Abstract
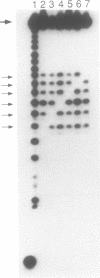
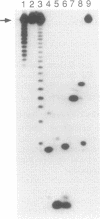
The synthesis of base protected 5'-O-dimethoxytrityl-2'-O-[1-(2- fluorophenyl)-4-methoxypiperidin-4-yl]-3'-O-(2-cyanoethyl N,N-diisopropylphosphoramidites) is described, using phenoxyacetyl protection for the exocyclic amino groups of guanosine and adenosine and acetyl protection of the amino group of cytidine. High yield assembly of these building blocks into oligoribonucleotides on aminopropyl controlled pore glass was achieved using 5-(4-nitrophenyl)-1H-tetrazole as activator. Mixed sequences containing selected 2'-O-methylation were also synthesised and their significance for the study of RNA biochemistry is discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cathala G., Brunel C. Use of n-butanol for efficient recovery of minute amounts of small RNA fragments and branched nucleotides from dilute solutions. Nucleic Acids Res. 1990 Jan 11;18(1):201–201. doi: 10.1093/nar/18.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S. H., Flynn P., Reid B. Solid-phase synthesis and high-resolution NMR studies of two synthetic double-helical RNA dodecamers: r(CGCGAAUUCGCG) and r(CGCGUAUACGCG). Biochemistry. 1989 Mar 21;28(6):2422–2435. doi: 10.1021/bi00432a013. [DOI] [PubMed] [Google Scholar]
- Farrance I. K., Eadie J. S., Ivarie R. Improved chemistry for oligodeoxyribonucleotide synthesis substantially improves restriction enzyme cleavage of a synthetic 35mer. Nucleic Acids Res. 1989 Feb 11;17(3):1231–1245. doi: 10.1093/nar/17.3.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai S., Ohtsuka E. 5'-Levulinyl and 2'-tetrahydrofuranyl protection for the synthesis of oligoribonucleotides by the phosphoramidite approach. Nucleic Acids Res. 1988 Oct 25;16(20):9443–9456. doi: 10.1093/nar/16.20.9443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai S., Yamada E., Asaka M., Hayase Y., Inoue H., Ohtsuka E. A new solid-phase synthesis of oligoribonucleotides by the phosphoro-p-anisidate method using tetrahydrofuranyl protection of 2'-hydroxyl groups. Nucleic Acids Res. 1987 May 11;15(9):3761–3772. doi: 10.1093/nar/15.9.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierzek R., Caruthers M. H., Longfellow C. E., Swinton D., Turner D. H., Freier S. M. Polymer-supported RNA synthesis and its application to test the nearest-neighbor model for duplex stability. Biochemistry. 1986 Dec 2;25(24):7840–7846. doi: 10.1021/bi00372a009. [DOI] [PubMed] [Google Scholar]
- Koizumi M., Hayase Y., Iwai S., Kamiya H., Inoue H., Ohtsuka E. Design of RNA enzymes distinguishing a single base mutation in RNA. Nucleic Acids Res. 1989 Sep 12;17(17):7059–7071. doi: 10.1093/nar/17.17.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann C., Xu Y. Z., Christodoulou C., Tan Z. K., Gait M. J. Solid-phase synthesis of oligoribonucleotides using 9-fluorenylmethoxycarbonyl (Fmoc) for 5'-hydroxyl protection. Nucleic Acids Res. 1989 Apr 11;17(7):2379–2390. doi: 10.1093/nar/17.7.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan J. F., Groebe D. R., Witherell G. W., Uhlenbeck O. C. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987 Nov 11;15(21):8783–8798. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka E., Tozuka Z., Iwai S., Ikehara M. A new condensing reagent, 1-(2,4,6-triisopropylbenzenesulfonyl)-5-(pyridin-2-yl)tetrazolide and its use in the synthesis of lambda cro binding heptadecanucleotide on a polymer support. Nucleic Acids Res. 1982 Oct 25;10(20):6235–6241. doi: 10.1093/nar/10.20.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese C. B., Skone P. A. Action of acid on oligoribonucleotide phosphotriester intermediates. Effect of released vicinal hydroxy functions. Nucleic Acids Res. 1985 Jul 25;13(14):5215–5231. doi: 10.1093/nar/13.14.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese C. B., Stewart J. C., van Boom J. H., de Leeuw H. P., Nagel J., de Rooy J. F. The synthesis of oligoribonucleotides. Part XI. Preparation of ribonucleoside 2'-acetal 3'-esters by selective deacylation. J Chem Soc Perkin 1. 1975;(10):934–942. doi: 10.1039/p19750000934. [DOI] [PubMed] [Google Scholar]
