Abstract
The function of the sigma-1 receptor (S1R) has been linked to modulating the activities of ion channels and G-protein coupled receptors (GPCR). In the CNS the S1R is expressed ubiquitously but is enriched in mouse motoneurons (MN), where it is localized to subsurface cisternae of cholinergic postsynaptic densities, also known as C-terminals. We found that S1R is enriched in mouse spinal MN at late stages of embryonic development when it is first visualized in the endoplasmic reticulum. S1Rs appear to concentrate at C-terminals of mouse MN only on the second week of postnatal development. We found that Indole-N-methyl transferase (INMT), an enzyme that converts tryptamine into the sigma-1 ligand dimethyltryptamine (DMT), is also localized to postsynaptic sites of C-terminals in close proximity to the S1R. This close association of INMT and SIRs suggest that DMT is synthesized locally to effectively activate S1R in MN.
Introduction
The Sigma-1 receptor (S1R), a 26 kDa protein, is expressed in many tissues. Initially it was classified as an opioid receptor because of its ability to bind dextromorphanes (Martin et al., 1976, Su, 1982), but it was later recognized as a unique pharmacological receptor (Quirion et al., 1992). The S1R binds to steroids with moderate affinity (Su et al., 1988) and to a wide variety of psychotomimetic compounds including cocaine (Sharkey et al., 1988), (+)-pentazocine (Su, 1982), methamphetamine (Nguyen et al., 2005), and dimethyltryptamine (DMT) (Fontanilla et al., 2009). DMT occurs endogenously (Barker et al., 1981) in a variety of tissues; it has been proposed to be a S1R endogenous ligand (Fontanilla et al., 2009) in addition to the steroids pregnenolone and progesterone (Su et al., 1988, Su et al., 2010) and the sphingolipids, sphingosine and sphinganine (Ramachandran et al., 2009).
Low levels of the S1R are found in all CNS regions, but it is most abundant in the MN of the brainstem and the spinal cord (Mavlyutov et al., 2010). At the subcellular level the S1R is localized in MN of cholinergic postsynaptic densities, also known as C-terminals. S1Rs are uniquely distributed in the subsurface cisternae, a few nanometers beneath the plasma membrane.
C-terminals are located in MN of the spinal cord and facial and hypoglossal nuclei of the brainstem (Houser et al., 1983, Connaughton et al., 1986). In rat MN, C-boutons (pre and postsynaptic elements) rapidly increase in size and number during postnatal development and the mean C-bouton size continues to increase slowly with age (Wetts and Vaughn, 2001). Ultrastructurally a large presynaptic bouton and the presence of postsynaptic cisternae serve as MN markers (Conradi, 1969). C-terminals were discovered 40 years ago (Conradi, 1969), but their function has only recently been revealed . C-terminals were found to increase the excitability of MN, particularly under stressful conditions such as swimming (Zagoraiou et al., 2009). C-terminals possess muscarinic type 2 acetylcholine receptor (M2AChR) (Hellstrom et al., 2003), Kv2.1 (Muennich and Fyffe, 2004), and SK potassium channels (Miles et al., 2007) located in the postsynaptic plasma membrane while the S1R receptor is localized in the subsurface cisternae (Mavlyutov et al., 2010). Activation of the M2AChR in MN inhibits certain potassium channels, likely the SK type, which might reduce the duration of afterhyperpolarization (Miles et al., 2007). Therefore it is conceivable that activation of M2AChR will result in a higher frequency of firing of MN.
In the present study we extend our primary work to examine the potential sources of endogenous ligands for S1R in MN. We found that the enzyme Indole N-methyl transferase (INMT) that produces the S1R ligand, DMT, is also localized to the postsynaptic C-terminals of MN, at sites that are in close juxtaposition to the S1R. Together these data support the hypothesis that locally produced DMT activates S1Rs that may regulate MN excitability.
Materials and methods
Animals used
Mice heterozygous for the S1R :Oprs1 mutant (+/−)
OprsGt(IRESBetageo)33Lex litters on a C57BL/6J×129s/SvEv mixed background were purchased from the Mutant Mouse Regional Resource Center, UC Davis, CA, USA. All mice were maintained on a normal 12-h light/dark cycle and handled in accordance with animal care and use guidelines of the University of Wisconsin, Madison. Procedures were optimized to minimize suffering and to reduce the number of animals used.
Source of drugs
Beuthanasia, Heparin were obtained from Midwest Veterinary Supply (Madison, WI). 1,3-Di(2-tolyl)guanidine (DTG) was from Research Biochemicals International (Natick, MA). (+)-Pentazocine was from Sigma-Aldrich. [125I]-Iodoazidophenpropimorph ([125I]-IAF) was prepared as previously described (Pal et al., 2007).
Immunocytochemistry
Mice were anesthetized with an intraperitoneal injection of beuthanasia and perfused through the left ventricle with phosphate buffered saline (PBS) containing heparin followed by fixative for 30 min. The data obtained for all the figures was performed by routine perfusion with 4% paraformaldehyde (PFA) and 0.1% glutaraldehyde (GA) except for the data in figures 3A, 3C and 4 where 0.1% GA was omitted. The spinal cords were then dissected and postfixed with the same fixative overnight. 4% PFA perfused Rhesus Macaque spinal cord tissue was purchased from the Primate Research Center, University of Wisconsin, Madison. The tissue was rinsed for 10 h in PBS, and cryoprotected in 30% sucrose in PBS overnight, all at 4°C. The data for most of the figures was obtained from 40 micron thick sections on the sliding microtome, except for the data in figure 3C which was obtained with 7-8 micron thick sections on a cryostat. The data for the movies were obtained from 60 micron thick slices on a vibratome (EMS Sciences). The following procedures were performed on free-floating slices: sections were blocked in 10% normal goat serum and primary antibodies (see supplementary table) were applied for 48 h. Secondary antibodies conjugated to fluorophore were applied for 4-12 hours.
