Abstract
Introduction
Monocyte chemoattractant protein-1 (MCP-1) is a bioactive molecule that is expressed in significant amounts in all stages of atherosclerosis. The role of MCP-1 in this disease is to recruit monocytes across the endothelium and into the arterial tissue. Eventually, the monocytes differentiate into cholesterol-engorged macrophages called “foam cells” that result in atherosclerotic plaque formation. The mechanism that MCP-1 uses to mediate monocyte transendothelial migration is believed to be via its concentration gradient. However, the formation of the MCP-1 concentration gradient in the extracellular matrix is still poorly understood.
Methods
A 3D in vitro vascular tissue model has been developed to study the cellular mechanisms involved in the early stages of atherosclerosis. In the present study, a mathematical model is used to determine the gradient of MCP-1 in the collagen matrix of the 3D in vitro vascular tissue model. Experiments were performed to investigate the stability of MCP-1 and the interaction between MCP-1 and the collagen matrix.
Results and Conclusions
MCP-1 is stable for at least 24 hours under experimental conditions and MCP-1 interacts with the collagen matrix. The diffusion coefficient for the transport of MCP-1 in the collagen matrix and the rate constant for the binding of MCP-1 to collagen were determined to be 0.108 mm2 hr−1 and 0.858 hr−1, respectively. Numerical results from the model indicate that the concentration gradients of both soluble and matrix-bound (or static) MCP-1 are formed inside the collagen matrix.
Keywords: atherosclerosis, cell migration, concentration gradient, chemotaxis, haptotaxis, diffusivity coefficient
1. Introduction
Atherosclerosis is an inflammatory disease (1), characterized by endothelial dysfunction, immune cell migration and differentiation, extracellular matrix (ECM) remodeling, and smooth muscle cell migration (2). The steps in atherosclerotic plaque formation begin with the subendothelial accumulation of lipid substances, followed by the adhesion of monocytes and lymphocytes to endothelial cells and their subsequent migration across the endothelium (3, 4). Once localized in the subendothelial space, monocytes differentiate into active macrophages and form foam cells by interacting with oxidized low-density lipoprotein and consuming lipid substances (1, 5). The foam cells continue to produce inflammatory signals, which results in more monocyte recruitment to the area and the development of an atherosclerotic lesion (5).
Morphologic studies have established that after leukocytes adhere to the endothelium, they enter the intima by diapedesis between endothelial cells at their junctions. This phenomenon of directed migration of leukocytes through an endothelium has been well known for over a century, but only recently has yielded to molecular analysis to identify the key players. Investigators have defined families of chemoattractant cytokines (chemokines) capable of recruiting leukocytes into the arterial intima. The best characterized of these chemokines is monocyte chemotactic protein-1 (MCP-1). MCP-1 is chemotactic for monocytes both in vitro and in vivo (6, 7). MCP-1 is overexpressed in human and experimental atheroma and appears to be essential for the recruitment of monocytes into the intima (8). MCP-1 is expressed in significant amounts in all stages of atherosclerosis (9, 10). Studies using compound mutant mice lacking MCP-1 or its receptor CCR2, and susceptible to atherosclerosis due to the absence of genes encoding apolipoprotein E or the low-density lipoprotein receptor, have shown significant decreases in mononuclear phagocyte accumulation and local lipid levels (11, 12).
Monocyte trafficking across the endothelial layer is believed to be mediated by an MCP-1 concentration gradient. This concept is supported by many studies (13, 14), including the work of Randolph and Furie (6), which illustrates in vitro that the transendothelial migration of monocytes depends on the soluble concentration gradient of MCP-1 across the endothelial layer. It was also shown in this latter study that MCP-1 is equally secreted from apical and basal sides of stimulated endothelial cells (6). This finding combined with the fact that MCP-1 is secreted in a soluble form suggests that in vivo, where the blood flow in the vascular space could prevent the formation of a MCP-1 concentration gradient on the luminal side, the concentration gradient of MCP-1 may be formed within the ECM via the diffusion of MCP-1 secreted from the basal side of the endothelial layer (15). However, information characterizing the effect of the diffusive gradient of MCP-1 in the subendothelial matrix on monocyte transmigration is still lacking. Whereas the previous studies measured a soluble concentration gradient in a liquid system, it is a challenge to characterize the diffusive gradient of MCP-1 in a matrix.
In addition to soluble gradients of chemokines that can direct cell migration through the tissues, it has been proposed that chemokines can interact with the ECM to form matrix-bound gradients that result in leukocyte haptotaxis (16-20). Studies have shown that some chemokines interact with glycosaminoglycans (GAGs) and related moieties within the ECM that result in matrix-bound gradients (18, 21). The various types of chemoattractants have also been shown to differ in their ability to induce cell chemotaxis or haptotaxis. A study by Patel et al. (18) did not show any detectable binding of MCP-1 with ECM components in situ.
We have used a 3D in vitro vascular tissue model to investigate immune cell trafficking and differentiation in response to stimuli (22). The vascular tissue model consists of a quiescent, confluent vascular endothelium over a collagen matrix. Monocytes from total peripheral blood mononuclear cells (PBMCs) selectively extravasate in and differentiate into either resident macrophages or migratory DCs with potent Ag-presenting capacity. Monocytes are known to migrate across an endothelium continuously but this migration is enhanced in case of certain inflammatory signals. In the present study, we investigate the formation of a diffusive gradient of MCP-1 in the collagen matrix of the 3D in vitro vascular tissue model, as a step towards understanding cell migration within the model. In contrast to a traditional 2D cell culture system, where MCP-1 that is secreted from a monolayer of endothelial cells and dissolves quickly into the surrounding media, the 3D tissue model more closely resembles in vivo conditions by providing a matrix in which the diffusive gradient of secreted MCP-1 can be formed. The main objective of this paper is to develop a mathematical model that can determine the MCP-1 concentration gradient within the collagen matrix. The mathematical model describes both diffusive and kinetic behaviors of MCP-1 in the matrix. The stability of MCP-1 under standard conditions and the interaction between MCP-1 and the collagen matrix were investigated to describe the behavior of MCP-1 in the matrix.
