Abstract
Regulatory T cells (Tregs) manipulated ex vivo have potential as cellular therapeutics in autoimmunity and transplantation. Although it is possible to expand naturally occurring Tregs, an attractive alternative possibility, particularly suited to solid organ and bone marrow transplantation, is the stimulation of total T cell populations with defined allogeneic antigen presenting cells under conditions that lead to the generation or expansion of donor-reactive, adaptive Tregs. Here we demonstrate that stimulation of mouse CD4+ T cells by immature allogeneic dendritic cells (DCs) combined with pharmacological inhibition of phosphodiesterase 3 (PDEi) results in a functional enrichment of Foxp3+ T cells. Without further manipulation or selection, the resultant population delayed skin allograft rejection mediated by polyclonal CD4+ effectors or donor-reactive CD8+ TCR transgenic T cells and inhibited both effector cell proliferation and T cell priming for IFN-γ production. Notably, PDE inhibition also enhanced the enrichment of human Foxp3+ CD4+ T cells driven by allogeneic APC. These cells inhibited T cell proliferation in a standard in vitro mixed lymphocyte assay and importantly, attenuated the development of vasculopathy mediated by autologous PBMC in a functionally relevant humanized mouse transplant model. These data establish a method for the ex vivo generation of graft-reactive, functional mouse and human Tregs that uses a clinically approved agent, making pharmacological PDE inhibition a potential strategy for Treg-based therapies
INTRODUCTION
Compelling evidence obtained from experimental models and from specific groups of patients indicates that regulatory T cells (Tregs) play an important role in T cell homeostasis and may offer therapeutic opportunities in autoimmune disease, bone marrow, and solid-organ transplantation (1-5). A number of current collaborative efforts are aimed at the identification of immunosuppressive protocols that might lead to Treg development in organ transplant patients, but these may be frustrated by the heterogeneity of immunosuppressive regimens and the paucity of reliable phenotypic markers of functional regulatory cells. An alternative approach is to develop ex-vivo protocols that generate or expand recipient-derived Tregs for administration to the patient as a cellular therapy, and several promising approaches depend on TCR signaling combined with the provision of specific cytokines or exogenous co-stimulation (6-8); (9) (10). However, both recent and historical data indicate that manipulation of specific metabolic pathways may also offer possibilities for promoting regulatory T cell development ex vivo.
The cyclic nucleoside monophosphates cAMP and cGMP contribute to cell growth, proliferation, differentiation, and survival, and phosphodiesterases (PDEs), which hydrolyse the 3′-5′ phosphodiester bond to yield free adenosine and guanosine, provide an important control point in these processes (11). Indeed, T cell responses in vitro can be inhibited by extracellular nucleosides such as adenosine (12), and membrane-permeable analogues have shown that this inhibition is mediated at least in part by cyclic AMP (cAMP) (13). Furthermore, elevation of intracellular cAMP in vitro using phosphodiesterase inhibitors such as theophyline and caffeine not only inhibits T cell proliferation and IL-2 secretion (14) but also generates populations of T cells that can inhibit proliferation of other T cells in secondary cultures (15, 16). However, these PDE inhibitors are broadly reactive against most PDE isoforms, making interpretation difficult particularly in the light of current knowledge of Treg differentiation and function.
Recent data have shown that the Treg-associated transcription factor Foxp3 binds to target sequences within the PDE3b gene (17, 18) and in a screen of genes regulated by Foxp3, pde3b has been identified as one of the most repressed (19). A further link between adenosine, cAMP and Treg function is provided by the observation that Foxp3+CD25+ T cells express ectoenzymes that yield free adenosine from ATP and AMP (20, 21) and it has been proposed that the local delivery of adenosine provides another mechanism by which Treg can provide relatively specific control of T cell responses (20). We have shown previously that stimulation of CD4+ T cells ex vivo with allogeneic BM DCs in the presence of IFN-γ enriches for Foxp3+ cells that regulate allograft rejection (9) (10). Importantly, this effect appears to be dependent on nitric oxide (NO) since enrichment is repressed in the presence of N-methyl-L-arginine (L-NMMA), an inhibitor of both constitutive and inducible forms of NO synthase (NOS) and induced in the absence of exogenous IFN-γ by provision of the NO donor S-nitroso-N-acetyl-penicillamine (SNAP) (9). The ability of exogenous NO to drive the development of functional Treg has also been shown using the NO donor NOC-18 (22). The role of NO in the generation of adaptive Foxp3+ Treg hinted at a possible connection with soluble guanylate cyclase (sGC), since this signal-transduction enzyme catalyses the conversion of GTP to cyclic GMP (cGMP) and is activated by nitric oxide (23). Cyclic GMP is an important second messenger mediating many physiological effects, but one of the consequences of elevated cGMP levels is an inhibition of phophodiesterase 3 (PDE3), resulting in a net increase in the intracellular concentration of cyclic AMP (cAMP) (24, 25).
The link between nitric oxide, phosphodiesterases and Tregs prompted us to ask whether functional alloreactive regulatory T cells could be induced by alloantigen stimulation in the presence of pharmacological phosphodiesterase inhibition. Foxp3+ cells enriched by this protocol prevent skin allograft rejection and development of transplant vasculopathy, suggesting that this method of alloreactive Treg enrichment may have potential in clinical transplantation.
RESULTS
PDE3 inhibition enriches for alloreactive Foxp3+ T cells
Elevated levels of cAMP have been linked to inhibition of T cell responses, prompting us to ask whether pharmacological inhibition of PDE3 might promote the enrichment of alloreactive Foxp3+ T cells. Naive CBA (H-2k) CD25−CD4+ T cells were co-cultured with B6 (H-2b) BM DCs in the presence of the PDE3 inhibitor cilostamide. Cells were re-stimulated on day 7, harvested on day 14, and intracellular Foxp3 expression analyzed. As shown in Figure 1A, the addition of cilostamide resulted in a dose-dependent increase in the proportion of Foxp3+ cells, reaching an approximate 10-fold enrichment at a concentration of 20μM (Figure 1B, left panel). A similar dose-dependent effect was seen in terms of the absolute number of Foxp3+ T cells recovered, with a decline above a concentration of 10μM, which probably reflects toxicity. These data indicate a potential route for the enrichment of alloreactive Foxp3+ cells ex vivo using a pharmacological agent in widespread clinical use.
Figure 1. Stimulation of CD4+ T cells with allogeneic APC combined with PDE3 inhibition leads to an enrichment of Foxp3+ T cells.
Naive CBA (H-2k) CD25−CD4+ T cells were co-cultured with B6 (H-2b) BM DCs in the presence of the PDE3 inhibitor cilostamide at the concentrations shown. Cells gated on CD4 and TCR-β for analysis of intracellular Foxp3 expression. (A) Representative plots. (B) Quantification.
