Abstract
Over the past few decades, neutrophils and macrophages had co-occupied center stage as the critical innate immune cells underlying the pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease and lung parenchymal destruction (i.e., emphysema). While chronic exposure to smoke facilitates the recruitment of innate immune cells into the lung, a clear role for adaptive immunity in emphysema has emerged. Evidence from human studies specifically point to a role for recruitment and activation of pathogenic lymphocytes and lung antigen-presenting cells in emphysema; similarly, animal models have confirmed a significant role for autoimumnity in progressive smoke-induced emphysema. Increased numbers of activated antigen-presenting cells, Th1 and Th17 cells, have been associated with smoke-induced lung inflammation and production of the canonical cytokines of these cells, IFN-γ and IL-17, correlates with disease severity. These exciting new breakthroughs could open new avenues for developing effective new therapies for smoke-induced emphysema.
Keywords: adaptive immunity, antigen-presenting cells, cytokines, Th1, Th17
Emphysema: an important & underdiagnosed feature of chronic obstructive pulmonary disease
Chronic obstructive pulmonary disease (COPD) broadly encompasses chronic bronchitis with or without lung parenchymal destruction (emphysema). COPD is currently the fourth leading cause of death in the world but is expected to become the third by 2020 [1,101]. Whereas tobacco smoking in western societies is considered the main causative factor in COPD, in developing nations chronic exposure to toxins (e.g., coal dust and biomass fuels) and chronic respiratory infections, together with a rapidly rising prevalence of tobacco smoking, all contribute significantly to the increase in the incidence of COPD. The most common clinical presentation of COPD includes symptoms of chronic bronchitis that consist of the presence of a daily cough productive of sputum for 3 months of a year for 2 consecutive years. The diagnosis of emphysema, on the other hand, is made based on histology or radiographic findings that describe the disappearance of lung tissue. Most often, the predominant location of emphysema is found in the upper lobes, but a more uniform distribution throughout the lung fields is found in a subset of smokers with α-1-antitrypsin (α-1AT) deficiency [2,3]. The lack of specific symptoms in smokers with milder forms of emphysema largely explains the often long delay in diagnosis. Compounding this issue, pulmonary function tests and conventional chest roentgenograms are insensitive means of detecting the early stages of emphysema [4,5]. Consequently, smokers all too often present at their initial clinical evaluation with severe physical limitation and end-stage disease.
Computed tomography (CT) scanning of the chest, the most reliable noninvasive method of detecting emphysema, is not conducted as a routine part of evaluating symptomatic smokers and most radiology reports do not commonly describe the presence or quantify the extent of emphysema (Figure 1). Consequently, a complete phenotypic characterization of lung disease in smokers is often delayed until the onset of self-reported clinical symptoms and findings of airflow obstruction on pulmonary function tests, which further obscures the true prevalence and extent of tissue damage in the smoking population. Furthermore, epidemiological studies have shown that lung cancer risk is strongly associated with radiographic emphysema, independent of airflow obstruction [6,7]. The immune-mediated pathophysiological processes in the lungs of smokers that lead to emphysema and lung cancer is currently under intense investigation. However, because not all smokers develop emphysema and/or lung cancer, the role of genetic susceptibility in combination with environmental factors is thought to play an important role in disease initiation.
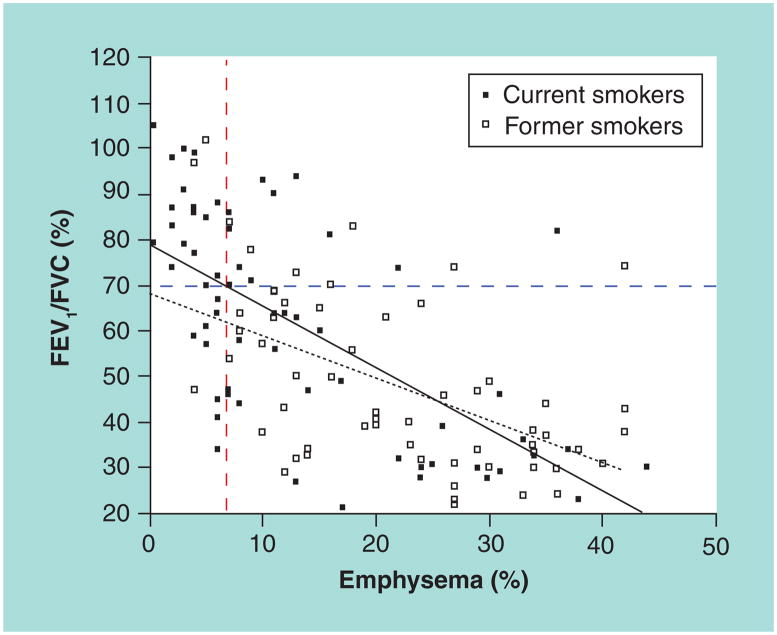
Figure 1. Positive correlation between emphysema (computerized tomography-based quantification) and airflow obstruction (pulmonary function tests) in ever-smokers.
