Abstract
Lipid-mediated signaling regulates a plethora of physiological processes, including critical aspects of brain function. In addition, dysregulation of lipid pathways has been implicated in a growing number of neurodegenerative disorders, such as Alzheimer’s disease (AD). Although much attention has been given to the link between cholesterol and AD pathogenesis, growing evidence suggests that other lipids, such as phosphoinositides, play an important role. Because regulators of lipid metabolism (e.g. statins) are a highly successful class of marketed drugs, exploration of lipid dysregulation in AD and identification of novel therapeutic agents acting through relevant lipid pathways offers new and effective options for the treatment of this devastating disorder.
Alzheimer’s disease (AD) is a fatal neurodegenerative disease characterized clinically by progressive memory loss as well as aberrant behavior 1,2, which, in the majority of cases occurs late in life and without a known cause (sporadic Alzheimer’s disease, SAD). Patients with AD display loss of synapses and neurons, as well as extracellular senile plaques and intracellular neurofibrillary tangles (NFTs). Senile plaques consist largely of aggregated amyloid β-peptide (Aβ), which is liberated from the holoprotein, amyloid precursor protein (APP), by sequential cleavages mediated by the β-secretase Beta-site APP cleavage enzyme 1 (BACE1) and the γ-secretase complex 1,2 (Box 1). Rare but highly penetrant mutations in the genes encoding APP and presenilins 1 and 2, the catalytic components of the γ-secretase, result in early-onset familial AD (FAD). Intraneuronal accumulation of insoluble aggregates of tau in the form of NFTs is also a pathological hallmark of AD 3. While under normal conditions tau binds and stabilizes microtubules in axons, a plethora of studies indicate that under pathological conditions, tau becomes hyperphosphorylated. This phenomenon causes the detachment of tau from microtubules and promotes the formation of insoluble tau aggregates, thus leading to the occurrence of paired helical filaments and NFTs present in AD brains 3. Although AD-associated cognitive dysfunction is shown to correlate strongly with the accumulation of Aβ and the severity of tau pathology, the precise relationship between these two pathological AD hallmarks remains poorly understood, particularly in SAD 4.
TEXT BOX 1: The amyloid cascade hypothesis.
The amyloid cascade hypothesis supports the idea that amyloid β-peptide (Aβ) plays a central and even causative role for AD 1,2. Cleavage of β-amyloid precursor protein (APP) by BACE1 liberates its soluble ectodomain (sAPPβ) into the extracellular space. The resulting cell-associated COOH-terminal fragments, which can be either 99 or 89 amino acids in length (termed “C99” or “C89”), are subjected to intramembrane proteolysis mediated by γ-secretase, which generates a spectrum of Aβ peptides of varying length at the COOH terminus as well as the APP intracellular COOH-terminal domain (AICD) 1,2. The predominant species of Aβ is 40 amino acids long (Aβ40), but the less abundant 42-amino acid variant (Aβ42) is more amyloidogenic and is the initial Aβ species that deposits into amyloid plaques in all forms of AD 1,2. Cleavage of APP within the Aβ sequence by α-secretase followed by γ-secretase cleavage, the non-amyloidogenic pathway, results in the production of a shorter, likely innocuous APP fragment (p3), along with the secreted ectodomain (sAPPα) and AICD 1,2. Although AD brains typically harbor senile plaques that consist of insoluble aggregates of Aβ, different assemblies of Aβ, including fibrils as well as soluble dimers, trimers and dodecamers, may differentially contribute to AD pathogenesis at various stages of this disorder 1,2. Importantly, elevation of soluble Aβ oligomers strongly correlates with cognitive decline, consistent with the synaptotoxic properties exhibited by these peptides in various systems 1,2. In sum, the modern amyloid cascade hypothesis takes into consideration the various assembly states of Aβ.
Text Box 1 Figure The amyloid cascade hypothesis. The cartoon illustrates the amyloidogenic proteolytic processing of APP leading to the production of Aβ and the role of this peptide in various assembly forms in the pathophysiological progression of AD.
Alois Alzheimer originally described a third pathological hallmark of AD, which was largely ignored by scientists in the field at its infancy, perhaps due to the lack of appropriate analytical tools. Indeed, AD brain displays a higher occurrence of “adipose inclusions” or “lipoid granules”, suggesting aberrant lipid metabolism5. Subsequently, biochemical alterations of lipid composition have been reported in post mortem brain tissue derived from individuals with AD. However, an intimate link between lipid metabolism and AD was only established when the ε4 allele of the apolipoprotein E (APOE) gene was identified as the strongest genetic risk factor for SAD6,7. ApoE encodes a ~34 kDa protein that serves as a crucial regulator of cholesterol metabolism in the brain and triglyceride metabolism throughout the body. It mediates the uptake of lipoprotein particles in the brain via the low-density lipoprotein receptor related protein (LRP) and the very low-density family lipoprotein receptor [recently reviewed by reference 8 and 9] (Fig. 1). The role of ApoE4 in amyloid pathology is supported by evidence that it binds Aβ and modulates the aggregation and clearance of Aβ. Additionally, several epidemiological studies now support a role for cholesterol in the pathogenesis of AD 8,9.
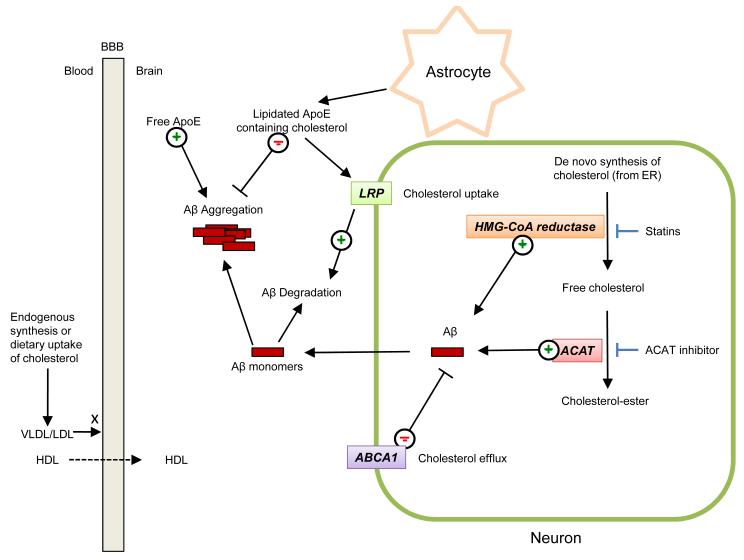
Figure 1. Contribution of cholesterol and apolipoprotein E metabolism to biogenesis, degradation and assembly of amyloid β-peptide.