Cilostamide promotes the development of functional regulatory T cells
To determine whether CD4+ T cells driven by alloantigen during PDE3 inhibition are functional in vivo, we used an established adoptive transfer MHC mismatched skin transplant model. Naive CBA (H2k) CD25−CD4+ T cells were co-cultured with GM-CSF/TGF-β differentiated B6 (H2b) BM DCs in the presence or absence of 10 μM cilostamide, re-stimulated under identical conditions on day 7, harvested on day 14 and transferred into CBA-Rag−/− mice with 1 × 105 naive CBA CD25−CD4+ T cells or 1 × 105 BM3 CD8+ T cells as effector populations followed by B6 skin grafting (day +1). As shown in Figure 2A, mice reconstituted with polyclonal CD25−CD4+ cells rejected their grafts acutely (MST 18 days, n=6) but co-transfer of 2×105 PDEi conditioned T cells (Tcilos), but not unconditioned T cells, prevented rejection with all animals accepting their grafts long-term (MST >100 days, n=5; MST 18.5, n=7 respectively, P=<0.05). We also examined regulation in a more stringent setting where rejection is mediated by H2Kb-reactive TCR transgenic BM3 T cells in which essentially 100% of the effector population is reactive against donor H2Kb. Reconstitution with 1×105 CD8+ BM3 T cells resulted in acute B6 skin graft rejection (MST 15 days, n=6; Figure 2B), but co-transfer of 2×105 Tcilos delayed rejection and resulted in long term survival in 2 out of 5 recipients (MST 51 days, n=5, P=0.02). Thus, the effect of alloantigen stimulation combined with PDE inhibition is not restricted to the phenotypic acquisition of Treg markers but also results in a population capable of functional regulation in vivo.
Figure 2. Ex vivo PDEi-conditioned CD4+ T cells prevent skin allograft rejection and inhibit T cell proliferation and priming in vivo.
(A) B6 (H2b) skin graft survival in CBA-Rag−/−(H2k) mice reconstituted with CBA CD25−CD4+ effector cells alone or with cultured CD4+ T cells. (B) B6 (H2b) skin graft survival in CBA-Rag−/− mice reconstituted with naive CD8+ TCR transgenic BM3 cells alone or with syngeneic CD4+ T cells. Syngeneic CD4+ T cells in A and B were stimulated with B6 bone marrow-derived DCs in the presence or absence of 10μM cilostamide. (C) Number of CD8+ T cells recovered from CBA-Rag−/− mice reconstituted with CFSE-labeled BM3 CD8+ T cells ± ex vivo PDEi-conditioned CBA CD25−CD4+ T cells (Tcilos) and transplanted with B6 (H2b) skin grafts the next day. (D) Proliferation of CD8+ T cells in C as determined by CFSE labeling. Histogram shows mean ± SD (5-6 mice per group) of CD8+ cells remaining CFSE positive defined according to the dot plot gate shown.
To explore potential regulatory mechanisms, we examined the effect of PDEi conditioned cells on the proliferation of BM3 T cells in vivo. CBA-Rag−/− mice were reconstituted with 1 × 105 CFSE labeled BM3 CD8+ T cells with or without 2×105 ex vivo PDEi conditioned CBA CD25−CD4+ T cells. The use of BM3 cells allowed unequivocal identification of the effector population. Reconstituted mice received a B6 (H2b) skin allograft the next day and 15 days later, splenocytes were stained for CD8 and TCR-β for determination of absolute numbers and for examination of in vivo proliferation history. BM3 cells were readily detected in mice reconstituted with this population alone but co-transfer of PDEi conditioned cells resulted in a 10-fold reduction in the number of BM3 cells recovered (Figure 2C, 33.6±13.0 × 103 vs. 3.2±0.8 × 103 CD8 cells per spleen, BM3 cells only and BM3 cells + Tcilos respectively, P=0.01), indicating that inhibition of expansion is one mechanism involved in regulation mediated by PDEi conditioned cells. This is further supported by division history analysis (Figure 2D). When transferred alone, almost 90% of this monoclonal alloreactive population was CFSE negative at day 15, reflecting extensive in vivo proliferation. In contrast, in the presence of PDEi-conditioned CD4+ T cells, a large proportion of BM3 cells remained CFSE positive (11.3%±2.4 vs. 43.7%±4.2 BM3 only and BM3 plus Tcilo respectively, P=0.004), thus providing evidence of inhibition of expansion by this ex vivo generated regulatory population.
To assess the impact of the PDEi population on defined T cell effector function, CBA-Rag−/− mice were reconstituted with either 1 × 105 naive CBA CD25−CD4 T cells or BM3 CD8 T cells as effector populations ± co-transfer of CBA Tcilos and transplanted with B6 (H2b) skin allografts the following day. Spleens were harvested 15 days after skin grafting. CD4+ cells or CD8+ cells were purified by magnetic positive selection from pooled splenocytes and challenged with T cell depleted splenocytes from B6 mice as stimulators in an IFN-γ ELISpot assay. The presence of PDEi conditioned cells at the time of skin grafting resulted in a striking reduction in the capacity of the responding polyclonal CD4+ population to secrete IFN-γ when re-challenged with alloantigen in vitro (Figure 3A, left panel), which translates to a 97% decrease in the frequency of donor-reactive IFN-γ producing cells (right panel, P=0.01 wrt CD25− cells only). PDEi-conditioned cells also had a striking effect on the ability of monoclonal BM3 cells to produce IFN-γ in response to alloantigen re-challenge, equating to a greater than 95% reduction when expressed as the number of IFN-γ producing cells per spleen (Figure 3B, P=0.01). Overall, these data indicate that the effect on graft outcome shown in Figures 2A and B most likely reflects an inhibition of both cell proliferation and acquisition of direct effector function mediated by this ex vivo stimulated regulatory population.
Figure 3. Cilostamide-induced Tregs inhibit IFN-γ production by effector T cells.
(A and B) Frequency of donor-reactive IFN-γ–secreting CD4+ (A) or BM3 (B) T cells per well (mean ± SD; 5-6 mice per group) with representative ELISpot images (left panels) from CBA-Rag−/− mice reconstituted with naive CBA CD25− CD4+ T cells or BM3 CD8+ T cells as effectors with or without ex vivo PDEi-conditioned CBA CD25−CD4+ T cells (Tcilos) transplanted with B6 (H2b) skin allografts. Right panels show the absolute number of IFN-γ positive cells per spleen.
