Abstract
Hypothesis
To test whether there are significant differences in pediatric and adult temporal bone anatomy as related to cochlear implant (CI) surgery
Background
Surgeons rely upon anatomical landmarks including the round window (RW) and facial recess (FR) to place CI electrodes within scala tympani. Anecdotally, clinicians report differences in orientation of such structures in children versus adults.
Methods
IRB approval was obtained. High resolution CT scans of 24 pediatric patient (46 ears) and 20 adult patients (40 ears) were evaluated using software consisting of a of a model-based segmentation algorithm that automatically localizes and segments temporal bone anatomy (e.g. facial nerve, chorda tympani, external auditory canal (EAC), and cochlea). On these scans, angles pertinent anatomy were manually delineated and measured blinded as to the age of the patient.
Results
The EAC and FR were more parallel to the basal turn (BT) of the cochlea in children versus adults (∠EAC:BT 20.55 ° vs. 24.28°, p = 0.003; ∠FR:BT 5.15° vs. 6.88°, p=0.009). And, the RW was more closely aligned with the FR in children versus adults (∠FR:RW 30.43° vs. 36.67°, p=0.009). Comparing the lateral portion of the EAC (using LatEAC as a marker) to the most medial portion (using ⊥TM as a marker), the measured angle in children was 136.57° and 172.20° in adults (p<0.001).
Conclusion
There are significant differences in temporal bone anatomy of children versus adults pertinent to CI electrode insertion.
Introduction
The frequency of cochlear implantation (CI) in the pediatric population has increased dramatically since the early 1990’s. While many surgeons feel CI can be more difficult in the pediatric population as compared to adults there is little evidence to support why this may be the case.
While embryologic growth of the temporal bone is fairly well understood, post-natal temporal bone growth is more highly debated. Historically, it was believed that the morphology and spatial orientation of the labyrinth did not change significantly after birth.1,2 It now appears that the cranium undergoes a bimodal growth curve occurring at ages 1-4 and then again during puberty which may have significant impact on temporal bone anatomy.3,4 It has been suggested that these changes occur in the mastoid process and tympanic and squamous portions of the temporal bone rather than in the bony labyrinth.4
With regard to CI relevant anatomy, the width of the facial recess (FR) does not appear to be significantly different in children and adults.5-8 Furthermore, there also does not appear to be differences in FR width in children less than one year old compared to those 2-3 years old.5 However, there is some evidence to show that the basal turn of the cochlea may change orientation with respect to the FR as an individual grows.9
While changes in one structure can alter a surgeon’s view, it is more often the relationship of multiple anatomic sites that can affect the ease or difficulty of surgery. Herein we discuss important anatomic relationships for CI surgery and how these relationships differ in children compared to adults.
Methods
Subjects
IRB approval was obtained. The pre-operative CT scans of patients undergoing CI were evaluated. Patients with cochlear malformations (e.g. common cavity, Mondini malformation) were excluded from this study. Each ear was treated as an independent variable in statistical analysis.
CT Scans
In constructing the models for the segmentation of the temporal bone anatomies, we used image volumes acquired from several scanners, including Philips Mx8000 IDT 16, Siemens Sensation Cardiac 64, and Philips Brilliance 64. The scans were acquired at 120-140 KVp and exposure times 265-1000 mA s. Typical scan resolution is 512 × 512 × 130 voxels for pediatric and 768 × 768 × 300 voxels for adult scans with typical voxel size of of 0.3 × 0.3 × 0.4 mm.
Automatic segmentation of anatomical structures
The ear canal, tympanic membrane, scala tympani, scala vestibuli, ossicles, facial nerve, chorda tympani, and the cochlea were automatically segmented in the CT images with a series of preiously-published algorithms. 10-15 While the detailed description of this is beyond the scope of the current paper., these techniques rely upon on refererence CT volumes, which are called atlases. For these atlases, models have been created using the manual delineation of structures of interest in a number of CT scans (12 scans for our pediatric model and 15 scans for our adult model). To segment a new image volume, the atlases are spatially aligned with the new image using automatic registration techniques, the models are projected from the atlases to the new volume, and automatically adjusted to precisely localize the structures of interest in this image volume using image intensity information.
