Abstract
Background
Critically ill patients require transfusions because of acute blood loss and the anemia of critical illness. In critically ill burn patients, typically, no distinction is made between transfusions related to acute surgical blood loss and those related to the anemia of critical illness. We sought to identify the percentage of blood transfusions due to the anemia of critical illness and the clinical characteristics associated with these transfusions in severely burned patients.
Methods
Sixty adult patients with ≥20% total body surface area (TBSA) burn who were transfused at least 1 unit of packed red blood cells during their hospitalization were studied. Clinical variables including age, %TBSA burn, Acute Physiology and Chronic Health Evaluation (APACHE) II score, number of ventilator days, inhalation injury, and number of operative events were correlated with the total number of packed red blood cell units and percentage of nonsurgical transfusions in these patients. Nonsurgical transfusions were defined as transfusions occurring after postoperative day 1 for each distinct operative event and were classified as being caused by the anemia of critical illness.
Results
Patients were transfused an average of 16.6 units ± 21.2 units. Nonsurgical transfusions accounted for 52% of these transfusions. APACHE II score, %TBSA burn, number of ventilator days, and number of operative events, all correlated with total transfusions. However, nonsurgical transfusions correlated with only APACHE II score (p = 0.01) and number of ventilator days (p = 0.03). There was no correlation between nonsurgical transfusions and other clinical variables.
Conclusion
The anemia of critical illness is responsible for >50% of all transfusions in severely burned patients. The initial severity of critical illness (APACHE II score) and duration of the critical illness (number of ventilator days) correlated with transfusions related to anemia of critical illness. Further investigation into the specific risk factors for these transfusions may help to develop strategies to further reduce transfusion rates.
Keywords: Anemia, Critical illness, Red blood cell transfusion, Burns
The anemia of critical illness afflicts medical and surgical intensive care patients both during their hospitalization1 and for months later.2 The anemia of critical illness is caused by a combination of persistent blood losses and decreased erythropoiesis.3 Blood losses occur with dressing changes, minor procedures, and phlebotomy, while decreased erythropoiesis stems from decreased erythropoietin response and production, bone marrow dysfunction,4-6 and poor nutrition.7 Despite attempts to characterize the causes and burden of the anemia of critical illness,3,8,9 this anemia has not been quantified in a strictly surgical population.
Measuring transfusions related to the anemia of critical illness is a challenging task as the exact onset of this anemia is difficult to predict and define. Previous work has suggested that this anemia occurs after 7 days8 or 21 days9 in medical and surgical intensive care units. Of all the surgical critical care patients, the injury, operative, and prolonged recovery periods of burn patients make them ideal candidates to study the anemia of critical illness. Burn patients suffer possibly the greatest initial hemodynamic and metabolic stress of all critically ill patients, and the duration of their critical illness is often weeks to months. Severely burned patients undergo multiple operative events, at times, over several weeks. As a result, they suffer both the acute blood loss caused by the initial injury and subsequent operations and the anemia of critical illness from prolonged hemodynamic and metabolic derangements both of which require transfusions as a corrective measure.
Because multiple blood transfusions are associated with considerable risk including immune suppression and increased risk of nosocomial infections, measures to reduce transfusions will potentially benefit patient care. Since conditions that necessitate transfusions related to the anemia of critical illness in burn patients are not well defined, we sought to determine the percentage of blood transfused due to the anemia of critical illness in severely burned patients and to identify the clinical factors associated with the anemia of critical illness. This study design proposes a new model to categorize and to quantify transfusions in severely burned patients that will enable strategies to reduce anemia and transfusions and may be applicable to other patient populations.
PATIENTS AND METHODS
Data Collection and Patient Selection
This study is a retrospective review of a prospectively collected database. Secondary use of the Inflammation and Host Response to Injury database, a multicenter study which includes epidemiologic and clinical data from several burn centers, was performed. Burn patients from Loyola University Medical Center (LUMC) were selected from the larger database. Patients were only selected from LUMC to ensure a uniform transfusion threshold and to limit operative variability as previous work has shown considerable differences in transfusion triggers among burn centers.10 Consent for epidemiologic data retrieval was obtained during the initial prospective collection of these data. Patient data were queried from those with data collected between March 2003 and March 2008. This study was approved by the Institutional Review Board of LUMC.