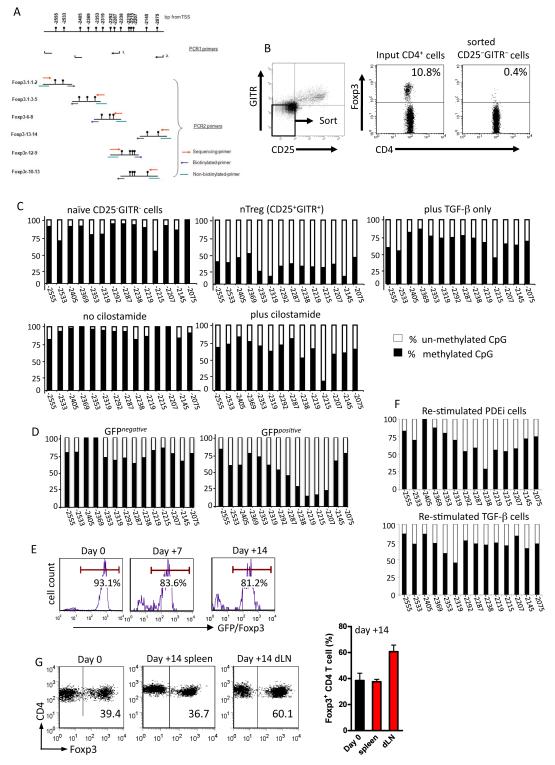
Phosphodiesterase inhibition results in de-methylation of the Foxp3 gene
Epigenetic modification leading to un-methylated CpGs in the Foxp3 Treg-specific de-methylated region (TSDR) has recently been shown to be a hallmark of naturally occurring regulatory T cells in both mice and humans and is thought to reflect stable, constitutive Foxp3 expression in this population (26). To examine epigenetic changes in the cilostamide-induced population that showed functional regulation in vivo (Figure 2), naïve CBA CD4+ T cells were stimulated with alloantigen in the presence of cilostamide as described above, harvested on day 14 and DNA isolated for bisulphate DNA pyro-sequencing. The TSDR region and PCR primers used are shown in Figure 4A. To provide a relevant comparator population in the same mouse strain, naïve CBA T cells were gated on CD4 and flow-sorted on the basis of GITR and CD25 expression. As shown in Figure 4B, this strategy provides a population depleted of naturally occurring Tregs (nTregs) and previous direct comparisons performed in this laboratory have shown that for naïve, un-stimulated CD4+ T cells, this approach is as effective as sorting cells on the basis of GFP expression from GFP-Foxp3 reporter mice (27). Naïve CD25−GITR− cells (containing <0.5% nTregs) showed high levels of methylation in the TSDR region (Figure 4C, upper panel) with levels at some CpG sites exceeding 90%, consistent with their Foxp3 protein signature. In contrast, the reciprocal CD25+GITR+ population showed extensive de-methylation within the same region in agreement with data reported previously for BALB/c mouse nTregs (26)
Figure 4. PDE3 inhibition leads to CpG de-methylation in the Foxp3 5′ UTR.
(A) CpG map of the Treg-specific de-methylated region (TSDR) and indication of the positions of the 8 primer sets. Replicate populations from two independent experiments were analyzed with 4 technical replicates per population.
(B) CBA CD4+ T cells were stained for CD25 and GITR and flow sorted on the basis of the gate shown (left panel). This results in a population of sorted cells essentially devoid of naturally occurring Tregs (right panel).
(C) Percent CpG methylation in the TSDR for each of the cell populations shown.
(D) Percent CpG methylation for CD4+CD25− cells from CBA Foxp3 GFP reporter mice that were stimulated with B6 BM-derived DC in the presence of 10μM cilostamide.
(E) CD4+CD25− T cells from CBA Foxp3 GFP reporter mice were stimulated with B6 BM DC in the presence of 10μM cilostamide and 14 days later, GFP+ cells isolated by flow sorting and re-stimulated with B6 BM DC under identical conditions without cilostamide. Cells were harvested on days 7 and 14 and stained for intracellular Foxp3.
(F) TSDR CpG methylation profiles of cells in 4(E) and that of sorted GFP+ cells re-stimulated under neutral conditions following initial simulation in the presence of TGF-β plus IL-2.
(G) Representative dot plots show Foxp3 expression in the gated CD4+ TCR+ input population (day 0; CBA CD4+ T cells stimulated with B6 BM DC for 14 days in the presence of cilostamide) and in the gated population in spleen and draining lymph node (dLN) 14 days after transfer to CBA-Rag−/− mice together with BM3 CD8+ T cells followed by B6 skin grafting. Histogram shows mean ± SD of data from 5 individual mice.
To examine the effect of PDE3 inhibition on TSDR methylation, naive CBA CD4+ T cells were stimulated with B6 BM-derived DC in the absence or presence of 10μM cilostamide. Cells were re-stimulated under identical conditions on day 7 then harvested on day 14 for DNA isolation. Cells were not sorted or enriched prior to DNA extraction because the intention was to determine whether the TSDR methylation status of the population without further manipulation regulates rejection responses in vivo (Figure 2). Cells stimulated with alloantigen in the absence of cilostamide remained heavily methylated in the TSDR region (Figure 4C, lower left) and in this respect were very similar to naïve non-Tregs. In contrast, T cells activated in the presence of cilostamide showed reduced TSDR methylation. Although the TSDR region in these cells is considerably more methylated than in nTregs, the levels are remarkably similar to those seen using the prototypic IL-2 + TGF-β protocol (Figure 4C, upper right) that has been reported elsewhere (6) (28) (29) and appear to be similar to those reported by Floess et al. (26). Therefore, although T cells driven by allogeneic APC in the presence of cilostamide are more highly methylated in the critical Foxp3 locus than their naturally occurring Treg counterparts, they show levels of de-methylation that are comparable to those seen in other adaptive or inducible Tregs.
Although the cilostamide treated cells showed less TSDR de-methylation than CD25+GITR+ nTregs, one possible explanation is that by design, the TSDR data for these cells was obtained from the total population that showed functional regulation in vivo (Figure2). As shown in Figure 1 these populations typically contain ~60% Foxp3-ve cells. To determine whether de-methylation of the TSDR in cilostamide-treated cells corresponded with Foxp3 expression, CD4+CD25− cells from CBA Foxp3.GFP reporter mice were stimulated with B6 BM-derived DCs in the presence of 10μM cilostamide, re-stimulated under identical conditions on day 7 then flow-sorted on day 14 on the basis of GFP(Foxp3) expression. Bi-sulphite sequencing was then performed on the isolated GFP− and GFP+ populations. As shown in Figure 4D, TSDR de-methylation was more pronounced in GFP+ than GFP− cells confirming that alloantigen stimulation combined with PDE inhibition induces TSDR epigenetic modifications preferentially in Foxp3+ cells.