After performing both intensity-based rigid registration—in which the atlas is, rotated, and translated to match the unknown CT scan—and intensity-based non-rigid registration—in which the images are stretched the external auditory canal (EAC), tympanic membrane (TM), ossicles, external boundary of the cochlea, and bone-encased regions of the FN (i.e. at the mastoid tip and at the 2nd genu) are accurately identified. To delineate the course of the FN and the chorda tympani (ChT), an additional algorithm is necessary which we term the NOMAD algorithm for navigated optimal medial axis and deformable model algorithm.10 The NOMAD algorithm was designed for segmenting tubular structures and begins with start and stop points for such structures which have been identified via the initial rigid and non-rigid registration. Starting with the facial nerve at the mastoid tip as a starting point, the algorithm analyzes each neighboring voxel and, based upon intensity and expected orientation, progresses through the image until the end point (2nd genu) is reached. In selecting which voxel to move to next, the algorithm uses information gathered from the models and determines the path in the images that matches best expected intensity values and direction at each point. This algorithm produces a central line for the FN and ChT. The complete structure is identified with a level-set based algorithm that expands the centerline.16 The expansion of the centerline is guided by a-priori information on structure diameter stored in the model and by the intensity values in the images, e.g., the algorithm stops where there is an edge in the image.
For the precise localization of the intracochlear anatomy, i.e., the scala tympani (ST) and scala vestibuli (SV) we use what is called a statistical shape model.17 We built these models using μCT scans (Scanco μCT scanner, voxel size 36 μm isotropic) of six cadaveric human cochleae in which the outline of ST and SV were manually delineated. These models capture anatomic variability in intracochlear structures and permit localizing the position of structure boundaries based on incomplete information, e.g., the positon of the ST and of the SV can be inferred from the position of the external boundary of the cochlea. Published work has shown the accuracy and reliability of these methods.10,11,14 An example of segmentation results are seen in figure 1.
FIG. 1.
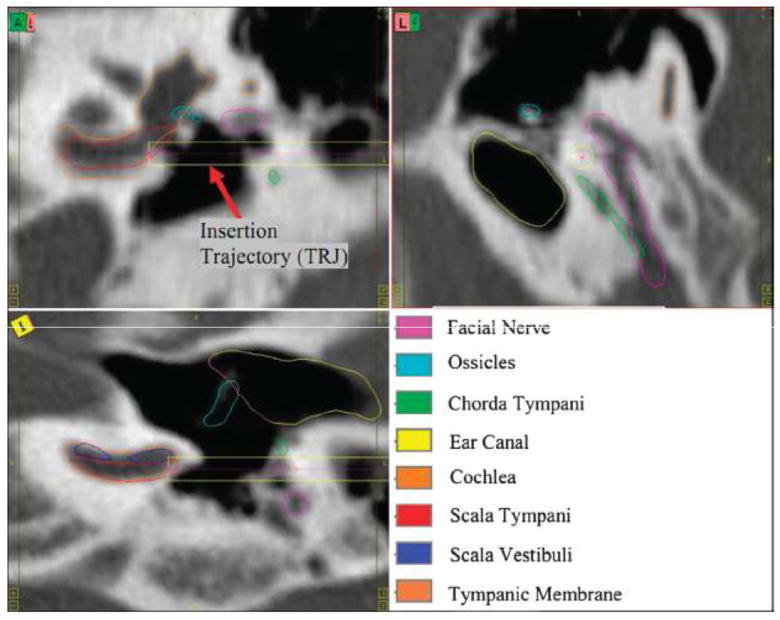
Contours of the temporal bone anatomy and trajectory computed using these segmentations.
Determination of anatomical relationships
In order to compare the orientations of the structures, the following lines were computed with no regard to the patient’s age (figure 2):
Drilling trajectory line (TRJ) – This line was computed using a method we have developed for computing an optimally safe trajectory for CI insertion that passes through the facial recess and through the round window into the scala tympani.12
Most lateral ear canal center line (LatEAC) – The start and end of the most lateral region of the bony ear canal were manually delineated. The start point is defined as the center of the most external portion of the ear canal and the second point as the center of the region of the ear canal just before the ear drum intersects it. As a result, a set of 3D points were produced in the reference volume. The 3D points were then projected onto each subject CT scan. Finally, the centers of mass of the projected points were used to mark the start and end points of the LatEAC line.
