Abstract
The current study was aimed at investigating the effect of HIV-1 protein Tat on the retinal neurosensory cell line R28. Exposure of Tat resulted in induction of pro-inflammatory mediators such as CXCL10 and TNF-α in addition to the activation marker GFAP in these cells. Conditioned media from Tat-treated R28 cells was able to induce monocyte migration, an effect that was blocked by CXCR3 antagonist. Complementary studies in the HIV-1 Tat-transgenic mice, showed a complete absence of the nuclear layer and the outer photoreceptor segments of the retina with a concomitant increase in glial activation. These findings lend support to the observation in post-HAART era of increased incidence of immune response-mediated retinal degeneration. These findings have direct relevance to diseases such as immune response uveitis and patients recovering from CMV retinitis.
Keywords: Retina, HIV, Chemokine
Introduction
The ophthalmic manifestations of human immunodeficiency virus (HIV) infection are easy to overlook since these severe symptoms appear at the later stages of the acquired immunodeficiency syndrome (AIDS). Regardless of the reduction in the number of absolute cases in the most debilitating eye conditions such as cytomegalovirus (CMV) retinitis, highly active anti-retroviral therapy (HAART) has neither eliminated the appearance of new cases nor reduced the development of complications such as retinal detachment or immune recovery uveitis (IRU). IRU in its most severe form is often characterized by an increase in intraocular inflammatory reactions and by the presence of inflammatory responses including macular edema, epiretinal membranes, neovascularization of the retina or optic disk, posterior synechiae, and/or cataracts (Holland 2008). Increased levels of pro-inflammatory cytokines have also been documented in tissues of patients who have recovered from CMV retinitis (Rios et al. 2005).
Infection and consequent activation of immune system cells therefore remain the most common cause of ocular damage. The ocular microenvironment is both immunosuppressive and anti-inflammatory in nature. The retina is, however, the only ocular tissue truly derived from the cells of the neural plate sharing the same lineage as the central nervous system (CNS) (Deshpande et al. 2005; Sanyal and Zeilmaker 1977) and protected by the blood-retinal barrier. Exposure to HIV-1 antigens in the retina occurs only following the breach of the blood-retinal barrier. While the exact mechanisms by which HIV-1 causes these neuropathologies is not completely understood, increasing evidence leads to the speculation that neuronal damage results, in part, from inflammation-mediated through activated glia (Deshpande et al. 2005; Martin-Garcia et al. 2006; Minagar et al. 2002).
Chemokines in the brain have been recognized as essential elements in the progression of neurodegenerative diseases and related neuroinflammation through their regulation of inflammatory responses (Conant et al. 1998; Kelder et al. 1998; Luster et al. 1987) thereby contributing to injury and eventual loss of neurons (Asensio and Campbell 1999; Miller and Meucci 1999). Increased levels of CXCL10 have been detected in the CSF and plasma of individuals with HIV-1 infection (Kolb et al. 1999) and in the brains of individuals with HIV-associated dementia (HAD) (Kolson and Pomerantz 1996; McArthur et al. 1993; Sanders et al. 1998). The presence of pro-inflammatory chemokines such as CXCL10 in the CNS of HIV-1 infected individuals correlated positively with disease progression (Dhillon et al. 2008b; Durudas et al. 2009). There is also evidence that CXCL10 participates in the neuropathogenesis of simian HIV-infected macaques (Sasseville et al. 1996; Westmoreland et al. 1998) by contributing to the degeneration of neurons possibly through activation of a calcium dependent apoptotic pathway (Sui et al. 2004; Sui et al. 2006). Additionally, the pro-inflammatory cytokines interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α) are also markedly increased in CNS tissues during HIV-1 infection and have been implicated in the pathophysiology of HAD (Saha and Pahan 2003; Shapshak et al. 2004; Wesselingh et al. 1997). Besides its induction by host factors, CXCL10 can also be induced by HIV-1 itself or by several HIV-1-associated proteins including gp120 and Tat (Asensio and Campbell 1999; Kutsch et al. 2000; van Marle et al. 2004).
In addition to chemokines, high levels of pro-inflammatory cytokines and viral proteins are also known to contribute to retinal degeneration (Madigan et al. 1996; Schrier et al. 2006). Specifically, HIV-1 Tat, known to be released from infected cells in the CNS, has been shown to be neurotoxic both in cell culture (Woodman et al. 1999) and in animal models of the disease (Hudson et al. 2000). Tat has been shown to transactivate HIV-1 gene expression through interactions with the transactivation responsive element TAR within the HIV-1 long terminal repeat promoter, human cyclin T1, and CDK9. Furthermore, it has been documented to exert pleiotropic effects on host cells (Kim et al. 2003). In this study, a retinal cell line has been used to examine the effect of HIV-1 Tat on the release of inflammatory mediators that could contribute to disease pathology. The rat retinal precursor neurosensory cell line R28 expresses both neuronal (Seigel et al. 1997) as well as glial cell markers (Adamus et al. 1997; Seigel 1996; Seigel and Liu 1997; Seigel et al. 1996). The R28 cells have been shown to be comprised of subpopulations of neuronal cells resembling properties of retinal cells. These cells have been used for studies on gene expression (Seigel et al. 2004), electrophysiology (Sun et al. 2002), and apoptosis (Ong et al. 2003; Seigel et al. 2000; Ragaiey et al. 1997). Their heterogenous nature and expression of markers for retinal ganglion cells, horizontal cells, amacrine cells, glial cells, photoreceptors, and bipolar cells make them a relevant model system replicating the in vivo milieu.
Our findings indicated that the exposure of retinal neurosensory cells to HIV Tat results in increased activation and release of pro-inflammatory mediators, predominantly the chemokine and neurotoxic factor CXCL10, which in turn, leads to increased monocyte migration. Intriguingly, retinas from HIV-1 Tat-transgenic mice with constitutive Tat expression in multiple tissues, (Kim et al. 2003; Prakash et al. 1997) also demonstrated increased loss of neurons as evidenced by a lack of microtubule-associated protein 2 (MAP2) staining in the outer photoreceptor segments with a concomitant increase in activation of glial cells shown by enhanced GFAP staining.
These findings provide evidence that the retina is similar to other parts of the CNS where CXCL10 is a major player in HIV-1 associated pathology and could be a potential target for therapeutic interventions.
Methods and materials
Cell culture and treatments
Immortalized R28 retinal precursor cells derived from postnatal day 6 Sprague–Dawley rats were maintained in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (Invitrogen, Gaithersburg, MD), ×1 minimal essential medium (MEM) nonessential amino acids (Invitrogen), ×1 MEM vitamins (Invitrogen), 0.37% sodium bicarbonate, 0.058% L-glutamine and 100 μg/ml penicillin–streptomycin (Invitrogen). Cells were maintained in tissue culture flasks in DMEM (containing 4,500 mg/L glucose). Cells were not grown on subtrates except when seeding on glass coverslips. Poly-D-lysine (50 μg/ml, Invitrogen) was used as a substrate on coverslips. When confluent, the cells were washed once with phosphate-buffered saline (PBS), detached from the flask by treatment with trypsin (Invitrogen), washed with complete cell culture medium and split 1:5 into fresh flasks.