Although exact hemoglobin concentrations at the time of transfusion are not supplied, our burn unit maintains a strict policy of transfusion for a hemoglobin concentration of 7 g/dL. In addition, no significant changes in our protocol for time to burn wound excision and grafting or operative technique were implemented during this time period. All burn wound excision and skin grafting were performed by one surgeon. The decision to transfuse packed red blood cells (pRBCs) in the operating room was made by the operating burn surgeon using the same thresholds. No transfusions were given immediately before excision and grafting in anticipation of surgical blood loss.
Clinical and epidemiologic data from 71 patients were collected of which 60 patients met the inclusion criteria (Fig. 1). Inclusion criteria included adults (aged >18 years), burn size ≥20% total body surface area (TBSA), survival of at least 10 days postburn, and at least transfusion of one unit of pRBCs. Exclusion criteria included blunt or penetrating trauma and excessive blood loss from anticoagulant or platelet inhibitor use. Of these 71 patients, 2 were excluded from our analysis because of incomplete data. Eight patients were not transfused. Four of these patients died early in the hospital course (postburn days [PBDs] 5–8) and the remaining four, although requiring excision and grafting, were not transfused (TBSA, 20–24%). One transfused patient was excluded because of extensive blood loss from the wound bed secondary to clopidogrel use. There were no cases of significant gastrointestinal bleeding.
Figure 1.
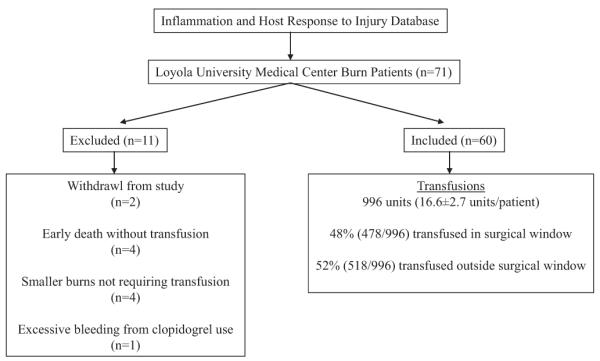
Study design. Excluded and included patients and transfusion data of included patients are shown.
Classification of Time of Transfusion
Most studies on transfusion focus solely on total transfusions and do not differentiate between transfusions related to acute surgical blood loss and the anemia of critical illness. For this study, transfusions related to acute surgical blood loss anemia were defined as those transfusions given in the operating room, on the remainder of the operative day, and on postoperative day 1. This period is considered as the surgical window. The surgical window allows for ample time postoperatively for the correction of acute surgical blood loss. The surgical window is unique to this study and has not been previously validated. Transfusions given outside this surgical window are considered nonsurgical and are considered to be related to the anemia of critical illness. Transfusions given before the first operative event, although rare, were attributed to the acute blood loss anemia. In addition, the number of transfusions given after the last surgical window was collected to illustrate the length of time after the last surgical window for which transfusions are still necessary.
Collection of Clinical Variables
Clinical data collected included age, %TBSA burn, Acute Physiology and Chronic Health Evaluation (APACHE) II score within 24 hours of admission, number of ventilator days, presence or absence of inhalation injury, number of operative events, additional clinical factors that may contribute to blood loss, survival, cause of death, and PBD of death. Inhalation injury was diagnosed via bronchoscopy and was not graded for these purposes.
Statistical Analysis
Linear correlation through the Pearson correlation coefficient was used to compare clinical and transfusion variables. To gauge the range of values for which transfusion data may change, these clinical variables were divided into groups of approximately equal thirds. This classification scheme is listed in Table 1. Data are reported as mean ± standard deviation, and significance was attributed to p < 0.05. Statistical analysis was performed using SAS Software (SAS Institute, Cary, NC).
TABLE 1.
Classification of Clinical Variables Into Approximately Equal Thirds
| Clinical Variable | Classification |
|---|---|
| %TBSA burn | Low: 20–29 Medium: 30–44 High: >45 |
| APACHE II score | Mild: 5–17 Moderate: 18–27 Severe: 28–41 |
| No. ventilator days | Low: 0–9 Medium: 10–39 High: >40 |
| Inhalation injury | No: absent Yes: present |
| No. operative events | Low: 0–1 Medium: 2–3 High:>4 |
RESULTS
Patient Data, Clinical Variables, and Infection Profile
For the 60 patients included in this analysis, the average age was 45 ± 16 years. Twenty-two patients were women and 38 were men. Eighty-two percent (49 of 60) of the patients survived. The mean %TBSA burn was 39.8% ± 16.1%. The mean APACHE II score was 21.8 ± 9.4. Forty-eight percent (29 of 60) of the patients had an inhalation injury diagnosed by bronchoscopy. Eighty percent (48 of 60) of patients were intubated at some point during their hospitalization. For patients who were intubated, the average number of ventilator days was 35.3 days ± 37.7 days. The average length of hospitalization was 55.8 days ± 46.7 days. Patients went to the operating room for excision and grafting 3.0 ± 3.0 times during their hospitalization.