One of the striking observations made by Floess et al. (26) was that although naturally occurring Tregs (nTregs) stimulated in the absence of cytokines retained Foxp3 expression (~99.4% Foxp3+ at day 0, 92% after 6 days), re-stimulation of TGF-β + IL-2-induced Tregs in the absence of TGF-β resulted in a dramatic loss of Foxp3 expression (~98% Foxp3+ at day 0, <10% after 6 days). To examine the stability of cilostamide-induced alloreactive Tregs, CD4+CD25− T cells from CBA-Foxp3 GFP reporter mice were stimulated with B6 BM DC in the presence of 10μM cilostamide as described above, flow-sorted on the basis of GFP (Foxp3) expression (>96% purity), then re-stimulated with B6 BM DC under identical conditions without cilostamide. This population retained Foxp3 expression 7 and 14 days after re-stimulation (Figure 4E). To ask whether this stability reflects epigenetic modifications, cells were prepared and isolated as described for Figure 4E and DNA was isolated 14 days after re-stimulation for TSDR bi-sulphite sequencing. To provide a comparator population, cells from CBA-Foxp3 GFP reporter mice were stimulated in the presence of IL-2 + TGF-β (Figure 2C) then isolated on the basis of GFP expression. These cells were re-stimulated without cytokine modification then harvested 14 days later for DNA isolation. As shown in Figure 4F, PDEi cells re-stimulated in the absence of further cilostamide treatment retained substantial levels of TSDR de-methylation, and the overall TSDR profile was similar to that seen in re-stimulated TGF-β cells. Although the TSDR in the re-stimulated PDEi cells was slightly less de-methylated than that in equivalent cells at the time of initial harvest (Figure 4D), when seen in combination with the Foxp3 expression data (Figure 4E), the data suggest that cilostamide-induced Tregs maintain a relatively stable phenotype under conditions of antigen re-exposure.
To assess functional stability of this population under conditions of alloantigen challenge in vivo, B6-reactive, CBA Tcilos were transferred to CBA-Rag−/− mice (day 0), together with Kb specific BM3 CD8+ TCR transgenic T− cells as described in Figure 2B. One day later, these mice were transplanted with B6 skin grafts. On day 14, spleen and draining lymph node cells (left axillary) were analysed phenotypically. Foxp3+ cells were readily detected in both compartments (Figure 4G), but were especially enriched in the draining lymph nodes (P=0.01 wrt spleen), known to be a site of early Treg recruitment and function in a comparable skin graft model (30). Thus, under conditions of active in vivo regulation (Figure 2A,B), alloreactive CD4+ cilostamide induced Treg retain Foxp3 stability during the critical period where their presence leads to long-term skin graft survival instead of acute transplant rejection.
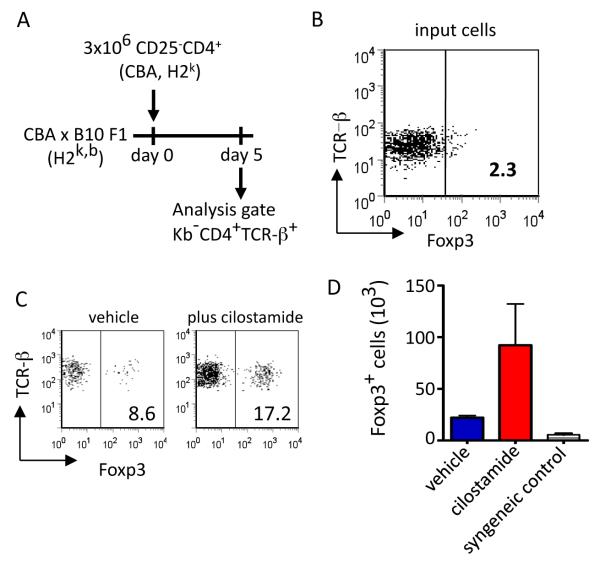
PDE3 inhibition increases Foxp3 expression in vivo
The demonstration that PDE3 inhibition in vitro leads to an enrichment of alloreactive Foxp3+ cells prompted us to ask whether a similar phenomenon might occur in vivo. To explore this possibility, CBA CD4+CD25− cells (input day 0 population, Figure 5B) were adoptively transferred to normal CBAxB.10 F1 mice, which were then treated with cilostamide or vehicle only. On day 5, spleen cells were harvested and analyzed for Foxp3 expression. Transfer of CBA cells into these F1 mice established an in vivo MLR where the transferred T cells respond to H2b alloantigens without NK killing of the input population and where staining for Kb allowed endogenous Foxp3+ cells to be gated out allowing Foxp3 expression to be determined in the transferred T cell population alone. Relative to vehicle-only controls, the administration of cilostamide resulted in a two-fold increase in both the percentage and absolute number of Foxp3+ T cells in the input population (Figure 5C and D), providing in vivo support for the effects of cilostamide demonstrated in vitro. Importantly, transfer of the same population to cilostamide-treated syngeniec CBA mice in which transgenic T cell expression of hCD52 allowed host hCD52+Foxp3+ cells to be excluded from the analysis did not result in an increase in Foxp3+ cells (D) indicating that this effect is dependent on direct T cell activation.
Figure 5. Phosphodiesterase inhibition in vivo enhances Foxp3 expression.
(A) CBA (H2k) CD4+CD25− cells were adoptively transferred on day 0 (B), to normal CBAxB.10 F1 (H2k+b) mice, which were then treated with cilostamide (6.4mg/kg per day) or vehicle (PBS) for 5 consecutive days. On day 5, spleen cells were analyzed for Foxp3 expression. (C) Representative dot plots, (D) absolute numbers of Foxp3+ T cells in the spleen; mean ± SD, n=3 per group.
PDE3 inhibition ex vivo results in functional human Foxp3+ regulatory T cells
To ask whether inhibition of PDE3 would also be an effective means for the generation ex vivo of human Tregs, CD45RO−CD25−CD4+ T cells were co-cultured with GM-CSF/IL-4/TGF-β-differentiated allogeneic monocyte-derived DCs in the presence of cilostamide, re-stimulated on day 7 under identical conditions and harvested on day 14 for phenotypic analysis and for use in a regulation assay based on a standard MLR. As in the mouse, between 5-10% of resting human CD4+ T cells are positive for Foxp3 but unlike the mouse, Foxp3 is up-regulated nearly three-fold after activation (Figure 6A). However, as with mouse cells, the addition of cilostamide resulted in a significant increase in Foxp3 expression such that the majority of cells became Foxp3 positive (Figure 6A). This was also reflected by the up-regulation of cell surface markers previously associated with a regulatory phenotype, including CD25, CD62L and CD152 (solid curves, Figure 6B).