Cholesterol in the brain is mainly derived from de novo synthesis from the endoplasmic reticulum. Small amounts of cholesterol can also be delivered to the brain from the periphery, via high density liproproteins (HDL), which can cross the blood-brain-barrier (BBB) unlike larger lipoproteins, such as low density lipoproteins (LDL) and very low density lipoproteins (VLDL). Hydroxymethyl glutaryl-coenzyme A (HMG-CoA) reductase mediates the rate-limiting step in de novo cholesterol biosynthesis. Excess free cholesterol is converted into cholesterol-ester by acyl-coenzyme A:cholesterol acyltransferase (ACAT). Inhibition of HMG-CoA reductase by statins leads to decreased levels of Aβ in animal models and ACAT inhibition has been also shown to reduce Aβ levels by a mechanism that remains as yet poorly defined. ApoE-containing HDL-like particles inhibit the aggregation of Aβ, while free ApoE has been shown to promote Aβ aggregation. LDL receptor-related protein (LRP) serves as a neuronal receptor for astrocyte-produced ApoE-containing lipid particles, thus mediating their internalization into neurons where they are broken down. ApoE-containing HDL-like particles inhibit the aggregation of Aβ, while free ApoE has been shown to promote Aβ aggregation in vitro. ABAC1, a regulator of cholesterol efflux, has been also shown to modulate Aβ levels in neurons. For simplicity, Aβ is drawn as free-floating in the cytoplasm, although it is produced in the lumen of neuronal organelles of the late secretory and endolysosomal systems, from where it can be secreted.
It is now well established that most, if not all, classes of lipids are implicated in AD pathogenesis, which can be reconciled with the following facts. First, lipids regulate the trafficking and/or proteolytic activity of membrane-bound proteins which play a fundamental role in this disorder, including APP, BACE1 and presenilins. Second, cytotoxic entities responsible for key phenotypic manifestations of AD, such as Aβ, exert their effects primarily by perturbing cellular membranes through direct and indirect mechanisms. Third, lipids themselves modulate the pathogenic potential of Aβ and perhaps tau as well, by affecting their propensity to aggregate. Thus, the scope of this review is to address the role of lipid dyshomeostasis in either mediating or modulating key pathological processes associated with AD. Specifically, this review elaborates on the roles of cellular lipids in the production and aggregation of Aβ, in mediating AD-associated synaptic dysfunction and in tau pathology.
Regulation of amyloidogenesis by lipids
APP, BACE1 and the components of the γ-secretase complex are transmembrane proteins. Consequently, lipid bilayer composition and organization have a significant impact on trafficking properties, as well as the proteolytic activities of β- and γ-secretases 10. Here, the role of cholesterol and other membrane lipid families in APP metabolism and secretase activity will be reviewed.
Role of cholesterol metabolism and transport in amyloidogenesis
In the brain, cholesterol is present mainly in its unesterified form in myelin sheaths and the cellular membranes of glial cells and neurons 11. Within the cells, the biggest reservoirs of cholesterol are found at the plasma membrane as well as in the endocytic recycling compartment 12. Since the blood brain barrier prevents any efficient exchange between brain and plasma lipoproteins, the majority of brain cholesterol is derived from de novo biosynthesis, rather than acquiring it from plasma LDL (Fig. 1) 11,13. Excess free cholesterol in the cell is converted into cholesteryl-esters by the enzyme acyl-coenzymeA:cholesterol acyltransferase (ACAT) (Fig. 1; Suppl. Fig. 1b), followed by accumulation in intracellular lipid droplets or efflux through the plasma membrane into the extracellular environment 14. Increasing levels of cholesteryl-esters enhances Aβ release in cultured cells, while pharmacological inhibition of ACAT (e.g., using CP-113818) leads to the reduction of both Aβ and cholesteryl-ester 15-17 (Fig. 1). Genetic ablation of ACAT1 reduces both Aβ pathology and cognitive impairments in a mouse model of AD18. However, complicating the interpretation, ACAT1 ablation also increases levels of oxysterol, 24(S)-hydroxy cholesterol (Suppl. Fig. 1c), suggesting a potential role of this cholesterol metabolite in decreasing amyloidogenesis 16,18. One putative mechanism consistent with these results is that the excess free brain cholesterol resulting from ACAT1 ablation, can be converted into 24(S)-hydroxycholesterol and subsequently cross the blood-brain barrier to reach the periphery, thus leading to reduced brain cholesterol levels. Together, these data suggest that the balance between free cholesterol and cholesterol esters is a key parameter controlling amyloidogenesis, although the molecular basis underlying this relationship is unclear 16.
Several lines of evidence indicate that cholesterol efflux also controls Aβ generation (Fig. 1). ATP-binding cassette transporter A1 (ABCA1) serves as an important regulator of the levels and lipidation status of ApoE by stimulating efflux of excess intracellular cholesterol to extracellular lipid acceptors, including unlipidated ApoE 19,20. Increased levels of ABCA1 were found to lower Aβ levels in cultured cells 21. Conversely, in vivo studies showed that deletion of ABCA1 gene in mouse models of AD dramatically decreases the levels of ApoE both in the brain and in the periphery, which correlates with greater Aβ deposits 22-24. Because residual ApoE in ABCA1-deficient mice is poorly lipidated, it was speculated that the low levels of ApoE lipidation, rather than the low levels of ApoE per se, promote amyloidogenesis 19. Alternatively, the latter phenomenon may be accounted for by accumulation of intracellular cholesterol as a result of decreased efflux 19.