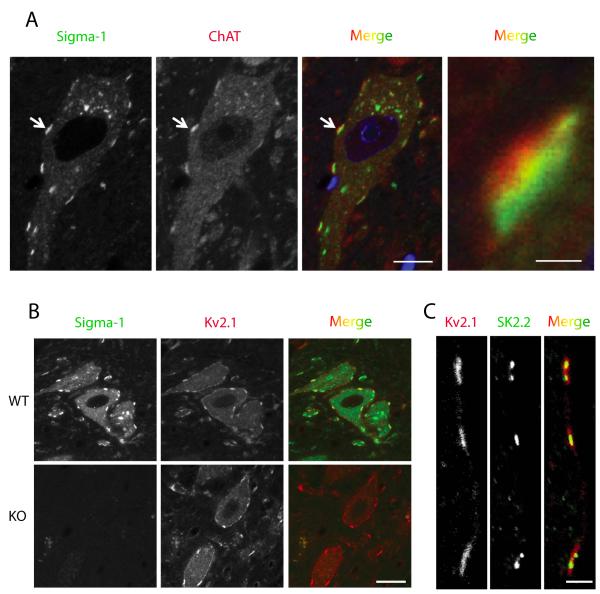
Figure 3.
Adult mouse MN. The Sigma-1 receptor is found in C-terminals where it colocalizes with potassium channels. A. Sigma-1 Receptor (green) in mouse MN is localized to cholinergic postsynaptic densities juxtaposed to presynaptic boutons positive for choline acetyltransferase labeling (ChAT, red). Scale=10μm (left). The image on the right is a magnified region of the synaptic contact indicated by the arrow in the images on the left. Note that the ChAT positive bouton is adjacent to the Sigma-1 receptor but remains separate. Scale=1μm (right). B. Sigma-1 receptor colocalizes with the Kv2.1channel. In Sigma-1 knockout mice (KO) the distribution of Kv2.1 channels did not appear to change compared to wildtype mice. Scale=20μm. C. In WT mouse MN potassium SK2.2 channels are present in C-terminals and colocalize with Kv2.1 potassium channels. Scale=3μm.
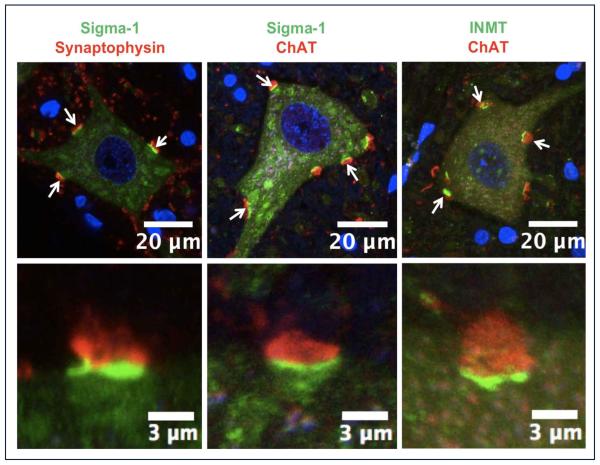
Figure 4.
The sigma-1 receptor (green signal in the left and middle panels) and the DMT producing enzyme INMT (green in the right panel) are localized to postsynaptic sites of C-terminals and juxtaposed to presynaptic cholinergic (ChAT positive) boutons (red). To demonstrate that the sigma-1 receptor is juxtaposed only to cholinergic postsynaptic densities of MNs we performed double labeling with antibodies against synaptophysin (a universal marker for different types of chemical synapses). Notice that not all synaptophysin-positive synapses are juxtaposed to the sigma-1 receptor. Blue (DAPI stain) indicates cell nuclei.
A mixture of anti-sigma-1 antibody (Ramachandran et al., 2007) at a concentration of 0.25 •g/ml and a variety of monoclonal antibodies at concentrations of 1–3 •g/ml were applied to the sections. Sections were rinsed and secondary antibodies of Alexa-488 conjugated goat-anti-rabbit and Alexa 568 conjugated goat-anti-mouse at a concentration of 2 •g/ml were applied overnight. Sections were rinsed, counterstained with DAPI and embedded into Prolong Gold mounting media (Invitrogen) and sealed with a coverslip. Images were taken with a Nikon A1R laser confocal microscope (Nikon, Tokyo, Japan) through a Apo60X VC oil-immersion objective with NIS elements software. A confocal system was equipped with a green 488 nm Argon laser and a red 561 nm DPSS laser. Acquired Z-stacks were analyzed with Image J software for a single plane selection. Single planes were saved as TIF files and final figures were constructed in Adobe Illustrator.
Photolabeling of spinal cord homogenates and ligand protection studies
Three of the 3 month old S1R WT mice were sacrificed by exposure to CO2 and the spinal cords were dissected from the spinal columns. A homogenate was prepared by combining the three spinal cords in 1-2 ml of PBS with a rotating Teflon pestle homogenizer at 30,000 rpm. The protein concentration was measured by the Bradford Biorad assay. For photolabeling, each sample contained 100μl of 5μg/μl protein. Where applicable (+)-pentazocine, 1,3-Di(2-tolyl)guanidine (DTG), tryptamine and DMT were preincubated with the homogenate for 30 min at room temperature (RT). Then carrier free [125I]-IAF (Pal et al., 2007) (approximately 1 nMolar) was added for 30 min and the membrane preparations further incubated in the dark at RT. After incubation, the samples were illuminated with an AH-6 mercury lamp for 7.5 sec and Laemmli buffer was added to each sample. Samples were separated by SDS PAGE, the gel was stained and dried, and exposed to a phosphorscreen (Molecular Dynamics) for 24 hours. The cassette was scanned on a Storm Phosphoimager (Molecular Dynamics) to produce the resulting autoradiogram.