Figure 6. Phosphodiesterase inhibition generates functional human regulatory cells.
(A) Frequency of Foxp3 positive cells derived from CD25−CD45RO−CD4+ T cells from human PBMC cultured with irradiated allogeneic APC in the absence (control) or presence of 20μM cilostamide.
(B) The same populations were examined for expression of CD25, CD127, CD62L and CD152; bold curves. Data representative of two independent experiments.
(C) PBMC were stimulated by allogeneic APC ± cilostamide as in A, re-isolated (conditioned T cells) and co-cultured with autologous PBMC (responders) driven by allogeneic APC. Responder proliferation was determined by thymidine incorporation. Results expressed as mean cpm ± SD of triplicate wells.
(D) BALB/c Rag2−/−/cγ−/− mice were transplanted with human arterial interposition grafts on day 0 and reconstituted i.p. the next day with 107 PBMC alone or together with 107 cilostamide-induced Tcilos driven by APC from the same PBMC donor. Grafts were harvested 30 days later. Representative EvG stained sections (left) and intimal expansion analysis from two independent experiments (right) are shown. Results are mean ± SEM.
These ex vivo conditioned CD4+ T cells were then tested in an MLR to determine their ability to suppress the proliferation of autologous PBMC against allogeneic stimulation. As shown in Figure 6C, when autologous responder PBMC were stimulated by irradiated allogeneic PBMC, the presence of cells conditioned in the primary culture with cilostamide resulted in a striking inhibition of proliferation in the secondary suppression assay. This inhibition could be detected at a conditioned T cell:responder ratio of 1:64 but increased in a dose-dependent manner such that at a ratio of 1:2, the proliferation was some 15-fold lower than that of responders alone (P<0.05, ratios 1:32 and above). By contrast, cells conditioned in the absence of cilostamide had no impact on proliferation except at a ratio of 1:1, and even here, proliferation was reduced by less than a factor of two (P<0.05 at 1:1, >0.05 all other groups). To ask whether these conditioned human cells have the capacity for regulation in vivo, we took advantage of a human artery transplantation model established in our laboratory. BALB/c Rag2−/−/cγ−/− mice were transplanted with human arterial interposition grafts then reconstituted the next day with 107 PBMC allogeneic to the vessel with or without 107 cilostamide-induced Treg from the same cell donor. Thirty days post transplant the grafts were harvested, sectioned, stained and evaluated for transplant vasculopathy. As shown in Figure 6D, although neither the procurement process nor transplantation itself results in vasculopathy, reconstitution of these immunodeficient recipients with PBMC alone results in a significant degree of intimal hyperplasia as indicated by the open arrows in the upper representative section. In clear contrast, co-transfer of cilostamide-treated T cells resulted in a significant reduction in vasculopathy, which on average was approximately 2-fold but in some cases, reduced intimal expansion almost to that seen in un-reconstituted animals (Figure 6D, right panel). Thus, as in the mouse, allogeneic stimulation of human CD4+ T cells in the presence of cilostamide promotes the enrichment functional regulatory T cells.
Discussion
The observation that regulatory T cells can control destructive T cell responses in numerous transplantation and autoimmunity models has recently been given new focus by the demonstration that naturally occurring Tregs (nTregs) play an essential role in peripheral self tolerance in normal, un-manipulated animals (31) (5). The fact that selective depletion of these cells leads to widespread autoimmune disease underlines the potential of such populations and has re-kindled attempts to identify protocols in which Tregs could be manipulated for therapeutic benefit. Although it is possible that some forms of current immunotherapy may induce the selection or development of human Tregs in vivo (32) (33), an alternative possibility is to develop protocols that expand and or generate Tregs ex vivo for use as a cellular therapy.
Much of the current research focus is on the expansion of nTregs using polyclonal stimulation that can result in a several thousand-fold expansion of the starting population. These cells can regulate alloreactivity in vivo, but more importantly, the initial observations made with mouse T cells (6) (34) have now been replicated in our laboratory with expanded human nTregs in a clinically relevant model of human chronic allograft vasculopathy (35). Thus, expanded nTregs clearly have considerable potential for regulating alloreactivity and several protocols now exist for the use of such populations in haematopoietic stem cell transplantation for the control of graft versus host disease (GVHD) and encouraging preliminary results have recently been published (36) (37). However, the impact on normal immune responses and immune homeostasis of administering large numbers of polyclonally activated Tregs is not known and the uncertainty is compounded by the fact that methods do not yet exist to eliminate potential effector T cells from the expanded population. An alternative approach is to promote the enrichment, expansion or generation of alloreactive induced Tregs by stimulation of recipient CD4+ T cells with donor antigen presenting cells under defined culture conditions. In this study we have demonstrated that stimulation of both mouse and human CD4+ T cells in the presence of a PDE3 inhibitor results in a population with the phenotypic characteristics of regulatory T cells and the functional ability to prevent allograft rejection in vivo. We have also demonstrated that the administration of cilostamide can promote the development of Tregs in vivo where the importance of T cell activation for the emergence of Foxp3+ cells is highlighted by the fact that whilst transfer of CD4+ T cells to F1 recipients results in an expanded Foxp3+ population, this does not occur on transfer to syngeneic recipients (Figure 5C-D). This is consistent with results of previously published work examining the role of TCR ligation and in vivo development of nTregs (38) (39) (40) and with our own experiments where alloreactive adaptive Tregs develop in vivo after tolerance induction (27).
In an attempt to understand the origin and development of alloreactive Tregs demonstrably responsible for allograft protection after tolerance induction in vivo (41), we have adoptively transferred mouse CD4+ T cells purged of nTregs by stringent flow-sorting to syngeneic Rag−/− recipients. These mice were then either tolerized with an established induction protocol based on donor alloantigen challenge or left untreated before delivery of an effector T cell population followed by transplantation with donor-strain skin grafts. Mice transplanted without the tolerance induction rejected their grafts acutely but in contrast, mice that had received the donor alloantigen induction protocol all accepted their grafts long term. Critically, this engraftment was associated with the emergence of a Foxp3+ T cell population (27). Thus, as with the development of nTregs, the development of adaptive Tregs in the periphery is critically dependent on TCR signaling. Although this is an important mechanistic observation, its relevance to the cilostamide data shown in Figure 5 is that an increase in the proportion of Foxp3+ Tregs is unlikely to be a clinical consequence in patients receiving phosphodiesterase inhibitors, for example for the treatment of claudication. However, we are actively exploring the possibility that when combined with established alloantigen-dependent tolerance induction protocols, cilostamide treatment may be a useful adjunctive strategy for the development of functional Tregs in vivo.