Role of cellular cholesterol and lipid rafts in amyloidogenesis
Cholesterol can also directly modulate secretase activities leading to altered Aβ generation (Fig. 2). Reducing membrane cholesterol levels via cholesterol-extracting compounds, such as beta-methyl-cyclodextrin (βMCD), decreases activity of both BACE1 and γ-secretase, leading to an additive reduction in Aβ generation 10,25,26. Additionally, cell-free assays indicate that high cholesterol content directly influences the activity of recombinant BACE1 in reconstituted unilamellar vesicles 27 (Fig. 2a,c). Treatment of cholesterol-enriched vesicles containing BACE1 with βMCD reduces the amount of membrane-associated BACE1, suggesting that cholesterol-enriched microenvironments could directly influence the activity of BACE1. Cholesterol levels also directly regulate γ-secretase-mediated production of Aβ (Fig. 2c) 25,28,29. Inclusion of cholesterol or sphingolipids in PC-containing vesicles leads to increased γ-secretase activity 30,31. Together, these in vitro data are consistent with a role of cholesterol- and sphingolipid-enriched membrane microdomains called “lipid rafts” (Box 2) in the amyloidogenic processing of APP.
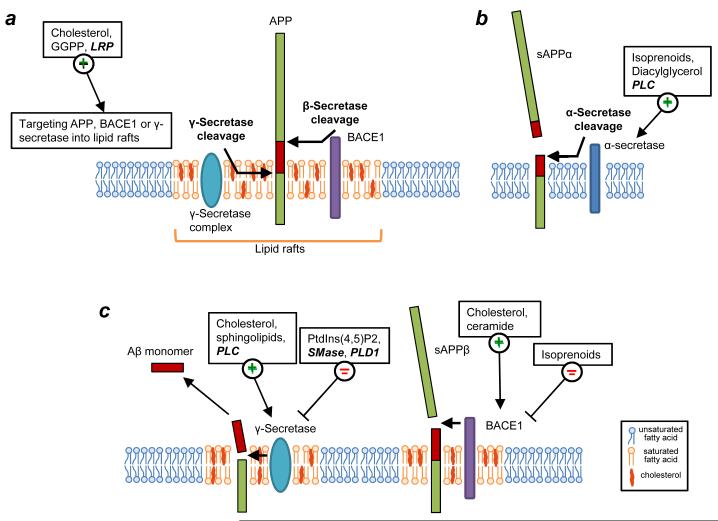
Figure 2. Modulation of proteolytic processing of β-amyloid precursor protein (APP) by lipids.
(a) The β-amyloid precursor protein (APP) undergoes amyloidogenic processing mediated either by β- and γ-secretases to yield amyloid β-peptide (Aβ) (purple rectangles). Amyloidogenic processing of APP largely occurs in lipid rafts. Cholesterol and LRP, an ApoE receptor, promote the localization of BACE1 to lipid rafts. GGPP (a short chain isoprenoid) has been shown to promote the association of the γ-secretase complex with lipid rafts. Several lipids (shown in bold text) and lipid metabolizing proteins (italicized bold text) influence APP processing through a variety of mechanisms. (b) APP is also subjected in neurons to a non-amyloidogenic pathway mediated by α-secretase. Localization of APP to non-lipid raft compartments favors processing by α-secretase. Isoprenoids, diaglycerol and phospholipase C (PLC) have been shown to promote the non-amyloidogenic pathway. (c) The relative abundance or absence of the lipids listed on the figure directly influences the activity of BACE1 or γ-secretase. Increased levels of cholesterol or ceramide enhance the activity of BACE1. Cholesterol and sphingolipids are positive modulators of γ-secretase activity while SMase and PLD1 have been identified as negative modulators of γ-secretase activity. PLC-mediated hydrolysis of PtdIns(4,5)P2 promotes amyloidogenesis by stimulating γ-secretase activity and PtdIns(4,5)P2 has been shown to directly inhibit γ-secretase activity.
TEXT BOX 2: Lipid rafts.
Lipid rafts are heterogenous, cholesterol- and sphingolipid-rich membrane microdomains that mediate compartmentalized cellular processes by clustering receptors and signaling molecules 140-143. In addition to cholesterol and sphingolipids, these dynamic lipid-protein assemblies are enriched in saturated glycerophospholipids and protein molecules with a high inherent affinity for ordered lipid domains. Raft lipids are believed to be held together by relatively weak covalent bonds, establishing a dynamic equilibrium of raft and non-raft regions within the plasma membrane 140. The sphingolipids interact laterally through van der Waals interactions and extensive hydrogen bonding between the sphingosine backbones and between the sugar head groups 144. Moreover, the majority of sphingolipids have saturated, and therefore unkinked, acyl chains that allow tighter packing of laterally associated lipids and a higher gel-liquid phase transition temperature 145. These interactions lead to segregation of sphingolipid-rich domains from their glycerophospholipid-rich surroundings. The degree of lateral association is further increased by the presence of cholesterol. The 3-β-hydroxyl group of cholesterol hydrogen bonds with the ceramide group of sphingolipids, while its planar sterol ring interacts with the saturated acyl chain 144,146. Growing evidence indicates that the amyloidogenic processing of APP occurs in lipid rafts, largely because functionally active pools of BACE1 and γ-secretase are present in these microdomains, alongside a pool of APP. A popular idea is that decreasing the association of these proteins with lipid rafts may be beneficial in AD, although the extent to which this approach would interfere with their physiological functions must be considered.