FEV1 and FVC ratios were plotted against quantified measurement of emphysema using a CT scan of the chest in former (open squares) and current (closed squares) ever-smokers (n = 128). There are significant correlations between a decrease in airway obstruction (FEV1/FVC) ratio and an increase in percentage of emphysema in each group (p < 0.0001; r2 = 0.41 and r2 = 0.31 for current and former smokers, respectively). Note, however, that significant emphysema (>7% emphysema) is detected in 18 subjects with no evidence of airway obstruction (FEV1/FVC >70%). The dashed vertical bar indicates the threshold for emphysema (>7%) and the dashed horizontal bar represents the threshold for airway obstruction (FEV1/FVC <70%). Solid black line is correlation line (smokers) and dotted black line represents correlation in former smokers. FEV1: Forced expiratory volume in 1 s; FVC: Forced vital capacity.
Genetic basis of emphysema & autoimmunity
The genetic basis of emphysema is consistent with a heritable multifactorial trait that is reminiscent of a number of autoimmune genetic disorders [8]. Although cigarette smoke can provoke activation and recruitment of inflammatory cells to the lung, not all smokers develop the clinical features of smoker’s lung disease. The exact prevalence of emphysema is currently unknown and, to date, the only known gene linked to increased risk of early onset emphysema is mutations in the α-1AT protein [9], encoded by SERPINA1 gene, but many other susceptibility loci have been considered [10,11]. Allelic variance in SERPINA1 accounts for only 1–2% of all diagnosed cases of emphysema, suggesting that many additional genes, as yet undiscovered, influence the expression of the disease. Furthermore, even among carriers of different forms of α-1AT proteins, there is a large phenotypic variability, suggesting that together modifier genes and environmental exposure exert a combined effect.
Recent technological advances in high-throughput genetic analyses have promoted the collection of new information about the genetics of COPD and emphysema. The three basic approaches include:
Candidate gene-association studies;
Linkage analysis in multiplex families;
Genome-wide association studies.
To date, only a few genetic studies have explicitly examined emphysema as the main diagnostic criteria to determine susceptibility loci in human smokers. One such study attempted to identify genetic determinants of emphysema using genome-wide association studies in a large cohort (using CT-based diagnosis of emphysema). Here they described a SNP at the BICD1 locus, encoding a protein critical in dynein–dynactin interaction that was significantly associated with emphysema and was verified in two different cohorts [12]. Most of the other reports, however, have examined the genetics of COPD without distinguishing the individual component of emphysema as a risk factor [13]. In particular, scanning the entire genome for SNPs associated with COPD (irrespective of the presence or absence of emphysema), has led to the identification of several new risk genes, such as the α-nicotinic acetylcholine receptor (CHRNA 3/5) and HHIP, involved in human COPD [14]. It should be noted, however, that some of the identified risk loci (e.g., CHRNA) have also been linked to smoking behavior and risk of lung cancer [15,16], indicting the complexity of the genetic-association studies in humans.
Despite some new insights into the genetics of smoke-induced lung disease, predictably, a complex genetic basis is emerging where multiple genes contribute to the increased disease risk, and a number of genes (with the exception of α-1AT) generally introduce a modest independent effect [11]. This type of heterogeneity among different phenotypes within smokers is quite reminiscent of the common genes that underlie multiple autoimmune disorders because, much like genetic associations with autoimmunity, COPD gene associations are extremely modest. These findings further indicate that the utility of discovering the array of new genes may be to generate new hypothesis about the pathogenesis of such diseases with complex heritable multifactorial trait [8].
Critical innate immune cells & enzymes in emphysema
Collectively, several classes of proteinases display elastolytic properties because when administered intratracheally under experimental conditions, they can induce lung parenchymal lesions that mimic smoke-induced emphysema [17]. The most frequently associated and well-studied classes of proteinases implicated in human emphysema belong to the MMPs and serine proteinase (neutrophil elastase [NE]) that are primarily produced by macrophages and neutrophils, respectively [18]. In particular MMP9, MMP12 and NE are among the most abundantly expressed enzymes reported in the lungs of smokers and are required for induction of emphysema [19,20]. The activity of most of these enzymes is controlled by endogenous inhibitors such as tissue inhibitors of metalloproteinases and α-1AT, the principal antagonist of elastases. Beside their potent elastin-degrading property, MMP12 could neutralize the action of α-1AT, re-enforcing the potent elastolytic function of NE, while cigarette smoke exposure is sufficient to enhance elastase and reduce the activity of elastase inhibitors [21]. These biochemical events are critically important to the development of emphysema because elastin is an important matrix protein that is, in part, responsible for both the structural integrity of the lung and its elastance (ability to stretch). Loss of lung integrity in emphysema is due to the unopposed overproduction of elastases expressed by inflammatory cells present in the lungs and the persistence of chronic autoimmune inflammation directed against self-antigens in the lung [22,23].
In parallel to the possible role of smoke-induced recruitment of inflammatory cells to the lungs, proteolytic cleavage of matrix molecules have been shown to provide the necessary environmental factors associated with human COPD [24,25]. In particular, collagen degradation can elaborate N-acetyl Pro-Gly-Pro tripeptides that act as a potent neutrophil chemoattractant in vivo, a process that is mediated through activation of CXCR2 [26].
Emphysema & autoimmune inflammation
A role for autoimmune-mediated inflammation in COPD was first suspected when histological studies of human lung tissue revealed a preponderance of CD8+ T cells in the small and large airway biopsies in ever-smokers with COPD [27]. The Lung Health Study, a multicenter longitudinal study of ever-smokers in the USA and Canada revealed a subset of smokers who develop a more rapid decline in lung function despite a long period of abstinence from smoking [28]. Together, these experimental and clinical findings provided a strong rationale for a focused search for the presence of a dysregulated or systemic autoimmune inflammatory disorder in smokers with emphysema [29].