Line perpendicular to tympanic membrane(TM) plane (⊥TM) – The TM surface was manually segmented in the reference volume. Then, points on the TM surface were projected onto each subject CT scan. A plane was subsequently fitted to the projected points to define the tympanic plane (TP) in each subject CT scan. Finally, the normal to the TP (⊥TM) that passes through its center, which is defined as the center of mass of the projected points, was computed.
Line perpendicular to the round window (⊥RW) – The RW surface was manually delineated in the reference volume. Then, the points on this surface were projected onto each subject CT scan. A plane was fitted to the set of projected points to define the RW plane. Finally, the normal to this plane (⊥RW) that passes through its center, which is defined as the center of mass of the projected points, was computed.
Long axis of the basal turn of the cochlea (BT) – The first point was manually localized near the round window at the end of the scala tympani surface, and its location saved in the reference volume. Next, the central axis of the cochlea was manually determined in the same volume. Then, a second point was localized 180 degree away from this point also in the reference volume. The angle was measured as follows: (1) a line perpendicular to the central axis was computed from the first point to the central axis, (2) the second point was moved along the centerline of the scala tympani, (3) a line perpendicular to the central axis was computed from the current location of the second point to the central axis, (4) the desired angle was then computed between the lines computed in (1) and in (3). Finally, the two points are projected onto each subject CT scan to produce the line of the long axis of the scala tympani.
Line perpendicular to the plane that bisects the facial recess (⊥FR) – A plane that passes through the facial recess was defined using three points on each subject CT scan. Two of the points that are used in defining the plane are the start and end points of the insertion trajectory. The third point was estimated as the medial point (center of mass) of the region of the lower end of the chorda tympani and facial nerve. Finally, the line perpendicular to the defined plane (⊥FR) that passes through the center of mass of the three points was computed.
FIG. 2.
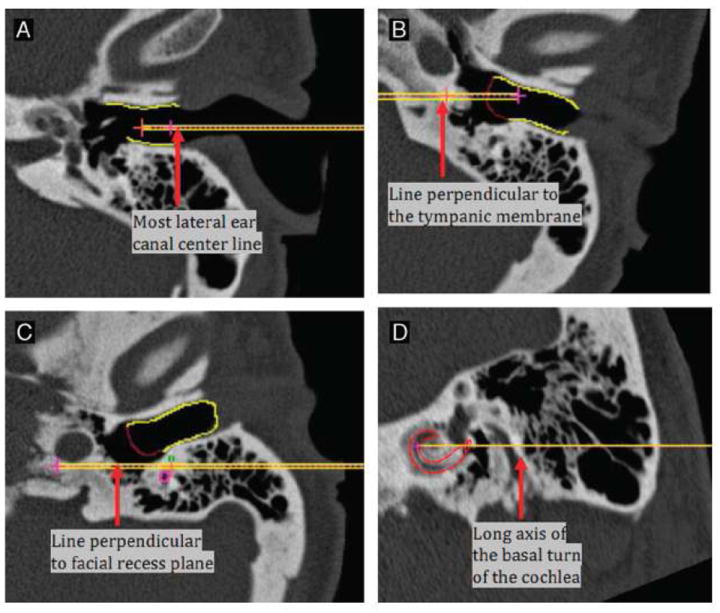
Examples of vectors identified. (A) Most lateral ear canal center line (Lat EAC): EAC is outlined in yellow. (B) Line perpendicular to TM (ˆTM): gain EAC is yellow and TM is outlined in red. (C) Line perpendicular to facial recess plane (ˆFR): facial nerve is outline in pink; chorda tympani is outlined in green. (D) Long axis of the basal turn of the cochlea (BT): scala tympani is outlined in red.
The angles between the lines were used as features. These are obtained by computing the arc cosine of the normalized dot product of the lines (vectors). Although labeling is difficult in two dimensional radiographs, figure 3 provides examples of the angles compared for this paper.
Figure 3.
