment had more than 1 occurrence in the same preferred term event category, only the most severe occurrence is taken. Event counts are adjusted to 100 years of patient exposure. Includes data up to 7 days after last dose of study drug. RTI, respiratory tract infection.
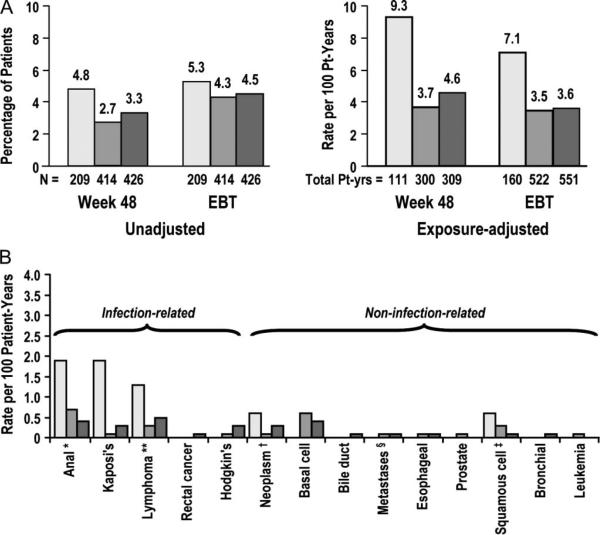
The analysis of malignancies observed after week 48 did not demonstrate a difference between maraviroc and placebo recipients in both the unadjusted (4.3%–4.5% versus 5.3%, respectively) and exposure-adjusted (3.3–3.5 versus 7.1 per 100 patient-years) incidences at EBT (Fig. 4A). Exposure-adjusted incidences of malignancies were noted to be decreasing after week 48. There was no association of any neoplasm with maraviroc treatment compared with placebo (Fig. 4B).
FIGURE 4.
Incidence of A, total malignancies at week 48 and at end of blinded therapy, and B, exposure-adjusted malignancies by type at end of blinded therapy. *Includes anal cancer stage 0; **T-cell, B-cell and diffuse large B-cell lymphoma; †Includes testicular and tongue neoplasms; §Includes bone, liver and peritoneum; ‡Includes squamous cell carcinoma of the skin.
Finally, the incidence of grade 3 or 4 liver transaminase or total bilirubin elevations (Table 2) was low overall and lower in maraviroc recipients than in placebo recipients at EBTwhen adjusted for study–drug exposure.
TABLE 2.
Incidence of LFT Abnormalities (Without Regard to Baseline) at Week 48 and EBT
| Incidence (Unadjusted), n(%) |
Incidence (Adjusted) Event Counts Adjusted to 100 Years of Patient Exposure, n (%) |
|||||
|---|---|---|---|---|---|---|
| Placebo + OBT | MVC Every Day + OBT | MVC Twice a Day + OBT | Placebo + OBT | MVC Every Day + OBT | MVC Twice a Day + OBT | |
| Week 48 | ||||||
| AST: >3.0 × ULN | 17 (8) | 39 (10) | 45 (11) | 16.2 | 13.8 | 15.7 |
| ALT: >3.0 × ULN | 13 (6) | 29 (7) | 37 (9) | 12.4 | 10.1 | 12.7 |
| Total bilirubin: >1.5 × ULN | 30 (14) | 66 (16) | 51 (12) | 31.9 | 25.3 | 18.5 |
| EBT | ||||||
| AST: >3.0 × ULN | 19 (9) | 45 (11) | 46 (11) | 13.3 | 9.4 | 9.5 |
| ALT: >3.0 × ULN | 15 (7) | 37 (9) | 39 (9) | 10.0 | 7.8 | 7.8 |
| Total bilirubin: >1.5 × ULN | 31 (15) | 68 (17) | 54 (13) | 23.8 | 16.0 | 11.3 |
ULN, upper limit of normal; MVC, maraviroc.
Total patient-years of exposure to study drug at week 48: placebo + OBT, 111, MVC, QD + OBT 300, MVC BID + OBT, 309. Total years of exposure to study drug at EBT: placebo + OBT, 160, MVC QD + OBT, 522, MVC BID + OBT, 551.
DISCUSSION
We have shown that maraviroc, when combined with an optimized background antiretroviral regimen, is safe and well tolerated and associated with durable virologic and immuno-logic responses in treatment-experienced patients through at least 96 weeks.