The cells (triplicate or quadruplicate wells) were treated for 0–48 h with Tat (50, 100, 200 ng/ml). Control treatments included incubation with heat-inactivated Tat at aforementioned concentrations. Tat was inactivated by denaturing in boiling water for 15 min.
R28 cells were cultured in 24-well plates (2 ml per well), grown in 2% serum overnight, and then treated with Tat (200 ng/ml) for 24 h prior to supernatant collection for chemotactic assay. Concentrations of Tat and incubation periods were based on previous publications (Dhillon et al. 2007; Hegg et al. 2000). All disposable plasticware were from Fisher Scientific (Fisher Scientific, Pittsburgh, PA).
Cytotoxicity indices
Cell proliferation/viability in cell lines was determined by 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyltetrazolium bromide (MTT; Sigma, St. Louis, MO) conversion as described previously (Ding and Keller 2001a, b). Briefly, after treatment in vitro, cells were exposed to 0.5 mg/ml MTT in serum-free and phenol red-free medium for 1–4 h at 37°C.
Following exposure, medium was aspirated and formazan precipitates were extracted by addition of 200 μl dimethylsulfoxide (DMSO; Sigma, St. Louis, MO). The amount of formazan formation was analyzed within an hour by determination of optical density at 570 nm with reference wavelength of 630 nm on a spectrophotometric UV/VIS plate reader (SynergyMx, Biotek). At least five cultures were utilized for each data point.
R28 cells were assayed for cell death using the well-established protocol of propidium iodide (Sigma) staining for dead cells (Steinkamp et al. 1999). Propidium iodide emission was at 617 nm, excitation at 490 nm. Fluorescence is only from the highly disordered membranes of necrotic, apoptotic, and dead cells. Briefly, cells were resuspended at least 0.5×106 cells in 12×75 mm tubes at a concentration of 106 cells/ml. Propidium iodide was added to a final concentration of 1 μg/ml. Cells were then incubated on ice for 5 min. Analysis was performed by a FACSCalibur (Becton Dickinson, Franklin Lakes, NJ) at the emission wavelength 617 nm.
Cytokine mRNA analysis
RNA was extracted from tissue samples using Trizol reagent (Life Technologies, Grand Island, NY). Quantitative analysis of mRNA of pro-inflammatory mediators from cells treated with 200 ng/ml Tat was performed by real-time reverse transcription polymerase chain reaction (RT-PCR) using the SYBR Green (ABI, Foster City, CA) detection method. RT-PCR primer pair sets were obtained from SABiosciences (Frederick, MD) and amplification of CXCL10 from first-strand cDNA was performed using an ABI Prism 7,700 sequence detector (ABI, Foster City, CA). Data were normalized using Ct values for the housekeeping gene Glyceraldehyde phosphate dehydrogenase (GAPDH) in each sample.
In order to calculate relative amounts of CXCL10, TNF-α and GFAP, the average Ct value of the GAPDH was subtracted from that of each target gene to provide changes in Ct (ΔCt) value. The fold-change in gene expression (differences in ΔCt, or ΔΔCt) was subsequently determined as log2 relative units.
Chemotactic assay
Migration assays were performed in a disposable ChemoTX microplate (NeuroProbe, Cabin John, MD) with a pore size of 8 μm. Supernatants from R28 cell cultures treated with either Tat or IFN-γ were collected after 24 h of incubation in a serum-free medium. These supernatants were then added in each lower compartment of the microplate and 2×104 human monocytes resuspended in 30-μl volume of DMEM, were added to the top chamber. The microplate was then incubated at 37°C with 5% CO2 for 4 h. Afterwards the cells in the upper chamber were removed by washing with PBS. Cells that had migrated to the lower chamber were counted using the Cell Titer 96 Aqueous One Solution Assay (Promega, Madison, WI). The positive control chemoattractant was 200 ng/ml IFN-γ and the negative control chemoattractant was base medium alone. Two percent fetal bovine serum in medium was also included as a negative control.
AMG487 was kindly provided by Amgen (San Francisco, CA). The in vitro formulation of AMG487 was prepared as a 10 mM stock with DMSO. Monocytes in suspension were washed and fresh growth medium containing 200 nM AMG487, 1 μM AMG487, or DMSO vehicle was added for 2 h of incubation at 37°C. The monocytes were washed and resuspended in serum-free medium before proceeding according to manufacturer’s instructions (Neuroprobe). All assays were performed in triplicate. Migration index was calculated as the number of cells migrating towards the treated supernatants divided by the number of cells migrating toward the medium only.
Immunohistochemical studies
Immunohistochemical analyses were carried out on paraffin-fixed tissue sections from 6 weeks old transgenic mouse eyeballs. The eyeballs were obtained from HIV-1 Tat-transgenic mice. These mice were derived from two transgene constructs (Prakash et al. 1997). Tat-transgenic mice showed expression of Tat mRNA in most tissues and the Tat protein was also found to be biologically active (Prakash et al. 1997). Eyeballs from six animals (three males, three females) were harvested. Sections of eyeballs from HIV Tat-transgenic animals and control animals were stained for MAP2 (for neurons), GFAP (for astrocytes or activated Mueller glia), Iba1 (for microglia), or vimentin (for Mueller glia). Sections were also stained with hemotoxylin–eosin for ascertaining gross structural changes.
Briefly, sections were deparaffinized in xylene, rehydrated in alcohol, and then rinsed in 0.1 M PBS. Afterwards, the slides were placed into 10 mM citrate buffer (pH 6) retrieval solution and boiled for 20 min followed by blockage by appropriate serum for 2 h. The primary antibodies were applied overnight, followed by treatment with repeated washes and application of the appropriate secondary antibody conjugated to chromophores for another 2 h (Molecular Probes). Images were recorded with a Zeiss 510 Meta Confocal Laser Scanning Microscope equipped with four lasers: a Blue Diode 405 nm; an Argon Laser 458/477/488 514 nm; a DPSS 561 nm and a HeNe 633 nm. Images were captured at ×40 and ×63 magnifications. All processing of images was performed on the Axiovision software (Version 4.6).
Statistical analysis
All statistical analyses were performed by using a two-tail, independent, t test. Results were judged as statistically significant at p values ≤0.05.
