Abstract
Proton-based chemical shift imaging (CSI) probes were encapsulated inside nano-carriers to increase the sensivitity of the reporters. Co-encapsulation with a relaxation agent results in improved sensitivity and suppresses background signals. Simultaneous imaging of different chemical shift reporters allows multiplexed detection.
In conventional biomedical magnetic resonance imaging (MRI), water and lipid protons are imaged. MRI is an insensitive technique and water and fat represent the largest concentrations of mobile protons in the body. Contrast agents based on gadolinium complexes or iron oxide nanoparticles shorten the relaxation times of biological water and induce image contrast. Limitations of these contrast agents are the inability to quantify the agent concentration in vivo and to simultaneously detect more than one type of contrast agent, i.e. multiplexed imaging.
More specific biochemical information can be obtained from magnetic resonance spectroscopy (MRS) or chemical shift imaging (CSI) of small molecules which reports directly on the imaging agent and its concentration. However this technique suffers from the inherent low sensitivity of NMR and millimolar (mM) concentrations of agent are required. Higher field strength scanners provide improved sensitivity and this has led to an increased interest in chemical shift agents. Numerous 19F-MRI agents have been proposed on the basis of the relatively high sensitivity of 19Fand the lack of endogenous 19F signal.(1) More sensitive still is to use the 1H nucleus, but here a challenge is the narrow 1H chemical shift window with its high background of water, fat, and endogenous metabolites.
Some notable 1H CSI probes include YbDOTMA (Figure 1) or its thulium analogue,(2,3) hexamethyldisiloxane (HMDS), and (±)imidazol-1-yl)succinic acid (ISUCA) that have been proposed as responsive agents for temperature, partial oxygen pressure (pO2) or pH, respectively.(2,4,5) YbDOTMA is a highly soluble, inert complex that has 12 equivalent methyl protons for higher sensitivity. The paramagnetic Yb(III) shifts these protons outside the typical diamagnetic proton chemical shift range, with its high endogenous background signal, without causing significant line broadening. However on its own, YbDOTMA has no specific biological targeting and is rapidly excreted. HMDS has 18 equivalent protons at 0 ppm. Injection of neat, water insoluble HMDS directly into muscle or tumours in rats enabled quantitative studies of tissue oxygenation, but the invasive route of administration and the very slow clearance of the agent limit the applicability of this agent to animal studies.(5) The water soluble ISUCA has a very short blood half-life and contains only one proton that is shifted from the natural background. Therefore, a continuous infusion is required to maintain a concentration sufficiently high for pH determination.(4) In general, the sensitivity of these contrast agents is low and concentrations in the millimolar range are required for imaging.
Figure 1.
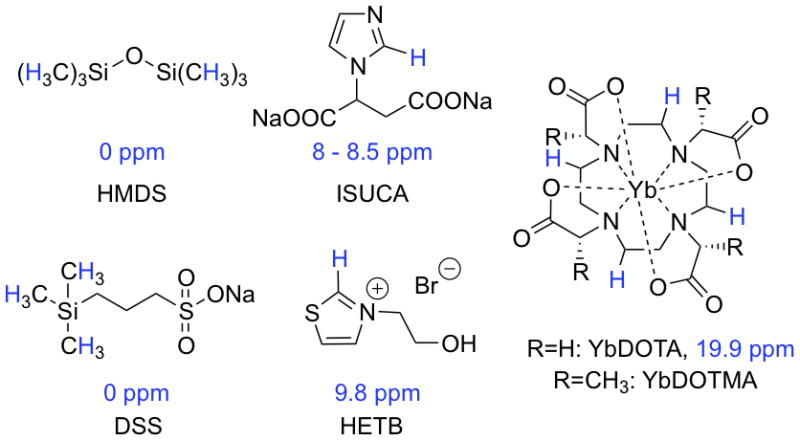
1H-CSI imaging agents. Protons with relevant chemical shifts are highlighted in blue.
Here, we describe approaches to increase the sensitivity of 1H-CSI probes by encapsulation of the chemical shift reporters inside liposomal nano-carriers. We demonstrate the potential for multiplexed imaging using three different probes with chemical shift signatures that are outside the biological proton shift window. We further show that incorporating a relaxation agent within the nano-carrier results in improved detection sensitivity.