Despite the fact that pools of APP, BACE1 and presenilin are present in both raft and non-raft regions of the membranes, APP processing occurring within lipid rafts appears to be largely amyloidogenic, while outside lipid rafts, APP is processed predominantly by the non-amyloidogenic, α-secretase pathway (Fig. 2) 1,2,26. A significant pool of BACE1 is localized to lipid rafts 32,33 mainly through palmitoylation of its transmembrane and cytoplasmic domains 34,35. Cholesterol depletion decreases the association of BACE1 with lipid rafts, which correlates with decreased amyloidogenic processing of APP (Fig. 2a) 32,33,36. In contrast, acute cell exposure to cholesterol promotes the co-clustering of APP and BACE1 in lipid raft domains and their rapid endocytosis 37. Further supporting the key role of the BACE1-raft association in Aβ generation, introduction of a glycosylphosphatidylinositol (GPI) anchor, a targeting motif for lipid raft localization, into BACE1 sequence strongly promotes amyloidogenic processing of APP 26,35. Collectively, these studies suggest that cholesterol-sensitive Aβ production is determined, at least in part, by the levels of BACE1 present in lipid rafts. However, there may be cell-type specific differences dictating the extent of this relationship. Enhancing the association of APP with lipid rafts also favors amyloidogenic processing 26,33,38,39. Finally, core components of the γ-secretase complex, including presenilins, are also associated with lipid rafts (Fig. 2) 26,29,40. Accordingly, inhibition of γ-secretase activity leads to the accumulation of APP COOH-terminal products in lipid rafts (Fig. 2a,c) 26. In contrast, other γ-secretase substrates, such as Notch 1 and Jagged 2, are largely processed in non-raft compartments 41. Collectively, these studies suggest that modulating the biophysical properties of rafts to decrease association of APP, BACE1 or presenilins with these lipid microdomains, may offer a therapeutic opportunity to reduce amyloidogenic processing of APP, thus delaying the progression of AD.
Role of sphingolipids in Aβ production
In addition to cholesterol, sphingolipids, including ceramide, sphingomyelin (SM) and glycosphingolipids (GSLs) (Suppl. Fig. 2a), are major components of lipid rafts, playing a number of crucial roles in cell functions associated with normal as well as diseased states 42,43. Ceramide is a central component in sphingolipid metabolism and serves as the backbone to generate sphingomyelin (SM) or more complex glycosphingolipids (GSLs) via the addition of phosphocholine or sugars at the hydroxyl group, respectively. SM and GSLs are abundant in the brain, and gangliosides, which are GSLs containing sialic acids, are the major components of neuronal membranes (see below). Early reports show that ceramide levels are elevated at the earliest clinically recognizable stage of AD, perhaps mediating oxidative stress-induced neuronal death 44. However, ceramide also regulates BACE1-mediated processing of APP independently of its role in oxidative cell death. The mechanism appears to be related to the enhancement of BACE1 stability in cells, perhaps through the formation of ceramide-enriched platforms (Fig. 2c) 45,46.
Sphingolipids are also directly involved in APP metabolism. Inhibition of sphingomyelinase (SMase), the enzyme that mediates the conversion of SM to ceramide, and resulting SM accumulation, reduces Aβ secretion due to inhibition of γ-secretase activity (Fig. 2c) 47. Enhanced SMase activity is also detected in cells harboring FAD mutations in PS1, further indicating an important role for sphingolipids in AD 47. However, suppression of the entire sphingolipid biosynthetic pathway by inhibition of serine-palmitoyl-transferase leads to increased Aβ42 production while Aβ40 levels remain unchanged. Subsequent addition of sphingosine restores the normal Aβ42/Aβ40 ratio indicating sphingolipids may act as γ-secretase modulators 48. Finally, the V3 loop-domain of the Aβ peptide has been described as a sphingolipid-binding sequence 49 and confers affinity for this peptide to raft-like sphingolipid- and cholesterol-rich regions of cellular membranes, with potentially important implications for the aggregation, internalization and intracellular sorting of Aβ, all of which can affect its pathogenic potential 50. Collectively, these studies show that sphingolipids modulate activity of γ-secretase and BACE1 as well the microdomain localization of Aβ, although further work is needed to elucidate the mechanistic details and to validate these lipid-dependent models in vivo.
Isoprenoids and small GTPases
The HMG-CoA reductase pathway, also called the mevalonate pathway, not only leads to the synthesis of cholesterol, but also provides eukaryotic cells with essential lipids, such as isoprenoids (recently reviewed in ref. 51) (Suppl. Fig. 1a). Thus, statins, which block HMG-CoA reductase, may also exert their effects through cholesterol-independent mechanisms 52. Long-chain isoprenoids play a role in membrane organization and protein glycosylation (e.g., dolichol) as well as mitochondrion respiration (e.g., ubiquinone or coenzyme Q). In contrast, short-chain isoprenoids farnesylpyrophosphate (FPP) and its metabolite geranylgeranylpyrophosphate (GGPP) are utilized for the isoprenylation of a wide variety of proteins, including nuclear lamins and small GTPases of the Ras, Rho and Rab families. Small GTPases act as molecular switches in a myriad of signaling and trafficking pathways and their isoprenylation, referred to as farnesylation and geranylgeranylation, allows for recruitment to the cytosolically-exposed leaflets of cellular membranes, where they exert their signaling actions. Recent evidence suggests that a perturbation of the metabolism of FPP and GGPP and thus GTPase signaling occurs in AD 51,53. This phenomenon is likely to be relevant for AD pathogenesis because GTPase signaling can control multiple aspects of amyloidogenesis, including the trafficking of APP, BACE1 and γ-secretase.