Results
1. HIV-1 Tat increases release of pro-inflammatory mediators in retinal precursor cell line R28
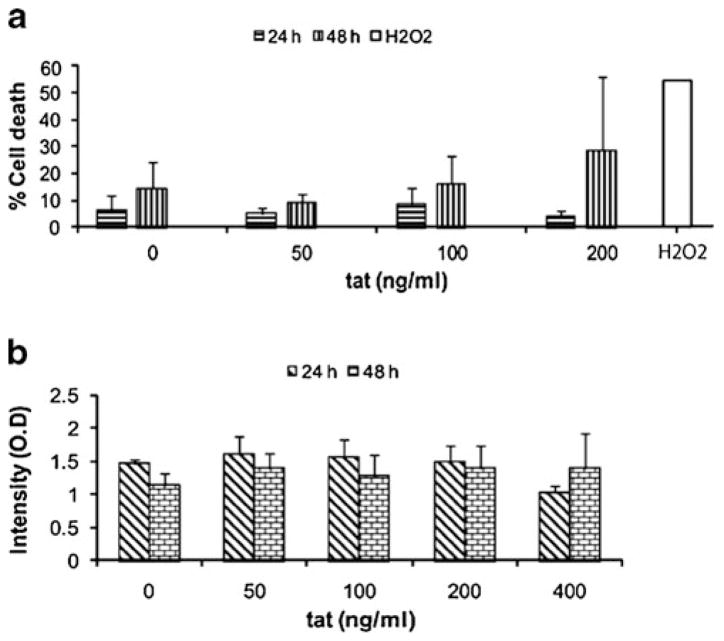
Before embarking on the effect of HIV Tat on the expression of pro-inflammatory mediators, it was critical to determine whether Tat exerted any toxicity in these cells. We therefore exposed the R28 retinal cell line to varying concentrations of HIV-1 Tat (50, 100, and 200 ng/ml) for 24 and 48 h. As shown in Fig 1, in the R28 cell line, exposure to HIV-1 Tat had no toxic effect on these cells when assessed by both propidium iodide live/dead (Fig 1a) and MTT (Fig 1b) assays. Since the cells showed no adverse effects on exposure to Tat, all further experiments were performed with 200 ng/ml.
Fig. 1.
Effect of Tat on R28 cell survival and proliferation. a R28 cells were exposed to increasing doses of Tat for 24 and 48 h; 3% H2O2 was used as a positive control. Cells were exposed to three concentrations of Tat; 50, 100, 200 ng/ml. Cells were then harvested and analyzed for cell death using flow cytometry. Cell death was not significantly higher on treatment with Tat. Increasing concentrations up to 200 ng/ml did not show significant variation. b R28 cells were treated with increasing doses of Tat for 24 h and 48 h. Cells were then treated with 0.5 mg/ml MTT for 4 h, after which the medium was aspirated, the formazan precipitates dissolved in DMSO, and the amount of formazan conversion analyzed at 570 nm for proliferation/viability. Cell proliferation was not significantly higher on treatment with Tat. Increasing concentrations up to 200 ng/ml did not show variation in results
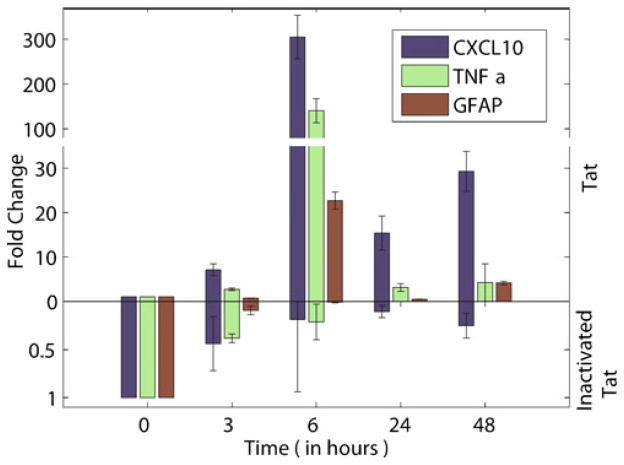
Since HIV-1 infection/proteins (Poluektova et al. 2001; Wetzel et al. 2002) are known to induce the expression of pro-inflammatory mediators such as MCP-1, CXCL10 and TNF-α in glial cells and it is known that both CXCL10 and TNF-α are up-regulated in CMV retinitis and IRU (Momma et al. 2003; Rios et al. 2005), we sought to examine the effect of HIV Tat on the expression of these mediators in the retinal cell line. R28 cells were treated for a period of up to 48 h with 200 ng/ml Tat and monitored for the expression of these mediators by quantitative Real-time PCR. Cells cultured in the presence of HIV-1 Tat were harvested at varying times (0, 3, 6, 24, and 48 h) followed by RNA extraction. As shown in Fig. 2, there was increased expression of both CXCL10 and TNF-α that peaked 6 h following Tat exposure. Intriguingly, CXCL10 levels increased more than 300-fold in Tat-treated cells compared with untreated cells. Levels of TNF-α increased to almost 150-fold at 6-h post-treatment in Tat-exposed cultures. Cells exposed to heat inactivated Tat failed to demonstrate the induction of these pro-inflamamtory mediators as expected.
Fig. 2.
Exposure to Tat induces pro-inflammatory factors CXCL10, TNF-α, and GFAP in retinal R28 cells. a CXCL10 and TNF-α showed dramatic rise in R28 cells when incubated with Tat. Expression of these two inflammatory mediators peaked after 6 h of incubation. GFAP expression was also highest after 6 h of incubation. The mRNA expression levels subsequently decreased over the next 48 h. R28 retinal cells were incubated with 200 ng/ml Tat, for up to 48 h, after which the cells were harvested and analyzed for mRNA levels of CXCL10, TNF-α, and GFAP. Data were obtained from four separate experiments, and were analyzed by Students t test. Asterisk indicates statistically significant increases (p<0.05) in factors expressed in cells treated with Tat as compared with vehicle-treated (control) cells. Exposure to inactivated Tat did not induce production of these cytokines or GFAP. Tat was inactivated by boiling for 15 min
In addition to pro-inflammatory mediators, it was also of interest to examine the expression of GFAP (Adamus et al. 1997), which is a known marker for activation of the R28 cells. In the retina, GFAP-positive astrocytes make up a small percentage of all glial cells; the predominant cell type being Mueller glia. However, damage and degeneration of the retina has been shown to be coupled with severe astrogliosis in the optic nerve and activation of Mueller glia (Madigan et al. 1996) leading to increased levels of GFAP. Similar to several pro-inflammatory mediators, GFAP levels also increased following Tat treatment (more than 20-fold after 6 h of Tat exposure). Another inflammatory mediator implicated in HIV-1 related pathology, MCP-1, however, showed no change in level after cells were exposed to Tat protein (data not shown).
The dramatic rise in pro-inflammatory cytokines correlates well with the protective role these cytokines serve during the early stages of infection. In addition, increased levels of these cytokines also allow them to function as chemoattractant molecules which can attract HIV-1-infected and/or immune competent mononuclear phagocytes and microglia which could lead to further neuronal dysfunction.