The trimethylsilyl (TMS) group containing nine magnetically equivalent protons with a chemical shift of 0 ppm is used in NMR experiments as a standard since it is shifted from most common resonances. Therefore, it is not surprising that compounds containing this moiety were already used as sensors in MRS.(5,6) We reasoned that sodium 3-(trimethylsilyl)-1-propanesulfonate (DSS, Figure 1) would offer high water solubility for nano-encapsulation. Furthermore, small hydrophilic anions are often well tolerated and we performed a cell toxicity assay on human umbilical vein endothelial cells (HUVEC, see supporting information) that confirmed the low toxicity of this compound (EC50 = 5 mM).
Protons exhibiting chemical shifts downfield of natural background are usually acidic and exchange with bulk water. This leads to line broadening and loss of signal. However, azolium salts bearing aliphatic substituents on the heteroatom(s) undergo slow proton exchange of the C2-proton in protic solvents and, therefore, can be considered as CSI probes as exemplified by ISUCA.(7) 3-(2-Hydroxyethyl)thiazolium bromide (HETB, Figure 1) was chosen as a model compound for this family of probes because the C2-proton of thiazolium salts have larger chemical shifts than their imidazolium analogues (9.8 ppm in this case).
Paramagnetic lanthanide ions shift protons in their proximity as a function of distance and orientation of the lanthanide-proton vector. Here, we investigate the chemically inert ytterbium complex of the symmetric ligand 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (YbDOTA, Figure 1) since it has a resonance at 19.9 ppm corresponding to four magnetically equivalent protons.(8)
To demonstrate the possibility for multiplex detection of these probes, phantoms were placed in an agar gel and imaged on a 9.4T small bore animal scanner with a STEAM sequence and VAPOR water suppression.(9,10) The five phantoms contained equimolar proton concentrations of a) DSS, b) HETB, c) HETB, DSS, YbDOTA, d) blank, and e) YbDOTA. To compensate for the bulk magnetic susceptibility effect of YbDOTA on the chemical shifts of DSS and HETB, equimolar YbCl3 was added to the samples that did not contain YbDOTA. Color overlays represent the chemical shift images generated at the characteristic resonance frequencies of each of the probes and show that simultaneous detection of all investigated compounds is possible.
On their own, small molecules like YbDOTA have limited applicability for in vivo CSI because of their non-specific distribution and their rapid blood clearance. However, it is known that large payloads of small molecules can be encapsulated inside liposomes or emulsions.(11) Moreover stealth liposome formulations have very long circulation times resulting in persistent MR signal.(12) Nanoparticle platforms have been widely used in molecular imaging to enable target-specific imaging of inflammation, angiogenesis, apoptosis, and atherosclerosis, demonstrating the broad applicability of such systems.(13–16)
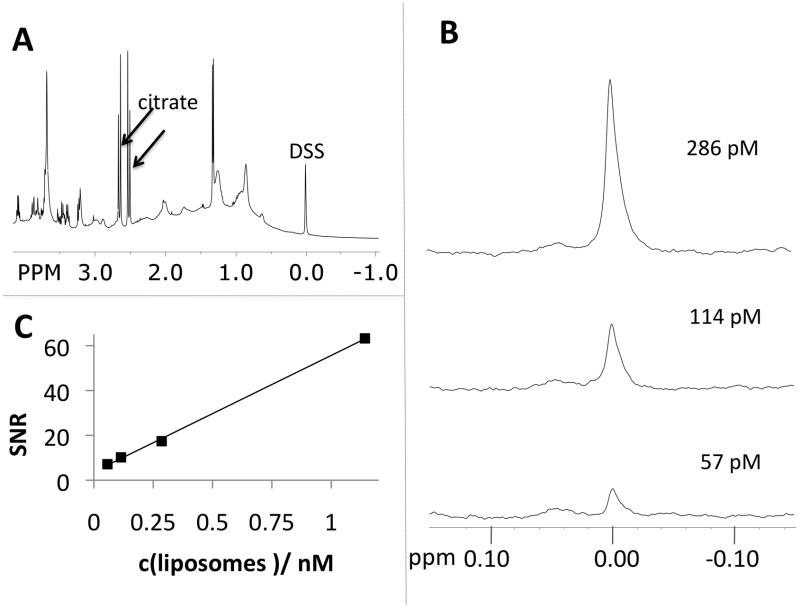
We encapsulated 135 mM DSS (isotonic) inside DSPC/cholesterol/PEG2000-PE liposomes resulting in a 1.2 M concentration of equivalent protons at 0 ppm in the liposome core. The vesicles showed a narrow size distribution (polydispersity index<0.11) and were stable over at least two weeks if stored at 4 °C. Figure 3A shows a 1H NMR spectrum of a 2.05 nM liposome suspension (6.3 μM DSS) in citrated blood plasma. We recorded spectra and calculated signal to noise ratios (SNR) for the TMS resonance for a series of dilutions (Figure 3C). The spectra show that the TMS resonance of DSS and the plasma resonances are well separated allowing for highly sensitive detection of liposomes; at the lowest dilution, a 57 pM liposome concentration was still readily apparent with SNR=7.1, Figure 3B. In vivo CSI will be less sensitive than ex vivo spectroscopy, but assuming a detection limit of 1 mM in protons for clinical CSI at 3T, this would represent a 40 nM detection limit for the DSS containing liposomes. This is well within the range of many vascular molecular imaging targets such as activated platelets, fibrin, the αvβ3 integrin, and the selectins. (17)
Figure 3.