Results
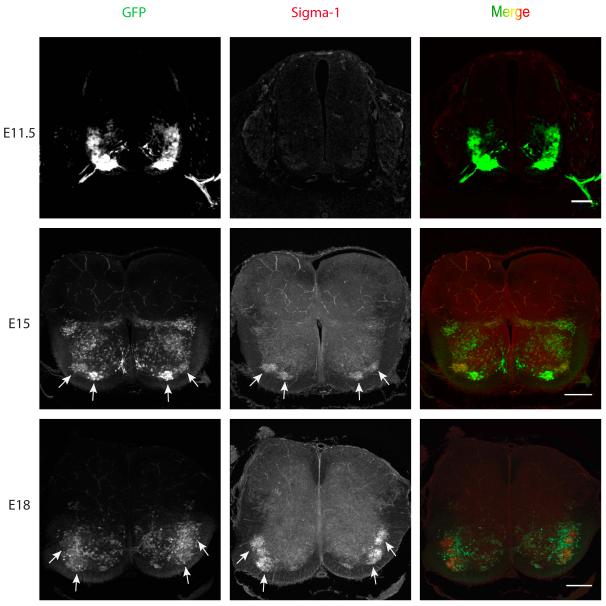
Previously we have reported that the S1R is abundant in mouse MN where it is localized in the subsurface cisternae that are closely apposed to the postsynaptic plasma membrane of the cholinergic postsynaptic densities, known as C-terminals (Mavlyutov et al., 2010) (also see supplemental movie 1). This region of the postsynaptic membrane is a specific marker found only in alpha MN and includes both the plasma membrane and subsurface cisternae (Conradi, 1969). The first goal of the current study was to determine at what stages of embryonic development does the S1R first appear in MN. We used the Hb9::GFP mouse line in which the transcription factor Hb9 was used to drive the expression of GFP in MN (Wichterle et al., 2002, Hinckley et al., 2005). However, it should be noted that not all somatic MNs express GFP (e.g., Fig. 2C in Hinckley et al., 2005). In this mouse line GFP-expressing glutamatergic interneurons are apparent in the ventral horn (Wilson et al., 2005). Precursors of MN are known to be present in the spinal cord at early developmental stages (Wichterle et al., 2002). At E11 S1Rs were not expressed in MNs, but they were visible at E15. S1R signal was more intense at E18 (Fig 1).
Figure 1.
Development of the Sigma-1 receptor in the embryonic mouse spinal cord. Sigma-1 receptor immunostaining is not visible in the mouse MN at embryonic stage E11.5. At E15 the Sigma-1 receptors concentrated in MNs in the lateral and medial motor columns in upper lumbar segments of the spinal cord. Note that not all MNs in the lateral column are GFP positive. The expression of S1Rs increased at E18 in the lateral motor column in the lower lumbar segments of the spinal cord. Not all MNs express GFP. Transgenic HB9-derived GFP mice were used for identification of MNs. Arrows point to clusters of ventral MNs. Scale bar = 100μm.
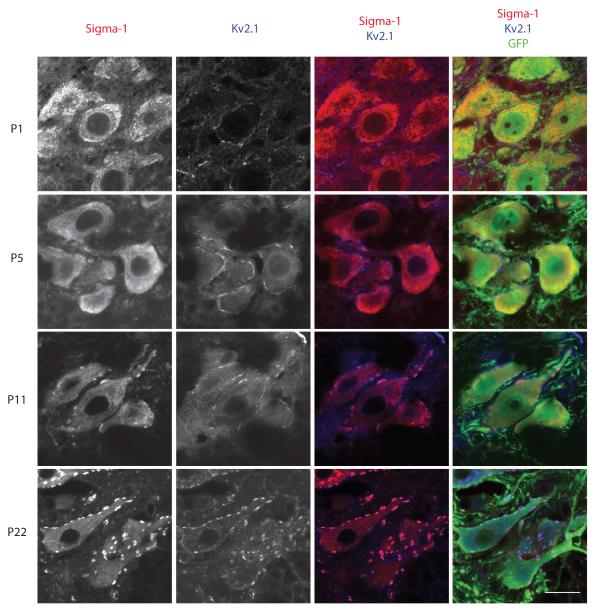
At early postnatal ages (P1, P5) the S1R was detected uniformly in the ER membranes without particular enrichment at the plasma membrane region. The S1R was first detected in the plasma membrane region, most likely in the C-terminal cisternae at P11, and assumed its mature form around P22. (Fig 2).
Figure 2.
Development of the Sigma-1 receptor in GFP-expressing MN in the spinal cord of the transgenic Hb9-GFP mouse. Immunohistochemical staining of the Sigma-1 receptor is difuse at early postnatal ages (P1, P5). However, an overlay of the staining of sigma-1 receptors and Kv2.1 that identifies the C-terminals demonstrated that the receptor is concentrated in C-terminals (purple) by postnatal day P11. Scale bar = 20μm.