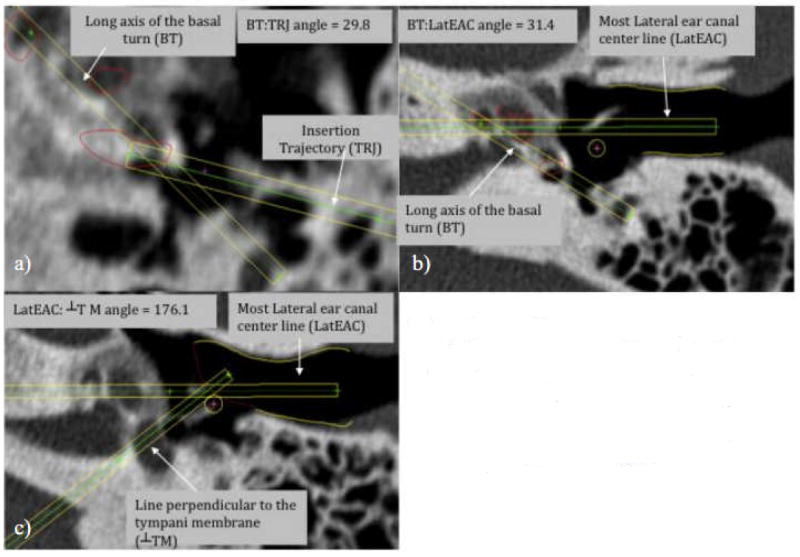
a) Comparison of BT and TRJ (scala tympani in red) b) Comparison of BT and LatEAC (scala tympani in red;lateral EAC in yellow) c) Comparison of LatEAC and line perpendicular to TM (TM in red; EAC in yellow)
Statistical methods
Comparisons between the pediatric and adult samples were conducted using mixed effects linear modeling analysis that included the ear within patient as a random effect. The distributions of the angle values were evaluated for normality prior to conducting tests of statistical significance. Some values were severely skewed and were rank transformed for appropriate use of the mixed effects linear modeling approach. Associations of age with the angle measures within each patient group were conducted using Spearman rank correlations.
Results
In total 46 pediatric ears (23 patients) and 40 adult ears (20 patients) were analyzed. The pediatric and adult median ages were 3.0 (range 0.8-16 year) and 53.0 (range 30-75 years) years respectively.
Orientation about BT of the cochlea
Orientation about the BT was chosen as it represents the target for CI electrode placement. Two of the major visual hurdles for CI are the relationships between the RW and the EAC and the RW and the FR. In this study, the BT serves as a marker for round window and cochlear orientation, while the LatEAC and ⊥FR are markers for the axis of the EAC and FR, respectively (figure 4). In comparing the angles between these structures in children versus adults, there was a clear statistical difference observed (table 1). The average angle between BT and LatEAC (∠BT:EAC) was 20.55° in children and 24.28° in adults (p=0.003) and the ∠BT:⊥FR was 5.15° in children and 6.88° in adults (p=0.009) (figure 1). Since there is difference in EAC orientation in children and adults (described later), we also measured the angle of the more medial portion of the EAC (⊥TM) and the BT. Similar to LatEAC, the difference in BT:⊥TM orientation was statistically significant (53.96° children vs. 31.47° adults; p<0.001). There was also a statistically significant difference when comparing the selected ideal drilling trajectory for scala tympani electrode insertion to the BT (32.56° children vs. 21.90° adults; p<0.001).
Figure 4.
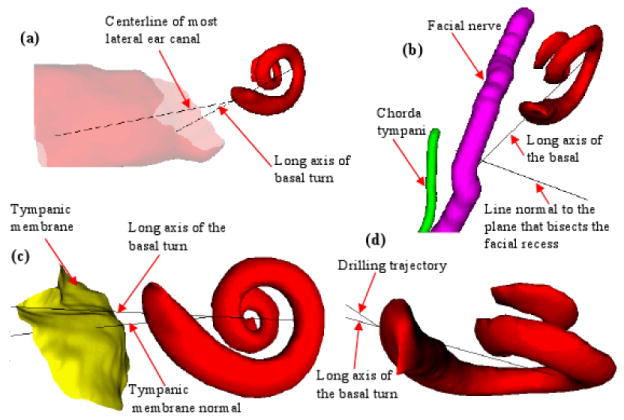
Orientation about the long axis of the basal turn of the cochlea. Demonstration of the angle between the: a) the center line of the most lateral ear canal (LatEAC) and the long axis of the basal turn of the cochlea (BT); b) line perpendicular to the facial recess (⊥FR) and the long axis of the basal turn of the cochlea (BT) (purple: facial nerve; green = chorda tympani); c) line perpendicular to the tympanic membrane (⊥TM) and the long axis of the basal turn of the cochlea (BT); d) the selected drilling trajectory (TRJ) and the long axis of the basal turn of the cochlea (BT).