While long-chain isoprenoids were reported to be altered in AD brains two decades ago 54, more recent studies have established that GGPP and to a lesser extent, FPP, are significantly elevated in the frontal cortex of AD patients, consistent with increased levels of their respective synthases 55. Importantly, pharmacological studies in various model systems, including in mice, have implicated short-chain isoprenoids as primary regulators of APP metabolism. Indeed, a search for the mechanism governing the Aβ42-lowering action of non-steroidal anti-inflammatory drugs (NSAIDs) has identified the activation of small GTPase Rho and its effector Rho-associated kinase (Rock) as potential mediators of this effect 56, although alternate mechanisms have also been proposed 57. While isoprenylation has been proposed to modulate the specificity or activity of γ-secretase in Aβ42 generation 40,58, statins were shown to promote ectodomain shedding of APP through the α-secretase pathway in a cholesterol-independent manner by inhibiting isoprenoid-mediated Rho/ROCK signaling, in favor of the non-amyloidogenic processing of APP (Fig. 2b) 59. However, lowering isoprenoid levels, yet, maintaining normal cholesterol levels, alters the traffic of APP leading to accumulation of β COOH-terminal fragments (βCTFs) and intracellular Aβ, suggesting that processing through the β-secretase pathway is enhanced (Fig. 2c) 60. Collectively, these studies indicate that isoprenylation differentially modulates all three secretase activities and underscores the pleiotropic effects of statins on APP metabolism by identifying cholesterol-and isoprenoid-specific processes.
Role of phospholipids in Aβ production
Early studies on AD brain tissue reported alterations of the composition and metabolism of several phospholipids, including phosphoinositides (i.e., phosphorylated derivatives of phosphatidylinositol), as well as changes in the levels of several phospholipid-metabolizing enzymes, such as phospholipases A2 (PLA2), C (PLC) and D (PLD), and phosphoinositide kinases and phosphatases 61,62. Additionally, wortmannin, a classical inhibitor of the phosphatidylinositol 3-kinase (PI3K) pathway was shown to reduce the levels of Aβ both in vitro and in vivo, thus further highlighting a potential role of phosphoinositides in AD pathogenesis 63,64, Phosphatidylinositol-4,5-bisphosphate [PtdIns(4,5)P2], the most abundant and best characterized species of phosphoinositides, is predominantly implicated in functions related to the plasma membrane 65. Insight into its role in amyloidogenesis was provided by the finding that reconstituted γ-secretase complex activity in liposomes is exquisitely sensitive to the presence of PtdIns(4,5)P2. This inhibitory effect on APP cleavage was proposed to reflect competitive interference between PtdIns(4,5)P2 and the substrate for their interaction with the γ-secretase complex 30,31. Further studies have shown that cellular PtdIns(4,5)P2 levels inversely correlate with secreted Aβ42 levels in cultured fibroblasts 66. The same study showed that enhanced turnover of PtdIns(4,5)P2 through the PLC pathway is a consequence of FAD-associated mutations in the presenilin genes. Reducing PtdIns(4,5)P2 turnover with a PLC inhibitor both decreases Aβ42 secretion and prevents Ca2+ entry, deficits commonly associated with these mutations (Fig. 2c) 66,67. While this raises the possibility of therapeutic targeting the PLC pathway, PLC-mediated PtdIns(4,5)P2 hydrolysis produces diacylglycerol, which, in turn, stimulates α-secretase via protein kinase C-dependent and independent mechanisms (Fig. 2b) 68. In the latter context, blocking PLC may thus indirectly enhance amyloidogenesis. Overall, it remains to be seen whether inhibition of PLC would be beneficial or not in AD.
The PLD pathway has also been shown to play a role in amyloidogenesis 62. Most studies on the link between PLD and amyloidogenesis have so far focused on PLD1, which, like related family member PLD2, hydrolyzes phosphatidylcholine to generate phosphatidic acid (PA) and free choline 62,69 (Fig. 3). PA is a bioactive lipid mediating signalling processes as well as membrane budding and fusion along the secretory, endolysosomal and autophagy pathway 69,70. PLD1 overexpression was shown to promote the cell surface delivery of APP and neurite outgrowth in primary hippocampal neurons expressing FAD mutant versions of PS1, two processes that are defective in mutant neurons. Additionally, PLD1 negatively regulates the processing of APP by PS1, likely through modulation of PS1 activity (Fig. 2b) 62,71,72. PLD1 also promotes the cell surface delivery of PS1 and physically interacts with this protein 71,73. Future studies are needed to establish whether PLD1 modulates AD pathogenesis through the abovementioned mechanisms in vivo and whether it involves crosstalk with the metabolism of PtdIns(4,5)P2, since its synthesis is stimulated by PA 69.
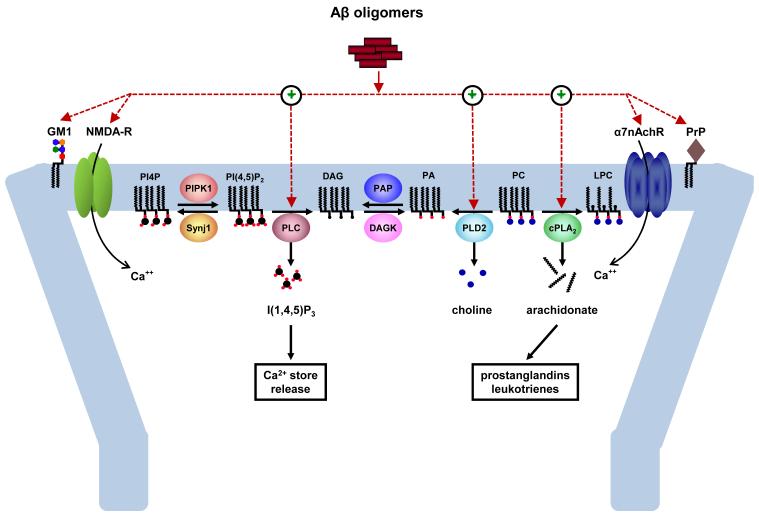
Figure 3. Role of lipids in Aβ-induced alterations in neuronal signaling and synaptic plasticity.
