Abstract
Purpose
Chronic Lymphocytic Leukemia (CLL), a malignancy of mature B-cells, is incurable with chemotherapy. Signals from the microenvironment support leukemic cell survival and proliferation, and may confer chemotherapy resistance. ON 01910.Na (Rigosertib) a multikinase PI3K inhibitor is entering phase III trials for myelodysplastic syndrome. Our aim was to analyze the efficacy of ON 01910.Na against CLL cells in vitro and investigate the molecular effects of this drug on tumor biology.
Experimental design
Cytotoxicity of ON 01910.Na against CLL cells from 34 patients was determined in vitro using flow cytometry of cells stained with Annexin V and CD19. Global gene expression profiling on Affymetrix microarrays, flow cytometry, western blotting, and co-cultures with stroma cells were used to delineate ON 01910.Na mechanism of action.
Results
ON 01910.Na induced apoptosis in CLL B-cells without significant toxicity against T-cells or normal B-cells. ON 01910.Na was equally active against leukemic cells associated with a more aggressive disease course (IGHV unmutated, adverse cytogenetics) than against cells without these features. Gene expression profiling revealed two main mechanisms of action: PI3K/AKT inhibition and induction of ROS that resulted in an oxidative stress response through activating protein 1 (AP-1), c-Jun NH2-terminal kinase, and ATF3 culminating in the upregulation of NOXA. ROS scavengers and shRNA mediated knockdown of ATF3 and NOXA protected cells from drug induced apoptosis. ON 01910.Na also abrogated the pro-survival effect of follicular dendritic cells on CLL cells and reduced SDF-1-induced migration of leukemic cells.
Conclusions
These data support the clinical development of ON 01910.Na in CLL.
INTRODUCTION
Chronic Lymphocytic Leukemia (CLL), the most common leukemia in the Western world, is characterized by the accumulation of monoclonal CD5+ mature B cells in the peripheral blood (PB), lymph nodes (LN) and bone marrow (BM). The majority of cases are diagnosed in asymptomatic patients with an incidental finding of lymphocytosis or lymphadenopathy. The standard of care for CLL is watchful waiting of asymptomatic patients and chemoimmunotherapy for patients with active disease (1). This clinical approach to the CLL patient is guided by the absence of a curative chemotherapy regimen, the results of clinical trials that have shown no benefit for early chemotherapy in asymptomatic patients, and the relatively long natural history of the disease with a median survival of 11 years (2). CLL is divided into two main subgroups based on the presence or absence of acquired somatic mutations in the immunoglobulin heavy-chain variable region (IGHV) expressed by the leukemic B cells. Patients with mutated IGHV have a more indolent disease and longer overall survival than patients whose tumors express an unmutated IGHV gene. High expression of ZAP70 and CD38 are additional markers indicating more rapid disease progression (3). Cytogenetic alterations are also strong predictors of outcome. In particular, deletion of TP53 locus on 17p and deletion of the ATM locus on 11q are associated with more rapidly progressive disease and inferior response to chemotherapy. Increasingly, risk stratified treatment approaches are pursued for patients with these adverse prognostic markers (4).
CLL has historically been considered an accumulative disease of quiescent cells arrested in the G0/G1 phase of the cell cycle and endowed with resistance to apoptosis. This notion has been based primarily on the study of leukemic cells from the peripheral blood. However, more recently it has been recognized that a proliferating subpopulation localized in lymphoid organs contributes to disease progression (5-7). Interactions with the tissue microenvironment are crucial for CLL cell proliferation and survival (6, 8). We recently demonstrated activation of the B-cell receptor (BCR) and nuclear factor-κB (NFκB) pathways in CLL cells isolated from the lymph node microenvironment. BCR signaling was stronger in the clinically more aggressive CLL subtype expressing unmutated IGHV genes and tumor proliferation in the lymph node correlated with clinical disease progression (7). Thus, targeting the tumor microenvironment interactions and BCR signaling in particular has become a priority in CLL. Inhibitors of SYK (9), BTK (10), and PI3K (11, 12) have shown promising preclinical and clinical activity in CLL. The PI3K/AKT pathway in particular is a key signaling node for several pathways implicated in CLL pathogenesis and interactions with the microenvironment including the BCR (13, 14), CXCR4 (15), CD40 (16), CD44 (17), and IL4 (13). Moreover it has been shown that the PI3K pathway is active in freshly isolated CLL cells (18). PI3K activates AKT, a serine-threonine kinase critically involved in cell growth and survival
ON 01910.Na, a styryl benzylsulfone is a non-ATP competitive multi-kinase inhibitor with marked anti-mitotic and anti-cancer activity. A phase I study in patients with solid tumors demonstrated good tolerability of the drug with an objective durable response in a patient with ovarian cancer (19). ON 01910.Na is currently being tested in a randomized phase III trial in patients with refractory myelodysplastic syndrome (MDS) (registered as NCT01241500). Initially it was thought that the anti-mitotic activity was due to inhibition of polo-like kinase 1 (20). Subsequent studies did not support a direct effect on polo-like kinases (21) and a recent study correlated hyperphosphorylation of Ran GTPase-activating protein 1 with ON 01910.Na induced cell cycle arrest (22). However, whether ON 01910.Na directly or indirectly contributes to mitotic arrest remains elusive. Focusing on mantle cell lymphoma (MCL), Prasad and colleagues showed direct inhibition of PI3K, preferentially of the p110α, p110β isoforms, resulting in decreased phosphorylation of AKT, inhibition of cyclin D1 translation, and induction of apoptosis in MCL cell lines (23). These results prompted us to explore the potential activity of ON 01910 against CLL cells. Here, we demonstrate that ON 01910.Na induces apoptosis in CLL cells irrespective of markers predicting resistance to classic chemotherapy while sparing normal lymphocytes. Using gene expression profiling we discovered that ON 01910.Na cytotoxicity is mediated by two main mechanisms: inhibition of the PI3K/AKT pathway and induction of an oxidative stress response. Moreover, ON 01910.Na remained active against CLL cells co-cultured with stroma cells and reduced SDF-1-induced migration. These studies provide a rationale for the development of ON 01910.Na as a therapeutic agent in CLL.
MATERIALS AND METHODS
Patients and clinical samples
Blood samples from 34 CLL patients (table 1) were obtained with written informed consent. Peripheral blood mononuclear cells (PBMCs) were isolated by gradient centrifugation using Lymphocyte Separation Medium (MP Biomedicals, Solon, OH) and used fresh or cryopreserved in liquid nitrogen in 10 % dimethyl sulfoxide, 90 % FCS. Analysis of IGHV gene status was performed as described (24). CLL samples were cultured in RPMI 1640 (Gibco-Invitrogen, Long Island, NY) supplemented with 10% fetal bovine serum (HyClone, Logan, UT), 2 mM L-glutamine, and penicillin/streptomycin (subsequently referred to as R10).
Table 1.