Due to significant differences in virologic efficacy between the maraviroc and placebo groups at 48 weeks, the MOTIVATE trials were unblinded after the last patient enrolled reached week 48.3,4 Thus, the efficacy data available through 96 weeks are derived from a combination of treatment with blinded study drug and open-label maraviroc dosed twice a day. Blinded safety data based on original randomization are available from all 3 study arms encompassing a wide range of treatment exposures ranging from 48 to more than 130 weeks, obtained before the week-48 visit of the last enrolled patient. Of note, the duration of blinded study treatment was almost 3 times longer in the maraviroc-treated versus placebo-treated patients.
The antiretroviral efficacy of maraviroc in combination with background therapy was durable beyond the initial 48 weeks of treatment. Overall, 41% and 39% of patients originally assigned to maraviroc twice a day or every day, respectively, had plasma HIV-1 RNA <50 copies per milliliter at 96 weeks. More specifically, 86.7% and 81.4% of maraviroc twice a day or every day recipients with <50 copies per milliliter at week 48 also had undetectable HIV-1 RNA at week 96. Moreover, well over half of the 10% of patients with plasma HIV-1 RNA between 50 and 400 copies per milliliter at week 96 subsequently resuppressed to <50 copies per milliliter beyond week 96.
Few subjects originally assigned to maraviroc treatment-experienced virologic failure between weeks 48 and 96. Coreceptor tropism screening before enrollment for this study was done using the original Trofile assay. During the first 48 weeks of the study, the suboptimal sensitivity of this assay may have contributed to virologic failures associated with emergence of previously undetected CXCR4-using virus.11,12 In the 83 subjects who had dual or mixed tropism at screening (n = 4) or at baseline (n = 79), the rate of virologic suppression (HIV-1 RNA <50 copies/mL) was 18% in the placebo group (3 of 17 patients), as compared with 30% in the group receiving maraviroc once daily (10 of 33 patients) and 27% in the group receiving maraviroc twice daily (9 of 33 patients). The test for an interaction between treatment and tropism at baseline was not significant (P = 0.54). Among 228 patients with an R5 tropism result at baseline who were deemed virologic failures, 76 of 133 patients who received maraviroc had a dual or mixed or X4 tropism result (57%) and 57 patients had an R5 tropism result (43%). By comparison, 6 of 95 patients who received placebo (6%) had virus binding to CXCR4 that was detectable at treatment failure.4
Results from detailed virologic analyses of isolates from study patients failing a maraviroc regimen through week 48 revealed the following: (1) 115 of 160 (72%) of patients receiving maraviroc with a wOBTSS (weighted OBT susceptibility score)_> 2 were TLOVR <50 responders with HIV-1 RNA <50 copies per milliliter at week 48; (2) excluding non-R5 failures and those without matched maraviroc susceptibility data, R5 virologic failure occurred in 62 of 331 (19%) patients, with maraviroc-resistant HIV-1 seen in 22 of 62 (35%) of these; (3) functional monotherapy or a single active nucleoside reverse transcriptase inhibitor (wOBTSS <1) accounted for 16 of 22 (73%) maraviroc resistance associated failures, and no maraviroc-resistant virus was found in patients with a wOBTSS of >2 who failed maraviroc treatment with an R5 tropism result; and (4) 16 of 80 (20%) patients who received functional monotherapy or a single active nucleoside reverse transcriptase inhibitor–experienced maraviroc resistance associated R5 virologic failure, and 50 of 80 (63%) of these patients responded through week 48.12
Due to the extremely low rate of virologic failure between week 48 and 96 (0.6% and 1.2% of maraviroc twice a day and every day patients, respectively), detailed virologic tropism and resistance analyses of second-year failures have not been completed.
It is of interest that the median increase from baseline in CD4+ T cells in maraviroc-treated subjects at week 96 did not appreciably differ from that seen at week 48. Reasons for this may stem from the older mean age of the study subjects (46 years) and/or the relatively low median CD4+ T-cell numbers at baseline (170 cells/mm3). In contrast, median CD4+ T-cell increases from baseline among treatment-naive patients receiving maraviroc or efavirenz with zidovudine–lamivudine in a post hoc reanalysis of the MERIT study were 174 cells/mm3 and 144/mm3, respectively, at 48 weeks compared to 212/mm3 and 171/mm3 at 96 weeks.13 Baseline mean age for these subjects was 37 years and median baseline CD4+ T-cell count was 245/mm3.