Transfusion Profile
A total of 996 units of pRBCs were transfused to these 60 patients. The mean number of units of pRBCs transfused per patient was 16.6 units ± 21.2 units with a range of 1 unit to 135 units. Forty-eight percent (478 of 996) of all units of pRBCs were transfused within the surgical window and 52% (518 of 996) outside the surgical window. Thirty-eight percent (383 of 996) of all units were transfused during the operative event. Approximately 10% of all transfusions occurred on postoperative day 1. By expanding the time attributed to surgical blood loss from the operating room time period to the surgical window ensures that these additional 10% of transfusions are assigned to the acute surgical blood loss anemia rather than the anemia of critical illness.
On average, the last transfusion was administered on PBD 44.0 ± 43.1. Seventeen percent of all units were transfused after the last surgical window. The average number of days after the last surgical window that a patient was transfused was 18.5 days ± 9.6 days. Sixty-one percent (30 of 49) of patients who survived to discharge were transfused after the last surgical window. For those patients who survived to discharge, 15% ± 21.2% of all blood was transfused after the last surgical window.
Clinical Variables
The relationship between the five parametric clinical variables examined (age, %TBSA burn, APACHE II score, number of ventilator days, and number of operative events) was measured using linear correlation (Table 2). As expected, the %TBSA burn correlated closely with number of operative events. Interestingly, the extent of the thermal injury (%TBSA burn) did not correlate with APACHE II score. The number of ventilator days correlated with %TBSA burn, APACHE II score, and number of operative events. Age did not correlate with any other variable.
TABLE 2.
Relationship Between Clinical Variables Using Linear Correlation With the Pearson Correlation Coefficient
| Age | %TBSA Burn | APACHE II | No. Ventilator Days | |
|---|---|---|---|---|
| Age | ||||
| %TBSA burn | 0.05; −0.26 (0.67) | |||
| APACHE II | 0.06; 0.25 (0.06) | 0.25; 0.15 (0.02) | ||
| No. ventilator days | 0.83; 0.03 (0.001) | <0.001*; 0.59 (0.35) | 0.0001*; 0.48 (0.23) | |
| No. OR events | 0.77; 0.04 (0.001) | 0.0009*; 0.42 (0.18) | 0.06; 0.24 (0.06) | 0.0009*; 0.42 (0.17) |
p < 0.05.
Data are supplied as p value; Pearson correlation coefficients and (r2) values
Clinical Variables and Transfusions
All clinical variables (%TBSA burn, APACHE II score, number of ventilator days, and number of operative events) except age and inhalation injury were significantly correlated to total transfusions. However, the percentage of nonsurgical transfusions were only correlated to APACHE II score (p = 0.01) and number of ventilator days (p = 0.03; Table 3).
TABLE 3.
Linear Correlation Between Total Transfusions and Percentage of Nonsurgical Transfusions and Clinical Variables
| Total Transfusions | Percentage of Nonsurgical Transfusions |
|
|---|---|---|
| Age | 0.24;−0.15 (0.02) | 0.08; 0.23 (0.05) |
| %TBSA burn | <0.0001*; 0.58 (0.33) | 0.75; 0.04 (0.002) |
| APACHE II score | 0.008*; 0.34 (0.12) | 0.01*; 0.32 (0.10) |
| No. ventilator days | <0.0001*; 0.81 (0.65) | 0.03*; 0.28 (0.08) |
| Inhalation injury | 0.49; −0.13 (0.02) | 0.36;−0.17 (0.03) |
| No. operative events | <0.0001*; 0.64 (0.41) | 0.44; 0.10 (0.01) |
p < 0.05.
Data are supplied as p value; Pearson correlation coefficients and (r2) values.