Table 1.
Comparison of relationship about the long axis of the basal turn of the cochlea.
| Relationship | Average Pediatric Angle (SD) | Average Adult Angle (SD) | P value |
|---|---|---|---|
| BT:LatEAC | 20.55 (0.82) | 24.28 (0.88) | 0.003 |
| BT:⊥FR | 5.15 (0.62) | 6.88 (0.66) | 0.009 |
| BT:⊥TM | 53.96 (0.94) | 31.47 (1.01) | <0.001 |
| BT:TRJ | 32.56 (1.98) | 21.90 (2.13) | <0.001 |
(SD=standard deviation; BT=long axis of the basal turn of the cochlea; LatEAC=center line of the external auditory canal; ⊥FR=line perpendicular to the facial recess; ⊥TM =line perpendicular to the tympanic membrane; TRJ=ideal drilling trajectory for cochleostomy)
Orientation about the centerline of the lateral most portion of the EAC
In anecdotal reports, the lateral portion of the EAC in children can be a substantial impediment to the visualization necessary for cochlear implantation. Indeed there does appear to be statistical differences in EAC orientation in children compared to adults (figure 5, table 2). When comparing the lateral portion of the EAC (using LatEAC as a marker) to the most medial portion (using ⊥TM as a marker), the measured angle in children was 136.57° and 172.20° in adults (p<0.001).
Figure 5.
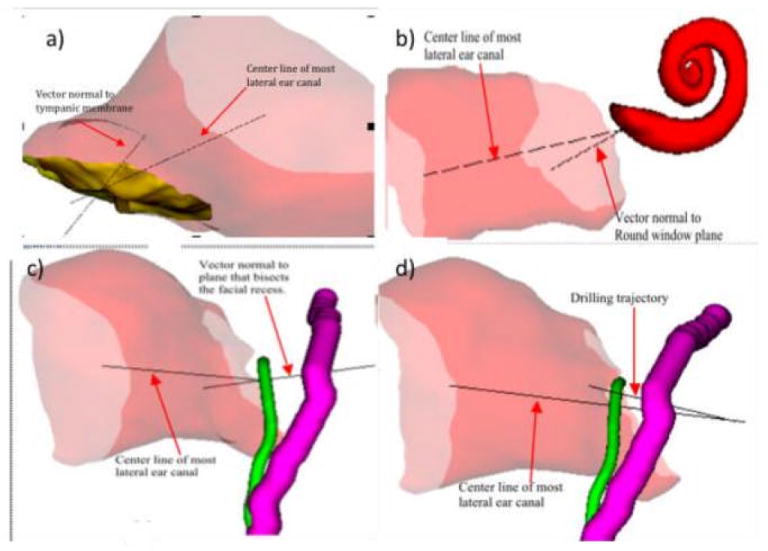
Orientation about the center line of the lateral most portion of the EAC. Demonstration of the angle between the: a) line perpendicular to the TM (⊥TM) and the center line of the lateral most portion of the EAC (LatEAC); b) line perpendicular to the round window plane (⊥RW) and the center line of the lateral most portion of the EAC (LatEAC); c) line perpendicular to the plane of the facial recess and the center line of the lateral most portion of the EAC (LatEAC); d) the drilling trajectory line (TRJ) and the center line of the lateral most portion of the EAC (LatEAC) (purple=facial nerve; green=chorda tympani).
Table 2.
Comparison of relationships about the lateral most portion of the EAC.
| Relationship | Average Pediatric Angle (SD) | Average Adult Angle (SD) | P value |
|---|---|---|---|
| LatEAC:⊥TM | 136.57 (1.08) | 172.20 (1.15) | 0.003 |
| LatEAC:⊥RW | 43.41 (1.09) | 52.34 (1.17) | 0.009 |
| LatEAC:⊥FR | 10.06 (0.81) | 14.42 (0.87) | <0.001 |
| LatEAC:TRJ | 23.51 (2.47) | 19.40 (2.65) | <0.001 |
(SD=standard deviation; LatEAC=center line of the external auditory canal; ⊥TM =line perpendicular to the tympanic membrane; ⊥RW =line perpendicular to the plane of the round window; ⊥FR =line perpendicular to the facial recess; TRJ=ideal drilling trajectory for cochleostomy)
We then compared the LatEAC to other important anatomical sites important for cochlear implantation (table 2). With regard to RW visualization, the angle between Lat EAC and a plane perpendicular to the RW (∠LatEAC:⊥RW) was 43.41° in children and 52.34 in adults (p<0.001). The difference in orientation of LatEAC and the plane perpendicular to the (⊥FR) was statistically significant (10.06° children and 14.42° adults; p=0.01) as was the difference in LatEAC and TRJ orientation (23.51° children and 19.40° adult; p=0.005).