Long-term blinded safety data failed to identify any new or unexpected events after week 48. The most commonly reported all-cause clinical adverse events were well balanced between the maraviroc and placebo treatment groups through EBT. It is notable that the incidences of upper respiratory tract infections, nasopharyngitis, dizziness, and rash that were higher in the maraviroc arms by the exposure-unadjusted analysis were similar in the maraviroc and placebo arms when adjusted for treatment exposure. It therefore seems that these adverse events were a function of the much longer exposures to blinded maraviroc treatment and not the drug itself (522 patient-years of every day exposure, 551 patient-years of twice a day exposure, versus 160 patient-years of placebo) or varying risk for the occurrence of these events over time.
The occurrence of AIDS-defining (CDC category C) events or AIDS-defining or non–AIDS-defining malignancies may be interpreted as a clinical surrogate of the immunologic function of HIV-1–infected patients. The incidences of all 3 of these were lower in the maraviroc arms compared with placebo at both week 48 and EBT by both unadjusted and exposure-adjusted analyses. This finding is especially notable given the concern for an increased incidence of malignancies seen in the phase IIb trial of vicriviroc, another CCR5 antagonist.7,9,10
Both the unadjusted and exposure-adjusted incidences of transaminase and bilirubin elevations were similar between the maraviroc and placebo-treated patients at week 48 and were decreasing in the maraviroc arms through EBT. The lack of this safety signal contrasts with the drug-related hepatotoxicity associated with aplaviroc, another CCR5 antagonist, which is no longer in clinical development.8 Animal treatment data suggests that aplaviroc-associated hepatotoxicity is drug specific, not class specific.7 The lack of evidence of hepatotoxicity associated with maraviroc in treatment-experienced patients contrasts with initial concern for this adverse event.2
These data provide 2 years of experience with maraviroc treatment and the potential immunologic consequences of blocking the human CCR5 coreceptor. Of note, the delta 32 deletion of the CCR5 gene in humans has been associated with protective sequelae such as a reduced incidence of rheumatoid arthritis and delayed progression of hepatitis C.14–17 Among HIV-infected individuals, the delta 32 deletion has been associated with reduced HIV disease progression and reduced risks of acquiring non-Hodgkin lymphoma and opportunistic infections such as toxoplasmosis, Pneumocystis jiroveci pneumonia, Mycobacterium avium complex, and cryptosporidiosis.18–22 On the other hand, this mutation has also been associated with deleterious outcomes, including more severe West Nile virus and yellow fever virus infections.23–25 To date, no such deleterious consequences have been seen with the long-term use of maraviroc in randomized controlled clinical studies.
In conclusion, the 96-week data from the combined MOTIVATE 1 and 2 studies demonstrate durable virologic suppression with maraviroc-containing regimens in treatment-experienced patients with R5 HIV-1. These data also indicate that no new or unexpected adverse events, AIDS-defining clinical events, AIDS-defining or non–AIDS-defining malignancies or hepatotoxicity were observed after the initial 48 weeks. The exposure-adjusted incidences of these markers of toxicity or adverse clinical events were found to be similar to or lower than those seen with placebo. We conclude that maraviroc is generally safe and well tolerated and associated with durable virologic and immunologic responses in HIV-infected treatment-experienced patients.
ACKNOWLEDGMENTS
The authors thank the study participants and the study investigators and site staff at the 239 participating study sites.
The MOTIVATE 1 and MOTIVATE 2 trials were sponsored by Pfizer Global Research and Development and were supported by Pfizer and by grants from the National Institutes of Health (AI-055032 to W.D.H. and AI-51966 to R.M.G.). Editorial assistance was provided by Nick Fitch of Health Interactions Ltd., with funding by Pfizer Inc.