2. Function of Tat-mediated induction of CXCL10: increased monocyte migration
Increased CXCL10 levels are known to be critical for the increased migration of inflammatory cells into the CNS, a hallmark feature of HIV induced neuro-degeneration (Nath 1999; Navia et al. 1986). The mechanism by which HIV enters the CNS has been assumed to be infection of macrophages or lymphocytes which cross the blood–brain barrier and further infect microglia and perivascular macrophages in the CNS. Alternatively, it has been suggested that CNS infiltration is through the choroid plexus with subsequent seeding of the brain by infected macrophages or CD4 lymphocytes harboring CCR5-using viral strains (Power et al. 2002). Macrophage infiltration and activation is frequently observed with detection of viral protein or nucleic acid in macrophages of 30–50% of biopsies in HIV patients with ocular manifestations (Holland 2008).
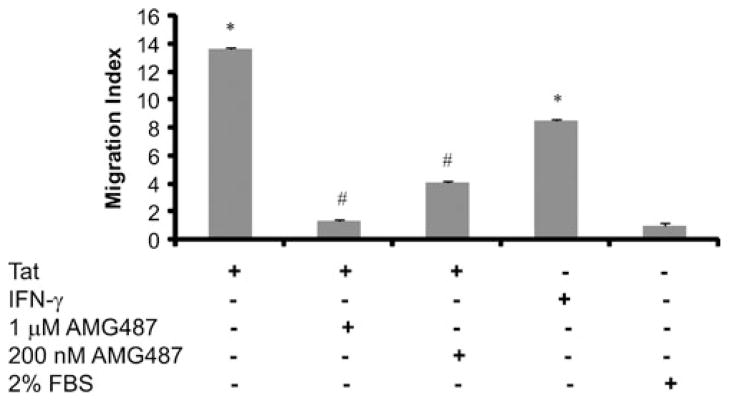
To test the functionality of CXCL10 released by R28 cells following exposure to Tat, we analyzed the migration of activated peripheral blood mononuclear monocytes (PBMCs) towards the gradient of CXCL10 released in the supernatant fluids of Tat-stimulated R28 cells. Chemotactic assays demonstrated that culture fluids from Tat-treated R28 demonstrated a higher chemotactic activity for monocytes compared with the media from the untreated cells (Fig. 3). Specificity of this response was confirmed by treating the culture fluids with a small molecule antagonist of the CXCR3, the receptor to which CXCL10 binds. CXCR3 belongs to the superfamily of the G-protein-coupled receptors (Proudfoot 2002). (R)-N-{1-[3-(4-Ethoxy-phenyl)-4-oxo-3,4-dihydro-pyrido [2,3-d]-pyrimidin-2-yl]-ethys-N-pyridin-3-yl-methyl-2-(4 trifluoromethoxyphenyl)-acetamide (AMG 487) is a potent and selective orally bio-available chemokine (C–X–C motif) known to block receptor 3 (CXCR3). It is known to function as an antagonist to lymphocyte migration mediated by CXCL9, CXCL10, or CXCL11 (Kryczek et al. 2005) at nanomolar ranges (IC50 8 nM for CXL10). As shown in Fig. 3, CXCL10 released from activated R28 cells had a substantial contribution in attracting monocytes. This effect on the monocytes was abrogated in the presence of CXCR3 antagonist, AMG487 (Fig. 3).
Fig. 3.
Tat induced increase in CXCL10 from R28 cells lead to increased migration of monocytes. Analysis of chemotactic activity of CXCL10 in supernatants collected from R28 cells treated with Tat, in absence and presence of AMG487, blocker of CXCL-10 receptor on monocytes, showed pronounced contribution of CXCL10. IFN-γ was used as positive control. Supernatant from unstimulated control cells and from IFN-γ and Tat-treated R28 cells were added to the lower chamber of ChemoTX microplate. 2×106 PBMCs were placed on the top of the membrane. In indicated wells, PBMCs were treated with two different concentrations of CXCR3 blocker, AMG487 for 2 h before loading in the top chamber of the ChemoTX microplate. After 4 h, PBMCs migrating towards supernatant collected from different treatments in lower chamber were counted by using Cell Titer 96 Aqueous One Solution Assay. Migratory response of PBMCs to supernatant fluids are shown as migration index, calculated as the number of cells migrating towards the treated supernatants divided by the number of cells migrating toward the medium only
3 Histopathological features of the retina in HIV-1 Tat-transgenic mice
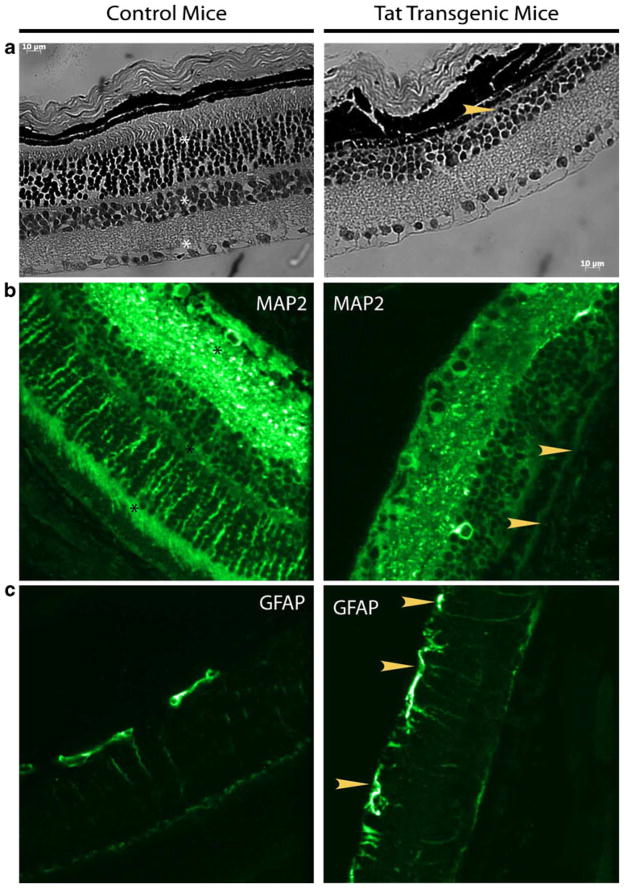
Tat expression in the CNS of mice is known to cause a hunched posture, tremors, ataxia, slow cognitive and motor movements, seizures, and premature death (Kim et al. 2003; Prakash et al. 1997). An underlying neurodegeneration has been shown to be a cause for these symptoms (Prakash et al. 1997). Neurodegeneration at the posterior segments of the eye is generally observed during the late stages of HIV infection in humans. In order to investigate the effects of constitutive expression of Tat in the retina, eyeballs harvested from HIV-1 Tat-transgenic mice and wild type control (C57BL/6 N; n=6) animals were sectioned and stained with hemotoxylin–eosin for gross pathology. As shown in Fig. 4, Tat-transgenic (Fig. 4b) animals exhibited a striking absence of the nuclear layers compared with the wild type controls (Fig. 4a) that demonstrated three distinct nuclear layers. Furthermore, the retinal thickness consequently in most parts was thinner in transgenic versus the control animals.