Previously we had shown in adult mice that S1R immunostaining was detected predominately in the postsynaptic C-terminals, where it colocalized with the C-terminal markers Kv2.1 and M2AChR (Mavlyutov et al., 2010). In addition, the S1R labeling was juxtaposed to choline acetyltransferase (ChAT) positive presynaptic boutons (Fig. 3A). Although the S1R was absent from the S1R KO mice, the distribution of the Kv2.1 channel visually did not appear to be altered in these mice (Fig 3B). We also found that SK 2.2 channels were localized to C-terminals (Fig.3C) and their distribution visually also did not appear to change in the S1R KO mice (data not shown). Is the S1R always linked to cholinergic postsynaptic densities? To answer this question we examined their distribution on Renshaw cells, inhibitory neurons innervated by MN and have cholinergic synaptic boutons (Alvarez and Fyffe, 2007). In contrast to MN, S1R labeling was absent in the Renshaw cells (supplemental movie 2), indicating that the presence of cholinergic synaptic boutons does not require the S1R and strengthens the case that the S1R is predominately localized to C-terminals of MN. To determine whether the S1R is present in C-terminals of a species related to humans, we studied the localization of the C-terminals in the spinal cord of the rhesus macaque. As in mice, the S1Rs were distributed in the C-terminals of primate MN (Fig. 4).
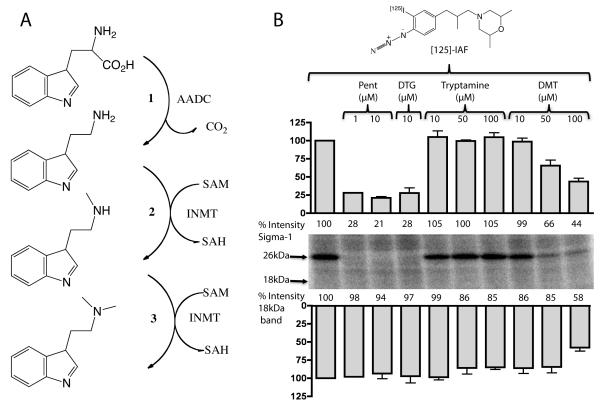
Recently we have proposed that N,N’-dimethyltryptamine (DMT) is an endogenous ligand for the S1R (Fontanilla et al., 2009). DMT is formed from tryptamine by the enzyme, indole-N-methyl-transferase (INMT) (Fig. 5A) (Jacob and Presti, 2005). Interestingly, in the spinal cord of rhesus macaque INMT is also localized on the postsynaptic sites of C-terminals (Fig. 4). Because this finding suggests that DMT could be produced in close juxtaposition to the S1R, we assessed the binding of DMT to the S1R in mouse spinal cord homogenates. In photolabeling experiments using the [125I]-IAF photoprobe on the whole spinal cord homogenates (Mavlyutov and Ruoho, 2007) S1R appeared as a 26kDa protein and the labeling by this photoprobe was blocked by preincubation with the synthetic S1R ligands (+)-pentazocine and DTG (Fig. 5B). In contrast, preincubation with tryptamine was not protective for S1R binding sites while 50μM DMT preincubation selectively protected the S1R. The 18 kDa region which is consistent with the position of the Sigma-2 receptor (S2R) was faintly labeled by [125I]-IAF in the spinal cord homogenates (Fig. 5B) but appeared to be much less prominent than the S1R band. Application of the sigma-2 ligand DTG did not reproducibly protect against [125I]-IAF photolabeling of the 18kDa region. Since the affinity of [125I]-IAF is greater for the S1R, and because the S1R is abundant in the spinal cord, the total levels of the S2R in the spinal cord cannot accurately be estimated by these photolabeling data. These data support the previous finding that DMT is an endogenous ligand for the S1R (Fontanilla et al., 2009) and that the site of DMT synthesis (by INMT) is in close proximity to the S1R.
Figure 5.
A. The enzymatic pathway for transformation of tryptophan into the S1R ligand, N,N’-dimethyltryptamine (DMT). 1. Aromatic Aminoacid Decarboxylase (AADC) forms tryptamine by removal of the carboxylic group from tryptophan. 2 and 3. Indole-N-Methyl transferase (INMT) adds methyl groups from S-Adenosyl-Methionine (SAM) to the nitrogen of tryptamine thus forming DMT. B. DMT binds to the S1R in spinal cord homogenates. The S1R photoprobe, [125I]-IAF revealed the S1R to be a prominent 26 kDa band which was selectively protected in photolabeling by 10μM (+)-pentazocine (P) and 10μM DTG (D). Protection of the S1R band is readily seen at 50μM DMT. The faint photolabeling in the 18kDa region by [125I]-IAF in the spinal cord homogenate was slightly protected by 50 and 100μM DMT. However protection by DTG of the 18 kDa region was not reproducibly seen. Thus the 18kDa region on the gel cannot be definitely assigned as the sigma-2 receptor. Pentazocine 1μM - n=1, 10μM - n=2; DTG 10μM – n=4; Tryptamine 10 and 50μM – n=4, 100μM – n=3; DMT 10, 50, 100μM – n=4.
Discussion
The role of S1R in MN is not clear even though a number of different scenarios have been proposed. The results presented provide a further characterization of the S1R in MN. We identified a putative source for an endogenous ligand for the S1R and determined the developmental stage when the S1R is expressed in its mature form. These results are consistent with a body of indirect evidence proposing that S1R affects motor function, probably through its action at the MN level (Luty et al., 2010, Mavlyutov et al., 2010, Al-Saif et al., 2011).