Aβ oligomers interact with a number of cell surface molecules, such as gangliosides (e.g., GM1) and Ca2+-permeable neurotransmitter receptor-channels [e.g., the NMDA and α7 nicotinic acetylcholine receptors (α7nAchR)], to influence neuronal activity and synaptic function in neurons. Some of these receptor-channels, such as the NMDA receptor, are critical for synaptic plasticity and are involved in learning and memory. A variety of Aβ oligomer species have been shown to potently alter synaptic function, in part by enhancing the activity of phospholipase C (PLC), cytosolic phospholipase A2 (cPLA2) and phospholipase D2 (PLD2). PLC hydrolyzes phosphatidylinositol-4,5-bisphosphate [PtdIns(4,5)P2] to diacylglycerol (DAG) and inositol-1,4,5-trisphosphate [Ins(1,4,5)P3]. cPLA2 hydrolyzes phosphatidylcholine (PC) to arachidonate and lysophosphatidylcholine (LPC). PLD2 hydrolyzes PC to phosphatidic acid (PA) and choline. The crosstalk and functional hierarchy among these phospholipases remain to be determined, although a common denominator for their activation appears to be elevation of intracellular Ca2+. Decreased dephosphorylation of PtdIns(4,5)P2 to phosphatidylinositol-4-phosphate (PtdIns4P) resulting from the heterozygous deletion of phosphoinositide phosphatase synaptojanin 1 (Synj1) confers protection against the synaptotoxic action of Aβ oligomers, indicating that phospholipid metabolism can directly influence the susceptibility of neurons to Aβ. PAP, phosphatidic acid phosphatase; DAGK, diacylglycerol kinase.
In summary, studies on phospholipids, such as phosphoinositides and PA, have implicated these lipids in several processes controlling Aβ generation, including the trafficking of APP and presenilin. This area thus has the potential to be explored for novel therapeutic interventions.
Role of lipids in Aβ misfolding and aggregation
Aβ is released in the lumen of organelles from the late secretory and endosomal systems as well as in the extracellular environment. Aβ is believed to exert its toxic effects by interacting with cellular membranes and their associated proteins, which, in turn, modulate the folding of this peptide into higher-order structures of varying cytotoxic potential 74,75. These Aβ species have the ability to perturb the integrity and signaling properties of membranes they interact with. Thus, membrane-bound assembly- (or aggregate-) promoting factors, such as gangliosides, have received considerable attention.
Gangliosides are abundant membrane glycosphingolipids that are primary modulators of Aβ aggregation 74,75 (Suppl. Fig. 2). They are concentrated in the luminal leaflet of various cellular organelles and the outer leaflet of the plasma membrane, where they are found in raft-like lipid microdomains that also contain cholesterol. The main gangliosides in the brain are GM1, GD1a (a-series) and GD1b and GT1b (b-series) 43. A link between gangliosides and AD was first suggested by studies showing aberrant expression levels and distribution of specific gangliosides in AD brain with significant regional differences 76,77. Importantly, gangliosides, such as GM1, bind Aβ and alter the conformation from random coils to more ordered structures with increased β-sheet content, which correlates with toxicity. Thus, cell–surface GM1 acts as a ‘seed’ for Aβ aggregation in neurons as well as in nerve terminal preparations. This property increases with the aging process and is facilitated by cholesterol-rich environments, indicating the process might occur in membrane rafts 75,78. Interestingly, the presence of GM1-bound Aβ (GAβ) is associated with early pathological changes in AD 79. FAD mutations within the Aβ sequence alter the ganglioside-binding specificity, perhaps in part, accounting for differences in the phenotypic manifestations associated with the respective families.
Genetic evidence now strongly supports the hypothesis that gangliosides may be key disease-modifiers and modulators of Aβ deposition. Ablation of GM2 synthase in a transgenic mouse model of AD leads to an accumulation of GM3 and a loss of GM1. This results in the accumulation of Aβ in brain parenchyma, and more interestingly, in vascular smooth muscle tissue. This vascular deposition is reminiscent of the amyloid angiopathy occurring in patients carrying the Dutch APP mutation, which lies within the Aβ sequence and enhances the affinity of Aβ for GM3 78. Perhaps more relevant for therapeutics, genetic ablation of GD3 synthase (GD3S) improves cognitive function and decreases the Aβ plaque burden in a bigenic mouse expressing mutant versions of the APP (swAPP) and PSEN1 (ΔE9) genes 80. Although Aβ fails to bind and exert neurotoxicity on GD3S null neurons and astrocytes, the precise nature of the protective mechanism conferred by GD3S ablation is unclear. In GD3S null cells, the decrease in b- and c-series gangliosides is concomitant with an increase in a-series, such as GM1 and GM3 80 (Suppl. Fig. 1). Potential benefits derived from ganglioside-based therapies are highlighted by studies in a transgenic model of AD, in which injections of GM1 were shown to decrease Aβ burden, likely by promoting degradation of Aβ in the periphery 81. Finally, gangliosides associated with Aβ in neuritic plaques may mediate evasion from immune surveillance by interacting with a negative immune receptor expressed in microglia, Siglec-11. Thus, gangliosides may indirectly facilitate the formation and maintenance of amyloid plaques by decreasing clearance through microglial degradation 82.
Lipid mediators and modulators of Aβ neurotoxicity and synaptic dysfunction
Aβ exerts its neurotoxicity primarily by interacting with cellular membranes leading to impairment of integrity and signaling properties. Synapses are particularly affected, not only because they are major sites of Aβ release, but also because Aβ release itself is enhanced by synaptic activity 83,84. Furthermore, oligomeric assemblies of Aβ appear to have high affinity for synaptic membranes, particularly dendritic spines 85. Because synaptic dysfunction is likely to be the primary cause of cognitive impairment in the early stage of AD 1,86, it is of paramount importance to fully understand the synaptotoxic signaling pathway dictated by Aβ and, in particular, the role of lipid changes in mediating this pathway. Central to the action of Aβ on lipid metabolism appears to be the ability of this peptide to perturb calcium homeostasis 87 and the subsequent dysregulation of calcium-sensitive phospholipid-metabolizing enzymes (Fig. 3).