Although all human autoimmune diseases differ greatly in terms of their clinical manifestations, where studied in detail, these same syndromes are also remarkably similar with respect to fundamental immune characteristics. In addition to unremitting inflammation without apparent cause, many autoimmune syndromes are characterized by the enhanced expression of a unique pattern of proinflammatory cytokines that include IFN-γ, TNF and the more recently described, IL-17A [30]. Importantly, IFN-γ and IL-17A are the canonical cytokines secreted by major subsets of effector T helper cells, Th1 and Th17 cells, respectively. Experimental models of human autoimmune diseases have confirmed that both Th1 and Th17 cells and cytokines are essential mediators of disease [31–33]. The importance of TNF is further underscored by the transformative clinical importance of inhibitors of the TNF signaling pathway that are now widely used to suppress debilitating pain and inflammation in diverse autoimmune disorders [34]. In contrast to these studies, mice lacking T and B cells develop smoke-induced emphysema, indicating that acute exposure to smoke in mice is sufficient to induce lung disease [35]. Paradoxically, however, not all smokers develop emphysema and, unlike the findings in humans, emphysematous changes in the lungs of mice resolve following smoke cessation [36]. Therefore the role of the adaptive immune system in development of lung parenchymal destruction in smokers is most likely dependent on the heritable multifactorial trait that is currently under intense investigation.
One of the first studies that detailed a potential role for autoimmunity in response to cigarette smoke showed that former smokers with emphysema who were free of active infection harbor lung Th1 cells [37]. The same study further revealed that while IFN-γ does not affect the production of MMP12, indirectly IFN-γ inducible protein of 10 kDa (IP-10; CXCL10) induces the expression of this MMP in lung tissue macrophages [37]. In agreement with these findings, studies using human bronchoalveolar cells have shown that Th1-specific chemokine receptors (e.g., CXCR3) are expressed in the lungs of humans with COPD but not in other inflammatory disorders such as asthma [38].
Additional evidence of autoimmunity in COPD came from the discovery of a specific memory T-cell recall response to elastin fragments in human smokers with emphysema [22]. T cells isolated from peripheral blood of ever-smokers showed a significant increase in secretion of IFN-γ, while no changes were noted in Th2 cytokines such as IL-4 and IL-13 [22]. Further analysis of T cells extracted from the peripheral lung of smokers with stable, uncomplicated emphysema confirmed that these cells secrete IFN-γ and IL-17A in the absence of IL-4 and IL-13 [39]. Thus, these studies were the first to confirm that human emphysema is characterized immunologically by the expression of the key autoimmune signature consisting of Th1 and Th17 cells and their canonical cytokines and related chemokine receptors.
The discovery of Th1 and Th17 cytokines in the emphysematous lung further proved to be the key to unlocking the immunological basis of how lung destruction results from smoking. IL-17A promotes secretion of neutrophil-attracting chemokines, in part accounting for the chronic neutrophilia of COPD [40]. IL-17A and IFN-γ further act together to promote a pro-elastolytic lung environment because chemokines induced by IFN-γ such as CXCL10 and IL-17A act directly to stimulate MMP12 secretion by macrophages. IL-17A further induces the production of the chemokine CCL20, which attracts monocytes and promotes an increase in recruitment of APCs in the lungs [33,39]. Thus, tobacco smoke acutely elicits elastase secretion from innate immune cells [18], but ultimately recruitment of the APCs and an increase in activation of lymphocytes that show a Th1/Th17 predominance underlies chronic inflammation and progressive emphysema, even in individuals who have stopped smoking.
The role of B cells and, in particular, a pathogenic role for autoreactive antibodies has further been investigated in human COPD [41,42]. Using a multiple dilution technique to measure anti-elastin antibodies in plasma based on an internal standard in a well-characterized group of smokers, we reported an increase in the titers of anti-elastin antibodies in subjects with emphysema when compared with smokers without emphysema [22]. Recently, using a less rigorous method of antibody measurement (single dilution) in stored serum samples in smokers or nonsmokers, no correlation was found between airway obstruction and antiproline or anti-elastin antibodies [43]. Similarly, a small case report of subjects with emphysema and fibrosis failed to show significant elevation of anti-elastin antibodies [44]. These studies detected anti-elastin antibodies in smokers but not all found associations between such antibodies and COPD, which could be due to the differences in methodological and or in stratification of smokers which, unlike the original report [22], were not based on CT evidence of clinically significant emphysema. Moreover, few, if any, autoimmune inflammatory diseases are driven exclusively by pathogenic antibodies and antibodies to self-proteins have been reported to exist in disease-free subjects [45,46]. Thus, because of the inherent difficulties in detecting autoreactive antibodies and interpretation of their clinical significance [47,48], our studies have focused on autoreactive T cells, which we have specifically shown to be pathogenic and, relative to autoantibodies, are more definitively identified using modern immunological techniques.