Significant changes occur in mouse MN during the first two postnatal weeks of development (Wilson et al., 2004). During that time the density of voltage-gated currents increase significantly in developing MN and greatly increase their excitability (Gao and Ziskind-Conhaim, 1998). At the same time pre- and postsynaptic components of C-terminals mature to acquire their adult localization pattern. Thus at postnatal day 2 (P2) Kv2.1 channel is found throughout the plasma membrane of MN and is only slightly enriched at the sites of developing C-terminals. Only at the end of the first week of postnatal development was Kv2.1 shown to form discrete punctae in postsynaptic sites of C-terminals (Wilson et al., 2004). In C-terminals the appearance of S1Rs is delayed compared to Kv2.1 and M2AChR (Fig 2). S1R translocates to C-terminals only after their maturation is complete. It is not clear why S1R appears in C-terminals only at P11 but it might be correlated with the ability of juvenile mice to start walking in an adult pattern.
The S1R is a major component of C-terminals although the role of S1R in C-terminals remains unknown. C-terminals appear to be important for increasing the firing rate of MN under stressful conditions (Miles et al., 2007, Zagoraiou et al., 2009). Additionally C-terminals are probably also involved in the operation of the central pattern generator that is essential for walking. Recent findings show that cholinergic neurons, located in lamina X, terminate synaptically as C-boutons on MN - 75% ipsilaterally and 25% contraterally (Stepien et al., 2010).
Presynaptically, C-boutons release acetylcholine (Hellstrom et al., 1999). However, a small number of large dense core vesicles (LDCV) are also present in C-boutons (Conradi, 1969). LDCV are known to contain catecholamines, monoamines or peptides. Thus, it is possible that an unknown neurotransmitter may also be co released from C-boutons that could be functionally important for overall activation of the S1R.
Our findings demonstrate that INMT is localized to postsynaptic C-terminals in close proximity to the S1R. INMT is known to produce DMT from tryptamine and DMT was shown previously to be an activator of the S1R (Fontanilla et al., 2009) with a low micromolar affinity. We propose two possible mechanisms whereby DMT is produced in close proximity to the S1R, thereby regulating the active state of the S1R. It is conceivable that endogenous tryptamine, is released either presynaptically or obtained from local stores and is converted into DMT inside the postsynaptic MN cell body. Another possibility is that tryptophan is decarboxylated locally by AADC to form tryptamine, which is further methylated by INMT to form DMT. A significant paradigm shift in endogenous neurotransmitter regulation of a receptor is offered by the colocalization of the sigma-1 receptor and INMT. In this case it is highly likely that endogenous DMT is locally produced rather than released presynaptically to regulate the S1R activity. The regulation of INMT enzymatic activity may also be another level of regulating sigma-1 receptor by altering the local levels of DMT.
Serotonin receptors that are abundant on the cell bodies and dendrites of MN (Alvarez et al., 1998) can also bind DMT with high affinity (Ray, 2004, Jacob and Presti, 2005). These receptors have not been shown to be present at C-terminals and therefore are unlikely to be targets for DMT activation. The postsynaptic machinery of C-terminals includes the M2AChR (Hellstrom et al., 2003), a GPCR, which acts to increase the frequency of MN firing in stressful conditions. This increase was shown to be mediated by inhibition of K+ channels, likely the SK type (Miles et al., 2007). Inhibition of this K+ channel results in larger depolarization and a decreased after hyperpolarization which together can increase MN firing frequency and generate stronger muscle contraction.
The S1R appears to influence motor behavior. Injection of the agonists DMT and SKF1047 has been shown to enhance locomotion in WT mice but not in S1R KO mice (Langa et al., 2003, Fontanilla et al., 2009). Tritiated S1R ligand binding as revealed by autoradiography in rat brain slices showed heavy labeling in the red nucleus, nucleus facialis, and the spinal cord ventral horn (Gundlach et al., 1986, Walker et al., 1990, Bouchard and Quirion, 1997). In rats, injection of S1R ligands into the red nucleus induced dystonia lasting for approximately one hour (Matsumoto et al., 1990). Furthemore some motor functions were observed to be altered in S1R KO mice compared to WT mice (Mavlyutov et al., 2010).
One of the possible mechanisms underlying S1R function in MN activity is that it regulates MN excitability. G-protein signaling with regard to the S1R deserves particular consideration because previous data has suggested the regulation of Gi/o signaling by S1R. Pretreatment of rat brain membranes by pertussis toxin, known to inhibit Gi/o pathways, significantly decreased binding of the agonist (+)-3-PPP to the S1R (Itzhak, 1989). In synaptoneurosomes the binding of the nonselective muscarinic agonist, oxotremorine, was attenuated by pretreatment with the specific sigma-1 agonist (+)-pentazocine (Walker et al., 1990). More recent data from Kim et al. showed that the antagonist, BD1047, when bound to the S1R increased the potency of activation of G proteins by agonists of opioid and muscarinic receptors, respectively (Kim et al., 2010). These data may indicate a possible close relationship between the S1R, the M2AChR and/or Gi/o proteins that are found in C-terminals. Thus, in the C-terminals of MN, a similar regulatory association may occur between an agonist activated S1R and the Gi/o linked M2AChR. In this scenario agonist activation of the S1R (perhaps by increased local DMT levels) leads to reduced sensitivity of the M2AChR to acetylcholine, inhibition of potassium channels (Miles et al., 2007) and reducing MN excitability, and firing frequency. It is also possible that a reduced Gi/o activation results in decreased PKA activity, a lower rate of phosphorylation of Kv2.1 and thus activation of the channel, and a larger afterhyperpolarization.