Inositol lipids, phospholipase C and synaptojanin
Early studies of AD brains showed a reduction in the levels of PtdIns 61 and an increase in PLC activity as a result of Aβ application on membrane fractions 88 or due to expression of FAD mutants of presenilin 66,89. Insights into the underlying mechanism have been provided by our recent study indicating that Aβ itself disrupts the metabolism of PtdIns(4,5)P2 and that this phenomenon is required for its synaptotoxic action (Fig. 3) 90. Indeed, PtdIns(4,5)P2 levels decreased upon acute and chronic exposures to oligomeric Aβ42 at nanomolar concentrations. This effect required extracellular Ca2+ as well as PLC activity and partially depended on NMDA receptor function (Fig. 3). Blocking PtdIns(4,5)P2 breakdown by genetically reducing the level of a phosphoinositide phosphatase, synaptojanin 1, prevented Aβ42 oligomers from suppressing long-term potentiation (LTP) in hippocampal slices (Fig. 3) 90. Because synaptojanin 1 regulates clathrin-mediated synaptic vesicle recycling and the internalization of AMPA receptors 65,91,92, haploinsufficiency of Synj1 may counteract the ability of Aβ42 oligomers to perturb the synaptic vesicle cycle presynaptically or promote the internalization of AMPA receptors (and perhaps also NMDA receptors) at the postsynapse, both of which are central to Aβ-mediated depression of synaptic transmission 93,94. In contrast, the overexpression of Synj1 due to triplication of chromosome 21, where it is located, and the resulting deficiency of PtdIns(4,5)P2, correlate with learning deficits in transgenic mouse models of trisomy 21 (or Down syndrome) 95. Because middle-aged individuals with Down syndrome develop AD pathology, including senile plaques, these studies suggest the intriguing possibility that trisomy 21-linked Synj1 overexpression may confer a sensitized background for the synaptotoxic effects of Aβ. The combined actions of Synj1 and Aβ may inflict a dual ‘hit” upon PtdIns(4,5)P2 in the brains of individuals with Down syndrome.
While conversion of PtdIns(4,5)P2 to PtdIns(3,4,5)P3 by class I PI3Ks is key to cell survival, recent evidence suggests that it may paradoxically mediate synapse- and memory-impairing actions of Aβ in a fly model of AD 96. Indeed, expression of a secretory form of Aβ42 in the fly brain was shown to decrease long-term depression and to induce an age-dependent increase in oligomeric and fibrillar Aβ42, which parallels memory loss and neurodegeneration. Whether these defects in PI3K signaling occur in mammalian neurons and more generally in AD brain remains to be discovered.
Arachidonic acid and phospholipase A2
Release of arachidonic acid (AA) from phospholipids through the PLA2 pathway (Fig. 3; Suppl. Fig. 3) produces a large family of metabolites, including prostaglandins and leukotrienes 97. PLA2 products and their metabolites have been implicated in several AD-relevant aspects, including inflammation, glutamatergic transmission, synaptic plasticity and oxidative stress. Important clues implicating dysregulation of the PLA2 pathway in AD stemmed from post mortem studies showing an increase in the immunoreactivity of cytosolic PLA2 (cPLA2) 98 and a reduction in the levels of phospholipid-bound AA in AD brain 99. Other studies have identified cPLA2 as a key mediator of Aβ oligomer-induced neuronal cell death acting in a cascade involving ROS formation and leading to downstream activation of a sphingomyelinase-ceramide pathway triggering apoptosis 100,101. Recently, Mucke and colleagues utilized an unbiased approach to profile essential fatty acid metabolism in various brain regions from a transgenic model of AD (‘J20’), which is associated with a significant age-dependent accumulation of Aβ 102,103. An increase in AA as well as in various metabolites of this fatty acid was found selectively in the hippocampus. This led to the identification of a specific isoform of PLA2, group IVA (GIVA), which is Ca2+-dependent, highly expressed in the brain and hyperactivated in AD 102,103. Importantly, genetic ablation of GIVA-PLA2 improved cognition, viability and behavior, in the context of the J20 APP transgenic model, despite the elevated Aβ burden 102. A partial mechanistic understanding of Aβ-mediated neurotoxicity was provided by data showing that GIVA-PLA2 activation by Aβ and direct applications of the product AA both stimulate AMPA-mediated excitotoxicity in acutely-treated primary neuronal cultures by elevating the cell surface levels of AMPA receptor 102,103. Thus, GIVA-PLA2 is likely to be one of the key Ca2+ effectors mediating the acute and chronic synaptotoxic actions of Aβ.
Phosphatidic acid and phospholipase D
Several early post mortem studies reported changes in PLD activity in AD brain 62, a link that was corroborated by studies in cell culture models showing that APP overexpression and extracellular Aβ applications enhance PLD activity 62. Recently, our lab has provided robust in vitro and in vivo evidence for a role for the PLD2 isoform in mediating the synaptotoxic actions of Aβ 104. Indeed, we found that Aβ42 oligomers enhance total PLD activity in primary cortical neurons and that this increase is abolished in mouse neurons lacking PLD2. Evidence indicating that PLD2 is required for the synaptotoxic action of Aβ42 was provided by data showing that genetic ablation of this enzyme confers protection against the suppressive effects of Aβ42 on LTP and memory impairment in a mouse model of AD expressing mutant APP (i.e., Swedish APP or SwAPP) (Fig. 3) 104}. Using mass spectrometry, this study also identified a molecular species of PA that is controlled by PLD2, selectively upregulated in response to SwAPP overexpression and has been previously linked to neurodegeneration 104,105. Thus, specific pools of PA and PLD2 are likely to play a key role in AD pathogenesis, although the molecular basis for this phenomenon is unknown.
Regulation of cholesterol homeostasis and sphingolipid metabolism by Aβ
Aβ is known to regulate the metabolism of cholesterol and sphingolipids, both of which also affect APP processing 47,106. Indeed, Aβ40 suppresses the mevalonate pathway by inhibiting HMG-coA reductase, thus decreasing the levels of cholesterol 106. Additionally, while micromolar concentrations of Aβ42 induce oxidative stress and neurotoxicity by enhancing ceramide production through activation of neutral sphingomyelinase (N-SMase) 101, physiologically-relevant concentrations of Aβ42 (i.e., low pM to nM range) stimulate SM hydrolysis and ceramide production via the same enzyme without affecting cell viability 47,106. In light of a growing number of studies pointing to the role of low, physiological (picomolar) Aβ concentrations in synaptic function (see for instance ref. 107), the latter study suggests that regulation of lipid metabolism by APP and its metabolites may be relevant to neuronal physiology, although the signaling pathways involved are poorly understood.