2. Initial assumptions
Fig. 1 shows the schematic diagram of the 3D in vitro vascular tissue model without cells, consisting of a collagen membrane formed in a membrane well. The cross-sectional area of the insert of the membrane well used in this study is 33 mm2. The membrane material is polystyrene with a pore size and thickness of 3 μm and 10 μm, respectively. The equation of continuity was used to derive the mathematical model to determine the MCP-1 concentration gradient in the collagen matrix. Several assumptions were applied to simplify the equation. First, the migration of MCP-1 through the collagen matrix in the 3D vascular tissue model is assumed to be due to diffusive mass transfer only (no convective mass transfer in the system). Second, because the focus of this study is on the gradient of MCP-1 in the perpendicular direction from the endothelial layer, or the top surface of the collagen matrix, the diffusion is assumed to be only in such direction (z-direction). Third, the effect of MCP-1 mass transfer across the membrane that supports the collagen matrix is assumed to be insignificant compared to mass transport across the collagen matrix, because the thickness of the membrane is significantly less than that of the collagen matrix and the pore size of the membrane is significantly larger than the size of the MCP-1 molecule. The wall effect within the membrane well is also neglected. By applying these assumptions to the equation of continuity, the main equation of the model becomes
| Eq. (1) |
where CM is the concentration of MCP-1, z is the distance from the top surface of the collagen matrix, t is time, DM|C is the diffusivity coefficient of MCP-1 in the matrix, which is assumed to be constant, and RM is a rate term.
FIGURE 1.
Schematic diagram of the 3D in vitro vascular tissue model without cells. A collagen matrix is formed within the well of a 24-well Transwell® plate.
The rate term describes the rate of MCP-1 that is reacted or consumed in the collagen matrix, and was still unknown at this point. Our initial hypothesis was that MCP-1 was stable under standard culture conditions (37°C, and humidified atmosphere of 5% CO2 and 95% air), since no loss has been shown using MCP-1 in other in vitro studies referenced above, and it did not bind with the collagen matrix, based on the investigation of MCP-1 with ECM by Patel et al. (18). The hypothesis was tested experimentally.
3. Material and Methods
3.1 Materials
Recombinant human MCP-1 was purchased from R&D Systems (Minneapolis, MN). Dulbecco’s phosphate-buffered saline (D-PBS), Medium 199, and penicillin-streptomycin-glutamine (PSG) solution were purchased from Invitrogen (Carlsbard, CA). Fetal bovine serum (FBS) was purchased from Hyclone (Logan, UT). PureCol® collagen (97% Type 1 collagen) was purchased from Advanced BioMatrix (San Diego, CA). Sodium hydroxide was purchased from VWR (West Chester, PA). Corning Transwell® permeable supports and Tween® 20 were purchased from Fisher Scientific (Pittsburgh, PA). Bovine serum albumin (BSA) was purchased from Sigma-Aldrich (St. Louis, MO). BD OptEIA™ Human MCP-1 enzyme-linked immunosorbent assay (ELISA) set was purchased from BD Biosciences (San Jose, CA).
3.2 MCP-1 stability test
Complete medium (Medium 199 with 10 vol% FBS and 1 vol% PSG) was used to prepare MCP-1 solutions at the following concentrations: 0.25, 5, 15, 25, and 50 ng mL−1. This concentration range was selected based on in vivo measurements (23) and in vitro experimental systems that study both the release of MCP-1 from cells (24, 25) and the use of MCP-1 to direct cell migration (26-28). The MCP-1 solutions were incubated at standard conditions for 24 hours. Samples were taken from the solutions every six hours during the incubation and stored at −81°C, until ready for analysis. The level of MCP-1 in the samples was determined by ELISA.
3.3 Development of the collagen matrix in the membrane microwell
Briefly, the collagen matrix was prepared by adding 50 μL of collagen solution containing 57.1 vol% PureCol® collagen (3 mg mL−1), 7.14 vol% Medium 199, and 35.7 vol% 0.1 M sodium hydroxide to each insert of a 24-well Transwell® plate. The plate was incubated at standard conditions for 60 minutes for the collagen to gel. Prior to testing, 100 μl and 650 μl of complete medium was added to the top and the bottom reservoirs of the plate, respectively, resulting in equal liquid heights in both reservoirs.
3.4 MCP-1 binding reaction test
Complete medium containing one of the following concentrations of MCP-1 was selected as a source of MCP-1 and added to the top reservoir of the complete 3D vascular tissue model without cells: 0.25, 5, 15, 25, or 50 ng mL−1. The bottom reservoir was filled with complete medium. The 3D vascular tissue model was incubated at standard conditions for 24 hours to allow MCP-1 to diffuse from the top reservoir through the collagen matrix to the bottom reservoir. During the incubation, samples were taken from both the top and the bottom reservoirs and were stored at −81°C, until ready for analysis. The MCP-1 concentration of the samples was analyzed by ELISA and was used to determine the binding reaction between MCP-1 and the collagen matrix.