A third mechanism may involve the activity of both SK and Kv2.1 channels by calcium via calmodulin (Misonou et al., 2005), resulting in a larger amplitude of afterhyperpolarization, and thus a reduced firing frequency. SK channels are directly linked to calcium calmodulin binding (Schumacher et al., 2001). The Kv2.1 channel is activated by calcineurin that is in turn activated by calcium calmodulin (Park et al., 2006). With regards to calcium signaling it has been shown that the S1R associates with and stabilizes the type 3 IP3 receptor (Hayashi and Su, 2007). Therefore, the subsurface cisternae may act as calcium reservoirs in C-terminals (Rosenbluth, 1962). Additionally Kv2.1 channels have been shown to be spatially associated with calcium releasing ryanodine receptors that are localized in subsurface cisternae in hippocampal neurons (Antonucci et al., 2001). At present, however, we have not found a particular enrichment of either IP3 or ryanodine receptors in the membranes of subsurface cisternae in MN (data not shown). A final mechanism to consider is a direct protein/protein interaction between the S1R in the ER cisternae and the SK2 or Kv2.1 channels in the plasma membrane of C-terminals. It has been shown that the S1R regulates the activity of some voltage-gated ion channels presumably via their direct interaction (Aydar et al., 2002, Renaudo et al., 2007). The S1R in C-terminals is localized to subsurface cisternae located less than 10 nm from the plasma membrane (Mavlyutov et al., 2010). Thus, it is reasonable to propose that a direct protein/protein interaction occurs between the receptor and potassium channels allowing for S1R regulation. Examples of such an interaction have been observed in skeletal muscle cells between the dihydropyridine receptor located in the plasma membrane and the ryanodine receptor in the vicinity of the sarcoplasmic reticulum and in cardiomyocytes between CRAC channels and the STIM protein (Clapham, 2009). Together this chain of events would result in an increased potassium efflux, larger afterhyperpolarization, and a decreased firing frequency. In this manner the S1R might serve as a brake that controls MN excitability.
Future pharmacological and electrophysiological experiments are needed to understand the enigmatic nature of C-terminals and the precise molecular mechanisms that the S1R may play in its role as a possible brake that controls MN excitability.
Supplementary Material
In this study we examined development and subcellular localization of the sigma-1 receptor (S1R) in motoneurons (MN).
We found that S1R is enriched in mouse spinal MN at late stages of embryonic development when it is first visualized in the endoplasmic reticulum.
In second postnatal week S1Rs appear to concentrate at cholinergic postsynaptic densities (C-terminals) of MN.
Indole-N-methyl transferase (INMT), an enzyme that converts tryptamine into the sigma-1 ligand dimethyltryptamine (DMT), is also localized to postsynaptic sites of C-terminals.
This close association of INMT and SIRs suggest that DMT is synthesized locally to effectively activate S1R in MN.
ACKNOWLEDGEMENTS
We are grateful to Anna Kowalkowski for help with preparation of cryosections and for support from Dr. Phil Smith (Department of Anatomy, UW Medical School). We would like to thank Lance Rodenkirch and Michael Hendrickson (UW, W.M. Keck Laboratory for Biological Imaging, UW Medical School) for help with confocal microscopy. We are thankful to Dr. Uyen Chu for help with the preparation of [125I]-IAF. This work was supported by NIH grant RO1 MH065503 to AER, RO1 DK081634 to MLE, RO1 NS-23808 to LZC and an Edwin and Dorothy Gamewell Retina Research Foundation/UW Eye Research Institute Professorship to AER.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Saif A, Al-Mohanna F, Bohlega S. A mutation in sigma-1 receptor causes juvenile amyotrophic lateral sclerosis. Ann Neurol. 2011 doi: 10.1002/ana.22534. [DOI] [PubMed] [Google Scholar]
- Alvarez FJ, Fyffe RE. The continuing case for the Renshaw cell. J Physiol. 2007;584:31–45. doi: 10.1113/jphysiol.2007.136200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez FJ, Pearson JC, Harrington D, Dewey D, Torbeck L, Fyffe RE. Distribution of 5-hydroxytryptamine-immunoreactive boutons on alpha-motoneurons in the lumbar spinal cord of adult cats. J Comp Neurol. 1998;393:69–83. [PubMed] [Google Scholar]
- Antonucci DE, Lim ST, Vassanelli S, Trimmer JS. Dynamic localization and clustering of dendritic Kv2.1 voltage-dependent potassium channels in developing hippocampal neurons. Neuroscience. 2001;108:69–81. doi: 10.1016/s0306-4522(01)00476-6. [DOI] [PubMed] [Google Scholar]
- Aydar E, Palmer CP, Klyachko VA, Jackson MB. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron. 2002;34:399–410. doi: 10.1016/s0896-6273(02)00677-3. [DOI] [PubMed] [Google Scholar]
- Barker SA, Monti JA, Christian ST. N, N-dimethyltryptamine: an endogenous hallucinogen. Int Rev Neurobiol. 1981;22:83–110. doi: 10.1016/s0074-7742(08)60291-3. [DOI] [PubMed] [Google Scholar]