The authors W.D.H. has received research grants (through Cedars-Sinai Medical Center) from Bionor Immuno, Boehringer-Ingelheim, Gilead, GlaxoSmithKline, Merck, Pfizer, Roche, and Tibotec; served as a consultant for Boehringer-Ingelheim, Gilead, GlaxoSmithKline, Merck, Monogram (Lab Corp), Pfizer, and Tibotec; and has served on a Data and Safety Monitoring Committee for Tibotec. R.M.G. received research grants (through Weill Cornell Medical College) to Merck, Pfizer, Schering, and Tibotec; served as an ad-hoc consultant to Boehringer-Ingelheim, Bristol-Myers, Gilead, GlaxoSmithKline, Merck, Pathway, Pfizer, Progenics, Schering, Tibotec, and Virostatics; and served as the Chair of a Data and Safety Monitoring Committee for Koronis. H.M. was at time of study analysis and manuscript preparation an employee of Pfizer with stock options in Pfizer. G.F. has received consultancy honoraria, lecture fees and grant support from Abbott, Boehringer Ingelheim, Bristol Myers Squibb, Gilead, Glaxo Smith Kline, Merck Sharp & Dohme, Monogram, Pfizer, Roche, Schering Plough, and Tibotec. M.N. has received consultancy fees/speaker fees from Bristol Myers Squibb, Gilead, GlaxoSmithKline, Pfizer, Roche, Tibotec, MSD, Abbott, Schering Plough, and Idenix. He has also received grant support from Pfizer, Gilead, MSD, Abbott, and Roche. J.H., N.R., and J.G. are employees of Pfizer and hold stock and stock options in Pfizer.
Footnotes
Trial registration: ClinicalTrials.gov identifiers NCT00098306, NCT00098722.
REFERENCES
- 1.Dorr P, Westby M, Dobbs S, et al. Maraviroc (UK-427,857): a potent, orally bioavailable and selective small-molecule inhibitor of the chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob Agents Chemother. 2005;49:4721–4732. doi: 10.1128/AAC.49.11.4721-4732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfizer Inc [November 16, 2009];Selzentry (maraviroc) prescribing information. Available at: http://www.selzentry.com/splashpage.aspx.
- 3.Gulick R, Lalezari J, Goodrich J, et al. the MOTIVATE Study Teams Maraviroc for previously treated patients with R5 HIV-1 infection. N Engl J Med. 2008;359:1429–1441. doi: 10.1056/NEJMoa0803152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fatkenheuer G, Nelson M, Lazzarin A, et al. the MOTIVATE 1 and MOTIVATE 2 Study Teams Subgroup analyses of maraviroc in previously treated R5 HIV-1 infection. N Engl J Med. 2008;359:1442–1555. doi: 10.1056/NEJMoa0803154. [DOI] [PubMed] [Google Scholar]
- 5.Ayoub A, Goodrich J, van der Ryst E, et al. Adverse event profile of maraviroc in treatment-experienced patients infected with R5 HIV-1.. Presented at: 48th Interscience Conference on Antimicrobial Agents and Chemotherapy/46th Annual Meeting of the Infectious Diseases Society of America; Washington, DC. October 25–28, 2008; Abstract H-1264. [Google Scholar]
- 6.Ayoub A, Goodrich J, Tressler R, et al. Incidence of infections in treatment-experienced patients infected with R5 HIV-1 in the MOTIVATE studies of maraviroc in combination with optimized background therapy.. Presented at: Ninth International Congress on Drug Therapy in HIV Infection; Glasgow, United Kingdom. November 9–13, 2008; Abstract P154. [Google Scholar]
- 7.Fry J. [August 14, 2009];FDA/FCHR collaborative public meeting on long-term safety concerns associated with CCR5 antagonist development. Available at: www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4283b1-02-03-FDA-draftreport.pdf.