Orientation about the round window
We chose to use a vector perpendicular to the round window plane (⊥RW) as a marker for RW orientation (figure 6). In comparing the relationship between ⊥RW and the ideal drilling trajectory (TRJ) we found no statistically significant difference between children and adults (41.39° vs. 39.47; p=0.93)(table3). However, when evaluating the differences in orientation between ⊥RW and ⊥FR, there was a statistically significant difference (30.43° children vs. 36.67° adults; p<0.001).
Figure 6.
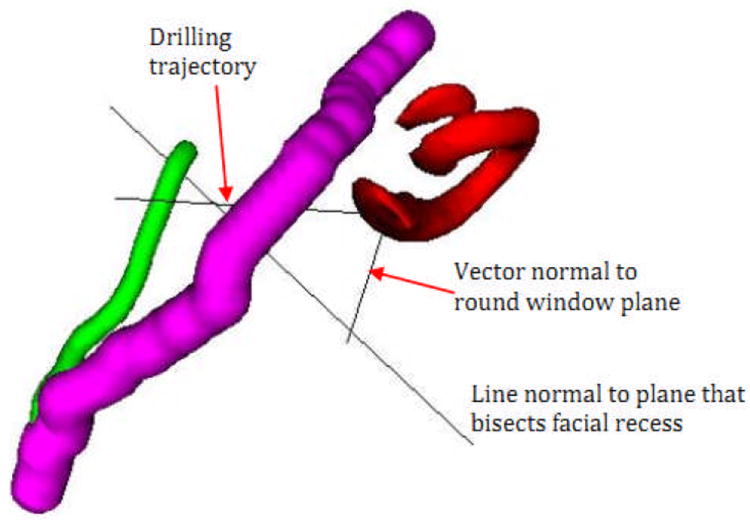
Orientation about the round window. Demonstration of the angle between the optimal drilling trajectory (TRJ) and the line perpendicular to the round window (⊥RW) as well as the line perpendicular to the facial recess plane and the line perpendicular to the round window (⊥RW).
Table 3.
Comparison of relationships about the round window.
| Relationship | Average Pediatric Angle (SD) | Average Adult Angle (SD) | P value |
|---|---|---|---|
| ⊥RW:TRJ | 41.39 (1.30) | 39.47 (1.39) | 0.003 |
| ⊥RW:⊥FR | 30.43 (0.94) | 36.67 (1.00) | 0.009 |
(SD=standard deviation; ⊥RW=line perpendicular to the plane of the round window; ⊥FR=line perpendicular to the facial recess; TRJ=ideal drilling trajectory for cochleostomy)
Discussion
Data pertaining to changes in cochlear orientation with increasing age are sparse, with multiple sources stating that no such changes occur.1,2 While this paper does not deal directly with single anatomical site variation with age, it does display significant changes in relationships in temporal bone anatomy relevant to CI. These orientations are important in otologic surgery as a surgeon depends heavily on anatomic relationships to estimate anatomy hidden by bone.
Exposure of the facial recess
The description of the differences in EAC anatomy in children versus adults is an interesting but not novel finding.5 While it is well known that the EAC orientation changes from an obtuse angle to more of a straight line, there is no objective data to describe this. We found that angle between the lateral EAC and the more medial EAC changes on average 35.63 (p=0.003) in the adult population compared to children. The more posterior orientation of the lateral EAC could have significant impact on visualization of the FR and the RW.