Role of lung APCs in emphysema
In search of factors that might operate as breaks or accelerators of inflammation in emphysema, our focus has shifted to lung APCs that are critical in activating lymphocytes required for durable immunological memory. CD4+ and CD8+ T cells traverse the body and are typically activated only by professional APCs, such as dendritic cells (DC), including myeloid DCs and plasmacytoid DC subtypes [49]. By virtue of their long dendritic processes, myeloid DCs are thought to pick up antigens through cell surface receptors (e.g., CD205), but the final antigen presentation in the context of MHC molecules to T cells is facilitated through receptor-mediated endocytosis and antigen processing in deep lysosomes or peripheral endosomes [50]. Additional studies to further characterize specific APC subsets in normal and diseased lung and their specific costimulatory molecules (CD80, CD86, CD83, CD40) showed a functional role for T-cell activation in human emphysema [39]. How DCs undergo this unique maturation process remains an intense area of investigation, but lack of robust costimulatory molecule expression has been shown to induce tolerance against foreign antigens (e.g., ovalbumin), which is marked by the induction of antigen-specific immunosuppressive cells or Tregs [51]. Although difficult to assess rigorously, under normal conditions, human lung DC are thought to lack costimulatory molecules and therefore favor immunosuppressive Treg development. Unfortunately for smokers, the tolerogenic mission of the lung is usurped by mature myeloid DCs replete with costimulatory molecules that readily activate proinflammatory T cells [39].
The immunophenotype of lung APCs in current and never smokers has been evaluated [52,53]. Further, clinical data derived from lung function studies and chest CT scans have shown positive correlations between disease severity and activated APCs (expressing costimulatory markers CD80, CD83, CD86) concomitant with a marker of activated CD4+ T cells (CD69) [39]. Mature APCs expressing costimulatory molecules could activate CD4+ T cells; however, members of the B7 family of costimulatory molecules (CD80 and CD86), which bind to CD28 to activate T cells, can also efficiently bind to CTLA-4 on CD4+T cells and deliver an inhibitory signal. Therefore, the complex interplay between T cells and DCs requires better understanding of the nature of T-cell activation status and DC maturation under normal and pathogenic conditions. These findings indicate that T cells become activated through signals from lung APCs, implying that a process more fundamental than activated T cells most likely is the ultimate endogenous governor of the pathobiology of smoke-induced emphysema. Future studies are needed to dissect the genes regulating activated lung APCs that play a key role in promoting T-cell activation.
Role of respiratory infections in progression of autoimmunity & emphysema
The otherwise stable deterioration in lung function that characterizes COPD is punctuated in many patients by bouts of disease exacerbation marked by sudden, but transient worsening of lung function in the setting of an acute and febrile pneumonic or bronchitic syndrome. These episodes are thought to represent bouts of airway infection with viruses, especially human rhinovirus, or bacteria such as Haemophilus influenza [54,55]. Patients usually respond to anti-inflammatory therapy with glucocorticoids and antibiotics, but may not return to baseline lung function and often require mechanical ventilatory support [56–58]. However, activation of the costimulatory signals through pathogen-recognition receptors and Toll-like receptors on APCs most likely can activate this critical cell population in the lung and may initiate or worsen extant autoimmune inflammation in smokers [59–61]. Thus, emphysema appears to be a Th1/Th17-driven lung inflammatory process that is initiated by cigarette smoke and possible recurrent infections in which both the innate and autoimmune components promote elastolysis, loss of lung integrity and impaired lung function [39,56,62].
Emerging hypothesis on the autoimmune inflammatory causes of emphysema
The intriguing clinical and pathological observations made over the past few years have opened the door to a new concept: in susceptible individuals, cigarette-smoke exposure may trigger long-lasting inflammatory memory T-cell responses that can persist beyond the immediate period of exposure to cigarette smoke. Given that not all smokers develop lung disease, we propose that the perpetual inflammation seen in ever-smokers with emphysema is driven by multiple insults that include:
T-cell activation to specific lung matrix and cell-derived antigens (e.g., elastin, collagen or endothelial fragments);
Presentation of lung-specific antigens that results in the accumulation of activated, autoreactive T cells;
Transient production of the cytokines that induce proliferation of autoreactive T cells (i.e., IL-6, IL-1 and IL-17A);
Positive-loop feedback, in part governed by genetic factors that shapes the host response and may increase the clonal T-cell sensitivity to Th1/Th17 cytokines in the lung.
Most importantly, the continuous recruitment of activated lung APCs could further ensure propagation of autoinflammatory T-cell responses and the development of chronic progressive lung destruction [33]. Although activation and clonal expansion of T cells most likely plays an exceedingly important role in chronic inflammation, individual genetic susceptibility factors that govern how the immune system responds to antigens most likely contribute importantly to the resulting degree of autoimmune inflammation. As a result, during the multistep activation and expansion of autoreactive T cells, a spectrum of disease severity is seen in human smokers (Figure 2). This model of autoimmune inflammation could provide a reason as to why despite smoking cessation, the lung inflammation rages on [63].
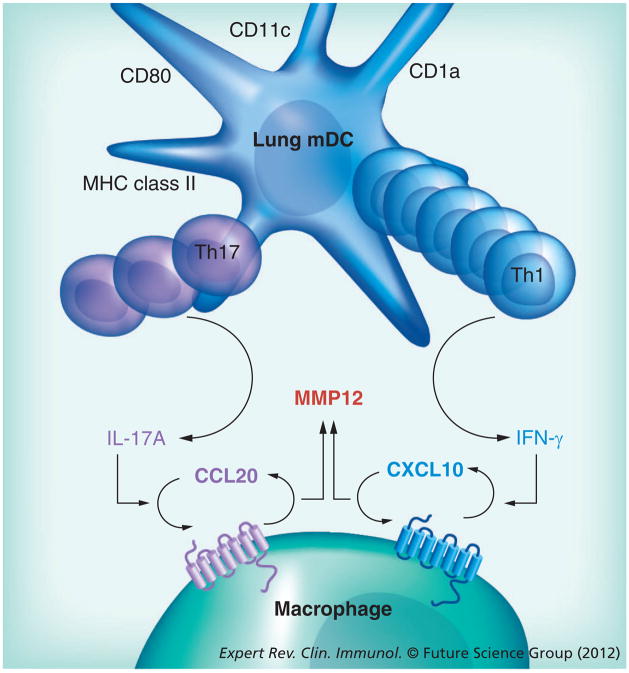
Figure 2. Autoimmune inflammation that causes emphysema.