- Sakatsume O., Ohtsuki M., Takaku H., Reese C. B. Solid phase synthesis of oligoribonucleotides using the 1-[(2-chloro-4-methyl)phenyl]-4-methoxypiperidin-4-yl (Ctmp) group for the protection of the 2'-hydroxy functions and the H-phosphonate approach. Nucleic Acids Res. 1989 May 25;17(10):3689–3697. doi: 10.1093/nar/17.10.3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandström A., Kwiatkowski M., Chattopadhyaya J. Chemical synthesis of a pentaribonucleoside tetraphosphate constituting the 3'-acceptor stem sequence of E. coli tRNAIle using 2'-O-(3-methoxy-1,5-dicarbomethoxypentan-3-yl)-ribonucleoside building blocks. Application of a new achiral and acid-labile 2'-hydroxyl protecting group in tRNA synthesis. Acta Chem Scand B. 1985;39(4):273–290. doi: 10.3891/acta.chem.scand.39b-0273. [DOI] [PubMed] [Google Scholar]
- Schulhof J. C., Molko D., Teoule R. The final deprotection step in oligonucleotide synthesis is reduced to a mild and rapid ammonia treatment by using labile base-protecting groups. Nucleic Acids Res. 1987 Jan 26;15(2):397–416. doi: 10.1093/nar/15.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharmeen L., Taylor J. Enzymatic synthesis of RNA oligonucleotides. Nucleic Acids Res. 1987 Aug 25;15(16):6705–6711. doi: 10.1093/nar/15.16.6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha N. D., Biernat J., McManus J., Köster H. Polymer support oligonucleotide synthesis XVIII: use of beta-cyanoethyl-N,N-dialkylamino-/N-morpholino phosphoramidite of deoxynucleosides for the synthesis of DNA fragments simplifying deprotection and isolation of the final product. Nucleic Acids Res. 1984 Jun 11;12(11):4539–4557. doi: 10.1093/nar/12.11.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sproat B. S., Beijer B., Iribarren A. New synthetic routes to protected purine 2'-O-methylriboside-3'-O-phosphoramidites using a novel alkylation procedure. Nucleic Acids Res. 1990 Jan 11;18(1):41–49. doi: 10.1093/nar/18.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sproat B. S., Lamond A. I., Beijer B., Neuner P., Ryder U. Highly efficient chemical synthesis of 2'-O-methyloligoribonucleotides and tetrabiotinylated derivatives; novel probes that are resistant to degradation by RNA or DNA specific nucleases. Nucleic Acids Res. 1989 May 11;17(9):3373–3386. doi: 10.1093/nar/17.9.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Tamatsukuri S., Ikehara M. Solid phase synthesis of oligoribonucleotides using o-nitrobenzyl protection of 2'-hydroxyl via a phosphite triester approach. Nucleic Acids Res. 1986 Aug 11;14(15):6265–6279. doi: 10.1093/nar/14.15.6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Tamatsukuri S., Ikehara M. Solid phase synthesis of oligoribonucleotides using the o-nitrobenzyl group for 2'-hydroxyl protection and H-phosphonate chemistry. Nucleic Acids Res. 1987 Sep 25;15(18):7235–7248. doi: 10.1093/nar/15.18.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Ogilvie K. K., Pon R. T. Prevention of chain cleavage in the chemical synthesis of 2'-silylated oligoribonucleotides. Nucleic Acids Res. 1989 May 11;17(9):3501–3517. doi: 10.1093/nar/17.9.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]