With regard to the relationship of the latEAC and the FR, we also found a statistically significant difference in this orientation in children and adults. This angle is more acute in children meaning that the surgeon has a more narrow view of the FR in the pediatric population; this suggests more difficulty placing CI electrodes via the FR. Based on our data, it is impossible to say whether this result is from changes solely in EAC or FR orientation. However, based on the above data, it is likely due to changes in both as there were significant difference in each orientation with regard to the ideal cochleostomy drilling trajectory and the long axis of the basal turn of the cochlea. This calls into question prior belief of minimal post-natal movement of the vertical segment of the facial nerve.7,18,19
Exposure of the round window and cochlea
We found several significant differences with orientation about the RW and the cochlea. The angles between the long axis of the basal turn of the cochlea and the lateral portion of the EAC as well as the angle between the long axis of the basal turn of the cochlea and the plane of the facial recess were statistically more acute in children compared to adults. Again, these more acute angles describe a narrower view of the round window and possibly increased difficulty with cochlear implantation.
Conversely, the orientation of the ideal cochleostomy drilling trajectory compared to the long axis of the basal turn of the cochlea is statistically more obtuse in children. From this information, we can infer that there is significant alteration in the facial recess or cochlea orientation as the ideal trajectory is moved more anteriorly in children making the angle more obtuse. Similarly, the view of the round window through the facial recess is significantly less of a straight line (10.66° greater) in children making electrode implantation more difficult. Post-natal alterations in cochlear orientation have been recently described9 calling into question prior data regarding cochlear stagnancy.19 Lloyd’s, et al. report of decreasing basal turn angle with regard to the sagital plane with age is consistent with the findings in our study.9
Drilling trajectory comparison
The data comparing the ideal cochleostomy drilling trajectory and the EAC orientation in adults and children are in harmony with above comments where we described how the adult EAC is significantly more obtuse when compared to children. Our data show that the drilling trajectory is significantly closer to paralleling the lateral EAC in adults as compared to children (19.40° vs. 23.51° respectively; p<0.001). This again suggests that the surgeon’s view of the round window through the facial recess is less obstructed in adults and more in- line with that view.
Martinez: wide variability in angulation of first and second turn in children; variation in cochlea with respect to midsagital line
Verbist: electrode identification in CI, system to detect CIs
While we have identified significant differences in pediatric and adult cochlear implant relevant anatomy we still do not yet know when such changes occur. Due to the relatively small sample size of this study, there were no statistically significant trends identified. We plan to further investigate when such changes occur using either larger populations of pediatric patients or, ideally, serial scans of pediatric patients. We also recognize that there may be unknown errors in the rigid and non-rigid registration processes in identifying important anatomical structures. While registration is manually confirmed and if necessary adjusted in each case, the accuracy of the verification is limited by the resolution of the imaging study as well as human visual acuity in identifying suboptimal registration. Nonetheless, our data represents a part of a growing body of literature suggesting that differences in labyrinthine anatomy do indeed exist.20-22
Conclusion
It has been widely shown that there are no statistically significant differences in facial recess width in children as compared to adults. This, however, does not do justice the differences in anatomical orientation that come into play during pediatric CI surgery. We have displayed a number of statistically significant differences in orientation among the EAC, facial recess, round window plane, and the long axis of the basal turn of the cochlea in children and adults that help to explain the difference between adult and pediatric CI.
Footnotes
This manuscript is to be presented at the American Neurotology Society Meeting in Chicago, IL (4/11)
References
- 1.Bast TH. Development of the otic capsule. VI. Histological changes and variations in the growing bony capsule of the vestibule and cochlea. Ann Otol Rhinol Laryngol. 1942;51:343–57. [Google Scholar]