Patient characteristics
| Study label |
Gender | IGHV status1 |
Cytogenetic alterations2 |
ON 01910.Na LD50 (48h, μM)3 |
|---|---|---|---|---|
| CLL 1 | F | M | 11q del; 13q del | 0.96 |
| CLL 2 | F | M | 13q del | 1.48 |
| CLL 3 | M | U | 17p del | 0.48 |
| CLL 4 | M | M | trisomy 12 | 5.43 |
| CLL 5 | M | U | trisomy 12 | 0.97 |
| CLL 6 | M | U | nd | 1.08 |
| CLL 7 | M | M | 17p del; 11q del; 13q del | 0.78 |
| CLL 8 | F | M | 13q del | 0.55 |
| CLL 9 | F | U | 13q del | 0.78 |
| CLL 10 | F | M | 17p del; 13q del | 0.55 |
| CLL 11 | F | U | 13q del | 0.58 |
| CLL 12 | M | U | 11q del; 13q del | 1.18 |
| CLL 13 | M | M | 17p del | 0.90 |
| CLL 14 | F | M | 13q del | 0.52 |
| CLL 15 | M | U | 13q del | 1.67 |
| CLL 16 | F | U | 11q del;13q del | 0.52 |
| CLL 17 | F | U | 17p del;11q del;13q del | 1.59 |
| CLL 18 | M | U | 11q del; 13q del | 0.92 |
| CLL 19 | M | U | 11q del; 13q del | 1.06 |
| CLL 20 | M | U | 17p del | 0.43 |
| CLL 21 | F | U | 13q del | >8 |
| CLL 22 | M | M | 13q del | 0.2 |
| CLL 23 | F | U | 17p del | 3.20 |
| CLL 24 | M | M | 13q del | 1.94 |
| CLL 25 | M | U | normal | 4.15 |
| CLL 26 | F | U | 17p del | 0.21 |
| CLL 27 | M | U | 11q del | 0.88 |
| CLL 28 | M | U | 11q del | 1.19 |
| CLL 29 | M | U | 6q del | >8 |
| CLL 30 | M | U | 11q del; trisomy 12 | 0.8 |
| CLL 31 | M | U | 13q del | 0.95 |
| CLL 32 | M | U | 13q del | 1.10 |
| CLL 33 | M | U | 11q del | 0.55 |
| CLL 34 | M | U | normal | 1.24 |
IGHV sequence is considered mutated when its homology to the germline IGHV sequence is below 98%
Determined by interphase fluorescence in situ hybridization
Calculated by non linear regression using Prism 4 software
Stromal cell coculture
The follicular dendritic cell line (FDC) HK kindly provided by Dr. Yong Sung Choi (25), was cultured in IMDM (Gibco-Invitrogen, Long Island, NY) supplemented with 20% fetal bovine serum (HyClone), 2 mM L-glutamine, and penicillin/streptomycin. HK cells were seeded in 12-well plates on day 0. The next day IMDM was removed and CLL cells (2 x106 cells/ml in R10) were added onto confluent stroma layers and cultured for the times indicated in the presence or absence of ON 01910.Na. CLL cells were recovered by gentle agitation, and the percentage of apoptotic cells was determined by flow cytometry after staining with CD19-FITC and AnnexinV–PE.
BCR stimulation by IgM crosslinking
CLL cells were reacted at 4°C for 30 minutes with 25μg/ml Goat F(ab’)2 anti-human IgM-biotin (Southern Biotech) followed by the addition of 10μg/ml streptavidin (Jackson ImmunoResearch Laboratories) for 20 min. Cells were transferred to 37°C for 30min and stimulation was stopped with cold PBS. Where indicated cells were pre-incubated with ON 01910.Na for 1 hour.
Migration assays
SDF-1α/CXCL12-induced migration was evaluated in 24 well chemotaxis chambers containing polycarbonate inserts of 5 μM pore size (Corning, Life Science). 100 μl cell suspensions at 5×106 cells/ml pretreated or not with ON-01910 for 2 hour were added to the top chamber and CXCL12 (Peprotech, Rocky Hill, NJ) to the lower chamber. Media without CXCL12 was used as baseline. After 3 hours, cells in both chambers were counted separately in an Attune flow cytometer at high flow rate for 1 minute. The migration index was calculated as the ratio between cells that migrated in response to CXCL12 divided by those that passively migrated without the chemokine. The MI of ON-01910.Na-treated cells was normalized to vehicle (DMSO) treated cells.
ATF3 and NOXA silencing by retroviral transfection of short hairpin RNA (shRNA)
The MCL cell line Jeko was transfected with retrovirus to generate shATF3 and shNoxa clones. Parental cells and shRNAs clones were cultured in R10. shPRS (non targeting control), shATF3 and shNOXA constructs were generated inserting the following sequences (shATF3: GGGTTAGGACTCTCCACTCAA and TTGAGTGGAGAGTCCTAACCC; s h N O X A : GGTGCACGTTTCATCAATTTG and CAAATTGATGAAACGTGCACC) into the pRetroSuper plasmid containing also GFP and puromycin-resistant elements (kind gift of Dr. Louis M Staudt). Viral particles were produced in Human Embryonic Kidney 293T cells (HEK-293T), and used to infect target cells by centrifugation (2500 rpm for 90 minutes) in the presence of DOTAP (Roche, Indianapolis, IN) followed by puromycin selection (2 μg/ml) for 7 days . The percentage of knock down was assessed by qRT-PCR using SYBR green specific oligos designed for ATF3 and NOXA genes, using beta-2-microglobulin (B2M) as housekeeping control (Qiagen)
Treatments and cytofluorimetric assessment of apoptosis
ON 01910.Na (gift from Onconova Therapeutics, Newtown, PA) was dissolved in DMSO and stored at -80°C. To assessed the lethal dose 50 (LD50) PBMCs were exposed to increasing concentrations of ON 01910.Na ranging from 0.25 μM to 8 μM for 48 hours. Cytotoxicity against B-CLL cells was evaluated by flow cytometry after staining with Annexin V-PE (BD-Pharmingen) and CD19-FITC. In CLL PBMCs <1% of CD19+ cells are typically normal B-cells. To evaluate toxicity against T-cells CD3-APC was added. LD50 was calculated by non-linear regression using Prism 4.0 (GraphPad Software, La Jolla, CA). Changes in the mitochondrial transmembrane potential (ΔΨm) were assessed either by MitoTracker Green FM and MitoTracker Red CMX Ros (100nM each) or by 20nM 3,3-diexyloxacarbocyanine iodide (DiOC6[3]) (Invitrogen). The generation of reactive oxygen species (ROS) was determined by staining cells with 2 μM dihydroethidine (DHE; Invitrogen). Cells were incubated with each probe for 30 min at 37 °C, and 10,000 cells per sample were analyzed in a FACS CANTO flow cytometer using FACS DIVA software (BD Biosciences). DiOC6 and DHE staining was done simultaneously.
To detect activation of BAX, BAK, and caspase-3, 0.5×106 cells were fixed with 4% paraformaldehyde, permeabilized with saponin 0.1% (Sigma), stained for 30 minutes with 1 μg/ml of antibodies against the active form of caspase-3 (BD Pharmingen), BAX (clone 6A7; BD Pharmingen) or BAK (clone Ab-1; Oncogene Research, Boston, MA) for 30 minutes at room temperature, followed by goat anti–rabbit FITC or goat anti–mouse FITC (Jackson ImmunoResearch, West Grove, PA,) and then analyzed in FACS CANTO flow cytometer.
MTT assay
5×104 cells per well were exposed to serial doubling concentrations of ON 01910.NA for 48 hours in flat bottom 96-well plates. For the last 4 hours, MTT reagent (Chemicon, Temecula, CA) at 0.5 mg/ml was added and analyzed as described (26).
Gene expression profiling and gene set enrichment analysis (GSEA)
2.5μg total RNA was subjected to gene expression profiling on Human Genome U133 plus 2.0 chips (Affymetrix, Palo Alto, CA). Processing, data extraction and normalization was done as described (27). Significant gene signatures were identified using GSEA v2.0 (Broad Institute at MIT, Cambridge, MA, http://www.broadinstitute.org/gsea/) using the C3 (motif gene sets) from the Molecular Signature Database v2.5, and experimentally derived custom gene sets (28). An increasing profile analysis with 1000 permutations of gene sets and a weighted metric was used. Bonferoni correction for multiple testing was applied and only gene sets with a False Discovery Rate (FDR) ≤0.2 and a normalized enrichment score (NES) of >1.4 or <-1.4 were considered significant. The leading edge genes, i.e. the subset of genes that contributed most significantly to the identification of a gene set, were displayed using Cluster (v2.11) and TreeView (v1.6) softwares (Eisen Laboratory, Stanford University, USA).