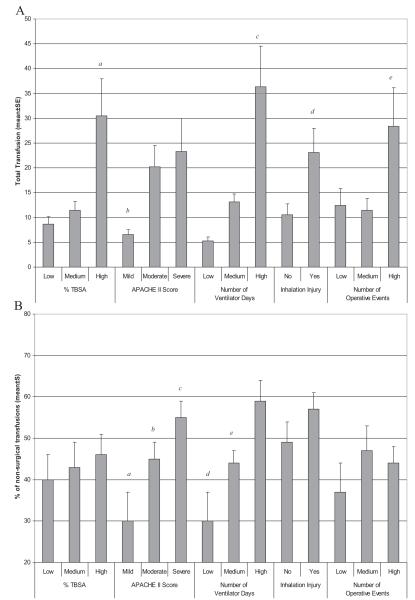
The relationship between transfusions and the different measures of these clinical variables are summarized in Figure 2, A and B. In general, these comparisons match the linear correlation data mentioned above. The total units transfused significantly increases with increasing %TBSA burn, number of ventilator days, and presence of an inhalation injury. For patients with mild APACHE II scores, the total transfusions were significantly less than for patients with moderate or severe scores. As expected, total transfusions also increased with number of operative events.
Figure 2.
Correlation between transfusion and clinical variables (A) Total transfusions administered to patients based on clinical variables. p<0.05 for a=% TBSA high v. low and high v. medium, b=APACHE II score mild v. moderate, and mild v. severe, c=number of ventilator days low v. medium, low v. high and medium v. high, d=inhalation injury no v. yes, e=number of operative events low v. high and medium v. high. (B) Percentage of non-surgical transfusions administered to patients based on clinical variables. p<0.05 for a= APACHE II score mild v. moderate; b=mild v. severe, c= moderate v. severe, d=number of ventilator days low v. high, e=medium v. high.
The percentage of nonsurgical transfusions was significantly increased with higher APACHE II scores and number of ventilator days. Although there was a trend, the percentage of nonsurgical transfusions did not significantly differ based on %TBSA burn, inhalation injury, or number of operative events.
DISCUSSION
In this study, the surgical window of operative day plus 1 postoperative day delineated transfusions related to the acute surgical blood loss anemia and the anemia of critical illness. By using this classification scheme, 52% of all transfusions are related to the anemia of critical illness, and these transfusions correlated with APACHE II score and number of ventilator days. However, total transfusions are associated with %TBSA burn, APACHE II score, number of ventilator days, and number of operative events. Taken together, total transfusions are associated with the cutaneous injury and the critical illness, whereas transfusions specifically related to the anemia of critical illness are associated with the initial severity of injury (APACHE II score) and duration of the critical illness (number of ventilator days).
Our use of the surgical window of operative day plus one postoperative day as the basis for delineating transfusions attributable to acute surgical blood loss versus anemia of critical illness is subjective. Obviously, transfusions on the operative day are because of acute surgical blood loss. The addition of one postoperative day should allow enough time for equilibration of hemoglobin concentration from surgical blood loss and after the respective transfusions. In fact, the initial hemoglobin concentration increase after transfusion should persist for up to 24 hours if there is no additional blood loss.11,12 Some overlap between transfusions associated with each anemia surely exists, especially given the multiple operative events that these patients undergo. Despite this, our classification scheme brings to light the high percentage of total transfusions that are not temporally related to surgical blood loss. In fact, the correlation between clinical variables and type of transfusion supports this classification as transfusions related to the anemia of critical illness correlated with indicators of critical illness, whereas the remaining transfusions correlated with the degree of the cutaneous injury and subsequent surgical care.
Transfusion rates associated with acute surgical blood loss anemia have decreased with the incorporation of surgical techniques including tourniquet placement13,14 and subdermal tumescence with vasoconstricting agents.15,16 Overall transfusion rates have also declined with stricter transfusion thresholds.17,18 Still, in our population, 16.6 units of pRBCs were transfused per patient. Because 52% of these transfusions are related to the anemia of critical illness, further study into the causes and possible prevention of this anemia is essential to reduce overall transfusions.
The anemia of critical illness is a complex, multifactorial phenomenon.19,20 Some data suggest that erythropoiesis in the bone marrow is dampened after a burn injury, leading to a decrease in overall erythrocyte production.4,5 Studies in the critical care literature indicate that blood loss from phlebotomy may contribute as well.21-23 For the burn population, blood is lost with dressing changes, although this has not yet been quantified. Taking into account these known contributors to the anemia of critical illness and those factors that still remain unknown easily demonstrates its multifactorial nature. In fact, the diverse mechanisms for the anemia of critical illness will probably make therapeutic interventions difficult and cumbersome. Our findings recognize the high rate of these transfusions providing an impetus for their study and provide a method of quantifying these transfusions allowing for measurement of interventions and prevention methods.