Fig. 4.
HIV-1 Tat-transgenic animals show large scale organizational changes in the retina. Immunocytochemical changes are observed in the retina of HIV-1 Tat-transgenic mice. Representative staining of paraffin-embedded retina sections. Analyses of sections from HIV-1 Tat-transgenic animals show pronounced changes in MAP2 b and GFAP c staining patterns. MAP2 staining is conspicuously missing from the outer plexiform layer and outer photoreceptor segments (arrowhead), of HIV-1 Tat-transgenic animals. GFAP which functions as an activation marker in the retina, showed increased expression with many more processes visible (arrowhead), compared with control animals. Hemotoxylin–eosin staining demonstrated the absence of an entire nuclear layer (arrowhead), absence of the outer photoreceptor segments and thinner retina (a). Scale bar 10 μm. Layers in control animals are marked by asterisks. Eyeballs were harvested from both control animals (n=6) and HIV-1 Tat-transgenic mice. Images shown were captured at ×40 magnification
We next stained the retinal sections with cell marker specific antibodies; MAP2 (neurons), GFAP (astrocytes or activated Mueller glial cells), Iba-1 (microglia), and vimentin (Mueller glia) were used to identify the cell type changes within the retina in Tat-transgenic versus control animals. MAP2, which identifies stable cytoskeletal structures, clearly stained processes of all the retinal neurons in sections from control animals. MAP2 stained neuronal processes in the ganglion cell layers and the inner and outer plexiform layers as well as the outer segments of the photoreceptors (Fig. 4c). Interestingly, this pattern of staining was notably absent in the retina from HIV-1 Tat-transgenic animals (Fig. 4d).
We next examined the pattern of GFAP staining in the retina of both HIV-1 Tat-transgenic and control animals. Compared with the control animals (Fig. 4e), considerably more processes were positively stained for GFAP in the HIV-1 Tat-transgenic mice (Fig. 4f). GFAP staining in control animals was limited to the cell bodies of GFAP-positive cells in the ganglion cell layer. However, in HIV-1 Tat-transgenic mice, the processes showed pronounced staining.
Tissues stained for Iba-1 and vimentin showed less salient differences. There were no significant changes noted between the two groups of animals (data not shown).
Discussion
The advent of HAART has improved outcomes in patients with CMV retinitis and other AIDS related opportunistic infections. However, the very recovery of the immune system in the initial stages of therapy can endanger immune privileged sites like the retina and initiate a chain of events which could eliminate susceptible cells such as retinal neurons. Despite the overwhelming success of HAART in dampening virus load in the systemic compartment, the prevalence of ophthalmic manifestations of HIV infection especially those of the posterior segment such as IRU, are actually on the rise. Increased cases of IRU in patients on HAART have been consistently observed (Venkatesh et al. 2008).
Chemokines in the brain have been recognized as essential mediators of neurodegenerative diseases and related neuroinflammation through their regulation of inflammatory responses (Conant et al. 1998; Kelder et al. 1998; Luster et al. 1987) thereby contributing to injury and eventual loss of neurons (Asensio and Campbell 1999; Miller and Meucci 1999). Specifically, increased levels of CXCL10 have been detected in the CSF and plasma of individuals with HIV-1 infection (Kolb et al. 1999) and in the brains of individuals with HAD and shown to be a chemoattractant (Kolson and Pomerantz 1996; McArthur et al. 1993; Sanders et al. 1998). Based on the chemotactic and neurotoxic potential of CXCL10 and the toxic effects of TNF-α and the induction of these mediators by viral proteins, we hypothesized that it was likely that the exposure of retinal glial cells to HIV Tat could lead to the release of these toxic mediators resulting in retinal pathology, exemplified by increased astrocytosis and/or neuronal degeneration.
Using the retinal R28 cell line that expresses both neuronal (Seigel et al. 1997) and glial markers (Adamus et al. 1997; Seigel 1996; Seigel and Liu 1997; Seigel et al. 1996) as our model system, we examined the release of pro-inflammatory mediators by these cells in response to HIV Tat. The precursor nature of R28 may make it more robust than neurons, a possible reason why Tat toxicity did not get reflected as cell death in R28 cells.
Similar to that reported by others (Sui et al. 2006; van Marle et al. 2004) in various cell types, our findings indicated that the exposure of retinal neurosensory and glial cells to HIV Tat resulted in increased activation and release of pro-inflammatory mediators, predominantly the chemokine and neurotoxic factor CXCL10 and the cytokine TNF-α. These findings provide evidence that the retina is similar to other parts of the CNS where CXCL10 is a major player in HIV-1 associated pathology. The chemotactic role of CXCL10 was also confirmed in the in vitro chemotactic assays wherein conditioned media from Tat-treated R28 cells was able to induce increased monocyte chemotaxis. This effect was specific for CXCL10 as monocytes treated with the CXCR3 antagonist failed to demonstrate significant migration in response to the conditioned media from Tat-exposed cells. This characteristic of CXCL10 is in agreement with previously published reports demonstrating the chemotactic properties of CXCL10 where migration of activated PBMCs was enhanced towards CXCL10 released in cell supernatants (Dhillon et al. 2008a).
The fact that inflammatory and activation responses are induced in retinal progenitor neurosensory cells such as R28 as well as in Tat-transgenic mice, indicates that similar to the rest of the CNS, many, if not all of the cells in the retina maintain the intrinsic property of expressing pro-inflammatory mediators and activation markers. It is likely that similar to the brain, irrespective of the virus replication process, the presence of viral proteins such as HIV-1 Tat can exacerbate and amplify the injury by initiating a cascade of pro-inflammatory mediators, which in turn, can have toxic consequences for the tissue. The presence in the retina of a chemokine ligand–receptor network could therefore, extend well beyond the regulation of leukocyte trafficking in immunoinflammatory diseases like IRU. It is particularly interesting that microglia, the immune system cell counterpart in the CNS does not show significant changes in the retina of HIV-1 Tat-transgenic mice. It may indicate that activation of other glial cell types contribute to a pro-inflammatory milieu and subsequent neuronal death. The ability of CXCL10, a pro-inflammatory cytokine to act as a neurotoxin is also borne out by similar effects seen with TNF-α. In TNF-α-induced optic neuropathy (Madigan et al. 1996) it was shown that TNF-α can cause neuronal and axonal damage in the optic nerve without greatly affecting astrocytes, microglia and oligodendrocytes.
It could thus be extrapolated that Tat-mediated induction of chemokines could in turn attract more monocytes which are well-known potential reservoirs of infection. Transmigration across the endothelial layer is a routine event, the final step of which is diapedesis between the tightly apposing endothelial cells (Nottet 1999). Monocyte trafficking is therefore of considerable interest. Our observations show that increased levels of CXCL10 alone are sufficient to function as a chemoattractant for the monocytes. However, it should be noted that constitutive expression of Tat, itself can largely contribute towards neuronal damage since we did not see enhanced presence of microglia and macrophage-like cells (data not shown).