Protein isolation and western blot
CLL cells were lysed in 1% Triton buffer containing PhosphoSTOP and the protease inhibitors Complete EDTA-free (Roche, Indianapolis, IN). Nuclear and cytosol fractions were obtained using a commercial kit (Biovision, Mountain View, CA). Western blot analysis was performed with the NuPAGE Bis-Tris electrophoresis system on PVDF membranes (Invitrogen) blocked in 5% non-fat milk or BSA with 0.05% Tween-20. Membranes were incubated with the primary antibodies (1:500 to 1:2000 dilutions) in 5% milk or BSA with 0.05% Tween-20 overnight at 4C or 1 hour at room temperature. Membranes were developed with enhanced chemiluminescence substrate (SuperSignal, Thermo Fisher Scientific) and visualized on a LAS4000 Fujifilm device. Protein quantification was done with Image Gauge Fujifilm software (Fujifilm Corporation, Tokyo, Japan). The following antibodies were used: pAKT (Ser 473) and pSAPK/JNK (Tyr183/Thr185) from Cell Signaling Technology (Beverly, MA) ;anti-c-JUN (H-79), anti MCL-1 (S19), anti-ATF3 (C-19) and anti-TBP (58C9) from Santa Cruz Biotechnology (Santa Cruz, CA); anti-NOXA from Calbiochem (San Diego, CA): anti α-tubulin and anti β-actin from Sigma.
Statistical analysis
Unpaired and paired T-tests were used to assess differences between two groups. P <0.05 was considered significant.
RESULTS
ON 01910.Na activates the mitochondrial cell death pathway and is selectively toxic against CLL cells independent of IGHV mutational status and cytogenetic profile
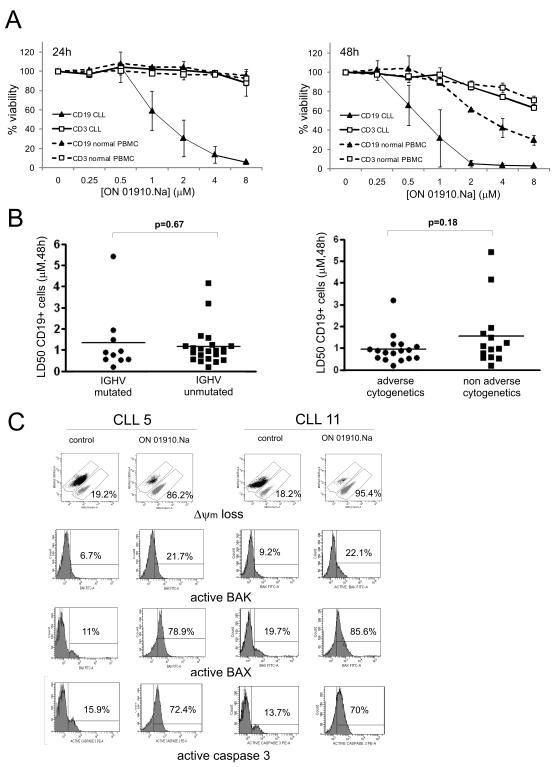
To assess the cytotoxic effect of ON 01910.Na against CLL cells, we treated PBMC from CLL patients and healthy donors ex vivo with increasing concentrations of drug. We measured drug induced apoptosis in B and T cell populations separately using AnnexinV staining in combination with antibodies against the lineage specific markers CD19 and CD3. ON 01910.Na induced time- and dose-dependent cytotoxicity against B cells from CLL patients (Fig. 1A). In contrast, there was no cytotoxic effect on T cells from CLL patients or B and T cells from healthy donors at 24 hours. At 48 hours normal B cells showed somewhat reduced viability albeit only at concentrations higher than those required to kill CLL cells. T cells from both normal donors and CLL patients remained largely unaffected even at concentrations of more than 8-fold the LD50 of CLL cells. Thus, ON 01910.Na is selectively toxic against CLL B cells.
Figure 1. ON 01910.Na is selectively cytotoxic for CLL cells independent of IGHV mutational status or cytogenetic subgroups and activates the mitochondrial apoptotic pathway.
A, PBMCs from CLL patients or healthy volunteers (duplicates of n=2 each) were treated with increasing doses of ON 01910.Na. Apoptosis in T-cells (CD3+) and B-cells (CD19+) cells was assessed by flow cytometry (Annexin V-PE/CD19-FITC/CD3-APC) at 24 and 48 hours. The percentage of viable cells normalized to the untreated control is shown for a representative experiment of at least 3. B, summary of CLL samples tested (n=32) as in A. Adverse cytogenetics (11q and/or 17p deletions); non-adverse cytogenetics (trisomy 12, 13q deletion or normal karyotype). C, CLL cells were treated with 2 μM ON 01910.Na for 24 hours and flow cytometry was used to assess the loss of mitochondrial membrane potential (ΔΨm) using mitotracker green/red staining. Activation of BAX, BAK, and caspase 3, was measured using confirmation specific antibodies. The numbers inside each plot indicate the percentage of apoptotic cells (mitotracker green positive) or the percentage of positive cells for activated BAX, BAK or caspase-3 above isotype control, respectively.
We next determined the activity of ON 01910.Na against a panel of CLL samples from 34 patients stratified for disease markers that are associated with more aggressive disease and inferior survival (Table 1). We determined the percentage of CD19 positive cells staining for Annexin V and calculated the LD50 for each sample at 48 hours. As shown in figure 1B, most of the patients had LD50 values between 1-2 μM, a concentration that is achieved in vivo (19). CLL cells expressing mutated or unmutated IGHV genes were equally sensitive to ON 01910.Na and most cases with 17p or 11q deletions had LD50 values comparable to samples without these adverse cytogenetic findings (Table 1, Fig. 1B).
To investigate whether ON 01910.Na activates the mitochondrial apoptotic pathway, CLL cells were treated with 2 μM ON 01910.Na for 24 hours. We used the mitotracker cell viability assay that measures mitochondrial depolarization to quantify drug induced apoptosis (Fig. 1C). In parallel, we assessed activation of the proapoptotic proteins BAK and BAX using confirmation specific antibodies. Oligomerization of BAX and BAK enables the formation of the mitochondrial pore leading to mitochondrial depolarization, followed by the release of apoptogenic factors, and finally activation of effector caspases (29). Both mitotracker staining and conformational changes in BAX and BAK indicate that ON 1910.Na activated the mitochondrial apoptotic pathway.