- 8.Nichols WG, Steel HM, Bonny T, et al. Hepatotoxicity observed in clinical trials of aplaviroc (GW873140). Antimicrob Agents Chemother. 2008;52:858–865. doi: 10.1128/AAC.00821-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gulick RM, Su Z, Flexner C, et al. Phase 2 study of the safety and efficacy of vicriviroc, a CCR5 inhibitor, in HIV-1-infected, treatment-experienced patients: AIDS clinical trials group 5211. J Infect Dis. 2007;196:304–312. doi: 10.1086/518797. [DOI] [PubMed] [Google Scholar]
- 10.Tsibris AM, Paredes R, Chadburn A, et al. Lymphoma diagnosis and plasma Epstein-Barr virus load during vicriviroc therapy: results of the AIDS Clinical Trials Group A5211. Clin Infect Dis. 2009;48:642–649. doi: 10.1086/597007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valdez H, Lewis M, Delogne C, et al. Weighted OBT susceptibility score (wOBTSS) is a stronger predictor of virologic response at 48 weeks than baseline tropism result in MOTIVATE 1 and 2.. Presented at: 48th ICAAC/IDSA 46th Meeting; Washington, DC. October 25–28 2008; Abstract H-1221. [Google Scholar]
- 12.Jubb R, Lewis M, Simpson P, et al. CCR5-tropic resistance to maraviroc is uncommon even among patients on functional MVC monotherapy or with ongoing low-level replication.. Presented at: 16th Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. February 8–11, 2009; Abstract M199. [Google Scholar]
- 13.Heera J, Ive P, Botes M, et al. The MERIT study of maraviroc in antiretroviral-naive patients with R5 HIV-1: 96-week results.. Presented at: 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention; Cape Town, South Africa. July 19–22, 2009; Abstract TUAB103. [Google Scholar]
- 14.Gomez-Reino JJ, Pablos JL, Carreira PE, et al. Association of rheumatoid arthritis with a functional chemokine receptor, CCR5. Arthritis Rheum. 1999;42:989–992. doi: 10.1002/1529-0131(199905)42:5<989::AID-ANR18>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 15.Pokorny V, McQueen F, Yeoman S, et al. Evidence for negative association of the chemokine receptor CCR5 d32 polymorphism with rheumatoid arthritis. Ann Rheum Dis. 2005;64:487–490. doi: 10.1136/ard.2004.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zapico I, Coto E, Rodriguez A, et al. CCR5 (chemokine receptor-5) DNA-polymorphism influences the severity of rheumatoid arthritis. J Rheumatol. 2000;27:2308–2311. doi: 10.1038/sj.gene.6363673. [DOI] [PubMed] [Google Scholar]
- 17.Goulding C, McManus R, Murphy A, et al. The CCR5-delta32 mutation: impact on disease outcome in individuals with hepatitis C infection from a single source. Gut. 2005;54:1157–1161. doi: 10.1136/gut.2004.055699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang Y, Paxton WA, Wolinsky SM, et al. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med. 1996;2:1240–1243. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- 19.de Roda Husman A, Koot M, Cornelisson M, et al. Association between CCR5 genotype and the clinical course of HIV-1 infection. Ann Intern Med. 1997;127:882–890. doi: 10.7326/0003-4819-127-10-199711150-00004. [DOI] [PubMed] [Google Scholar]
- 20.Dean M, Lacobson LP, McFarlane G, et al. Reduced risk of AIDS lymphoma in individuals heterozygous for the CCR5-Δ32 mutation. Cancer Res. 1999;59:3561–3564. [PubMed] [Google Scholar]
- 21.Meyer L, Magierowska M, Hubert J-B, et al. CCR5 Δ32 deletion and reduced risk of toxoplasmosis in persons infected with human immunodeficiency virus type 1. J Infect Dis. 1999;180:920–924. doi: 10.1086/314933. [DOI] [PubMed] [Google Scholar]
- 22.Ashton LJ, Stewart GJ, Biti R, et al. Heterozygosity for CCR5-Delta32 but not CCR2b-64I protects against certain intracellular pathogens. HIV Med. 2002;3:91–96. doi: 10.1046/j.1468-1293.2002.00106.x. [DOI] [PubMed] [Google Scholar]
- 23.Glass WG, McDermott DH, Lim JK, et al. CCR5 deficiency increases risk of symptomatic West Nile virus infection. J Exp Med. 2006;203:35–40. doi: 10.1084/jem.20051970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim JK, Louie CY, Glaser C, et al. Genetic deficiency of chemokine receptor CCR5 is a strong risk factor for symptomatic West Nile virus infection: a meta-analysis of 4 cohorts in the US epidemic. J Infect Dis. 2008;197:262–265. doi: 10.1086/524691. [DOI] [PubMed] [Google Scholar]
- 25.Pulendran B, Miller J, Querec TD. Case of yellow fever vaccine-associated viscerotropic disease with prolonged viremia, robust adaptive immune responses, and polymorphisms in CCR5 and RANTES genes. J Infect Dis. 2008;198:500–507. doi: 10.1086/590187. [DOI] [PMC free article] [PubMed] [Google Scholar]