In addition to pro-inflamamtory mediators, retinal cells also demonstrated increased cell activation as evidenced by augmented expression of GFAP. Increased GFAP expression is a correlate of increased astrocytosis, a hallmark feature of HIV-1 associated CNS pathology. Furthermore, these cell culture findings were also corroborated in the Tat-transgenic mice that demonstrated an increase in GFAP staining in the retina compared with the wild-type controls. Increased GFAP staining could suggest either increased astrocyte entry or greater activation of Mueller glia. Clinical manifestation of astrogliosis is known to be an important component of ocular disorders resulting from HIV infection (Holland 2008). Interestingly, immunostaining for the Mueller glial cell marker, vimentin, did not show significant differences between control and HIV-1 Tat-transgenic mice (data not shown). This could indicate that the increased GFAP is due to activation of Mueller glial cells rather than the proliferation of astrocytes.
Furthermore, increased GFAP staining correlated with a decreased expression of the neuronal cytoskeletal marker, MAP2, in the processes of retinal neurons leading to the speculation that HIV-1 Tat-mediated activation of the glia resulted in release of neurotoxic and inflammatory mediators that could subsequently lead to the damage of the inner plexiform neuronal layer. What was striking in the Tat-transgenic mice was the cellular composition and orderly structure of the retina had been dramatically obliterated compared with the retinas of the wild type animals. It is interesting to speculate how exposure to a single soluble HIV protein can regulate a complex cytokine network, which in turn can affect functional properties of both infected monocytes and their noninfected tissue-specific targets. In summary, these findings for the first time explore the role of HIV Tat in retinal neuro–glial interaction with the specific roles of CXCL10 and GFAP as mediators of neuroinflammation and neurotoxicity.
Acknowledgments
The authors thank Dr. Sabita Roy, University of Minnesota for providing the HIV-1 Tat-transgenic mice eyeballs. The authors also thank the Confocal Laser Scanning Microscope Core Facility at the University of Nebraska Medical Center for providing assistance with confocal microscopy and the Nebraska Research Initiative and the Eppley Cancer Center for their support of the Core Facility. We thank Dr. Sitabhra Sinha for his help in generating Fig. 2 with MATLAB. NC was supported by the Indian Council of Medical Research Young Biomedical Scientist Fellowship while working at UNMC on this work. GMS is supported by R21CA127061, a departmental challenge grant from Research to Prevent Blindness and a Vision Research core infrastructure grant 1R24EY016662 and by U54CA143876 from the National Cancer Institute.
Footnotes
Guarantors Nivedita Chatterjee, Shilpa J. Buch
Disclaimers The authors have no conflicting interests.
Contributor Information
Nivedita Chatterjee, Email: drnc@snmail.org, Department of Ocular Pathology, Vision Research Foundation, Sankara Nethralaya, 41 College Road, Chennai 600006, India.
Shannon Callen, Department of Pharmacology and Experimental Neuroscience, Nebraska Medical Center, University of Nebraska Medical Center, Omaha, NE 68198-5880, USA.
Gail M. Seigel, Center for Hearing and Deafness, University at Buffalo, 3435 Main Street, Cary 137, Buffalo, NY 14214, USA
Shilpa J. Buch, Department of Pharmacology and Experimental Neuroscience, Nebraska Medical Center, University of Nebraska Medical Center, Omaha, NE 68198-5880, USA
References
- Adamus G, Machnicki M, Seigel GM. Apoptotic retinal cell death induced by antirecoverin autoantibodies of cancer-associated retinopathy. Invest Ophthalmol Vis Sci. 1997;38:283–291. [PubMed] [Google Scholar]
- Asensio VC, Campbell IL. Chemokines in the CNS: plurifunctional mediators in diverse states. Trends Neurosci. 1999;22:504–512. doi: 10.1016/s0166-2236(99)01453-8. [DOI] [PubMed] [Google Scholar]
- Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, Gallo RC, Major EO. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci USA. 1998;95:3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande M, Zheng J, Borgmann K, Persidsky R, Wu L, Schellpeper C, Ghorpade A. Role of activated astrocytes in neuronal damage: potential links to HIV-1-associated dementia. Neurotox Res. 2005;7:183–192. doi: 10.1007/BF03036448. [DOI] [PubMed] [Google Scholar]
- Dhillon NK, Peng F, Ransohoff RM, Buch S. PDGF synergistically enhances IFN-gamma-induced expression of CXCL10 in blood-derived macrophages: implications for HIV dementia. J Immunol. 2007;179:2722–2730. doi: 10.4049/jimmunol.179.5.2722. [DOI] [PubMed] [Google Scholar]
- Dhillon N, Zhu X, Peng F, Yao H, Williams R, Qiu J, Callen S, Ladner AO, Buch S. Molecular mechanism(s) involved in the synergistic induction of CXCL10 by human immunodeficiency virus type 1 Tat and interferon-gamma in macrophages. J Neurovirol. 2008a;14:196–204. doi: 10.1080/13550280801993648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon NK, Williams R, Callen S, Zien C, Narayan O, Buch S. Roles of MCP-1 in development of HIV-dementia. Front Biosci. 2008b;13:3913–3918. doi: 10.2741/2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Keller JN. Proteasome inhibition in oxidative stress neurotoxicity: implications for heat shock proteins. J Neurochem. 2001a;77:1010–1017. doi: 10.1046/j.1471-4159.2001.00302.x. [DOI] [PubMed] [Google Scholar]
- Ding Q, Keller JN. Proteasomes and proteasome inhibition in the central nervous system. Free Radic Biol Med. 2001b;31:574–584. doi: 10.1016/s0891-5849(01)00635-9. [DOI] [PubMed] [Google Scholar]