- Bouchard P, Quirion R. [3H]1,3-di(2-tolyl)guanidine and [3H](+)pentazocine binding sites in the rat brain: autoradiographic visualization of the putative sigma1 and sigma2 receptor subtypes. Neuroscience. 1997;76:467–477. doi: 10.1016/s0306-4522(96)00221-7. [DOI] [PubMed] [Google Scholar]
- Clapham DE. A STIMulus Package puts orai calcium channels to work. Cell. 2009;136:814–816. doi: 10.1016/j.cell.2009.02.022. [DOI] [PubMed] [Google Scholar]
- Connaughton M, Priestley JV, Sofroniew MV, Eckenstein F, Cuello AC. Inputs to motoneurones in the hypoglossal nucleus of the rat: light and electron microscopic immunocytochemistry for choline acetyltransferase, substance P and enkephalins using monoclonal antibodies. Neuroscience. 1986;17:205–224. doi: 10.1016/0306-4522(86)90237-x. [DOI] [PubMed] [Google Scholar]
- Conradi S. Ultrastructure and distribution of neuronal and glial elements on the motoneuron surface in the lumbosacral spinal cord of the adult cat. Acta Physiol Scand Suppl. 1969;332:5–48. [PubMed] [Google Scholar]
- Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science. 2009;323:934–937. doi: 10.1126/science.1166127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao BX, Ziskind-Conhaim L. Development of ionic currents underlying changes in action potential waveforms in rat spinal motoneurons. J Neurophysiol. 1998;80:3047–3061. doi: 10.1152/jn.1998.80.6.3047. [DOI] [PubMed] [Google Scholar]
- Gundlach AL, Largent BL, Snyder SH. Autoradiographic localization of sigma receptor binding sites in guinea pig and rat central nervous system with (+)3H-3-(3-hydroxyphenyl)-N-(1-propyl)piperidine. J Neurosci. 1986;6:1757–1770. doi: 10.1523/JNEUROSCI.06-06-01757.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Hellstrom J, Arvidsson U, Elde R, Cullheim S, Meister B. Differential expression of nerve terminal protein isoforms in VAChT-containing varicosities of the spinal cord ventral horn. J Comp Neurol. 1999;411:578–590. [PubMed] [Google Scholar]
- Hellstrom J, Oliveira AL, Meister B, Cullheim S. Large cholinergic nerve terminals on subsets of motoneurons and their relation to muscarinic receptor type 2. J Comp Neurol. 2003;460:476–486. doi: 10.1002/cne.10648. [DOI] [PubMed] [Google Scholar]
- Hinckley CA, Hartley R, Wu L, Todd A, Ziskind-Conhaim L. Locomotor-like rhythms in a genetically distinct cluster of interneurons in the mammalian spinal cord. J Neurophysiol. 2005;93:1439–1449. doi: 10.1152/jn.00647.2004. [DOI] [PubMed] [Google Scholar]
- Houser CR, Crawford GD, Barber RP, Salvaterra PM, Vaughn JE. Organization and morphological characteristics of cholinergic neurons: an immunocytochemical study with a monoclonal antibody to choline acetyltransferase. Brain Res. 1983;266:97–119. doi: 10.1016/0006-8993(83)91312-4. [DOI] [PubMed] [Google Scholar]
- Itzhak Y. Multiple affinity binding states of the sigma receptor: effect of GTP-binding protein-modifying agents. Mol Pharmacol. 1989;36:512–517. [PubMed] [Google Scholar]
- Jacob MS, Presti DE. Endogenous psychoactive tryptamines reconsidered: an anxiolytic role for dimethyltryptamine. Med Hypotheses. 2005;64:930–937. doi: 10.1016/j.mehy.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Kim FJ, Kovalyshyn I, Burgman M, Neilan C, Chien CC, Pasternak GW. Sigma 1 receptor modulation of G-protein-coupled receptor signaling: potentiation of opioid transduction independent from receptor binding. Mol Pharmacol. 2010;77:695–703. doi: 10.1124/mol.109.057083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langa F, Codony X, Tovar V, Lavado A, Gimenez E, Cozar P, Cantero M, Dordal A, Hernandez E, Perez R, Monroy X, Zamanillo D, Guitart X, Montoliu L. Generation and phenotypic analysis of sigma receptor type I (sigma 1) knockout mice. Eur J Neurosci. 2003;18:2188–2196. doi: 10.1046/j.1460-9568.2003.02950.x. [DOI] [PubMed] [Google Scholar]
- Luty AA, Kwok JB, Dobson-Stone C, Loy CT, Coupland KG, Karlstrom H, Sobow T, Tchorzewska J, Maruszak A, Barcikowska M, Panegyres PK, Zekanowski C, Brooks WS, Williams KL, Blair IP, Mather KA, Sachdev PS, Halliday GM, Schofield PR. Sigma nonopioid intracellular receptor 1 mutations cause frontotemporal lobar degeneration-motor neuron disease. Ann Neurol. 2010;68:639–649. doi: 10.1002/ana.22274. [DOI] [PubMed] [Google Scholar]
- Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- Matsumoto RR, Hemstreet MK, Lai NL, Thurkauf A, De Costa BR, Rice KC, Hellewell SB, Bowen WD, Walker JM. Drug specificity of pharmacological dystonia. Pharmacol Biochem Behav. 1990;36:151–155. doi: 10.1016/0091-3057(90)90141-4. [DOI] [PubMed] [Google Scholar]