In the protocol described, both CD4+ T cells and allogeneic DC are exposed to cilostamide in the 14-day co-culture period (Figure 1) and so either population could be potentially affected by the inhibitor. The reduced PDE3b expression in nTregs (17, 18) suggests that T cells rather than APC are the likely primary targets of cilostamide in the culture system described; however, further studies will need to be performed to confirm this hypothesis. The observation that cilostamide-induced enrichment of Foxp3+ Tregs is dependent on T cell activation suggests that stimulation of bulk CD4+ T cells with CD3/CD28 beads in the presence of cilostamide might be an alternative route to promote the development of regulatory T cells in vitro. However, polyclonal stimulation in the presence of cilostamide results in an out-growth of CD25+Foxp3negative cells. Although it might be possible to modify this outcome by using alternative or weaker agonists, the potential risk of expanding non-Tregs probably means that polyclonal stimulation of total CD4+ cells is less attractive than polyclonal expansion of isolated Treg populations.
In an attempt to determine whether the enrichment of Tregs extends beyond alloreactive T cells in our system, we have asked whether the vitro cilostamide protocol can promote Treg development in a polyclonal response to human gamma globulins, known to be capable of inducing tolerance in vivo (42) (43). However, attempts to detect an enrichment of Foxp3+ cells in this setting have been unsuccessful, possibly due to the low frequency of T cells responding to nominal antigens. To overcome this problem we have used ovalbumin reactive DO11.10 TCR transgenic T cells. These preliminary experiments have been performed using total CD4+ T cells from DO11.10 mice on a Rag−/− background and even though positive controls proliferate vigorously as anticipated, the addition of cilostamide does not result in the development of Foxp3+ cells. It is known that TCR transgenic Rag−/− mice frequently have low numbers of endogenous nTregs, probably due to a failure of positive selection (39) (40) and this is also a feature of DO11.10 TCR transgenics (44). We have previously shown that the enrichment of alloreactive Foxp3+ T cells in vitro depends at least in part, on the presence of pre-existing nTregs in the input T cell population (10) perhaps representing an in vitro correlate of in vivo infectious tolerance (45) suggesting that our inability to drive the enrichment of Foxp3+ DO11.10 T cells may be a direct reflection of this nTreg deficit. Although the contribution that pre-existing nTregs make to this process is unknown, one intriguing possibility is that that conversion of non-Tregs depends on direct delivery of cAMP through gap junctions as has been shown elsewhere (46).
A growing understanding of how Foxp3 influences regulatory cell differentiation combined with the fact that PDE inhibitors are widely used to treat clinical conditions such as claudication, pulmonary hypertention and asthma highlights the relevance of targeting phosphodiesterases as a potential route toward Treg cellular therapy. Indeed, although the focus of this investigation was the inhibition of PDE3, additional phosphodiesterase isoforms exist, some of which show differential expression in specific cell populations (11). For example, although both PDE3 and PDE4 are expressed in human and mouse T cells, human T cells also express PDE7 (47) (48) and the availability of selective inhibitors for defined isoforms suggests further options for manipulating T cell responses. Inhibition of PDEs may contribute to the development of a number of therapeutic approaches for the generation of regulatory T cells for use in autoimmune disease and transplantation.
Materials and methods
Mice
CBA.Ca (CBA, H2k), C57BL/6 (B6, H2b), BM3 (H2k) were bred and maintained at our institution. CBA-recombination-activating gene 1 knockout (CBA-Rag−/−) H2k; were kindly provided by Dr D. Kioussis, National Institute for Medical Research, London). CBA transgenic mice expressing human CD52 were a kind gift from Professor Herman Waldmann, Sir William Dunn School of Pathology, Oxford. BALB/c-recombination-activating gene 2 knockout and common γ chain knockout (BALB/c Rag2−/−/cγ−/−) mice were obtained Charles River Laboratories. All mice were maintained in the BMSU, John Radcliffe Hospital.
Reagents and monoclonal antibodies
The antibodies to Foxp3, FJK-16s and PCH101, were obtained from eBioscience. Other conjugated antibodies were from BD Pharmingen. Cilostamide was from Sigma-Aldrich.
Cell purification
CD25−CD4+ T cells and CD14+ monocytes were isolated using CD4, CD25 and CD14 MicroBeads (Miltenyi). CD45RO− CD25−CD4+ T cells were isolated using DynaBeads (Invitrogen). On re-analysis, all populations were >95% pure.
In vitro generation of dendritic cells
Mouse bone marrow derived DCs (BM DCs) were generated using rmGM-CSF and rhTGF-β1 as described (10). Human monocyte derived DCs were generated from peripheral blood CD14+ cells by culture for 6 days with rhGM-CSF, rhIL-4, and rhTGF-β1.
Ex vivo conditioning protocol
Purified naive CD25−CD4+ T cells or CD45RO−CD25−CD4+ T cells were co-cultured with allogeneic DCs (5×105 T cells plus 5×104 DC per 2 ml well) in complete medium in the presence of additions specified. On day 7, the T cells were re-stimulated under identical conditions and harvested 7 days later.
Adoptive transfer and skin transplantation
CBA-Rag−/− mice were reconstituted intravenously with 1×105 CD25−CD4+ cells from naive CBA or BM3 CD8+ cells with or without 2×105 ex vivo conditioned cells and transplanted the next day with full thickness H2b skin grafts.
CpG methylation analysis
DNA methylation in the T reg-specific demethylated region (TSDR) of the Foxp3 5’UTR was assessed using bisulphite DNA pyro-sequencing. A nested PCR approach was applied with 2 overlapping larger (<400bp) and 6 smaller (>200bp) amplicons for PCR1. Pyro-sequencing amplicons covered all 14 CpG sites in the TSDR for PCR2. Genomic DNA (20ng) was bisulphite converted using the EZ DNA Methylation Kit (Zymo Research). DNA thermocycler settings were: Temperature (95°C 30sec, 50°C 15min) for 20 cycles. PCR 1 on two oligo sets, was performed according to standard protocols (49) with minor changes to the cycling conditions: 95°C 5:00 min (94°C 0:30 min, 57°C 1:00 min, 72°C 1:00 min) for 40 cycles, 72°C 5:00 min.
PCR1 primers
Foxp3.1fAGGAAGAGAAGGGGGTAGATA
Foxp3.1rAAACTAACATTCCAAAACCAAC
Foxp3.2fATTTGAATTGGATATGGTTTGT
Foxp3.2rAACCTTAAACCCCTCTAACATC
Six oligo sets were designed for pyro sequencing using Biotage primer design software (Biotage, Sweden) in conjunction with published protocols for DNA methylation pyrosequencing assays (49). A universal M13 tag was added to the opposite oligo of the sequencing primer, as described (50). Two primer sets were designed for FoxP3_1 and 4 for FoxP3_2. Cycling conditions were: 95°C 5:00 min (95°C 0:30 min, 58°C 0:30 min, 72°C 0:30 min) for 45 cycles, 72°C 5:00 min.