3.5 MCP-1 ELISA
ELISA was performed according to the manufacturer’s instructions, and similar to other studies (29-31). Complete medium was used as diluents for standards and samples instead of Assay Diluent. Briefly, samples and standards were added to each well of a microtiter plate, which was precoated with anti-human MCP-1 monoclonal antibody, and incubated for two hours at room temperature. Each well was washed with washing buffer and incubated with the working detector (detection antibody specific for human MCP-1 and streptavidin-horseradish peroxidase) for 1 hour at room temperature. The wells were washed to remove unbound antibody-enzyme reagent, and a substrate solution was added to each well. After incubation for 30 minutes at room temperature and in the dark, the enzyme reaction was stopped and the optical density of the samples was read at 450 nm. The optical density was measured with an Emax Precision microplate reader (Molecular Devices, Sunnyvale, CA). The standard curve for the assay resulted in an average absolute percent deviation between measured and actual values of 11.5%.
3.6 Statistical analysis
MCP-1 concentrations are expressed as mean ± SD of three samples or more. Student’s t test was used to determine significant differences in the MCP-1 stability test results, and the estimated MCP-1 concentration in collagen matrix results. A value of p < 0.05 was considered significant. Absolute-average-percentage deviation (%AAD) was used to compare the difference between experimental results and results from the mathematical model.
4. Experimental results
4.1 MCP-1 stability test
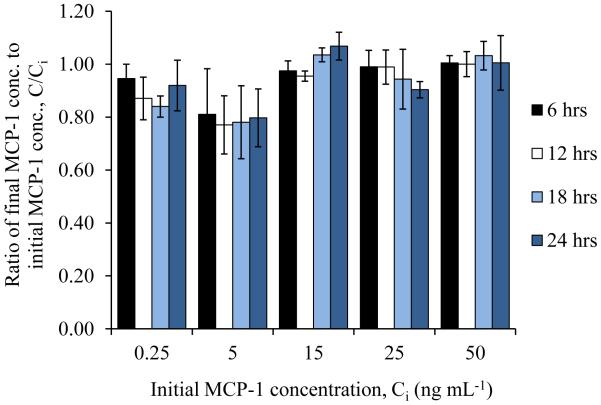
Initially, it was assumed that MCP-1 is stable at standard conditions. To test this assumption, complete medium containing various concentrations of MCP-1 was incubated at standard conditions for 24 hours. Samples were collected from the complete medium during that time and analyzed for the concentration of MCP-1. The results in Fig. 2 show that there is no significant loss of MCP-1 for the concentrations of MCP-1 (0.25, 5, 15, 25, and 50 ng/ml) for any of the time points (6, 12, 18, and 24 hours), taking into consideration the average absolute percent deviation of the assay. Thus, we concluded that MCP-1 is stable at standard conditions for at least 24 hours.
FIGURE 2.
The stability of MCP-1 at standard culture conditions. Complete medium containing different concentrations of MCP-1 was incubated at standard conditions. Samples were collected from the medium every six hours and analyzed for MCP-1 concentration. Values are presented as mean ± SD. Taking into consideration the average absolute percent deviation of the assay, no significant change in concentration at any time points was observed.
4.2 MCP-1 binding reaction test
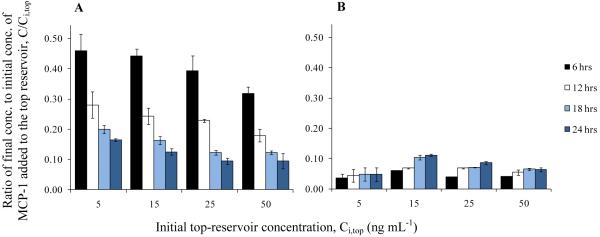
Another initial assumption was that MCP-1 does not bind with the collagen matrix. Complete medium containing a set concentration of MCP-1 was added to the top reservoir above the collagen matrix. The following concentrations of MCP-1 were tested: 0.25, 5, 15, 25, or 50 ng mL−1. The system was incubated at standard conditions to allow MCP-1 to diffuse from the top reservoir, through the collagen matrix, and into the bottom reservoir, which initially contained only complete medium. Samples taken from the top and the bottom reservoirs at different time points were analyzed for the concentration of MCP-1. The results are shown in Fig. 3. Since MCP-1 concentrations in both the top and the bottom reservoirs of samples with initial top-reservoir concentration (Ci,top) of 0.25 ng mL−1 were lower than the detection range of the ELISA, there is no data from those samples displayed in the figure.
FIGURE 3.
Concentration of MCP-1 in the top and the bottom reservoirs of the 3D vascular tissue model without cells. Complete medium with different concentrations of MCP-1 was added to the top reservoir, and the tissue model was incubated at standard conditions to allow for the diffusion of MCP-1 to the bottom reservoir. Samples were collected from the tissue model during the incubation period. Fig. 3A shows the ratio of the concentration of MCP-1 in the top reservoir, to the initial concentration of MCP-1 added to the top reservoir, Ci,top. Fig. 3B shows the ratio of the concentration of MCP-1 in the bottom reservoir, to the initial concentration of MCP-1 added to the top reservoir, Ci,top. Values are presented as mean ± SD.
If the volume of the collagen matrix is known, then the data in Fig. 3 can be used to calculate the average concentration of MCP-1 in the collagen matrix at each time point. The collagen volume was found to be 13 μL, estimated by using the cross-sectional area of the membrane insert and the thickness of the collagen matrix, which was measured microscopically to be 0.37 ± 0.06 mm. Additional assumptions that were used to calculate the concentration of MCP-1 in the collagen matrix include the following: (1) the volumes of the top and the bottom reservoirs, and the collagen matrix are constant, and (2) the contents in the top and the bottom reservoirs are well mixed. Results of the calculation showed that the average concentration of MCP-1 in the collagen matrix is higher than the concentration of MCP-1 in the top reservoir for all time points (Table 1). However, for this experimental system, the concentration of MCP-1 in the collagen matrix cannot be higher than the concentration in the top reservoir, if there is no reaction inside the collagen matrix. The addition of MCP-1 to the top reservoir produces a concentration gradient, which drives the diffusion of MCP-1 through the collagen matrix and into the bottom reservoir. The diffusion will continue until MCP-1 concentration in the system is uniformed. Therefore, it is not possible that the concentration of MCP-1 in the collagen matrix is higher than in the top reservoir unless there is a reaction that occurs inside the collagen matrix, which consumes MCP-1 or transforms it from a soluble to a nonsoluble form. Hence, this conclusion suggests that our initial assumption about MCP-1 binding is not accurate and that MCP-1 does bind with the collagen matrix.