ON 01910.Na inhibits the PI3K/AKT pathway
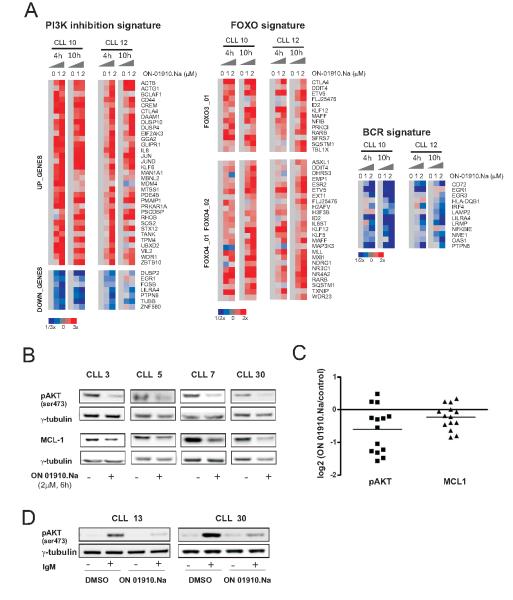
ON 01910.Na has been shown to inhibit PI3K activity in vitro in cell free assays and in MCL cell lines (23). In contrast to CAL-101 (11), another clinical grade PI3K inhibitor, we noted apronounced pro-apoptotic effect of ON 1910.Na against CLL cells already at 24 hours even in the low μM range. Thus, we sought to better characterize the cellular stress response triggered by ON 01910.Na. We first performed gene expression profiling (GEP) of CLL cells treated with 1 and 2 μM ON 01910.Na for 4 and 10 hours on Affymetrix whole genome arrays. In order to investigate the functional correlates of drug induced gene expression changes, we used GSEA (28). This observer-independent statistical method can dissect gene expression data into defined signatures that correlate with cellular functions or the activity of defined transcription factors. Among the genes differentially expressed in ON 01910.Na treated CLL cells GSEA identified several significantly enriched gene sets (FDR<0.2; Supplementary Table S1). ON 01910.Na-treated CLL cells showed gene expression changes significantly related to gene signatures derived from HL60 cells treated with the PI3K inhibitor LY-294002 (Fig. 2A). Thus, our gene expression data indicates inhibition of the PI3K pathway and is in agreement with findings by Prasad et al in MCL cell lines (23). PI3K activation leads to activation of AKT which in turn phosphorylates and thereby inactivates forkhead transcription factors. Conversely, when PI3K is inhibited, these factors can translocate to the nucleus and initiate the transcription of proapoptotic genes (30). As shown in Fig. 2A target genes of the transcription factors forkead box O3 (FOXO3) and forkhead box O4 (FOXO4) were upregulated in ON 01910.Na treated cells. In addition, we also observed a decrease in genes induced by B-cell receptor activation, consistent with a role of PI3K in BCR signaling (Supplementary Table S1, Figure 2A)
Figure 2. ON 01910.Na inhibits PI3K/AKT and activates FOXOs transcriptional activity.
A, cells from patients CLL10 and CLL12 treated with 1 and 2 μM ON 01910.Na for 4 and 10 hours and untreated cells cultured for the same time periods were analyzed on Affymetrix arrays. The leading edge genes of gene expression signatures that were significantly regulated by ON 01910.Na (FDR <0.2, supplementary table 1) are shown in a heat map representation scaled as indicated. B, the effect of ON 01910.Na (2 μM for 6 hours) on AKT phosphorylation and MCL-1 expression in CLL cells from 4 representative patients is shown by Western blotting. C, pAKT and MCL-1 expression normalized to γ-tubulin in samples treated as in B (n=14) was quantified by densitometry. The log2 of the ratio between treated and untreated cells is depicted. D, CLL patients were stimulated with IgM for 30 minutes in the presence or absence of 2 μM ON 01910.Na. Protein lysates were analyzed by Western blot for pAKT expression using γ-tubulin as loading control.
To validate the inhibition of PI3K/AKT pathway at protein level, we treated cells from CLL patients with 2 μM ON 01910.Na for 6 hours and assessed the phosphorylation status of AKT by western blot. pAKT was significantly reduced in most of the drug treated samples (Fig 2B and 2C). Furthermore, expression of the antiapoptotic protein MCL-1, which is in part regulated through increased translation downstream of PI3K/AKT signaling, also decreased in cells treated with ON 01910.Na (Fig. 2B and C). While about half of the samples showed >50% downregulation of pAKT in response to ON 01910.Na another half showed only minimal changes. This could be due to heterogeneity in cellular response to drug treatment or due to a relatively low baseline pAKT level in circulating CLL cells that makes it difficult to detect any further decrease. CLL cells in the peripheral blood are resting cells and, as we have recently shown, activation through signaling pathways, in particular the BCR occurs primarily in the lymph node microenvironment (7). Thus, having seen that treatment with ON 01910.Na down regulates BCR target genes, we next tested whether ON 01910.Na is able to block BCR activation in vitro. Indeed, ON 01910.Na was able to inhibit phosphorylation of AKT in response to BCR engagement by IgM crosslinking (Fig. 2D).
ON 01910.Na induces an oxidative stress response through JNK and AP-1 leading to the upregulation of the proapoptotic BH3-only protein Noxa
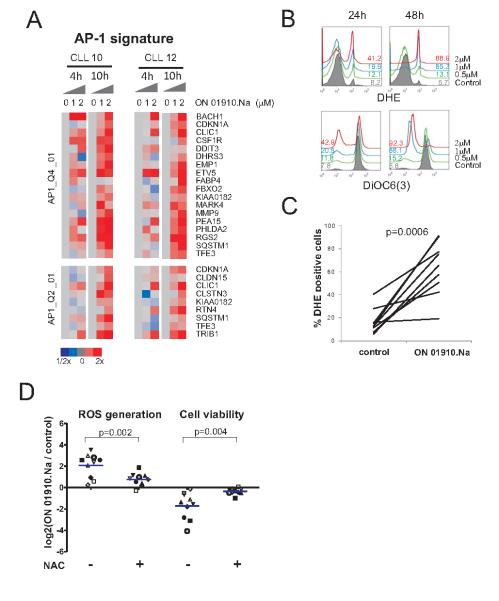
Although inhibition of the PI3K/AKT pathway may contribute to ON 01910.Na cytotoxic activity, this is unlikely to be the sole mechanism because cytotoxicity did not correlate well with the extent of PI3K/AKT blockade and the degree of apoptosis observed with ON 01910.Na was higher than what has been reported for other PI3K inhibitors (11, 15). Further indicating a second mechanism of cytotoxic activity, GSEA identified a significant upregulation of genes controlled by the AP-1 transcription factor (Supplementary Table S1, Fig. 3A). AP-1 protein dimmers most commonly composed of JUN, FOS, or members of the ATF family, upon activation by JNK kinase, act as transcriptional transducers of stress pathways. The JNK/AP-1 pathway is an integral part of the cellular response to reactive oxygen species (ROS) (31). In keeping with the gene expression data, we detected a dose- and time-dependent increase in ROS in response to ON 01910.Na that paralleled mitochondrial depolarization (Fig. 3B and C). Thus, ON 01910.Na induces an oxidative stress response that activates the JNK pathway leading to the induction of AP-1 target genes.
Figure 3. ON 01910.Na induces ROS and activates a stress response mediated by AP-1.
A, purified CLL cells (CD19+ selection) from patients CLL10 and CLL12 treated without and with 1 and 2 μM ON 01910.Na for 4 and 10 hours were analyzed on Affymetrix arrays. The leading edge genes of AP-1 gene signatures (FDR <0.2, Supplementary Table 1) are shown in a heat map representation scaled as indicated. B, ON 1910.Na induced generation of ROS and loss of ΔΨm measured by flow cytometry using DHE and DiOC6 are shown for a representative patient. The percent of DHE positive and DiOC6 low cells is indicated. C, CLL cells from 9 patients were treated with 2 μM ON 01910.Na for 48 hours and ROS generation was measured using DHE staining. The percentage of DHE positive cells at 48 hours is given for ON 01910.Na and control (DMSO treated) cells. D, cells from 10 CLL patients were treated with 1 μM ON 01910.Na for 48 hours in the presence or absence of 25 mM N-acetylcysteine (NAC). ROS generation and cell viability was measured as in B. The log2 of the ratio between treated and untreated cells is depicted.