1H NMR (11.7T, NS=32, LB=1.5Hz) of suspensions containing different concentrations of liposomes in citrated bovine plasma and phosphate buffered saline. A) spectrum showing plasma and DSS resonances, B) expansion of the TMS peak in DSS at liposome concentrations below 300 pM, C) SNR values as a function of liposome concentration.
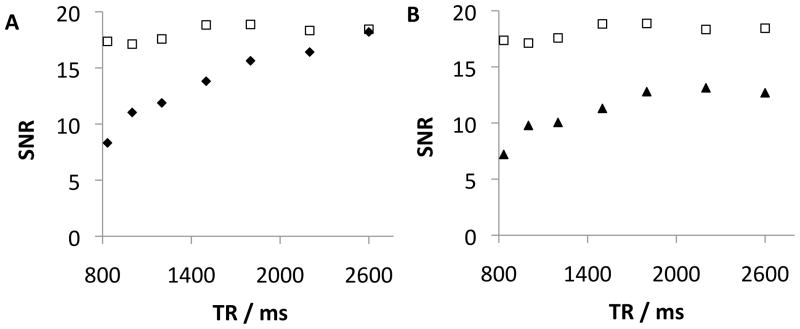
An additional problem in CSI experiments is the relatively long longitudinal relaxation times (T1) of nuclei under investigation. Such long T1 values require long repetition times (TR) to allow for recovery of the magnetization, and this in turn increases acquisition times. Reduction of T1 would reduce scan times or allow more scans to be performed in the same time period leading to even higher sensitivity.(1,18,19) A benefit of the liposome approach is that relaxation agents can also be encapsulated along with the chemical shift reporter. To demonstrate this, a dilute solution of the gadolinium(III) complex of 10-(2-hydroxypropyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triacetic acid (Gd-HPDO3A), a clinically approved MRI contrast agent, was co-encapsulated into liposomes together with DSS. CSI imaging with varying TR was performed on two phantoms containing liposome-encapsulated DSS with and without 2 mM Gd-HPDO3A inside the liposomes. As shown in Figure 4A, there is a significant signal loss for diamagnetic liposomes when TR is shortened whereas the relaxation agent counteracts this loss. This short TR allows for >3-fold reduction in acquisition time.
Figure 4.
SNR values as a function of repetition time (TR) for liposome suspensions in bovine plasma. A) SNR of DSS resonance at 0 ppm with (open squares) and without (diamonds) 2 mM Gd-HPDO3A in the aqueous core, B) SNR of 0 ppm DSS resonance (open squares) and the most intense plasma resonance (1.3 ppm, filled triangles) for liposomes containing 2 mM Gd-HPDO3A. 9.4T MR scanner, TE=3ms, NS=512, voxel size 0.125×0.125×0.2 cm. VAPOR water suppression.
Additionally, the ability of Gd(III) to shorten T1 is a function of the distance between the Gd(III) ion and the nucleus. Since Gd is entrapped within liposomes in the proximity of DSS, only the DSS protons are affected; endogenous nuclei will not experience significant changes in relaxation and thus loose signal faster with shortened TR than the imaging probe. This is illustrated in Figure 4B where the SNR of DSS co-encapsulated with Gd-HPDO3A is compared with the SNR of the most intense peak of plasma in the same voxel. The plasma signal relaxes slower than the DSS signal due to the local separation of Gd from the plasma components. Thus, the addition of small amounts of relaxation agent not only allows shorter imaging times, it also enables more selective detection of the imaging agent at a favorably shorter TR by suppressing the background signal.