Schematic diagram of possible mechanisms in lung CD1a+ dendritic cells, lung macrophages and CD4+ T-cell interaction in autoimmune-mediated destruction of lung parenchyma. Cigarette smoke could activate macrophages and induce CCL20 expression, which in turn, could recruit and activate CD1a+ lung dendritic cells. CD4+ T cells receive positive costimulatory signals from lung dendritic cells that present antigen through MHC II molecules and induce development of Th1 and Th17 subsets of T cells in the lung. The chronic presence of the relevant Th1 and Th17 chemokines could further induce MMP12 production and result in uncontrolled elastolysis in human lungs. mDC: Myeloid dendritic cell.
Expert commentary
The presence of chronic autoreactive systemic inflammation increasingly appears to be most strongly associated with emphysema in ever-smokers. Unresolved questions regarding the wide spectrum of lung diseases seen in smokers have prompted a large global mobilization of resources to assess different subphenotypes of human smokers. Most likely differences in response to antigens (self or foreign) by the major immune effector cells in the peripheral blood and lung APCs will provide a clue to the pathogenesis of emphysema. Although the fundamental mechanism by which lung inflammation is sustained in humans remains unknown, the finding that autoimmunity involving elastin and perhaps other endogenous antigens presents a solid platform to begin this important search [22,23]. Even more fundamentally, however, we can postulate that the act of smoking triggers an as yet unknown innate inflammatory response that activates lung APCs. This signaling pathway could comprise adjuvant-like ligands generated during smoking-induced lung damage that signal through APC-activating TLRs. The many chemically reactive molecules identified in cigarette smoke could further sufficiently alter the antigenic character of endogenous molecules such as elastin to render them more reactive to T and B cells, thereby breaking the lung tolerogenic state that normally precludes such autoreactivity. Obviously, much work remains before our understanding of emphysema pathogenesis begins to impact how we predict, diagnose and treat this devastating malady. However, we may now have a roadmap for dissecting the relevant cells and molecular pathways that probably play key roles in gaining this insight.
Five-year view
Current estimates are that the prevalence of COPD in the world will continue to rise despite a steady decline in cigarette consumption in western countries; this is in part owing to the lag in time for disease manifestation and a sharp rise in smoking habits in developing countries. In the next 5 years, the results of several ongoing multicenter studies will provide a better understanding of the pathobiology of human emphysema. Predictably, characterizing distinct clinical phenotypes and identifying the susceptibility genes will provide new guidance to the clinicians to not only provide personalized treatment to those with pure emphysema, airway obstruction or mixed types, but also will provide means to screen and identify smokers who are at a higher risk of developing COPD, and more specifically, lung destruction seen in emphysema. This goal will aid in implementation and delivery of personalized medicine to those at risk, and will potentially bring to light smokers with emphysema who may be at a higher risk of developing lung cancer who should be screened more effectively. Detection of a number of proteins or their modified forms that could pose as self-antigens in humans could provide useful tools for screening smokers with lung parenchymal damage, and offer therapy aimed to halt the destructive action of the pathogenic T cells in the lungs. In mice, evidence is mounting that many of the early immunological changes occur in response to pathogenic APCs prior to the onset of lung disease; while this could also be true in humans, there is no evidence to support this contention at the present time. Therefore, longitudinal T-cell-based studies in early smokers could help identify whether development of pathogenic T cells take place at an early time point during chronic exposure to smoke.
Key issues.
Susceptible ever-smokers continue to have progressive lung parenchymal disease, indicating a memory response to self-antigens.
The conundrum of progressive and destructive lung disease includes the need to find the possible threshold phenomenon in susceptibility genes that may dictate which smokers break tolerance to self-antigens.
While identification of those at high risk for development of emphysema is critical, developing personalized medicine to halt the progression of disease is dependent on early intervention.
Activation of proinflammatory genes in lung APCs most likely underlies the pathophysiology of emphysema and could potentially be targeted for effective therapy.
A key finding in autoimmune-mediated destruction of the lung would include delineation of the relevant antigen(s) in humans.