- Mavlyutov TA, Epstein ML, Andersen KA, Ziskind-Conhaim L, Ruoho AE. The sigma-1 receptor is enriched in postsynaptic sites of C-terminals in mouse motoneurons. An anatomical and behavioral study. Neuroscience. 2010;167:247–255. doi: 10.1016/j.neuroscience.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavlyutov TA, Ruoho AE. Ligand-dependent localization and intracellular stability of sigma-1 receptors in CHO-K1 cells. J Mol Signal. 2007;2:8. doi: 10.1186/1750-2187-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles GB, Hartley R, Todd AJ, Brownstone RM. Spinal cholinergic interneurons regulate the excitability of motoneurons during locomotion. Proc Natl Acad Sci U S A. 2007;104:2448–2453. doi: 10.1073/pnas.0611134104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misonou H, Mohapatra DP, Trimmer JS. Kv2.1: a voltage-gated k+ channel critical to dynamic control of neuronal excitability. Neurotoxicology. 2005;26:743–752. doi: 10.1016/j.neuro.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Muennich EA, Fyffe RE. Focal aggregation of voltage-gated, Kv2.1 subunit-containing, potassium channels at synaptic sites in rat spinal motoneurones. J Physiol. 2004;554:673–685. doi: 10.1113/jphysiol.2003.056192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen EC, McCracken KA, Liu Y, Pouw B, Matsumoto RR. Involvement of sigma (sigma) receptors in the acute actions of methamphetamine: receptor binding and behavioral studies. Neuropharmacology. 2005;49:638–645. doi: 10.1016/j.neuropharm.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Pal A, Hajipour AR, Fontanilla D, Ramachandran S, Chu UB, Mavlyutov T, Ruoho AE. Identification of regions of the sigma-1 receptor ligand binding site using a novel photoprobe. Mol Pharmacol. 2007;72:921–933. doi: 10.1124/mol.107.038307. [DOI] [PubMed] [Google Scholar]
- Park KS, Mohapatra DP, Misonou H, Trimmer JS. Graded regulation of the Kv2.1 potassium channel by variable phosphorylation. Science. 2006;313:976–979. doi: 10.1126/science.1124254. [DOI] [PubMed] [Google Scholar]
- Quirion R, Bowen WD, Itzhak Y, Junien JL, Musacchio JM, Rothman RB, Su TP, Tam SW, Taylor DP. A proposal for the classification of sigma binding sites. Trends Pharmacol Sci. 1992;13:85–86. doi: 10.1016/0165-6147(92)90030-a. [DOI] [PubMed] [Google Scholar]
- Ramachandran S, Chu UB, Mavlyutov TA, Pal A, Pyne S, Ruoho AE. The sigma1 receptor interacts with N-alkyl amines and endogenous sphingolipids. Eur J Pharmacol. 2009;609:19–26. doi: 10.1016/j.ejphar.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran S, Lu H, Prabhu U, Ruoho AE. Purification and characterization of the guinea pig sigma-1 receptor functionally expressed in Escherichia coli. Protein Expr Purif. 2007;51:283–292. doi: 10.1016/j.pep.2006.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray TS. Psychedelics and the human receptorome. PLoS One. 2004;5:e9019. doi: 10.1371/journal.pone.0009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaudo A, L’Hoste S, Guizouarn H, Borgese F, Soriani O. Cancer cell cycle modulated by a functional coupling between sigma-1 receptors and Cl- channels. J Biol Chem. 2007;282:2259–2267. doi: 10.1074/jbc.M607915200. [DOI] [PubMed] [Google Scholar]
- Rosenbluth J. Subsurface cisterns and their relationship to the neuronal plasma membrane. J Cell Biol. 1962;13:405–421. doi: 10.1083/jcb.13.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher MA, Rivard AF, Bachinger HP, Adelman JP. Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin. Nature. 2001;410:1120–1124. doi: 10.1038/35074145. [DOI] [PubMed] [Google Scholar]
- Sharkey J, Glen KA, Wolfe S, Kuhar MJ. Cocaine binding at sigma receptors. Eur J Pharmacol. 1988;149:171–174. doi: 10.1016/0014-2999(88)90058-1. [DOI] [PubMed] [Google Scholar]
- Stepien AE, Tripodi M, Arber S. Monosynaptic rabies virus reveals premotor network organization and synaptic specificity of cholinergic partition cells. Neuron. 2010;68:456–472. doi: 10.1016/j.neuron.2010.10.019. [DOI] [PubMed] [Google Scholar]
- Su TP. Evidence for sigma opioid receptor: binding of [3H]SKF-10047 to etorphine-inaccessible sites in guinea-pig brain. J Pharmacol Exp Ther. 1982;223:284–290. [PubMed] [Google Scholar]
- Su TP, Hayashi T, Maurice T, Buch S, Ruoho AE. The sigma-1 receptor chaperone as an inter-organelle signaling modulator. Trends Pharmacol Sci. 2010;31:557–566. doi: 10.1016/j.tips.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TP, London ED, Jaffe JH. Steroid binding at sigma receptors suggests a link between endocrine, nervous, and immune systems. Science. 1988;240:219–221. doi: 10.1126/science.2832949. [DOI] [PubMed] [Google Scholar]
- Walker JM, Bowen WD, Walker FO, Matsumoto RR, De Costa B, Rice KC. Sigma receptors: biology and function. Pharmacol Rev. 1990;42:355–402. [PubMed] [Google Scholar]
- Wetts R, Vaughn JE. Development of cholinergic terminals around rat spinal motor neurons and their potential relationship to developmental cell death. J Comp Neurol. 2001;435:171–183. doi: 10.1002/cne.1200. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Hartley R, Maxwell DJ, Todd AJ, Lieberam I, Kaltschmidt JA, Yoshida Y, Jessell TM, Brownstone RM. Conditional rhythmicity of ventral spinal interneurons defined by expression of the Hb9 homeodomain protein. J Neurosci. 2005;25:5710–5719. doi: 10.1523/JNEUROSCI.0274-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM, Rempel J, Brownstone RM. Postnatal development of cholinergic synapses on mouse spinal motoneurons. J Comp Neurol. 2004;474:13–23. doi: 10.1002/cne.20089. [DOI] [PubMed] [Google Scholar]
- Zagoraiou L, Akay T, Martin JF, Brownstone RM, Jessell TM, Miles GB. A cluster of cholinergic premotor interneurons modulates mouse locomotor activity. Neuron. 2009;64:645–662. doi: 10.1016/j.neuron.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.