To further determine the role of ROS in ON 01910.Na induced apoptosis we conducted cytotoxicity assays in presence of the antioxidant N-acetylcysteine (NAC). NAC reduced ROS generation by ON 01910.Na > 50% (p=0.002) and protected cells from the cytotoxic effect of the drug; while ON1910.Na on average killed more than 70% of cells at 48 hours, NAC potently antagonized the cytotoxic effect and all samples showed >50% viability (P=0.004, Fig. 3D).
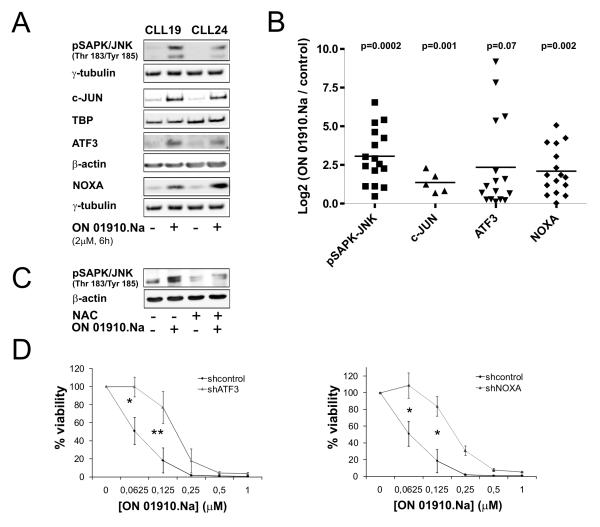
Next, we assessed the role of the JNK pathway in drug induced apoptosis by Western blotting and shRNA knockdown experiments. ON 01910.Na induced phosphorylation of JNK within 6 hours with concomitant upregulation of ATF3 and the nuclear accumulation of c-JUN (Fig. 4A and B). In keeping with the known link between ROS and JNK activation (31), the ROS scavenger NAC reduced ON1910.Na induced phosphorylation of JNK (Fig. 4C). We also found upregulation of the proapoptotic BH3-only protein NOXA in response to ON 01910.Na consistent with reports of a role for NOXA in the oxidative stress response (32, 33). Finally, we used shRNAs to knock down NOXA and ATF3 in Jeko-1 cells (Figure 4D). As there are no bona fide CLL cell lines we chose the mantle cell lymphoma cell line Jeko because the IC50 of ON1910.Na is comparable between the cell line and primary CLL cells. Efficient reduction of ATF3 and NOXA expression by shRNA was verified by RT-PCR (Supplementary Fig. 1). As shown in Figure 4D, knockdown of ATF3 or NOXA expression significantly diminished the cytotoxic effect of ON 01910.Na, further supporting the important contribution of the oxidative stress response and the JNK pathway to ON 01910.Na’ mechanism of action.
Figure 4. ON 01910.Na activates the JNK pathway and upregulates the BH3-only protein NOXA.
A, the effect of ON 01910.Na (2 μM for 6 hours) on SAP/JNK phosphorylation, nuclear c-JUN levels, and ATF3 and NOXA expression is shown by Western blotting in two representative patients. B, summary of pSAP/JNK(n=16), nuclear c-JUN (n=5), ATF3(n=16) and NOXA (n=16) levels quantified by densitometry and normalized to γ-tubulin, β-actin or TBP is shown. The log2 of the ratio between treated and untreated cells is depicted. Statistical significance was assessed by paired Student’s T-test. C, CLL cells (CLL 24) were pretreated with NAC (25mM) for 1hr, followed by ON-01910.Na (2μM) or vehicle (DMSO) incubation for 6h additional hours. Activation of pSAP/JNK was detected by Western blotting using β-actin as loading control. D, dose response to ON 01910.Na in Jeko cells transfected with a control non-targeting shRNA vector or a vector expressing shRNAs that knockdown ATF3 or NOXA Viability by MTT assay at 48 hours normalized to vehicle control is shown. Comparison of viability at the indicated dose levels done by paired Student’s T-test (*p<0.01; **p<0.001).
ON 01910.Na overcomes stroma mediated pro-survival effects on CLL cells
It is well documented that the host microenvironment contributes to CLL cell proliferation, survival, and drug resistance (7, 8). Thus, a critical need in CLL is the development of therapeutic approaches that remain highly active against tumor cells in the microenvironment. Given the prominent role of the lymph node microenvironment for CLL cells, we assessed the efficacy of ON 01910.Na in an in-vitro model mimicking the lymph node environment by culturing CLL cells on the follicular dendritic cell line (FDC) HK (25). As expected HK cells significantly increased the viability of co-cultured CLL cells compared to media alone (Fig. 5A) in agreement with previous work from other groups (34, 35). Next we tested the cytotoxic effect of ON 01910.Na on CLL cells in this model system. As shown in Fig. 5B, ON 01910.Na was able to completely abrogate the cytoprotective effect mediated by HK cells and demonstrated equal efficacy against CLL cells co-cultured on HK or in media alone. In separate experiments we verified that the viability of HK cells was not affected by ON 01910.Na at concentrations used in these experiments (data not shown).
Figure 5. ON 01910.Na overcomes stroma-derived survival signaling.
A, CLL cells (n= 15) were cultured with or without the follicular dendritic cell line HK (ratio CLL:HK 20:1) and viability was assessed by Annexin V-PE in CD19-FITC stained cells by flow cytometry. Differences between groups were assessed by paired Student’s T-test. B, cytotoxic effect of ON 01910.Na at 48 hours on CLL cells in the presence or absence of HK cells measured as in A. C, expression levels of MCL-1, pAKT, and pSAP/JNK in cells from two representative CLL patients treated with ON 01910.Na (2 μM for 24 hours) in the presence or absence of HK cells. D, migration towards SDF-1α (200ng/ml) was assessed for CLL cells pre-incubated with ON 01910 (2 μM) or vehicle (DMSO) for 2 hours. Cell counts were performed in triplicates and the mean(+/− SD) of the migration index normalized to control is shown. Statistical significance was assessed by non paired Student’s T-test
Previous work has shown that the pro-survival effect of HK cells on CLL cells is mediated at least in part through upregulation of MCL-1 (35). We therefore tested if ON 01910.Na was able to counteract HK induced upregulation of MCL-1. Indeed, ON 01910.Na greatly reduced MCL-1 expression in CLL cells cultured with HK cells (Fig. 5C). Moreover, ON 01910.Na also reduced of AKT phosphorylation equally in CLL cells cultured with or without HK cells. Finally, activation of SAP/JNK by ON 01910.Na was not affected by HK cells, consistent with the preserved cytotoxic activity of the drug against CLL cells cultured in this stroma model system.
Finally we addressed whether ON 01910.Na affects migration of CLL cells towards the chemokines SDF-1α/CXCL12. SDF-1 is a chemokine secreted by different types of stroma cells that may guide migration of CLL cells to the stroma microenvironment (36). It has been described recently that PI3K inhibitors targeting p110α or p110γ isoforms interfere with SDF-1α induced migration of CLL cells (15). As shown in Fig. 5D, ON 01910.Na was very potent in blocking the migration of CLL cells in an SDF-1α chemokine gradient.