- Durudas A, Milush JM, Chen HL, Engram JC, Silvestri G, Sodora DL. Elevated levels of innate immune modulators in lymph nodes and blood are associated with more-rapid disease progression in simian immunodeficiency virus-infected monkeys. J Virol. 2009;83:12229–12240. doi: 10.1128/JVI.01311-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegg CC, Hu S, Peterson PK, Thayer SA. Beta-chemokines and human immunodeficiency virus type-1 proteins evoke intracellular calcium increases in human microglia. Neuroscience. 2000;98:191–199. doi: 10.1016/s0306-4522(00)00101-9. [DOI] [PubMed] [Google Scholar]
- Holland GN. AIDS and ophthalmology: the first quarter century. Am J Ophthalmol. 2008;145:397–408. doi: 10.1016/j.ajo.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Hudson L, Liu J, Nath A, Jones M, Raghavan R, Narayan O, Male D, Everall I. Detection of the human immunodeficiency virus regulatory protein Tat in CNS tissues. J Neurovirol. 2000;6:145–155. doi: 10.3109/13550280009013158. [DOI] [PubMed] [Google Scholar]
- Kelder W, McArthur JC, Nance-Sproson T, McClernon D, Griffin DE. Beta-chemokines MCP-1 and RANTES are selectively increased in cerebrospinal fluid of patients with human immunodeficiency virus-associated dementia. Ann Neurol. 1998;44:831–835. doi: 10.1002/ana.410440521. [DOI] [PubMed] [Google Scholar]
- Kim BO, Liu Y, Ruan Y, Xu ZC, Schantz L, He JJ. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am J Pathol. 2003;162:1693–1707. doi: 10.1016/S0002-9440(10)64304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb SA, Sporer B, Lahrtz F, Koedel U, Pfister HW, Fontana A. Identification of a T cell chemotactic factor in the cerebrospinal fluid of HIV-1-infected individuals as interferon-gamma inducible protein 10. J Neuroimmunol. 1999;93:172–181. doi: 10.1016/s0165-5728(98)00223-9. [DOI] [PubMed] [Google Scholar]
- Kolson DL, Pomerantz RJ. AIDS dementia and HIV-1-induced neurotoxicity: possible pathogenic associations and mechanisms. J Biomed Sci. 1996;3:389–414. doi: 10.1007/BF02258044. [DOI] [PubMed] [Google Scholar]
- Kryczek I, Lange A, Mottram P, Alvarez X, Cheng P, Hogan M, Moons L, Wei S, Zou L, Machelon V, et al. CXCL12 and vascular endothelial growth factor synergistically induce neo-angiogenesis in human ovarian cancers. Cancer Res. 2005;65:465–472. [PubMed] [Google Scholar]
- Kutsch O, Oh J, Nath A, Benveniste EN. Induction of the chemokines interleukin-8 and IP-10 by human immunodeficiency virus type 1 Tat in astrocytes. J Virol. 2000;74:9214–9221. doi: 10.1128/jvi.74.19.9214-9221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster AD, Jhanwar SC, Chaganti RS, Kersey JH, Ravetch JV. Interferon-inducible gene maps to a chromosomal band associated with a (4;11) translocation in acute leukemia cells. Proc Natl Acad Sci USA. 1987;84:2868–2871. doi: 10.1073/pnas.84.9.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan MC, Sadun AA, Rao NS, Dugel PU, Tenhula WN, Gill PS. Tumor necrosis factor-alpha (TNF-alpha)-induced optic neuropathy in rabbits. Neurol Res. 1996;18:176–184. doi: 10.1080/01616412.1996.11740399. [DOI] [PubMed] [Google Scholar]
- Martin-Garcia J, Cao W, Varela-Rohena A, Plassmeyer ML, Gonzalez-Scarano F. HIV-1 tropism for the central nervous system: Brain-derived envelope glycoproteins with lower CD4 dependence and reduced sensitivity to a fusion inhibitor. Virology. 2006;346:169–179. doi: 10.1016/j.virol.2005.10.031. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Hoover DR, Bacellar H, Miller EN, Cohen BA, Becker JT, Graham NM, McArthur JH, Selnes OA, Jacobson LP, et al. Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS Cohort Study. Neurology. 1993;43:2245–2252. doi: 10.1212/wnl.43.11.2245. [DOI] [PubMed] [Google Scholar]
- Miller RJ, Meucci O. AIDS and the brain: is there a chemokine connection? Trends Neurosci. 1999;22:471–479. doi: 10.1016/s0166-2236(99)01408-3. [DOI] [PubMed] [Google Scholar]
- Minagar A, Shapshak P, Fujimura R, Ownby R, Heyes M, Eisdorfer C. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J Neurol Sci. 2002;202:13–23. doi: 10.1016/s0022-510x(02)00207-1. [DOI] [PubMed] [Google Scholar]
- Momma Y, Nagineni CN, Chin MS, Srinivasan K, Detrick B, Hooks JJ. Differential expression of chemokines by human retinal pigment epithelial cells infected with cytomegalovirus. Invest Ophthalmol Vis Sci. 2003;44:2026–2033. doi: 10.1167/iovs.02-0980. [DOI] [PubMed] [Google Scholar]
- Nath A. Pathobiology of human immunodeficiency virus dementia. Semin Neurol. 1999;19:113–127. doi: 10.1055/s-2008-1040830. [DOI] [PubMed] [Google Scholar]
- Navia BA, Jordan BD, Price RW. The AIDS dementia complex: I. Clin features Ann Neurol. 1986;19:517–524. doi: 10.1002/ana.410190602. [DOI] [PubMed] [Google Scholar]
- Nottet HS. Interactions between macrophages and brain microvascular endothelial cells: role in pathogenesis of HIV-1 infection and blood–brain barrier function. J Neurovirol. 1999;5:659–669. doi: 10.3109/13550289909021294. [DOI] [PubMed] [Google Scholar]
- Ong JM, Aoki AM, Seigel GM, Sacerio I, Castellon R, Nesburn AB, Kenney MC. Oxysterol-induced toxicity in R28 and ARPE-19 cells. Neurochem Res. 2003;28:883–891. doi: 10.1023/a:1023223409798. [DOI] [PubMed] [Google Scholar]
- Poluektova L, Moran T, Zelivyanskaya M, Swindells S, Gendelman HE, Persidsky Y. The regulation of alpha chemokines during HIV-1 infection and leukocyte activation: relevance for HIV-1-associated dementia. J Neuroimmunol. 2001;120:112–128. doi: 10.1016/s0165-5728(01)00413-1. [DOI] [PubMed] [Google Scholar]
- Power C, Gill MJ, Johnson RT. Progress in clinical neurosciences: the neuropathogenesis of HIV infection: host–virus interaction and the impact of therapy. Can J Neurol Sci. 2002;29:19–32. doi: 10.1017/s0317167100001682. [DOI] [PubMed] [Google Scholar]
- Prakash O, Teng S, Ali M, Zhu X, Coleman R, Dabdoub RA, Chambers R, Aw TY, Flores SC, Joshi BH. The human immunodeficiency virus type 1 Tat protein potentiates zidovudine-induced cellular toxicity in transgenic mice. Arch Biochem Biophys. 1997;343:173–180. doi: 10.1006/abbi.1997.0168. [DOI] [PubMed] [Google Scholar]