Conclusions
In conclusion, the sensitivity of 1H CSI probes with distinct chemical shifts that are well separated from the natural background was increased to the low picomolar range by encapsulation into liposomes. The probes presented here are examples of imaging agents that can serve in multiplex imaging. For paramagnetic probes (e.g. YbDOTA), using the same ligand to complex different trivalent lanthanides will result in a wide dispersion of chemical shifts and offers another possibility for multiplexed detection. This is conceptually similar to the simultaneous detection of various lanthanide chelates in PARACEST or LIPOCEST experiments.(20) Addition of relaxation agents to the liposome core shortens acquisition times and enhances contrast. This approach is generalizable for multiplexed targeted molecular imaging using MRS or CSI (e.g. imaging platelets and fibrin in the context of thrombosis). By using different chemical shift reporters inside different targeted liposomes, it may be feasible to image two or more molecular targets simultaneously.
Supplementary Material
Figure 2.
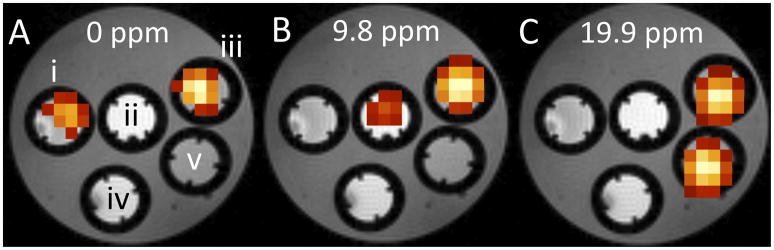
Color overlays showing the chemical shift image acquired on a 9.4T MR scanner for 13.5 mM equimolar proton concentrations. TE=3ms, TR=1500ms, NS=5096, voxel size 0.125×0.125×0.2 cm, VAPOR water suppression i) DSS and YbCl3, ii) HETB and YbCl3, iii) DSS, HETB, YbDOTA, iv) YbCl3, v) YbDOTA, A) overlay for CSI at 0 ppm (DSS resonance), B) overlay for CSI at 9.8 ppm (HETB resonance), C) overlay for CSI at 19.9 ppm (YbDOTA resonance).
Acknowledgments
This work was partially supported by a fellowship within the postdoc-program of the German Academic Exchange Service (DAAD), by grant R01EB009062 from the National Institute of Biomedical Imaging and Bioengineering, and by grants P41RR14075 and S10RR025563 from the National Center for Research Resources.
Footnotes
Electronic Supplementary Information (ESI) available: See DOI: 10.1039/b000000x/
Notes and references
- 1.Chalmers KH, De Luca E, Hogg NHM, Kenwright AM, Kuprov I, Parker D, Botta M, Wilson JI, Blamire AM. Design principles and theory of paramagnetic fluorine-labelled lanthanide complexes as probes for F-19 magnetic resonance: A proof-of-concept study. Chem - Eur J. 2010;16(1):134–148. doi: 10.1002/chem.200902300. [DOI] [PubMed] [Google Scholar]
- 2.Aime S, Botta M, Fasano M, Terreno E, Kinchesh P, Calabi L, Paleari L. A new ytterbium chelate as contrast agent in chemical shift imaging and temperature sensitive probe for MR spectroscopy. Magn Reson Med. 1996;35(5):648–651. doi: 10.1002/mrm.1910350504. [DOI] [PubMed] [Google Scholar]
- 3.Hekmatyar SK, Poptani H, Babsky A, Leeper DB, Bansal N. Non-invasive magnetic resonance thermometry using thulium-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetate (TmDOTMA−). Intern. J Hyperthermia. 2002;18(3):165–179. doi: 10.1080/02656730110098598. [DOI] [PubMed] [Google Scholar]
- 4.Provent P, Benito M, Hiba B, Farion R, Lopez-Larrubia P, Ballesteros P, Remy C, Segebarth C, Cerdan S, Coles JA, Garcia-Martin ML. Serial in vivo spectroscopic nuclear magnetic resonance imaging of lactate and extracellular ph in rat gliomas shows redistribution of protons away from sites of glycolysis. Cancer Research. 2007;67(16):7638–7645. doi: 10.1158/0008-5472.CAN-06-3459. [DOI] [PubMed] [Google Scholar]
- 5.Kodibagkar VD, Cui WN, Merritt ME, Mason RP. Novel H-1 NMR approach to quantitative tissue oximetry using hexamethyldisiloxane. Magn Reson Med. 2006;55(4):743–748. doi: 10.1002/mrm.20826. [DOI] [PubMed] [Google Scholar]