TABLE 1.
Estimated average concentration of MCP-1 in the collagen matrix. The average concentration of MCP-1 in the collagen matrix was estimated from the data shown in Fig. 3. Ci,top represents the initial MCP-1 concentration in the top reservoir. Values shown in the table are presented as mean ± SD, * p < 0.05 or ** p < 0.01 for change between MCP-1 concentration in the collagen matrix and the top reservoir at each time point.
| Samples | Ratio of MCP-1 concentration at current time point to initial MCP-1 concentration in top reservoir, C / Ci,top |
|||
|---|---|---|---|---|
| 6 hrs | 12 hrs | 18 hrs | 24 hrs | |
| Ci,top = 5 ng/mL | ||||
| Top reservoir | 0.460 ± 0.056 | 0.281 ± 0.044 | 0.199 ± 0.013 | 0.165 ± 0.005 |
| Collagen matrix | 2.36 ± 0.27** | 3.34 ± 0.72** | 3.77 ± 1.13** | 4.06 ± 1.13** |
| Ci,top = 15 ng/mL | ||||
| Top reservoir | 0.443 ± 0.024 | 0.243 ± 0.027 | 0.163 ± 0.013 | 0.125 ± 0.010 |
| Collagen matrix | 1.25 ± 0.17* | 2.39 ± 0.19** | 1.24 ± 0.42* | 1.17 ± 0.10** |
| Ci,top = 25 ng/mL | ||||
| Top reservoir | 0.394 ± 0.050 | 0.229 ± 0.005 | 0.123 ± 0.009 | 0.094 ± 0.010 |
| Collagen matrix | 2.66 ± 0.38** | 2.51 ± 0.09** | 3.21 ± 0.06** | 2.68 ± 0.21** |
| Ci,top = 50 ng/mL | ||||
| Top reservoir | 0.318 ± 0.023 | 0.179 ± 0.020 | 0.122 ± 0.006 | 0.094 ± 0.025 |
| Collagen matrix | 3.09 ± 0.08** | 3.48 ± 0.53** | 3.51 ± 0.15** | 3.61 ± 0.28** |
5. Model development
5.1 Governing equations
The experimental results showed that MCP-1 is stable at standard conditions for at least 24 hours and it binds with the collagen matrix. We assumed that the binding reaction between MCP-1 and the collagen matrix follows the general ligand-receptor reaction kinetics. Evidence from a study by Distler et al. (32) has shown that, if the binding reaction exists, it should be an irreversible reaction. Taking these experimental findings into consideration, the equation that represents the binding reaction between MCP-1 and the collagen matrix can be displayed as
| Eq.(2) |
where M is MCP-1, S is a binding site in the collagen matrix, and M·S is a MCP-1-binding-site complex. To derive the rate term of the mathematical model, it was assumed further that the concentration of binding sites in the collagen matrix is much more than the concentration of MCP-1. This assumption was based on experimental measurements that show changes in the MCP-1 concentration during the 24-hour time period. So, the rate of consumption of MCP-1 in this reaction depends only on the concentration of MCP-1, as shown below.
| Eq. (3) |
In Eq. (3), Kb is the rate constant for the binding reaction. By substituting Eq. (3) into Eq. (1), the model equation becomes
| Eq. (4) |
Eq. (4) can also be referred to as the mass balance equation of MCP-1 in the collagen matrix. The concentration gradient of MCP-1 in the matrix can be determined by solving this equation.
After determining that MCP-1 can bind with the collagen matrix to form MCP-1-binding-site (M·S) complex, we would also like to determine the concentration profile of the M·S complex in the matrix, as it may have a haptotactic effect on monocyte migration. The equation of continuity was used again to develop the equation to represent the concentration of the M·S complex. However, unlike MCP-1, which can diffuse through the collagen matrix, the M·S complex is assumed to be fixed inside the matrix. Thus, the kinetics is the only concern in this case, and the equation to determine the concentration of the M·S complex was derived as
| Eq. (5) |
where CM·S is the concentration of the M·S complex, and RM·S is the rate of reaction of the M·S complex. We know from Eq. (2) that the rate of production of the M·S complex is equal to the negative value of the rate of consumption of MCP-1, or
| Eq. (6) |
Combining Eqs. (5) and (6), the equation to determine the concentration of the M·S complex, or the mass balance equation of the M·S complex in the collagen matrix, becomes
| Eq. (7) |
5.2 Boundary conditions
To solve Eqs. (4) and (7), two initial conditions for CM and CM·S and two boundary conditions for CM are required. All conditions were based on the assumptions that the top and bottom reservoirs are well mixed, and the concentrations of MCP-1 at the top and the bottom surface of the collagen matrix are equal to the concentrations in the top and the bottom reservoirs, respectively. Initial conditions were derived from the fact that initially, there was no MCP-1 in the collagen matrix or the bottom reservoir, and the concentration of MCP-1 in the top reservoir was known.
| Eq. (8) |
Because MCP-1 was added into the top reservoir, there was no binding reaction taking place at the beginning and the concentration of the M·S complex was equal to zero for all locations.