Pyrosequencing primers
Foxp3.1_2-1F1 AAGGTTGGATGTTTGGTGAGTATT
Foxp3.1_2-1R1 gacGGGACACCGCTGATCGTTTAAAATCCATACACCCTACAAAATCT
Foxp3.1_2-1S1 GTAATAGAAATTTAGAATTG
Foxp3.1_3-5F1 gacGGGACACCGCTGATCGTTTATTAGGTAGGGTGATGTGGGTGTTA
Foxp3.1_3-5R1 ACATCCAACCTTAAACCCCTCTA
Foxp3.1_3-5S1 TCCAAAAAAAACAAAAT
Foxp3.2_6-8F1 gacGGGACACCGCTGATCGTTTAAAGGAGGAAGAGAAGGGGGTAGAT
Foxp3.2_6-8R1 CACCCACATCACCCTACCTAAA
Foxp3.2_6-8S1 CCCTACCTAAACCTATCC
Foxp3.2_12-9F1 GGAGGTTGTTTTTGGGATATAGAA
Foxp3.2_12-9R1 gacGGGACACCGCTGATCGTTTAAAATTATCTACCCCCTTCTCTTCC
Foxp3.2_12-9S1 TTAGATTTTTTTGTTATTGA
Foxp3.2_10-13F1 gacGGGACACCGCTGATCGTTTAGTATGGAGGTTGTTTTTGGGATAT
Foxp3.2_10-13R1 ACCCCCTTCTCTTCCTCCTTATTA
Foxp3.2_10-13S1 ATAAAACCCAATACATCC
Foxp3.2_13-14F1 gacGGGACACCGCTGATCGTTTAGGTTGGGTTGGTTAGTTAGTTTTT
Foxp3.2_13-14R1 ACCCCCTTCTCTTCCTCCTTATTA
Foxp3.2_13-14S1 CAAAACCCAAATATAAACC
Universal M13Biotin
5′-BIOTAG-GGGACACCGCTGATCGTTTA
Pyrosequencing reactions were performed using the manufacturers protocol (PSQ96, Biotage Uppsala Sweden) and CpG methylation was analyzed using Q-CpG (Biotage) software.
Mixed lymphocyte reaction
PBMCs (1×105) and syngeneic irradiated CD45RO−CD25−CD4+ T cells conditioned ex vivo in the absence or presence of cilostamide were co-cultured with irradiated allogeneic PBMC (1 × 105) for 7 days, with 0.5 mCi/well 3H-thymidine added for the last 16 hours. Thymidine incorporation was measured by scintillation counting and results expressed as the mean of triplicate wells ± SD.
Transplant vasculopathy in human vessels
Human internal mammary artery side-branches retrieved with informed consent (REC Ref No. 07/H0605/130) were transplanted into BALB/c Rag2−/−/cγ−/− mice as aortic interposition grafts. The day after transplantation, mice were reconstituted intraperitoneally with human PBMCs with or without PDEi-conditioned T cells. Grafts were harvested on POD 30. Data were analyzed only from mice displaying >1% human CD45+ cells as judged by phenotypic analysis of the spleen. Intimal expansion on Elastin van Gieson (EvG)-stained sections was measured using Adobe Photoshop and calculated as: (Arealamina-Areaintima)/Arealamina) x100.
Statistical analysis
Two-tailed comparisons were made using the Mann-Whitney test except for transplant outcome data (Log-rank test) and ELISPOT analyses (Unpaired t-test).
One sentence summary.
A clinically approved agent can generate ex vivo graft-reactive, functional mouse and human regulatory T cells.
Acknowledgements
We are indebted to Professor David Taggart and the cardiac surgery team at the Nuffield Department of Surgery for providing human vessels from patients undergoing coronary artery bypass surgery, Dr. Dr Dimitris Kioussis for providing CBA-Rag−/−mice, Professor Herman Waldman for providing hCD25 transgenic mice, the staff of the BMS-JR for animal care, Dr. Nick Jones for advice and discussion of the data obtained using BM3 TCR transgenic mice and Dr. Jochen Huehn for critical appraisal of the manuscript. Funding: This work was supported by The Wellcome Trust, the European Union Framework 6 Integrated Project, RISET, and the British Heart Foundation (PG/06/050). GF received a Dorothy Hodgkin Post-graduate award and support from the China-Oxford Scholarship Fund. RF received a Kidney Research UK Training Fellowship. SN received an American Society of Transplantation Research Fellowship Award. AS is supported by the Swedish Heart and Lung Foundation and the Swedish Research Council. KJW holds a Royal Society Wolfson Research Merit Award. SB is funded by the Wellcome Trust (084071).
Footnotes
Competing interests: The authors declare that they have no competing interests.