DISCUSSION
We chose to investigate ON 01910.Na because of its reported activity against MCL cell lines through inhibition of the PI3K pathway (23) and its promising clinical activity in MDS, for which it has now entered a pivotal phase III study (NCT01241500). In initial experiments, we observed rapid induction of apoptosis selectively in CLL cells, while T cells and normal B cells were not or only minimally affected. We were surprised by the strong proapoptotic effect of ON 01910.Na given reports of only minimal apoptosis in response to other clinical grade PI3K inhibitors (11). Based on its unique chemical structure and mechanism of kinase inhibition (20), we considered that ON 01910.Na may have additional effects. We chose to investigate the molecular effects and resultant cellular stress response using global gene expression profiling because this approach does not require prior assumptions. The transcriptional profile in ON 01910.Na treated CLL cells was consistent with inhibition of PI3K signaling. In addition we observed upregulation of gene signatures indicating a ROS induced oxidative stress response. We went on to confirm a key role of ROS in ON 01910.Na induced apoptosis in CLL cells: first, we demonstrated a significant accumulation of ROS in response to drug that paralleled onset of apoptosis. Second, the antioxidant NAC effectively antagonized ON 01910.Na mediated apoptosis. Third, we found that ON 01910.Na treatment induced a classic ROS triggered stress response pathway involving activation of pSAP/JNK, nuclear accumulation of c-JUN, and induction of ATF3 and NOXA in all CLL samples tested. It is notable that this stress response differs in some aspects from the NRF2 dominated response we described in MCL cells treated with the proteasome inhibitor bortezomib (33). NRF2, a key regulator of the protective antioxidant response, is in part regulated through PI3K. The combination of a PI3K inhibitor with a ROS inducing agent has been shown to inhibit the translocation of NRF2 to the nucleus and block upregulation of NRF2 target genes (37, 38). In keeping with this, ON 1910.Na treated cells mounted no significant NRF2 response (FDR>0.2, data not shown).
Pharmacologic induction of ROS is increasingly recognized as a therapeutic principle to selectively kill transformed cells (39, 40). Cancer cells are particularly sensitive to disruption of redox homeostasis; a potential vulnerability thought to be due to increased ROS levels originating from oncogene activation (39). One of the first agents shown to have selective anti-tumor activity through upregulation of ROS is beta-phenylethyl isothiocyanate (PEITC) (41). This compound was subsequently shown to be effective against fludarabine-resistant CLL cells (42). Similarly, apoptosis induced in CLL cells by adaphostin, a tyrosine kinase inhibitor of the tyrphostin class (43), has been shown to depend on the induction of ROS. In vitro, these agents remain active against chemotherapy-resistant CLL cells with dysfunctional TP53; an effect that seems to be primarily due to the induction of NOXA in response to ROS (32). Despite their encouraging in-vitro activity, these agents have no established clinical benefit in CLL; adaphostin has not entered clinical testing, and a phase I study of PEITC is only about to begin (NCT00968461). In contrast, ON 01910.Na has recently entered a pivotal phase III clinical trials in MDS and is in phase I/II studies for other indications. Interestingly, ON 01910.Na has been reported to be selectively cytotoxic for the myeloid tumor cells without affecting normal hematopoiesis (44). While induction of oxidative stress has not been described as a therapeutic principle in MDS, ROS plays an important role in the pathogenesis and is increased in MDS cells compared to normal bone marrow cells (reviewed in (45)). Given our findings, it thus appears plausible that generation of ROS contributes to the selective anti-tumor activity of ON 01910.Na in MDS.
Consistent with a report that ON 01910.Na inhibits PI3K signaling we detected a transcriptional response in drug treated CLL cells indicative of substantial PI3K inhibition. To do this, we selected two representative patients for gene expression analysis and exposed PBMCs to ON 01910.Na in a dose and time dependent fashion. In this way we generated 4 replicates of drug treated cells in each patient that also incorporate dose and time effects. Gene expression in treated cells was compared to untreated cells obtained at the same time points. We then tested whether ON 01910.Na induces the gene expression fingerprint of a PI3K inhibitor using GSEA to estimate the connection of ON 01910.Na induced gene expression changes with previously established signatures of the pan-PI3K inhibitor LY 294002. This approach termed “connectivity mapping” has been developed and used successfully to identify compounds affecting the same cellular pathways (46). Indeed, the connection between the gene signatures of LY 0294002 and ON 01910.Na was highly significant (FDR 0.01 for downregulated genes; FDR 0.17 for upregulated genes; Supplementary Table S1, Fig. 2A). Further supporting “on target” effects are the upregulation of genes controlled by FOXO. Transcription factors of the FOXO family are retained in the cytoplasm by PI3K and/or ERK dependent phosphorylation. Upon downregulation of PI3K activity, unphosphorylated FOXO translocates to the nucleus and upregulates numerous genes involved in cell cycle arrest, apoptosis, and stress response (47). Finally, we also observed downregulation of a subset of genes that we have previously identified as target genes of BCR signaling in CLL cells (7). Possible reasons why we observed only a partial downregulation of the BCR signature include PI3K independent regulation of some BCR target genes and incomplete inhibition of the pathway by ON 01910.Na, which has been reported to primarily inhibit PI3Kα and PI3Kβ (23), while BCR signaling activates PI3Kδ (11). As reported by Prasad and colleagues, we did observe a decrease in pAKT in most samples (Fig. 2C), while effects on p-mTOR, pS6K, and p4EBP1 were apparent in some but not other CLL samples tested (data not shown). These differences may be due to the former study testing only two cell lines, or could reflect differences between cell lines and primary samples.
The tumor microenvironment is increasingly recognized as a significant factor contributing to treatment resistance in CLL (8). Strategies to target cells in the protective environment are therefore a priority. ON 01910.Na combines two complementary activities that could endow it with significant clinical activity. ROS activation can induce apoptosis, particularly in cells with high baseline ROS levels. CLL cells are reported to have high baseline ROS levels (42) and some of the key signals activated in the secondary lymphoid structures including BCR and CD40 signaling further increase ROS (48, 49). In addition to increasing oxidative stress through inactivation of NRF2, PI3K inhibition may combine with ROS to target MCL-1, an important survival factor upregulated by stroma contact. MCL-1 protein stability is increased through phosphorylation by AKT (14) and glutathionylation (42). Thus, ROS may destabilize MCL-1 through depletion of glutathione while PI3K inhibition blocks the effect of stroma (Fig. 5C, and (50)). While, treatment with a pan-PI3K inhibitor is sufficient to reverse the increase in CLL viability observed in co-culture with stroma cells (50), or conferred by CD44 activation (17), it alone does not induce significant apoptosis. In contrast, ON 01910.Na not only blocked the pro-survival effect of HK cells but induced apoptosis in >50% of cells even in the presence of stroma (Fig. 5B). ON 01901.Na is also more cytotoxic in vitro than more specific inhibitors such as CAL-101 (11) or the BTK inhibitor PCI-32765 (10) that on average reduce CLL cell viability by 10-20% even at concentrations in excess of what is required to inhibit the specific target. These considerations suggest that the combination of a PI3K inhibitor with an agent inducing ROS may confer synergistic anti-tumor effects.
In summary, our studies identify ON 01910.Na as an agent with important preclinical activity in CLL. Specifically, ON 01910.Na is active against CLL cells with loss of TP53 and against cells sheltered by a protective stroma environment. Our results also provide new insights into the mechanism of action of this drug showing that a combination of ROS induction and inhibition of PI3K signaling can effectively combine to induce apoptosis. Insights generated from these studies help identify biomarkers to correlate with clinical activity. Based on these results and work by Prasad and colleagues (23) we have initiated the first clinical trial with ON 01901.Na in patients with lymphoid malignancies (NCT008615100).
Supplementary Material
TRANSLATIONAL RELEVANCE.