| Eq. (9) |
Next, the boundary conditions of CM were derived from the mass balance equations of MCP-1 in the top and the bottom reservoirs when t > 0. Since the concentration of MCP-1 in the top reservoir is assumed to be equal to the concentration at the top surface of the collagen matrix, the convective mass transfer of MCP-1 from the bulk of the top reservoir to the collagen surface is neglected. Thus, the rate of change of MCP-1 concentration in the top reservoir depends only on the rate of MCP-1 that diffuses through the top surface of the collagen matrix.
| Eq. (10) |
The relationship is the same for the change of MCP-1 concentration in the bottom reservoir and the diffusion of MCP-1 through the bottom surface of the collagen matrix. A negative sign was added to the following equation, to show the direction of MCP-1 diffusing from the collagen matrix to the bottom reservoir.
| Eq. (11) |
In Eqs. (10) and (11), A is the surface area of either the top or the bottom surface of the collagen matrix, V is the volume of the top or the bottom reservoir, and zf is the thickness of the collagen matrix. The area of the top and the bottom surface of the collagen matrix is assumed to be equal to the cross-sectional area of the membrane insert of the Transwell® plate. The volumes of the top and the bottom reservoirs are the values set for the experiments.
6. Numerical solution
There were two unknown constants, DM|C and Kb, in the mathematical model. Their values were estimated by fitting the results of the mathematical model to experimental data. Data from Fig. 3, when the initial concentration of MCP-1 in the top reservoir was 5, 15, or 50 ng mL−1, were selected for the regression. The mathematical model was solved in MS-Excel with Visual Basic for Applications (VBA) using the Crank-Nicolson numerical method. The values of DM|C and Kb were determined by fitting the calculated results to the selected experimental data, using the weighted least squares method.
To validate the estimated values of DM|C and Kb, and to evaluate the mathematical model, the initial concentration of MCP-1 in the top reservoir of 25 ng mL−1 was used to compare the model results to the experimental results.
7. Numerical results
7.1 Parameter estimation
We found that the values of DM|C and Kb that gave the best fit to the selected experimental data are 0.108 mm2 hr−1 and 0.858 hr−1, respectively. Results of the regression are displayed in Fig. 4. From the figure, the data calculated from the mathematical model are about the average of the experimental data with the overall %AAD of 18.0%. When considering the correlation results of the MCP-1 concentration in the top and the bottom reservoirs separately, the %AADs for the top- and bottom-reservoir concentrations are 21.0% and 15.1%, respectively.
FIGURE 4.
Experimental and correlated concentrations of MCP-1 in the top and the bottom reservoirs. Fig. 4A shows the ratio of the MCP-1 concentration in the top reservoir to the initial concentration of MCP-1 added to the top reservoir for each time point. Fig 4B shows the ratio of the MCP-1 concentration in the bottom reservoir to the initial concentration of MCP-1 added to the top reservoir for each time point. The initial MCP-1 concentrations of 5, 15, or 50 ng ml−1, were selected from data in Fig. 3 and used in the correlation. Experimental data are displayed as open shape markers, while data from the mathematical model are displayed as a solid line. The values of two unknown constants; DM|C and Kb, in the mathematical model were estimated from the correlation to be 0.108 mm2 hr−1 and 0.858 hr−1, respectively.
7.2 Validation of the mathematical model
The mathematical model, together with the estimated values of DM|C and Kb, were validated with the experimental data shown in Fig. 3, when the initial concentration of MCP-1 in the top reservoir was 25 ng mL−1. The validation results are displayed in Fig. 5. As observed from the figure, the mathematical model demonstrated a good prediction of MCP-1 concentrations in both the top and the bottom reservoirs. The overall %AAD between the predicted and experimental results is 10.8%, with %AAD equal to 13.7% for the top-reservoir calculation and 6.8% for the bottom-reservoir calculation. This low value of deviation shows that the transportation of MCP-1 in the collagen matrix in the first 24 hours can be represented by the formulated mathematical model for the initial concentration of MCP-1 in the top reservoir between 5 and 50 ng mL−1.
FIGURE 5.
Predicted and experimental concentrations in the top and the bottom reservoirs. The MCP-1 concentrations in the top (A) and the bottom (B) reservoirs, when the initial concentration of MCP-1 in the top reservoir was 25 ng ml−1, were predicted using the mathematical model and compared to the data selected from Fig. 3. Experimental data are displayed as open diamond markers, while data from the model are displayed as a solid line.
7.3 MCP-1 concentration gradient in the collagen matrix
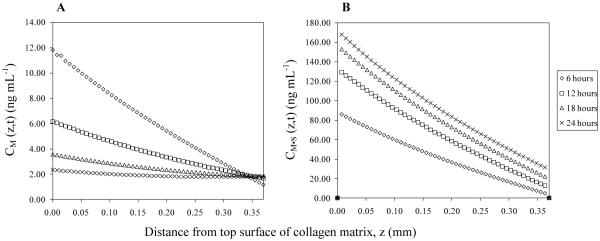
The concentration gradients of MCP-1, both soluble and static (bound), were calculated using the mathematical model. Plots showing the concentration of MCP-1 as a function of distance from the top surface of the collagen matrix are presented in Fig. 6. The concentration gradient of soluble MCP-1 appears to be linearly dependent on the distance at any time point, and such gradient diminishes over time. Contrarily, the concentration gradient of MCP-1 that binds with collagen, or the static gradient of MCP-1, increases as time passed and overcomes the soluble gradient of MCP-1 after two hours (data are not shown in the figure). This finding suggests that, apart from the soluble gradient of MCP-1, the static gradient of MCP-1 is another potent factor that may mediate monocyte transendothelial migration.
FIGURE 6.