- 6.Kodibagkar VD, Wang XH, Pacheco-Torres J, Gulaka P, Mason RP. Proton imaging of siloxanes to map tissue oxygenation levels: A tool for quantitative tissue oximetry. NMR in Biomed. 2008;21(8):899–907. doi: 10.1002/nbm.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fahlbusch T, Frank M, Schatz J, Schühle DT. Kinetic acidity of supramolecular imidazolium salts - effects of substituent, preorientation, and counterions on H/D exchange rates. J Org Chem. 2006;71(4):1688–1691. doi: 10.1021/jo052319d. [DOI] [PubMed] [Google Scholar]
- 8.Aime S, Botta M, Ermondi G. NMR-study of solution structures and dynamics of lanthanide(III) complexes of DOTA. Inorg Chem. 1992;31(21):4291–4299. [Google Scholar]
- 9.Tkac I, Starcuk Z, Choi IY, Gruetter R. In vivo H-1 NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Medi. 1999;41(4):649–656. doi: 10.1002/(sici)1522-2594(199904)41:4<649::aid-mrm2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 10.Frahm J, Merboldt KD, Hanicke W. Localized proton spectroscopy using stimulated echoes. J Magn Reson. 1987;72(3):502–508. doi: 10.1002/mrm.1910170113. [DOI] [PubMed] [Google Scholar]
- 11.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 12.Lasic DD, Needham D. The “Stealth” Liposome: A prototypical biomaterial. Chem Rev. 1995;95(8):2601–2628. [Google Scholar]
- 13.Heath TD, Fraley RT, Papahadjopoulos D. Antibody targeting of liposomes - cell specificity obtained by conjugation of F(ab′)2 to vesicle surface. Science. 1980;210(4469):539–541. doi: 10.1126/science.7423203. [DOI] [PubMed] [Google Scholar]
- 14.Lanza GM, Yu X, Winter PM, Abendschein DR, Karukstis KK, Scott MJ, Chinen LK, Fuhrhop RW, Scherrer DE, Wickline SA. Targeted antiproliferative drug delivery to vascular smooth muscle cells with a magnetic resonance imaging nanoparticle contrast agent implications for rational therapy of restenosis. Circulation. 2002;106(22):2842–2847. doi: 10.1161/01.cir.0000044020.27990.32. [DOI] [PubMed] [Google Scholar]
- 15.Mulder WJM, Strijkers GJ, van Tilborg GAF, Griffioen AW, Nicolay K. Lipid-based nanoparticles for contrast-enhanced MRI and molecular imaging. NMR Biomed. 2006;19(1):142–164. doi: 10.1002/nbm.1011. [DOI] [PubMed] [Google Scholar]
- 16.Vucic E, Sanders HM, Arena F, Terreno E, Aime S, Nicolay K, Leupold E, Dathe M, Sommerdijk NA, Fayad ZA, Mulder WJ. Well-defined, multifunctional nanostructures of a paramagnetic lipid and a lipopeptide for macrophage imaging. J Am Chem Soc. 2009;131(2):406–407. doi: 10.1021/ja808310u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uppal R, Caravan P. Targeted probes for cardiovascular MR imaging. Future Med Chem. 2010;2(3):451–470. doi: 10.4155/FMC.09.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neubauer AM, Myerson J, Caruthers SD, Hockett FD, Winter PM, Chen JJ, Gaffney PJ, Robertson JD, Lanza GM, Wickline SA. Gadolinium-modulated F-19 signals from perfluorocarbon nanoparticles as a new strategy for molecular imaging. Magn Reson Med. 2008;60(5):1066–1072. doi: 10.1002/mrm.21750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee H, Price RR, Holburn GE, Partain CL, Adams MD, Cacheris WP. In-vivo F-19 MR-imaging - relaxation enhancement with Gd-DTPA. JMRI. 1994;4(4):609–613. doi: 10.1002/jmri.1880040416. [DOI] [PubMed] [Google Scholar]
- 20.Terreno E, Castelli DD, Viale A, Aime S. Challenges for molecular magnetic resonance imaging. Chem Rev. 2010;110(5):3019–3042. doi: 10.1021/cr100025t. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.