- Proudfoot AE. Chemokine receptors: multifaceted therapeutic targets. Nat Rev Immunol. 2002;2:106–15. doi: 10.1038/nri722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragaiey T, Ma JX, Jiang WJ, Greene W, Seigel GM, Stewart WC. L-deprenyl protects injured retinal precursor cells in vitro. J Ocul Pharmacol Ther. 1997;13:479–88. doi: 10.1089/jop.1997.13.479. [DOI] [PubMed] [Google Scholar]
- Rios LS, Vallochi AL, Muccioli C, Campos-Machado MA, Belfort R, Rizzo LV. Cytokine profile in response to Cytomegalovirus associated with immune recovery syndrome after highly active antiretroviral therapy. Can J Ophthalmol. 2005;40:711–720. doi: 10.1016/S0008-4182(05)80087-0. [DOI] [PubMed] [Google Scholar]
- Saha RN, Pahan K. Tumor necrosis factor-alpha at the crossroads of neuronal life and death during HIV-associated dementia. J Neurochem. 2003;86:1057–1071. doi: 10.1046/j.1471-4159.2003.01942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders VJ, Pittman CA, White MG, Wang G, Wiley CA, Achim CL. Chemokines and receptors in HIV encephalitis. AIDS. 1998;12:1021–1026. [PubMed] [Google Scholar]
- Sanyal S, Zeilmaker GH. Cell lineage in retinal development of mice studied in experimental chimaeras. Nature. 1977;265:731–733. doi: 10.1038/265731a0. [DOI] [PubMed] [Google Scholar]
- Sasseville VG, Smith MM, Mackay CR, Pauley DR, Mansfield KG, Ringler DJ, Lackner AA. Chemokine expression in simian immunodeficiency virus-induced AIDS encephalitis. Am J Pathol. 1996;149:1459–1467. [PMC free article] [PubMed] [Google Scholar]
- Schrier RD, Song MK, Smith IL, Karavellas MP, Bartsch DU, Torriani FJ, Garcia CR, Freeman WR. Intraocular viral and immune pathogenesis of immune recovery uveitis in patients with healed cytomegalovirus retinitis. Retina. 2006;26:165–169. doi: 10.1097/00006982-200602000-00007. [DOI] [PubMed] [Google Scholar]
- Seigel GM. Establishment of an E1A-immortalized retinal cell culture. In Vitro Cell Dev Biol Anim. 1996;32:66–68. doi: 10.1007/BF02723034. [DOI] [PubMed] [Google Scholar]
- Seigel GM, Liu L. Inducible apoptosis-promoting activity in retinal cell-conditioned medium. Mol Vis. 1997;3:14. [PubMed] [Google Scholar]
- Seigel GM, Chiu L, Paxhia A. Inhibition of neuroretinal cell death by insulin-like growth factor-1 and its analogs. Mol Vis. 2000;6:157. [PubMed] [Google Scholar]
- Seigel GM, Mutchler AL, Imperato EL. Expression of glial markers in a retinal precursor cell line. Mol Vis. 1996;2:2. [PubMed] [Google Scholar]
- Seigel GM, Mutchler AL, Adamus G, Imperato-Kalmar EL. Recoverin expression in the R28 retinal precursor cell line. In Vitro Cell Dev Biol Anim. 1997;33:499–502. doi: 10.1007/s11626-997-0091-5. [DOI] [PubMed] [Google Scholar]
- Seigel GM, Sun W, Wang J, Hershberger DH, Campbell LM, Salvi RJ. Neuronal gene expression and function in the growth-stimulated R28 retinal precursor cell line. Curr Eye Res. 2004;28:257–269. doi: 10.1076/ceyr.28.4.257.27831. [DOI] [PubMed] [Google Scholar]
- Shapshak P, Duncan R, Minagar A, Rodriguez de la Vega P, Stewart RV, Goodkin K. Elevated expression of IFN-gamma in the HIV-1 infected brain. Front Biosci. 2004;9:1073–1081. doi: 10.2741/1271. [DOI] [PubMed] [Google Scholar]
- Steinkamp JA, Lehnert BE, Lehnert NM. Discrimination of damaged/dead cells by propidium iodide uptake in immunofluorescently labeled populations analyzed by phase-sensitive flow cytometry. J Immunol Meth. 1999;226:59–70. doi: 10.1016/s0022-1759(99)00053-8. [DOI] [PubMed] [Google Scholar]
- Sui Y, Potula R, Dhillon N, Pinson D, Li S, Nath A, Anderson C, Turchan J, Kolson D, Narayan O, Buch S. Neuronal apoptosis is mediated by CXCL10 overexpression in simian human immunodeficiency virus encephalitis. Am J Pathol. 2004;164:1557–1566. doi: 10.1016/S0002-9440(10)63714-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui Y, Stehno-Bittel L, Li S, Loganathan R, Dhillon NK, Pinson D, Nath A, Kolson D, Narayan O, Buch S. CXCL10-induced cell death in neurons: role of calcium dysregulation. Eur J Neurosci. 2006;23:957–964. doi: 10.1111/j.1460-9568.2006.04631.x. [DOI] [PubMed] [Google Scholar]
- Sun W, Seigel GM, Salvi RJ. Retinal precursor cells express functional ionotropic glutamate and GABA receptors. NeuroReport. 2002;13:2421–4. doi: 10.1097/00001756-200212200-00009. [DOI] [PubMed] [Google Scholar]
- van Marle G, Henry S, Todoruk T, Sullivan A, Silva C, Rourke SB, Holden J, McArthur JC, Gill MJ, Power C. Human immunodeficiency virus type 1 Nef protein mediates neural cell death: a neurotoxic role for IP-10. Virology. 2004;329:302–318. doi: 10.1016/j.virol.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Venkatesh KK, Biswas J, Kumarasamy N. Impact of highly active antiretroviral therapy on ophthalmic manifestations in human immunodeficiency virus/acquired immune deficiency syndrome. Indian J Ophthalmol. 2008;56:391–3. doi: 10.4103/0301-4738.42415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesselingh SL, Takahashi K, Glass JD, McArthur JC, Griffin JW, Griffin DE. Cellular localization of tumor necrosis factor mRNA in neurological tissue from HIV-infected patients by combined reverse transcriptase/polymerase chain reaction in situ hybridization and immunohistochemistry. J Neuroimmunol. 1997;74:1–8. doi: 10.1016/s0165-5728(96)00160-9. [DOI] [PubMed] [Google Scholar]
- Westmoreland SV, Rottman JB, Williams KC, Lackner AA, Sasseville VG. Chemokine receptor expression on resident and inflammatory cells in the brain of macaques with simian immunodeficiency virus encephalitis. Am J Pathol. 1998;152:659–665. [PMC free article] [PubMed] [Google Scholar]
- Wetzel MA, Steele AD, Henderson EE, Rogers TJ. The effect of X4 and R5 HIV-1 on C, C–C, and C–X–C chemokines during the early stages of infection in human PBMCs. Virology. 2002;292:6–15. doi: 10.1006/viro.2001.1249. [DOI] [PubMed] [Google Scholar]
- Woodman SE, Benveniste EN, Nath A, Berman JW. Human immunodeficiency virus type 1 TAT protein induces adhesion molecule expression in astrocytes. J Neurovirol. 1999;5:678–684. doi: 10.3109/13550289909021296. [DOI] [PubMed] [Google Scholar]