Concentration gradients of MCP-1 in the collagen matrix. The soluble and the static gradients of MCP-1, when the initial concentration of MCP-1 in the top reservoir was 25 ng ml−1, were calculated using the mathematical model and the estimated values of the constants, DM|C and Kb. Fig. 6A shows the concentration of MCP-1 that is soluble in the collagen matrix, CM (z, t). Fig. 6B shows the concentration of MCP-1 that binds with the collagen matrix, CM·S (z, t).
8. Discussion
In the present study, we developed a mathematical model to determine the concentration gradient of MCP-1 that forms in the collagen matrix of the 3D in vitro vascular tissue model. Both kinetic and transport behaviors were taken into account for the mathematical model development. For the kinetics, the mathematical model focused on the stability of MCP-1 at standard conditions and the reaction between MCP-1 and the collagen matrix. Our experimental results demonstrate that MCP-1 is stable for at least 24 hours when it is presented as a solute in complete medium. However, this finding applies for recombinant MCP-1 only. When endothelial cells are added to the tissue model, the stability of MCP-1 secreted from the cells will need to be evaluated, because other substances produced by the cells may affect MCP-1 stability, such as matrix metalloproteinases (33, 34).
A binding reaction between MCP-1 and the collagen matrix is another aspect that we considered during the development of the mathematical model. Studies have shown that chemokines can bind to ECM components that result in matrix-bound gradients (16-20). However, the study by Patel et al. (18) did not show any detectable binding of MCP-1 with ECM components in situ. Experiments were conducted with the collagen matrix of the 3D tissue model, in order to determine whether the binding reaction existed. We concluded from experimental results that MCP-1 does bind with the collagen matrix. To validate this conclusion, we attempted to determine the concentration of MCP-1 in the collagen matrix directly by dissolving the collagen matrix and measuring the MCP-1 by the ELISA method. This method resulted in no detectable MCP-1. We believe that the MCP-1 could not be detected due to the digested collagen interfering with the detection method and/or proteinase in the collagenase can degrade the MCP-1. However, in a separate study that investigates monocyte migration within the tissue model, we found that the number of monocytes that migrated into the collagen matrix increased significantly when the collagen matrix is pretreated with MCP-1, compared to when it is not (manuscript in review). The conflicting results of our study compared to the Patel el al. study is likely due to the following: i) they investigated a complex ECM system that contained many cells and bioactive components that may have interfered with MCP-1 binding and/or detection, and ii) MCP-1 was added to their system and incubated for just 45 minutes, which may not have been long enough for MCP-1 to bind to the ECM. For our study, we investigated a simplified system of MCP-1 and collagen, and we investigated MCP-1 binding over a 24 hour time period. We also determined from the mathematical model that significant MCP-1 binding within the collagen occurs after two hours. In addition, a study by Distler et al. (32) investigated the time-dependent release of MCP-1 from type 1 collagen, the same type of collagen used for this study. Results from this study showed that no release of MCP-1 was detected in the first 72 hours. This result agrees with the assumption that the binding reaction between MCP-1 and the collagen matrix can be considered as an irreversible reaction for the time period tested.
Based on the knowledge about the MCP-1 binding reaction, two equations that represent the mass balance of MCP-1 and the M·S complex in the collagen matrix were developed. The values of two unknown constants, DM|C and Kb, which appear in the mathematical model, were found to be 0.108 mm2 hr−1 and 0.858 hr−1, respectively. The value of DM|C in this study is lower than the one calculated from an empirical equation used in the study of Zhao et al. (35) (0.247 mm2 hr−1) or the one reported in the study of Fleury et al. (36) for bioactive molecules that are in the same family as MCP-1 and have molecular weights close to MCP-1 (0.468-0.576 mm2 hr−1). The difference in the values could be due to the methods used to determine the values. In this study, the parameter DM|C was determined based on experimental data, while empirical equations for the estimation of the diffusivity coefficient in water and correction factors were used to estimate DM|C in the other two studies.
Results of the mathematical model show that the concentration gradient of soluble MCP-1 decreases over time; whereas, the static gradient of MCP-1 increases. This finding is reasonable and expected for a system with a single administration of MCP-1 and with the irreversible binding reaction. Interestingly, the concentration of static MCP-1, or the MCP-1 that binds with the collagen matrix, is higher than that of the soluble MCP-1 after two hours. This observation is opposite to the results of a previous study, which showed that when applied to amnion membrane, most of the MCP-1 appears in a soluble form (6). This could be due to the fact that the amnion membrane is very thin, by an order of magnitude, when compared to the collagen matrix used in this study. Moreover, the type 1 collagen content in the amnion membrane is not as high as in the collagen matrix. Both differences could result in fewer possible binding sites for MCP-1 in the amnion membrane compared to the collagen matrix used in this study.
In conclusion, a mathematical model to determine the concentration gradients of MCP-1 in a collagen matrix of a 3D vascular tissue model without cells was developed in this study. Results of our experiments and the mathematical model suggest that there should be a binding reaction of MCP-1 with the collagen matrix, and such a reaction can produce a static gradient of MCP-1 across the matrix. We hypothesize that the binding is occurring through the interaction of GAGs with both the collagen matrix and with MCP-1. A static gradient may play a role during the transendothelial migration of monocytes, and we recommend that more investigations should be done to ensure the existence of the binding reaction. Furthermore, we expect that the use of this mathematical model, together with the 3D vascular tissue model, will provide a way to study the formation of the gradient of MCP-1 that is secreted from endothelial cells and the effect of the gradient on monocyte migration involved in the early stages of atherosclerosis.