- 2.Schonemann A. Schlafenbein und Schadelbasis, eine anatomischotiatrische Studie. N Denkschr algem Schweizer Gesellsch gesamt Naturwiss. 1906;40:95–160. [Google Scholar]
- 3.Farkas LG, Posnick JC, Hreczko TM. Anthropometric growth study of the head. Cleft Palate Craniofac J. 1992;29:303–8. doi: 10.1597/1545-1569_1992_029_0303_agsoth_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 4.Eby TL. Development of the facial recess: implications for cochlear implantation. Laryngoscope. 1996;106(s80):1–7. doi: 10.1097/00005537-199605001-00001. [DOI] [PubMed] [Google Scholar]
- 5.Bielamowicz SA, Coker NJ, Jenkins HA, Igarashi M. Surgical dimensions of the facial recess in adults and children. Arch Otolaryngol Head Neck Surg. 1988;114:534–537. doi: 10.1001/archotol.1988.01860170064020. [DOI] [PubMed] [Google Scholar]
- 6.Eby TL, Nadol JB. Postnatal growth of the human temporal bone: Implications for cochlear implants in children. Ann Otol Rhinol Laryngol. 1986;95:356–364. doi: 10.1177/000348948609500407. [DOI] [PubMed] [Google Scholar]
- 7.Su W-Y, Marion MS, Hinojosa R, et al. Anatomical measurements of the cochlear aqueduct, round window membrane, round window niche, and facial recess. Laryngoscope. 1982;92:483–486. doi: 10.1288/00005537-198205000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Bettman RH, Appelman AM, van Olphen AF, et al. Cochlear orientation and dimensions of the facial recess in cochlear implantation. ORL. 2003;65:353–8. doi: 10.1159/000076054. [DOI] [PubMed] [Google Scholar]
- 9.Lloyd SKW, Kasbekar AV, Kenway B, et al. Developmental changes in cochlear orientation—implications for cochlear implantation. Otol-Neurotol. 2010;31:902–7. doi: 10.1097/MAO.0b013e3181db7146. [DOI] [PubMed] [Google Scholar]
- 10.Noble JH, Warren FM, Labadie RF, Dawant BM. Automatic segmentation of the facial nerve and chorda tympani in CT images using a spatially dependent feature values. Med Phys. 2008;35:5375–5384. doi: 10.1118/1.3005479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reda FA, Noble JH, Rivas A, McRackan TR, Labadie RF, Dawant BM. Automatic segmentation of the facial nerve and chorda tympani in pediatric CT scans. Med Physics. doi: 10.1118/1.3634048. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noble JH, Majdani O, Labadie RF, et al. Automatic determination of optimal linear drilling trajectories for cochlea access accounting for drill-positioning error. Int J Med Robotics and Computer Assist Surgery. 2010;6:281–290. doi: 10.1002/rcs.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noble JH, Rutherford RB, Labadie RF, Dawant BM. Modeling and segmentation of intra-cochlear anatomy in conventional CT. IEEE Trans On Biomedical Eng. in press. [Google Scholar]
- 14.Noble JH, Dawant BM. An atlas-navigated optimal medial axis and deformable model algorithm (NOMAD) for the segmentation of the optic nerves and chiasm in MR and CT images. Med Image Anal. 2011 doi: 10.1016/j.media.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noble JH, Dawant BM, Warren FM, Majdani O. Automatic Identification and 3-D Rendering of Temporal Bone Anatomy. Otol-Neurotol. 2009 Jun;30(4):436–442. doi: 10.1097/MAO.0b013e31819e61ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sethian J. Level Set Methods and Fast Marching Methods. 2. Cambridge University Press; Cambridge: 1999. [Google Scholar]
- 17.Cootes TF, Taylor CJ, Cooper DH, et al. Active shape models—Their training and application. Comp Vis And Image Unders. 1995;61:39–59. [Google Scholar]
- 18.Litton WB, Krause CJ, Anson BA, et al. The relationship of the facial canal to the annular sulcus. Laryngoscope. 1969;79:1584–1604. [PubMed] [Google Scholar]
- 19.Jeffery N, Spoor F. Prenatal growth and development of the modern human labyrinth. J Anat. 2004;204:71–92. doi: 10.1111/j.1469-7580.2004.00250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez-Monedero R, Niparko JK, Aygun N. Cochlear coiling pattern and orientation differences in cochlear implant candidates. Otol-Neurotol. 2011;32(7):1086–93. doi: 10.1097/MAO.0b013e31822a1ee2. [DOI] [PubMed] [Google Scholar]
- 21.Verbist BM, Joemai RM, Briaire JJ, Teeuwisse WM, Veldkamp WJ, Frijns JH. Cochlear coordinates in regard to cochlear implantation: a clinically individually applicable 3 dimensional CT-based method. Otol-Neurotol. 2010;31(5):738–44. doi: 10.1097/MAO.0b013e3181d8d3c4. [DOI] [PubMed] [Google Scholar]
- 22.Erixon E, Högstorp H, Wadin K, Rask-Andersen H. Variational anatomy of the human cochlea: implications for cochlear implantation. Otol-Neurotol. 2009;30(1):14–22. doi: 10.1097/MAO.0b013e31818a08e8. [DOI] [PubMed] [Google Scholar]