Acknowledgments
The authors thank the Corry/Kheradmand laboratory members for their insightful comments.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Financial & competing interests disclosure
Funding from the NIH (HL072419-A1) and Veterans Administration Medical Center paid part of F Kheradmand’s salary and postdoctoral research to study the role of T cells in human COPD and emphysema. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Brown DW, Croft JB, Greenlund KJ, Giles WH. Deaths from chronic obstructive pulmonary disease – United States, 2000–2005. J Am Med Assoc. 2009;301(13):1331–1333. [Google Scholar]
- 2.Takubo Y, Guerassimov A, Ghezzo H, et al. Alpha1-antitrypsin determines the pattern of emphysema and function in tobacco smoke-exposed mice: parallels with human disease. Am J Respir Crit Care Med. 2002;166(12 Pt 1):1596–1603. doi: 10.1164/rccm.2202001. [DOI] [PubMed] [Google Scholar]
- 3.Newell JD., Jr CT of emphysema. Radiol Clin North Am. 2002;40(1):31–42. doi: 10.1016/s0033-8389(03)00107-6. [DOI] [PubMed] [Google Scholar]
- 4•.Spaggiari E, Zompatori M, Verduri A, et al. Early smoking-induced lung lesions in asymptomatic subjects. Correlations between high resolution dynamic CT and pulmonary function testing. Radiol Med. 2005;109(1–2):27–39. One of the first studies in human smokers to show that emphysema could exist in asymptomatic smokers with normal spirometry. [PubMed] [Google Scholar]
- 5•.Hesselbacher SE, Ross R, Schabath MB, et al. Cross-sectional analysis of the utility of pulmonary function tests in predicting emphysema in ever-smokers. Int J Environ Res Public Health. 2011;8(5):1324–1340. doi: 10.3390/ijerph8051324. Study of a large population of ever-smokers that examined quantitative computed tomography-based emphysema and pulmonary function tests to show that routine screening studies using lung function tests underdiagnose emphysema. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spitz MR, Hong WK, Amos CI, et al. A risk model for prediction of lung cancer. J Natl Cancer Inst. 2007;99(9):715–726. doi: 10.1093/jnci/djk153. [DOI] [PubMed] [Google Scholar]
- 7•.Wilson DO, Weissfeld JL, Balkan A, et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med. 2008;178(7):738–744. doi: 10.1164/rccm.200803-435OC. Large human study that shows that independent of airflow obstruction, emphysema increases the risk of lung cancer. This type of epidemiological study makes an important point regarding the need to pursue different lung phenotypes in human smokers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gregersen PK, Olsson LM. Recent advances in the genetics of autoimmune disease. Annu Rev Immunol. 2009;27:363–391. doi: 10.1146/annurev.immunol.021908.132653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laurell CB, Eriksson S. The serum alpha-L-antitrypsin in families with hypo-alpha-L-antitrypsinemia. Clin Chim Acta. 1965;11:395–398. doi: 10.1016/0009-8981(65)90184-1. [DOI] [PubMed] [Google Scholar]
- 10.Castaldi PJ, Cho MH, Cohn M, et al. The COPD genetic association compendium: a comprehensive online database of COPD genetic associations. Hum Mol Genet. 2010;19(3):526–534. doi: 10.1093/hmg/ddp519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greene CM, Hassan T, Molloy K, McElvaney NG. The role of proteases, endoplasmic reticulum stress and SERPINA1 heterozygosity in lung disease and alpha-1 anti-trypsin deficiency. Expert Rev Respir Med. 2011;5(3):395–411. doi: 10.1586/ers.11.20. [DOI] [PubMed] [Google Scholar]
- 12.Kong X, Cho MH, Anderson W, et al. Genome-wide association study identifies BICD1 as a susceptibility gene for emphysema. Am J Respir Crit Care Med. 2011;183(1):43–49. doi: 10.1164/rccm.201004-0541OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. J Chron Obstruct Pulmon Dis. 2010;7(1):32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pillai SG, Ge D, Zhu G, et al. A genome-wide association study in chronic obstructive pulmonary disease (COPD): identification of two major susceptibility loci. PLoS Genet. 2009;5(3):e1000421. doi: 10.1371/journal.pgen.1000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thorgeirsson TE, Geller F, Sulem P, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452(7187):638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thorgeirsson TE, Gudbjartsson DF, Surakka I, et al. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat Genet. 2010;42(5):448–453. doi: 10.1038/ng.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan PD, Kuhn C, Pierce JA. The induction of emphysema with elastase. I The evolution of the lesion and the influence of serum. J Lab Clin Med. 1973;82(3):349–356. [PubMed] [Google Scholar]
- 18.Shapiro SD. Elastolytic metalloproteinases produced by human mononuclear phagocytes. Potential roles in destructive lung disease. Am J Respir Crit Care Med. 1994;150(6 Pt 2):S160–S164. doi: 10.1164/ajrccm/150.6_Pt_2.S160. [DOI] [PubMed] [Google Scholar]
- 19.Senior RM, Connolly NL, Cury JD, Welgus HG, Campbell EJ. Elastin degradation by human alveolar macrophages. A prominent role of metalloproteinase activity. Am Rev Respir Dis. 1989;139(5):1251–1256. doi: 10.1164/ajrccm/139.5.1251. [DOI] [PubMed] [Google Scholar]
- 20.Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol. 2009;4:435–459. doi: 10.1146/annurev.pathol.4.110807.092145. [DOI] [PubMed] [Google Scholar]