Our study is limited because of its retrospective nature, small sample size, and single-institution design. Given our findings, further prospective studies with a larger sample size that includes details of phlebotomy, fluid resuscitation volumes, blood loss because of dressing changes, and measures of erythropoiesis are needed to better delineate the causes of anemia of critical illness. Moreover, such studies are essential in our ability to find alternate methods to decrease the transfusion rate in these patients. As an advantage, one burn surgeon operating on all patients and dictating transfusion decisions ensured consistent clinical decision making, which would be otherwise eliminated with a larger, multicentered trial. In fact, despite modifications to transfusions thresholds over the past 20 years,17,24,25 resulting in the general acceptance of transfusing to maintain a hemoglobin of 7 g/dL, transfusion thresholds still differ between burn centers.10 Our strict thresholds and operative strategies eliminate the profound and unforeseen confounding effects of differing transfusion thresholds and operative techniques.
In conclusion, more than half of all transfusions are administered because of the anemia of critical illness in severely burned patients when using a surgical window of operative day plus one postoperative day to distinguish between transfusions related to acute surgical blood loss anemia and the anemia of critical illness. Given the association between transfusion of pRBCs and immune suppression and increased nosocomial infection risk, further work delineating the causes of the anemia of critical illness may help to reduce overall transfusions and possibly improve patient outcome.
ACKNOWLEDGMENTS
We thank Chindo Hicks, PhD, for his help with statistics. We acknowledge the contribution of the Inflammation and the Host Response to Injury Large-Scale Collaborative Project Award 2-U54-GM062119 from the National Institute of General Medical Sciences.
Supported by Dr. Ralph and Marian C. Falk Medical Research Trust grant and NIH T32 GM008750.
The Inflammation and the Host Response to Injury “Glue Grant” program is supported by the National Institute of General Medical Sciences. This article was prepared using a dataset obtained from the Glue Grant program and does not necessarily reflect the opinions or views of the Inflammation and the Host Response to Injury Investigators or the National Institute of General Medical Sciences.
REFERENCES
- 1.Walsh TS, Saleh EE, Lee RJ, McClelland DB. The prevalence and characteristics of anaemia at discharge home after intensive care. Intensive Care Med. 2006;32:1206–1213. doi: 10.1007/s00134-006-0213-7. [DOI] [PubMed] [Google Scholar]
- 2.Bateman AP, McArdle F, Walsh TS. Time course of anemia during six months follow up following intensive care discharge and factors associated with impaired recovery of erythropoiesis. Crit Care Med. 2009;37:1906–1912. doi: 10.1097/CCM.0b013e3181a000cf. [DOI] [PubMed] [Google Scholar]
- 3.Tinmouth AT, McIntyre LA, Fowler RA. Blood conservation strategies to reduce the need for red blood cell transfusion in critically ill patients. CMAJ. 2008;178:49–57. doi: 10.1503/cmaj.071298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallner SF, Vautrin R. The anemia of thermal injury: mechanism of inhibition of erythropoiesis. Proc Soc Exp Biol Med. 1986;181:144–150. doi: 10.3181/00379727-181-42236. [DOI] [PubMed] [Google Scholar]
- 5.Wallner SF, Warren GH. The haematopoietic response to burning: an autopsy study. Burns Incl Therm Inj. 1985;12:22–27. doi: 10.1016/0305-4179(85)90179-2. [DOI] [PubMed] [Google Scholar]
- 6.Wallner S, Vautrin R, Murphy J, Anderson S, Peterson V. The haematopoietic response to burning: studies in an animal model. Burns Incl Therm Inj. 1984;10:236–251. doi: 10.1016/0305-4179(84)90002-0. [DOI] [PubMed] [Google Scholar]
- 7.Pieracci FM, Henderson P, Rodney JR, et al. Randomized, double-blind, placebo-controlled trial of effects of enteral iron supplementation on anemia and risk of infection during surgical critical illness. Surg Infect (Larchmt) 2009;10:9–19. doi: 10.1089/sur.2008.043. [DOI] [PubMed] [Google Scholar]