Acknowledgement
This work was supported by a grant from the National Institute of Biomedical Imaging and Bioengineering (1R15EB009527-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ross R. Atherosclerosis is an inflammatory disease. American heart journal. 1999 Nov;138(5 Pt 2):S419–20. doi: 10.1016/s0002-8703(99)70266-8. [DOI] [PubMed] [Google Scholar]
- 2.Newby AC. An overview of the vascular response to injury: a tribute to the late Russell Ross. Toxicology letters. 2000 Mar 15;112-113:519–29. doi: 10.1016/s0378-4274(99)00212-x. [DOI] [PubMed] [Google Scholar]
- 3.Babaev VR, Bobryshev YV, Sukhova GK, Kasantseva IA. Monocyte/macrophage accumulation and smooth muscle cell phenotypes in early atherosclerotic lesions of human aorta. Atherosclerosis. 1993 May;100(2):237–48. doi: 10.1016/0021-9150(93)90210-l. [DOI] [PubMed] [Google Scholar]
- 4.Napoli C, D’Armiento FP, Mancini FP, Postiglione A, Witztum JL, Palumbo G, Palinski W. Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia. Intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions. The Journal of clinical investigation. 1997 Dec 1;100(11):2680–90. doi: 10.1172/JCI119813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navab M, Berliner JA, Watson AD, Hama SY, Territo MC, Lusis AJ, Shih DM, Van Lenten BJ, Frank JS, Demer LL, Edwards PA, Fogelman AM. The Yin and Yang of oxidation in the development of the fatty streak. A review based on the 1994 George Lyman Duff Memorial Lecture. Arteriosclerosis, thrombosis, and vascular biology. 1996 Jul;16(7):831–42. doi: 10.1161/01.atv.16.7.831. [DOI] [PubMed] [Google Scholar]
- 6.Randolph GJ, Furie MB. A soluble gradient of endogenous monocyte chemoattractant protein-1 promotes the transendothelial migration of monocytes in vitro. J Immunol. 1995 Oct 1;155(7):3610–8. [PubMed] [Google Scholar]
- 7.Navab M, Imes SS, Hama SY, Hough GP, Ross LA, Bork RW, Valente AJ, Berliner JA, Drinkwater DC, Laks H, et al. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. The Journal of clinical investigation. 1991 Dec;88(6):2039–46. doi: 10.1172/JCI115532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terkeltaub R, Boisvert WA, Curtiss LK. Chemokines and atherosclerosis. Current opinion in lipidology. 1998 Oct;9(5):397–405. doi: 10.1097/00041433-199810000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Yla-Herttuala S, Lipton BA, Rosenfeld ME, Sarkioja T, Yoshimura T, Leonard EJ, Witztum JL, Steinberg D. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proceedings of the National Academy of Sciences of the United States of America. 1991 Jun 15;88(12):5252–6. doi: 10.1073/pnas.88.12.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mackay CR. Chemokine receptors and T cell chemotaxis. The Journal of experimental medicine. 1996 Sep 1;184(3):799–802. doi: 10.1084/jem.184.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Molecular cell. 1998 Aug;2(2):275–81. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 12.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998 Aug 27;394(6696):894–7. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 13.Douglas MS, Ali S, Rix DA, Zhang JG, Kirby JA. Endothelial production of MCP-1: modulation by heparin and consequences for mononuclear cell activation. Immunology. 1997 Dec;92(4):512–8. doi: 10.1046/j.1365-2567.1997.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wain JH, Kirby JA, Ali S. Leucocyte chemotaxis: Examination of mitogen-activated protein kinase and phosphoinositide 3-kinase activation by Monocyte Chemoattractant Proteins-1, -2, -3 and -4. Clinical and experimental immunology. 2002 Mar;127(3):436–44. doi: 10.1046/j.1365-2249.2002.01764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber KS, von Hundelshausen P, Clark-Lewis I, Weber PC, Weber C. Differential immobilization and hierarchical involvement of chemokines in monocyte arrest and transmigration on inflamed endothelium in shear flow. European journal of immunology. 1999 Feb;29(2):700–12. doi: 10.1002/(SICI)1521-4141(199902)29:02<700::AID-IMMU700>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 16.Gilat D, Hershkoviz R, Mekori YA, Vlodavsky I, Lider O. Regulation of adhesion of CD4+ T lymphocytes to intact or heparinase-treated subendothelial extracellular matrix by diffusible or anchored RANTES and MIP-1 beta. Journal Of Immunology (Baltimore, Md: 1950) 1994;153(11):4899–906. [PubMed] [Google Scholar]
- 17.Huber AR, Kunkel SL, Todd RF, 3rd, Weiss SJ. Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science (New York, NY) 1991;254(5028):99–102. doi: 10.1126/science.1718038. [DOI] [PubMed] [Google Scholar]
- 18.Patel DD, Koopmann W, Imai T, Whichard LP, Yoshie O, Krangel MS. Chemokines have diverse abilities to form solid phase gradients. Clin Immunol. 2001 Apr;99(1):43–52. doi: 10.1006/clim.2000.4997. [DOI] [PubMed] [Google Scholar]
- 19.Rot A. Neutrophil attractant/activation protein-1 (interleukin-8) induces in vitro neutrophil migration by haptotactic mechanism. European journal of immunology. 1993 Jan;23(1):303–6. doi: 10.1002/eji.1830230150. [DOI] [PubMed] [Google Scholar]