- 21.Churg A, Zay K, Shay S, et al. Acute cigarette smoke-induced connective tissue breakdown requires both neutrophils and macrophage metalloelastase in mice. Am J Respir Cell Mol Biol. 2002;27(3):368–374. doi: 10.1165/rcmb.4791. [DOI] [PubMed] [Google Scholar]
- 22••.Lee SH, Goswami S, Grudo A, et al. Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat Med. 2007;13(5):567–569. doi: 10.1038/nm1583. Studies the autoreactive response of T cells present in the peripheral blood of smokers with emphysema to a specific lung-derived antigen. [DOI] [PubMed] [Google Scholar]
- 23.Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med. 2009;360(23):2445–2454. doi: 10.1056/NEJMra0804752. [DOI] [PubMed] [Google Scholar]
- 24.Senior RM, Griffin GL, Mecham RP, et al. Val-Gly-Val-Ala-Pro-Gly, a repeating peptide in elastin, is chemotactic for fibroblasts and monocytes. J Cell Biol. 1984;99(3):870–874. doi: 10.1083/jcb.99.3.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Houwelingen AH, Weathington NM, Verweij V, et al. Induction of lung emphysema is prevented by L-arginine-threonine-arginine. FASEB Journal. 2008;22(9):3403–3408. doi: 10.1096/fj.07-096230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weathington NM, van Houwelingen AH, Noerager BD, et al. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med. 2006;12(3):317–323. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- 27•.Saetta M, Baraldo S, Corbino L, et al. CD8+ve cells in the lungs of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(2):711–717. doi: 10.1164/ajrccm.160.2.9812020. One of the first reports that showed T cells, in addition to innate immune cells, are increased in smokers with chronic obstructive pulmonary disease (COPD) [DOI] [PubMed] [Google Scholar]
- 28••.Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of lung health study participants after 11 years. Am J Respir Crit Care Med. 2002;166(5):675–679. doi: 10.1164/rccm.2112096. A landmark paper that showed approximately 8–10% of former smokers continue to have a decline in lung function, indicating that genetic and epigenetic factors (other than acute exposure to smoke) play a role in human COPD. [DOI] [PubMed] [Google Scholar]
- 29.Cosio MG, Guerassimov A. Chronic obstructive pulmonary disease. Inflammation of small airways and lung parenchyma. Am J Respir Crit Care Med. 1999;160(5 Pt 2):S21–S25. doi: 10.1164/ajrccm.160.supplement_1.7. [DOI] [PubMed] [Google Scholar]
- 30.Palmer MT, Weaver CT. Autoimmunity: increasing suspects in the CD4+ T cell lineup. Nature Immunology. 2010;11(1):36–40. doi: 10.1038/ni.1802. [DOI] [PubMed] [Google Scholar]
- 31.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 32.Fletcher JM, Lalor SJ, Sweeney CM, Tubridy N, Mills KH. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2010;162(1):1–11. doi: 10.1111/j.1365-2249.2010.04143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33••.Shan M, Yuan X, Song L, et al. Cigarette smoke induction of osteopontin (SPP1) mediates Th17 inflammation in human and experimental emphysema. Sci Transl Med. 2012;(4):117. doi: 10.1126/scitranslmed.3003041. Demonstrates that lung APCs are capable of transferring disease in an animal model of emphysema. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanauer SB, Cohen RD, Becker RV, 3rd, Larson LR, Vreeland MG. Advances in the management of Crohn’s disease: economic and clinical potential of infliximab. Clin Ther. 1998;20(5):1009–1028. doi: 10.1016/s0149-2918(98)80082-9. [DOI] [PubMed] [Google Scholar]
- 35.D’Hulst AI, Maes T, Bracke KR, et al. Cigarette smoke-induced pulmonary emphysema in SCID-mice. Is the acquired immune system required? Respiratory Research. 2005;6:147. doi: 10.1186/1465-9921-6-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guerassimov A, Hoshino Y, Takubo Y, et al. The development of emphysema in cigarette smoke-exposed mice is strain dependent. Am J Respir Crit Care Med. 2004;170(9):974–980. doi: 10.1164/rccm.200309-1270OC. [DOI] [PubMed] [Google Scholar]
- 37.Grumelli S, Corry DB, Song LZ, et al. An immune basis for lung parenchymal destruction in chronic obstructive pulmonary disease and emphysema. PLoS Med. 2004;1(1):e8. doi: 10.1371/journal.pmed.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panina-Bordignon P, Papi A, Mariani M, et al. The C-C chemokine receptors CCR4 and CCR8 identify airway T cells of allergen-challenged atopic asthmatics. J Clin Invest. 2001;107(11):1357–1364. doi: 10.1172/JCI12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39••.Shan M, Han-Fang Cheng HF, Song LZ, et al. Lung myeloid dendritic cells coordinately induce Th1 and Th17 responses in human emphysema. Sci Transl Med. 2009;1:132–140. doi: 10.1126/scitranlsmed.3000154. Demonstrates that dendritic cells in the lungs of smokers with emphysema are capable of activating autologous T cells. These studies are key in understanding the role of adaptive immunity in the propagation of autoreactive T cells in smokers with emphysema. [DOI] [PubMed] [Google Scholar]