- 8.Milbrandt EB, Clermont G, Martinez J, Kersten A, Rahim MT, Angus DC. Predicting late anemia in critical illness. Crit Care. 2006;10:R39. doi: 10.1186/cc4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chant C, Wilson G, Friedrich JO. Anemia, transfusion, and phlebotomy practices in critically ill patients with prolonged ICU length of stay: a cohort study. Crit Care. 2006;10:R140. doi: 10.1186/cc5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palmieri TL, Greenhalgh DG. Blood transfusion in burns: what do we do? J Burn Care Rehabil. 2004;25:71–75. doi: 10.1097/01.BCR.0000105094.25999.0D. [DOI] [PubMed] [Google Scholar]
- 11.Wiesen AR, Hospenthal DR, Byrd JC, Glass KL, Howard RS, Diehl LF. Equilibration of hemoglobin concentration after transfusion in medical inpatients not actively bleeding. Ann Intern Med. 1994;121:278–230. doi: 10.7326/0003-4819-121-4-199408150-00009. [DOI] [PubMed] [Google Scholar]
- 12.Elizalde JI, Clemente J, Marin JL, et al. Early changes in hemoglobin and hematocrit levels after packed red cell transfusion in patients with acute anemia. Transfusion. 1997;37:573–576. doi: 10.1046/j.1537-2995.1997.37697335150.x. [DOI] [PubMed] [Google Scholar]
- 13.O’Mara MS, Goel A, Recio P, et al. The use of tourniquets in the excision of unexsanguinated extremity burn wounds. Burns. 2002;28:684–687. doi: 10.1016/s0305-4179(02)00186-9. [DOI] [PubMed] [Google Scholar]
- 14.Smoot EC., III Modified use of extremity tourniquets for burn wound debridement. J Burn Care Rehabil. 1996;17:334–337. doi: 10.1097/00004630-199607000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Barret JP, Dziewulski P, Wolf SE, Desai MH, Nichols RJ, II, Herndon DN. Effect of topical and subcutaneous epinephrine in combination with topical thrombin in blood loss during immediate near-total burn wound excision in pediatric burned patients. Burns. 1999;25:509–513. doi: 10.1016/s0305-4179(99)00038-8. [DOI] [PubMed] [Google Scholar]
- 16.Sheridan RL, Szyfelbein SK. Staged high-dose epinephrine clysis is safe and effective in extensive tangential burn excisions in children. Burns. 1999;25:745–748. doi: 10.1016/s0305-4179(99)00088-1. [DOI] [PubMed] [Google Scholar]
- 17.Kwan P, Gomez M, Cartotto R. Safe and successful restriction of transfusion in burn patients. J Burn Care Res. 2006;27:826–834. doi: 10.1097/01.BCR.0000245494.45125.3E. [DOI] [PubMed] [Google Scholar]
- 18.Palmieri TL, Lee T, O’Mara MS, Greenhalgh DG. Effects of a restrictive blood transfusion policy on outcomes in children with burn injury. J Burn Care Res. 2007;28:65–70. doi: 10.1097/BCR.0B013E31802C895E. [DOI] [PubMed] [Google Scholar]
- 19.Posluszny JA, Jr, Gamelli RL. Anemia of thermal injury: combined acute blood loss anemia and anemia of critical illness. J Burn Care Res. 2010;31:229–242. doi: 10.1097/BCR.0b013e3181d0f618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corwin HL, Krantz SB. Anemia of the critically ill: “acute” anemia of chronic disease. Crit Care Med. 2000;28:3098–3099. doi: 10.1097/00003246-200008000-00079. [DOI] [PubMed] [Google Scholar]
- 21.Vincent JL, Baron JF, Reinhart K, et al. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288:1499–1507. doi: 10.1001/jama.288.12.1499. [DOI] [PubMed] [Google Scholar]
- 22.von Ahsen N, Muller C, Serke S, Frei U, Eckardt KU. Important role of nondiagnostic blood loss and blunted erythropoietic response in the anemia of medical intensive care patients. Crit Care Med. 1999;27:2630–2639. doi: 10.1097/00003246-199912000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Fowler RA, Berenson M. Blood conservation in the intensive care unit. Crit Care Med. 2003;31:S715–S720. doi: 10.1097/01.CCM.0000099350.50651.46. [DOI] [PubMed] [Google Scholar]
- 24.Sittig KM, Deitch EA. Blood transfusions: for the thermally injured or for the doctor? J Trauma. 1994;36:369–372. [PubMed] [Google Scholar]
- 25.Mann R, Heimbach DM, Engrav LH, Foy H. Changes in transfusion practices in burn patients. J Trauma. 1994;37:220–222. doi: 10.1097/00005373-199408000-00012. [DOI] [PubMed] [Google Scholar]