CLL cells depend on signals from the microenvironment for proliferation and survival. Defective DNA damage response and stroma contact can protect CLL cells from chemotherapy induced cytotoxicity. Therapies efficacious against tumor cells located in a protective microenvironment are needed. ON 01910.Na is a non-ATP competitive multikinase inhibitor in advanced clinical development for myelodysplastic syndrome. Here we report that ON 01910.Na inhibits the PI3K/AKT pathway, generates ROS, and selectively induces apoptosis in tumor cells, while sparing normal lymphocytes. ON 01910.Na was effective against tumor cells with p53 deletion and cells cocultured with follicular dendritic cells, an in-vitro model of the tumor microenvironment. Quenching of ROS production with an anti-oxidant and knockdown of NOXA protected tumor cells from drug induced apoptosis. These data support PI3K inhibition concomitant with ROS induction as a therapeutic principle with good activity and tumor selectivity. A clinical trial investigating ON 01910.Na in lymphoid malignancies has been initiated.
ACKNOWLEDGEMENTS
Foremost we thank our patients for their willingness to participate in these research studies. We also thank Susan Soto, Therese White, and Megan Tweito for clinical research support; the department of transfusion medicine for lymphapheresis procedures; Keyvan Keyvanfar for his help with flow cytometry; Yong Sung Choi for providing HK cells; Jocabed Roldán and Laura Jimenez Martí for their expert technical assistance, Nalini Raghavachari and Poching Liu in the gene expression core facility of NHLBI for performing gene expression analysis, and Marc A. Weniger for his help with bioinformatics.
Research support: This research was supported by the Intramural Research Program of the the National, Heart, Lung and Blood Institute and the National Cancer Institute, National Institutes of Health, and the Spanish Ministry of Science and Innovation (RYC2009-05134)
Footnotes
AUTHORSHIP CONTRIBUTIONS Adrian Wiestner and Patricia Pérez-Galán designed the study, supervised and coordinated laboratory work, analyzed data, and wrote the paper. Colby M. Chapman, Xiameng Sun, and Patricia Pérez-Galán conducted molecular and cellular assays and performed data analysis. Mark Roschewski, Mohammed Farooqui, Georg Aue, and Adrian Wiestner collected clinical data. Lawrence Stennett and Federica Gibellini processed cell samples and conducted IGHV sequencing. Diane Arthur conducted and interpreted the cytogenetic analyses.
REFERENCES
- 1.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–56. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wierda WG, O’Brien S, Wang X, Faderl S, Ferrajoli A, Do KA, et al. Prognostic nomogram and index for overall survival in previously untreated patients with chronic lymphocytic leukemia. Blood. 2007;109:4679–85. doi: 10.1182/blood-2005-12-051458. [DOI] [PubMed] [Google Scholar]
- 3.Rassenti LZ, Jain S, Keating MJ, Wierda WG, Grever MR, Byrd JC, et al. Relative value of ZAP-70, CD38, and immunoglobulin mutation status in predicting aggressive disease in chronic lymphocytic leukemia. Blood. 2008;112:1923–30. doi: 10.1182/blood-2007-05-092882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gribben JG, O’Brien S. Update on therapy of chronic lymphocytic leukemia. J Clin Oncol. 2011;29:544–50. doi: 10.1200/JCO.2010.32.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Messmer BT, Messmer D, Allen SL, Kolitz JE, Kudalkar P, Cesar D, et al. In vivo measurements document the dynamic cellular kinetics of chronic lymphocytic leukemia B cells. J Clin Invest. 2005;115:755–64. doi: 10.1172/JCI23409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deaglio S, Malavasi F. Chronic lymphocytic leukemia microenvironment: shifting the balance from apoptosis to proliferation. Haematologica. 2009;94:752–6. doi: 10.3324/haematol.2009.006676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herishanu Y, Perez-Galan P, Liu D, Biancotto A, Pittaluga S, Vire B, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011;117:563–74. doi: 10.1182/blood-2010-05-284984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burger JA, Ghia P, Rosenwald A, Caligaris-Cappio F. The microenvironment in mature B-cell malignancies: a target for new treatment strategies. Blood. 2009;114:3367–75. doi: 10.1182/blood-2009-06-225326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedberg JW, Sharman J, Sweetenham J, Johnston PB, Vose JM, Lacasce A, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2010;115:2578–85. doi: 10.1182/blood-2009-08-236471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herman SE, Gordon AL, Hertlein E, Ramanunni A, Zhang X, Jaglowski S, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117:6287–96. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herman SE, Gordon AL, Wagner AJ, Heerema NA, Zhao W, Flynn JM, et al. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116:2078–88. doi: 10.1182/blood-2010-02-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoellenriegel J, Meadows SA, Sivina M, Wierda WG, Kantarjian H, Keating MJ, et al. The phosphoinositide 3′-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011 doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bilancio A, Okkenhaug K, Camps M, Emery JL, Ruckle T, Rommel C, et al. Key role of the p110delta isoform of PI3K in B-cell antigen and IL-4 receptor signaling: comparative analysis of genetic and pharmacologic interference with p110delta function in B cells. Blood. 2006;107:642–50. doi: 10.1182/blood-2005-07-3041. [DOI] [PubMed] [Google Scholar]
- 14.Longo PG, Laurenti L, Gobessi S, Sica S, Leone G, Efremov DG. The Akt/Mcl-1 pathway plays a prominent role in mediating antiapoptotic signals downstream of the B-cell receptor in chronic lymphocytic leukemia B cells. Blood. 2008;111:846–55. doi: 10.1182/blood-2007-05-089037. [DOI] [PubMed] [Google Scholar]
- 15.Niedermeier M, Hennessy BT, Knight ZA, Henneberg M, Hu J, Kurtova AV, et al. Isoform-selective phosphoinositide 3′-kinase inhibitors inhibit CXCR4 signaling and overcome stromal cell-mediated drug resistance in chronic lymphocytic leukemia: a novel therapeutic approach. Blood. 2009;113:5549–57. doi: 10.1182/blood-2008-06-165068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Omori SA, Rickert RC. Phosphatidylinositol 3-kinase (PI3K) signaling and regulation of the antibody response. Cell Cycle. 2007;6:397–402. doi: 10.4161/cc.6.4.3837. [DOI] [PubMed] [Google Scholar]
- 17.Herishanu Y, Gibellini F, Njuguna N, Hazan-Halevy I, Farooqui M, Bern S, et al. Activation of CD44, a receptor for extracellular matrix components, protects chronic lymphocytic leukemia cells from spontaneous and drug induced apoptosis through MCL-1. Leuk Lymphoma. 2011 doi: 10.3109/10428194.2011.569962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ringshausen I, Schneller F, Bogner C, Hipp S, Duyster J, Peschel C, et al. Constitutively activated phosphatidylinositol-3 kinase (PI-3K) is involved in the defect of apoptosis in B-CLL: association with protein kinase Cdelta. Blood. 2002;100:3741–8. doi: 10.1182/blood-2002-02-0539. [DOI] [PubMed] [Google Scholar]