- 20.Wiedermann CJ, Reinisch N, Bellmann R, Schratzberger P, Kowald E, Kähler CM. Different patterns of deactivation of chemotaxis and haptotaxis of human peripheral blood mononuclear leukocytes by soluble and surface-bound attractants. Journal Of Leukocyte Biology. 1995;58(4):438–44. doi: 10.1002/jlb.58.4.438. [DOI] [PubMed] [Google Scholar]
- 21.Witt DP, Lander AD. Differential binding of chemokines to glycosaminoglycan subpopulations. Current Biology. 1994;4(5):394–400. doi: 10.1016/s0960-9822(00)00088-9. [DOI] [PubMed] [Google Scholar]
- 22.Gappa-Fahlenkamp H, Shukla AS. The effect of short-term, high glucose concentration on endothelial cells and leukocytes in a 3D in vitro human vascular tissue model. In vitro cellular & developmental biology. 2009 May-Jun;45(5-6):234–42. doi: 10.1007/s11626-008-9171-4. [DOI] [PubMed] [Google Scholar]
- 23.Jilma-Stohlawetz P, Homoncik M, Drucker C, Marsik C, Rot A, Mayr WR, Seibold B, Jilma B. Fy phenotype and gender determine plasma levels of monocyte chemotactic protein. Transfusion. 2001 Mar;41(3):378–81. doi: 10.1046/j.1537-2995.2001.41030378.x. [DOI] [PubMed] [Google Scholar]
- 24.Holtkamp GM, De Vos AF, Peek R, Kijlsta A. Analysis of the secretion pattern of monocyte chemotactic protein-1 (MCP-1) and transforming growth factor-beta 2 (TGF-beta2) by human retinal pigment epithelial cells. Clinical and experimental immunology. 1999 Oct;118(1):35–40. doi: 10.1046/j.1365-2249.1999.01016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uriarte SM, Molestina RE, Miller RD, Bernabo J, Farinati A, Eiguchi K, Ramirez JA, Summersgill JT. Effects of fluoroquinolones on the migration of human phagocytes through Chlamydia pneumoniae-infected and tumor necrosis factor alpha-stimulated endothelial cells. Antimicrobial Agents And Chemotherapy. 2004;48(7):2538–43. doi: 10.1128/AAC.48.7.2538-2543.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krankel N, Kuschnerus K, Madeddu P, Luscher TF, Landmesser U. A novel flow cytometry-based assay to study leukocyte-endothelial cell interactions in vitro. Cytometry A. 2011 Apr;79(4):256–62. doi: 10.1002/cyto.a.21043. [DOI] [PubMed] [Google Scholar]
- 27.Lührmann A, Bargsten G, Kuzu M, Koslowski R, Pabst R, Tschernig T. The alveolar epithelial type I-like cell line as an adequate model for leukocyte migration studies in vitro. Experimental and Toxicologic Pathology. 2007;58(5):277–83. doi: 10.1016/j.etp.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Weber KS, Nelson PJ, Grone HJ, Weber C. Expression of CCR2 by endothelial cells: implications for MCP-1 mediated wound injury repair and In vivo inflammatory activation of endothelium. Arteriosclerosis, thrombosis, and vascular biology. 1999 Sep;19(9):2085–93. doi: 10.1161/01.atv.19.9.2085. [DOI] [PubMed] [Google Scholar]
- 29.Flores-Villanueva PO, Ruiz-Morales JA, Song CH, Flores LM, Jo EK, Montano M, Barnes PF, Selman M, Granados J. A functional promoter polymorphism in monocyte chemoattractant protein-1 is associated with increased susceptibility to pulmonary tuberculosis. The Journal of experimental medicine. 2005 Dec 19;202(12):1649–58. doi: 10.1084/jem.20050126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glushakova O, Kosugi T, Roncal C, Mu W, Heinig M, Cirillo P, Sanchez-Lozada LG, Johnson RJ, Nakagawa T. Fructose induces the inflammatory molecule ICAM-1 in endothelial cells. J Am Soc Nephrol. 2008 Sep;19(9):1712–20. doi: 10.1681/ASN.2007121304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hildebrandt GC, Duffner UA, Olkiewicz KM, Corrion LA, Willmarth NE, Williams DL, Clouthier SG, Hogaboam CM, Reddy PR, Moore BB, Kuziel WA, Liu C, Yanik G, Cooke KR. A critical role for CCR2/MCP-1 interactions in the development of idiopathic pneumonia syndrome after allogeneic bone marrow transplantation. Blood. 2004 Mar 15;103(6):2417–26. doi: 10.1182/blood-2003-08-2708. [DOI] [PubMed] [Google Scholar]
- 32.Distler JH, Jungel A, Caretto D, Schulze-Horsel U, Kowal-Bielecka O, Gay RE, Michel BA, Muller-Ladner U, Kalden JR, Gay S, Distler O. Monocyte chemoattractant protein 1 released from glycosaminoglycans mediates its profibrotic effects in systemic sclerosis via the release of interleukin-4 from T cells. Arthritis and rheumatism. 2006 Jan;54(1):214–25. doi: 10.1002/art.21497. [DOI] [PubMed] [Google Scholar]
- 33.Mauro A, Buscemi M, Gerbino A. Immunohistochemical and transcriptional expression of matrix metalloproteinases in full-term human umbilical cord and human umbilical vein endothelial cells. J Mol Histol. 2010 Dec;41(6):367–77. doi: 10.1007/s10735-010-9298-y. [DOI] [PubMed] [Google Scholar]
- 34.McQuibban GA, Gong JH, Wong JP, Wallace JL, Clark-Lewis I, Overall CM. Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood. 2002 Aug 15;100(4):1160–7. [PubMed] [Google Scholar]
- 35.Zhao X, Jain S, Benjamin Larman H, Gonzalez S, Irvine DJ. Directed cell migration via chemoattractants released from degradable microspheres. Biomaterials. 2005 Aug;26(24):5048–63. doi: 10.1016/j.biomaterials.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Fleury ME, Boardman KC, Swartz MA. Autologous morphogen gradients by subtle interstitial flow and matrix interactions. Biophysical journal. 2006 Jul 1;91(1):113–21. doi: 10.1529/biophysj.105.080192. [DOI] [PMC free article] [PubMed] [Google Scholar]