References and Notes
- 1.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. Nat. Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 2.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova J-L, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli F. Dagna, Byrne G, McEuen M, Proll S, Appleby M, Brunkow ME. Nat. Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 3.Hori S, Nomura T, Sakaguchi S. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 4.Williams LM, Rudensky AY. Nat. Immunol. 2007;8:277–284. doi: 10.1038/ni1437. [DOI] [PubMed] [Google Scholar]
- 5.Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, Eberl G, Hamann A, Wagner H, Huehn J, Sparwasser T. J. Exp. Med. 2007;204:57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor PA, Lees CJ, Blazar BR. Blood. 2002;99:3493–3499. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 7.Zheng SG, Gray JD, Ohtsuka K, Yamagiwa S, Horwitz DA. J. Immunol. 2002;169:4183–4189. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann P, Eder R, Kunz-Schughart LA, Andreesen R, Edinger M. Blood. 2004;104:895–903. doi: 10.1182/blood-2004-01-0086. [DOI] [PubMed] [Google Scholar]
- 9.Feng G, Gao W, Strom TB, Oukka M, Francis RS, Wood KJ, Bushell A. Eur. J. Immunol. 2008;38:2512–2527. doi: 10.1002/eji.200838411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng G, Wood KJ, Bushell A. Transplantation. 2008;86:578–589. doi: 10.1097/TP.0b013e3181806a60. [DOI] [PubMed] [Google Scholar]
- 11.Conti M, Beavo J. Annual Review of Biochemistry. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- 12.Kammer GM. Immunol. Today. 1988;9:222–229. doi: 10.1016/0167-5699(88)91220-0. [DOI] [PubMed] [Google Scholar]
- 13.Mandler R, Birch RE, Polmar SH, Kammer GM, Rudolph SA. Proc. Natl. Acad. Sci. U. S. A. 1982;79:7542–7546. doi: 10.1073/pnas.79.23.7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mary D, Aussel C, Ferrua B, Fehlmann M. J. Immunol. 1987;139:1179–1184. [PubMed] [Google Scholar]
- 15.Shore A, Dosch H-M, Gelfand EW. Nature. 1978;274:586–587. doi: 10.1038/274586a0. [DOI] [PubMed] [Google Scholar]
- 16.Goeken NE, Thompson JS. Human Immunol. 1982;4:37–45. doi: 10.1016/0198-8859(82)90048-9. [DOI] [PubMed] [Google Scholar]
- 17.Zheng Y, Rudensky AY. Nat. Immunol. 2007;8:457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 18.Hill JA, Feuerer M, Tash K, Haxhinasto S, Perez J, Melamed R, Mathis D, Benoist C. Immunity. 2007;27:786–800. doi: 10.1016/j.immuni.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 20.Kobie JJ, Shah PR, Yang L, Rebhahn JA, Fowell DJ, Mosmann TR. J. Immunol. 2006;177:6780–6786. doi: 10.4049/jimmunol.177.10.6780. [DOI] [PubMed] [Google Scholar]
- 21.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen J-F, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. J. Exp. Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niedbala W, Cai B, Liu H, Pitman N, Chang L, Liew FY. Proc. Natl. Acad. Sci. U. S. A. 2007;104:15478–15483. doi: 10.1073/pnas.0703725104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murad F. N. Engl. J. Med. 2006;355:2003–2011. doi: 10.1056/NEJMsa063904. [DOI] [PubMed] [Google Scholar]
- 24.Degerman E, Belfrage P, Manganiello VC. J. Biol. Chem. 1997;272:6823–6826. doi: 10.1074/jbc.272.11.6823. [DOI] [PubMed] [Google Scholar]
- 25.Bender AT, Beavo JA. Pharmacol. Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- 26.Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, Schlawe K, Chang H-D, Bopp T, Schmitt E, Klein-Hessling S, Serfling E, Hamann A, Huehn J. PLoS Biol. 2007;5:e38. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Francis RS, Feng G, Tha-in T, Lyons IS, Wood KJ, Bushell A. Eur. J. Immunol. 2011;41:726–738. doi: 10.1002/eji.201040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen W, Jin W, Hardegen N, Lei K.-j., Li L, Marinos N, McGrady G, Wahl SM. J. Exp. Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng SG, Meng L, Wang JH, Watanabe M, Barr ML, Cramer DV, Gray JD, Horwitz DA. Int. Immunol. 2006;18:279–289. doi: 10.1093/intimm/dxh368. [DOI] [PubMed] [Google Scholar]
- 30.Carvalho-Gaspar M, Jones ND, Luo S, Martin L, Brook MO, Wood KJ. J. Immunol. 2008;180:6640–6648. doi: 10.4049/jimmunol.180.10.6640. [DOI] [PubMed] [Google Scholar]
- 31.Kim JM, Rasmussen JP, Rudensky AY. Nature Immunol. 2006;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 32.Chatenoud L. Nat. Rev. Immunol. 2003;3:123–132. doi: 10.1038/nri1000. [DOI] [PubMed] [Google Scholar]
- 33.Bloom DD, Chang Z, Fechner JH, Dar W, Polster SP, Pascual J, Turka LA, Knechtle SJ. Am. J. Transplant. 2008;8:793–802. doi: 10.1111/j.1600-6143.2007.02134.x. [DOI] [PubMed] [Google Scholar]
- 34.Edinger M, Hoffman P, Ermann J, Drago K, Fathman CG, Strober S, Negrin RS. Nat. Med. 2003;9:1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 35.Nadig SN, Wieckiewicz J, Wu DC, Warnecke G, Zhang W, Schiopu A, Taggart DP, Wood KJ. Nat. Med. 2010;16:809–814. doi: 10.1038/nm.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trzonkowski P, Bieniaszewska M, Juscinska J, Dobyszuk A, Krzystyniak A, Marek N, Mysliwska J, Hellmann A. Clin. Immunol. 2009;133:22–26. doi: 10.1016/j.clim.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, DeFor T, Levine BL, June CH, Rubinstein P, McGlave PB, Blazar BR, Wagner JE. Blood. 2011;20:1061–1070. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luhder F, Katz J, Benoist C, Mathis D. J. Exp. Med. 1998;187:379–387. doi: 10.1084/jem.187.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. J. Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- 40.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 41.Bushell A, Wood K. Am. J. Transplant. 2007;7:759–768. doi: 10.1111/j.1600-6143.2006.01716.x. [DOI] [PubMed] [Google Scholar]
- 42.Benjamin RJ, Waldmann H. Nature. 1986;320:449–451. doi: 10.1038/320449a0. [DOI] [PubMed] [Google Scholar]
- 43.Karim M, Feng G, Wood KJ, Bushell AR. Blood. 2005;105:4871–4877. doi: 10.1182/blood-2004-10-3888. [DOI] [PubMed] [Google Scholar]
- 44.Coombes JL, Siddiqui KRR, Arancibia-Cárcamo CV, Hall J, Sun C-M, Belkaid Y, Powrie F. J. Exp. Med. 2008;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qin S, Cobbold S, Pope H, Elliott J, Kioussis D, Davies J, Waldmann H. Science. 1993;259:974–977. doi: 10.1126/science.8094901. [DOI] [PubMed] [Google Scholar]
- 46.Bopp T, Becker C, Klein M, Klein-Hessling S, Palmetshofer A, Serfling E, Heib V, Becker M, Kubach J, Schmitt S, Stoll S, Schild H, Staege MS, Stassen M, Jonuleit H, Schmitt E. J. Exp. Med. 2007;204:1303–1310. doi: 10.1084/jem.20062129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li L, Yee C, Beavo JA. Science. 1999;283:848–851. doi: 10.1126/science.283.5403.848. [DOI] [PubMed] [Google Scholar]
- 48.Nakata A, Ogawa K, Sasaki T, Koyama N, Wada K, Kotera J, Kikkawa H, Omori K, Kaminuma O. Clin Exp Immunol. 2002;128:460–466. doi: 10.1046/j.1365-2249.2002.01856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tost J, Gut IG. Nat. Protoc. 2007;2:2265–2275. doi: 10.1038/nprot.2007.314. [DOI] [PubMed] [Google Scholar]
- 50.Royo JL, Hidalgo M, Ruiz A. Nat. Protoc. 2007;2:1734–1739. doi: 10.1038/nprot.2007.244. [DOI] [PubMed] [Google Scholar]