- 19.Jimeno A, Li J, Messersmith WA, Laheru D, Rudek MA, Maniar M, et al. Phase I study of ON 01910.Na, a novel modulator of the Polo-like kinase 1 pathway, in adult patients with solid tumors. J Clin Oncol. 2008;26:5504–10. doi: 10.1200/JCO.2008.17.9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gumireddy K, Reddy MV, Cosenza SC, Boominathan R, Baker SJ, Papathi N, et al. ON01910, a non-ATP-competitive small molecule inhibitor of Plk1, is a potent anticancer agent. Cancer Cell. 2005;7:275–86. doi: 10.1016/j.ccr.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Lenart P, Petronczki M, Steegmaier M, Di Fiore B, Lipp JJ, Hoffmann M, et al. The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of polo-like kinase 1. Curr Biol. 2007;17:304–15. doi: 10.1016/j.cub.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 22.Oussenko IA, Holland JF, Reddy EP, Ohnuma T. Effect of ON 01910.Na, an anticancer mitotic inhibitor, on cell cycle progression correlates with RanGAP1 hyperphosphorylation. Cancer Res. 2011 doi: 10.1158/0008-5472.CAN-10-1603. [DOI] [PubMed] [Google Scholar]
- 23.Prasad A, Park IW, Allen H, Zhang X, Reddy MV, Boominathan R, et al. Styryl sulfonyl compounds inhibit translation of cyclin D1 in mantle cell lymphoma cells. Oncogene. 2009;28:1518–28. doi: 10.1038/onc.2008.502. [DOI] [PubMed] [Google Scholar]
- 24.Wiestner A, Rosenwald A, Barry TS, Wright G, Davis RE, Henrickson SE, et al. ZAP-70 expression identifies a chronic lymphocytic leukemia subtype with unmutated immunoglobulin genes, inferior clinical outcome, and distinct gene expression profile. Blood. 2003;101:4944–51. doi: 10.1182/blood-2002-10-3306. [DOI] [PubMed] [Google Scholar]
- 25.Kim HS, Zhang X, Klyushnenkova E, Choi YS. Stimulation of germinal center B lymphocyte proliferation by an FDC-like cell line, HK. J Immunol. 1995;155:1101–9. [PubMed] [Google Scholar]
- 26.Rizzatti EG, Mora-Jensen H, Weniger MA, Gibellini F, Lee E, Daibata M, et al. Noxa mediates bortezomib induced apoptosis in both sensitive and intrinsically resistant mantle cell lymphoma cells and this effect is independent of constitutive activity of the AKT and NF-kappaB pathways. Leuk Lymphoma. 2008;49:798–808. doi: 10.1080/10428190801910912. [DOI] [PubMed] [Google Scholar]
- 27.Perez-Galan P, Mora-Jensen H, Weniger MA, Shaffer AL, 3rd, Rizzatti EG, Chapman CM, et al. Bortezomib resistance in mantle cell lymphoma is associated with plasmacytic differentiation. Blood. 2011;117:542–52. doi: 10.1182/blood-2010-02-269514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–50. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–37. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Horst A, Burgering BM. Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol. 2007;8:440–50. doi: 10.1038/nrm2190. [DOI] [PubMed] [Google Scholar]
- 31.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–52. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 32.Tonino SH, van Laar J, van Oers MH, Wang JY, Eldering E, Kater AP. ROS-mediated upregulation of Noxa overcomes chemoresistance in chronic lymphocytic leukemia. Oncogene. 2011;30:701–13. doi: 10.1038/onc.2010.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weniger MA, Rizzatti EG, Perez-Galan P, Liu D, Wang Q, Munson PJ, et al. Treatment-induced oxidative stress and cellular antioxidant capacity determine response to bortezomib in mantle cell lymphoma. Clin Cancer Res. 2011 doi: 10.1158/1078-0432.CCR-10-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurtova AV, Balakrishnan K, Chen R, Ding W, Schnabl S, Quiroga MP, et al. Diverse marrow stromal cells protect CLL cells from spontaneous and drug-induced apoptosis: development of a reliable and reproducible system to assess stromal cell adhesion-mediated drug resistance. Blood. 2009;114:4441–50. doi: 10.1182/blood-2009-07-233718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pedersen IM, Kitada S, Leoni LM, Zapata JM, Karras JG, Tsukada N, et al. Protection of CLL B cells by a follicular dendritic cell line is dependent on induction of Mcl-1. Blood. 2002;100:1795–801. [PubMed] [Google Scholar]
- 36.Burger JA, Burger M, Kipps TJ. Chronic lymphocytic leukemia B cells express functional CXCR4 chemokine receptors that mediate spontaneous migration beneath bone marrow stromal cells. Blood. 1999;94:3658–67. [PubMed] [Google Scholar]
- 37.Fer ND, Shoemaker RH, Monks A. Adaphostin toxicity in a sensitive non-small cell lung cancer model is mediated through Nrf2 signaling and heme oxygenase 1. J Exp Clin Cancer Res. 2010;29:91. doi: 10.1186/1756-9966-29-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang KW, Lee SJ, Park JW, Kim SG. Phosphatidylinositol 3-kinase regulates nuclear translocation of NF-E2-related factor 2 through actin rearrangement in response to oxidative stress. Mol Pharmacol. 2002;62:1001–10. doi: 10.1124/mol.62.5.1001. [DOI] [PubMed] [Google Scholar]
- 39.Raj L, Ide T, Gurkar AU, Foley M, Schenone M, Li X, et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature. 2011;475:231–4. doi: 10.1038/nature10167. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–91. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 41.Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H, et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–52. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 42.Trachootham D, Zhang H, Zhang W, Feng L, Du M, Zhou Y, et al. Effective elimination of fludarabine-resistant CLL cells by PEITC through a redox-mediated mechanism. Blood. 2008;112:1912–22. doi: 10.1182/blood-2008-04-149815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shanafelt TD, Lee YK, Bone ND, Strege AK, Narayanan VL, Sausville EA, et al. Adaphostin-induced apoptosis in CLL B cells is associated with induction of oxidative stress and exhibits synergy with fludarabine. Blood. 2005;105:2099–106. doi: 10.1182/blood-2004-06-2205. [DOI] [PubMed] [Google Scholar]
- 44.Silverman LR, Raza A, Sloand EM, Greenberg PL, Wilhelm FE. Overall Survival In Myelodysplastic Syndrome or Acute Myeloid Leukemia Patients Treated with On 01910. Na Correlates with Bone Marrow Blast Response. ASH Annual Meeting Abstracts. 2010;116:3998. [Google Scholar]
- 45.Davids MS, Steensma DP. The molecular pathogenesis of myelodysplastic syndromes. Cancer Biol Ther. 2010;10:309–19. doi: 10.4161/cbt.10.4.12612. [DOI] [PubMed] [Google Scholar]
- 46.Lamb J, Crawford ED, Peck D, Modell JW, Blat IC, Wrobel MJ, et al. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science. 2006;313:1929–35. doi: 10.1126/science.1132939. [DOI] [PubMed] [Google Scholar]
- 47.Yang JY, Hung MC. A new fork for clinical application: targeting forkhead transcription factors in cancer. Clin Cancer Res. 2009;15:752–7. doi: 10.1158/1078-0432.CCR-08-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Capasso M, Bhamrah MK, Henley T, Boyd RS, Langlais C, Cain K, et al. HVCN1 modulates BCR signal strength via regulation of BCR-dependent generation of reactive oxygen species. Nat Immunol. 2010;11:265–72. doi: 10.1038/ni.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jak M, van Bochove GG, van Lier RA, Eldering E, van Oers MH. CD40 stimulation sensitizes CLL cells to rituximab-induced cell death. Leukemia. 2011;25:968–78. doi: 10.1038/leu.2011.39. [DOI] [PubMed] [Google Scholar]
- 50.Cuni S, Perez-Aciego P, Perez-Chacon G, Vargas JA, Sanchez A, Martin-Saavedra FM, et al. A sustained activation of PI3K/NF-kappaB pathway is critical for the survival of chronic lymphocytic leukemia B cells. Leukemia. 2004;18:1391–400. doi: 10.1038/sj.leu.2403398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.