- 40.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24(6):677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Feghali-Bostwick C, Gadgil A, Otterbein L, et al. Autoantibodies in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177(2):156–163. doi: 10.1164/rccm.200701-014OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nunez B, Sauleda J, Anto JM, et al. Anti-tissue antibodies are related to lung function in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;1183(8):1025–1031. doi: 10.1164/rccm.201001-0029OC. [DOI] [PubMed] [Google Scholar]
- 43.Greene CM, Low TB, O’Neill SJ, McElvaney NG. Anti-proline-glycine-proline or antielastin autoantibodies are not evident in chronic inflammatory lung disease. Am J Respir Crit Care Med. 2009;181(1):31–35. doi: 10.1164/rccm.200904-0545OC. [DOI] [PubMed] [Google Scholar]
- 44.Cottin V, Fabien N, Khouatra C, Moreira A, Cordier JF. Anti-elastin autoantibodies are not present in combined pulmonary fibrosis and emphysema. Eur Respir J. 2009;33(1):219–221. doi: 10.1183/09031936.00140208. [DOI] [PubMed] [Google Scholar]
- 45.Lacroix-Desmazes S, Kaveri SV, Mouthon L, et al. Self-reactive antibodies (natural autoantibodies) in healthy individuals. J Immunol Methods. 1998;216(1–2):117–137. doi: 10.1016/s0022-1759(98)00074-x. [DOI] [PubMed] [Google Scholar]
- 46.Zelenay S, Moraes Fontes MF, Fesel C, Demengeot J, Coutinho A. Physiopathology of natural auto-antibodies: the case for regulation. J Autoimmun. 2007;29(4):229–235. doi: 10.1016/j.jaut.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 47.da Mota LM, dos Santos Neto LL, de Carvalho JF. Autoantibodies and other serological markers in rheumatoid arthritis: predictors of disease activity? Clin Rheumatol. 2009;28(10):1127–1134. doi: 10.1007/s10067-009-1223-y. [DOI] [PubMed] [Google Scholar]
- 48.Jacob N, Stohl W. Autoantibody-dependent and autoantibody-independent roles for B cells in systemic lupus erythematosus: past, present, and future. Autoimmunity. 2010;43(1):84–97. doi: 10.3109/08916930903374600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.GeurtsvanKessel CH, Lambrecht BN. Division of labor between dendritic cell subsets of the lung. Mucosal Immunol. 2008;1(6):442–450. doi: 10.1038/mi.2008.39. [DOI] [PubMed] [Google Scholar]
- 50.Mahnke K, Guo M, Lee S, et al. The dendritic cell receptor for endocytosis, DEC-205, can recycle and enhance antigen presentation via major histocompatibility complex class II-positive lysosomal compartments. J Cell Biol. 2000;151(3):673–684. doi: 10.1083/jcb.151.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11(2):119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsoumakidou M, Zhu J, Wang Z, et al. Immunohistochemical detection of dendritic cells in human lung tissue. Histopathology. 2007;51(4):565–568. doi: 10.1111/j.1365-2559.2007.02813.x. [DOI] [PubMed] [Google Scholar]
- 53.Demedts IK, Bracke KR, Van Pottelberge G, et al. Accumulation of dendritic cells and increased CCL20 levels in the airways of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175(10):998–1005. doi: 10.1164/rccm.200608-1113OC. [DOI] [PubMed] [Google Scholar]
- 54.Singh M, Lee S-H, Porter P, et al. Human Rhinovirus proteinase 2A induces Th1 and Th2 immunity in COPD. J Allergy Clin Immunol. 2010;125:1369–1378. doi: 10.1016/j.jaci.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bandi V, Jakubowycz M, Kinyon C, et al. Infectious exacerbations of chronic obstructive pulmonary disease associated with respiratory viruses and non-typeable Haemophilus influenzae. FEMS Immunol Med Microbiol. 2003;37(1):69–75. doi: 10.1016/S0928-8244(03)00100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seemungal T, Harper-Owen R, Bhowmik A, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(9):1618–1623. doi: 10.1164/ajrccm.164.9.2105011. [DOI] [PubMed] [Google Scholar]
- 57.Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161(5):1608–1613. doi: 10.1164/ajrccm.161.5.9908022. [DOI] [PubMed] [Google Scholar]
- 58.Wilkinson TMA, Donaldson GC, Johnston SL, Openshaw PJM, Wedzicha JA. Respiratory syncytial virus, airway inflammation, and FEV1 decline in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173(8):871–876. doi: 10.1164/rccm.200509-1489OC. [DOI] [PubMed] [Google Scholar]
- 59.Takeuchi O, Hoshino K, Kawai T, et al. Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components. Immunity. 1999;11(4):443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 60.Doz E, Noulin N, Boichot E, et al. Cigarette smoke-induced pulmonary inflammation is TLR4/MyD88 and IL-1R1/MyD88 signaling dependent. J Immunol. 2008;180(2):1169–1178. doi: 10.4049/jimmunol.180.2.1169. [DOI] [PubMed] [Google Scholar]
- 61.Knobloch J, Schild K, Jungck D, et al. The T-helper cell type 1 immune response to Gram-negative bacterial infections is impaired in COPD. Am J Respir Crit Care Med. 2011;183(2):204–214. doi: 10.1164/rccm.201002-0199OC. [DOI] [PubMed] [Google Scholar]
- 62.Shapiro SD, Campbell EJ, Welgus HG, Senior RM. Elastin degradation by mononuclear phagocytes. Ann NY Acad Sci. 1991;624:69–80. doi: 10.1111/j.1749-6632.1991.tb17007.x. [DOI] [PubMed] [Google Scholar]
- 63.Shapiro SD. End-stage chronic obstructive pulmonary disease: the cigarette is burned out but inflammation rages on. Am J Respir Crit Care Med. 2001;164(3):339–340. doi: 10.1164/ajrccm.164.3.2105072c. [DOI] [PubMed] [Google Scholar]
Website
- 101.GOLD Executive Committee. [Accessed 21 May 2009];Global strategy for the diagnosis and prevention of COPD. www.goldcopd.org.