Linking lipid metabolism to tau pathology
In contrast to APP and the secretases (which are membrane-bound), tau is a cytoplasmic protein that interacts with and stabilizes microtubules and although several types of membranous organelles critically depend on microtubules for their subcellular localization and intracellular transport, the relationship of tau with lipids is not direct. However, tau (and tau pathology) can be modulated by lipids in several ways. First, the Aβ signaling pathway, as highlighted above, perturbs biochemical pathways involving lipid metabolizing enzymes and bioactive lipids, ultimately affecting tau phosphorylation 108. Second, cholesterol levels are a key parameter controlling Aβ-induced tau proteolysis by calpain 109 and proteolytic cleavage of tau by this protease, but also by caspases, appears to be an early step leading to tau pathology 110,111. A pool of hyperphosphorylated tau is also present in lipid rafts, along with APP metabolites (including dimeric Aβ), BACE1, the γ-secretase complex and ApoE, suggesting that significant crosstalk may exist between Aβ and tau at the raft interface 112. Furthermore, there is evidence that kinases implicated in tau phosphorylation, such as Cdk5 and GSK-3β, are activated on cellular membranes and thus dysregulation of lipid metabolism may affect the activity of these kinases 113,114. Cdk5, in turn, phosphorylates the lipid kinase Vps34 115, whose product PtdIns3P, may regulate the clearance of tau aggregates by stimulating the autophagy pathway 116. Finally, a growing scientific community is investigating the mechanisms through which tau aggregates may be secreted or exert their prion-like infectious properties across cell membranes, thus propagating the tau pathology 117.
Although the link between lipid dysregulation and tau pathology is not well understood in AD, the occurrence of neurofibrillary tangles in the brain of individuals with lipid storage disorders, such as Niemann-Pick type C (NPC) disease, suggests a tight relationship between lipid trafficking defects and tau pathology 118. NPC is an inherited autosomal recessive neurodegenerative disorder that causes childhood or early adulthood death and stems from mutation in either of two functionally related genes, NPC1 and NPC2, which encode late endosome/lysosome-enriched proteins proposed to be involved in the trafficking of cholesterol 119. Loss of function mutant versions of NPC1 or NPC2 results in higher endosomal levels of unesterified cholesterol and SM in peripheral tissues as well as of multiple glycosphingolipids in the central nervous system, likely reflecting the inability of these lipids to efficiently exit this compartment and traffic to their normal destinations in cells 119. Although NPC is not associated with increased Aβ plaque burden, the lipid trafficking defect in NPC has been linked to enhanced amyloidogenic processing of APP in endosomes. As in AD, the relationship between tau pathology and aberrant APP metabolism is unclear, although the emerging notion of a central role for lipid trafficking defects and endosomal dysfunction in NPC suggests the existence of shared pathogenic mechanisms between this disorder and AD.
Lipid-focused therapeutic approaches
Despite a myriad of studies connecting lipid metabolism to AD pathogenesis, relatively few therapeutic approaches have exploited this connection thus far, with the exception of drugs affecting cholesterol metabolism, such as statins and ACAT inhibitors. Indeed, current drug development strategies mainly rely on the repositioning of existing therapeutic agents that have been originally developed for other disease indications, such as atherosclerosis. While the progress in the latter area is summarized here, this section will also elaborate on promising new leads stemming from research on phospholipids as well as lipid-based nutritional supplements.
Statins and ACAT inhibitors
As mentioned above, statins inhibit the enzyme HMG-CoA reductase which catalyzes the rate-limiting step in cholesterol biosynthesis (Suppl. Fig. 1). Retrospective case control studies suggest that statins reduce the risk of developing AD 120,121, but clinical trials and prospective cohort studies have produced inconsistent, mixed results 122,123. One of the more encouraging studies showed that statins prevent brain-trauma-induced Aβ elevation in mice, raising the possibility that an activity/injury-dependent, statin-sensitive pool of Aβ may be present in the brain 124. This is relevant because brain trauma may be a significant cause of SAD. Overall, despite early promise maintaining statins may influence AD via inhibitory effects on amyloidogenesis, anti-inflammatory actions or modification of cardiovascular risk factors 106,125, results from clinical studies have been rather disappointing.
A more recent therapeutic approach involves small molecule inhibitors of ACAT1, an enzyme that mediates the esterification of cholesterol, as discussed above (Suppl. Fig. 1). CP-113,818 and CI-1011 have demonstrated efficacy in preclinical models of AD 16,17. However, important challenges associated with the development of ACAT inhibitors for AD indication are safety concerns and their ability to cross the blood-brain barrier. Additionally, more research is needed to better understand the molecular mechanisms underlying the amelioration of AD phenotypes by these compounds.
Emerging lipid-centric therapeutic approaches
Genetic, pharmacological and cell biological studies continue to explore the possibility of targeting effectors and pathways of lipid metabolism relevant to AD. While many molecular targets, including cPLA2, Synj1, PLD2 and nSMase, are being validated at the genetic level, further studies are needed to assess the “drugability” of these targets and identify chemical probes that are suitable for further therapeutic development. Positive modulation of the postsynaptic ApoE receptor 2 by reelin also represents a promising avenue, as reelin inhibits the synaptotoxic action of Aβ by promoting glutamatergic transmission in depressed synapses 126.
While major research efforts are in progress to identify cellular effectors and enzymes of lipid metabolism, lipids themselves have been considered for therapeutic use as well as preventive agents for AD. Epidemiological studies suggest that reduced intake of omega-3 fatty acids elevates the risk of AD 127. Docosahexaenoic acid (DHA), the most popular omega-3 fatty acid used as a nutritional supplement, has been shown to exert multiple cellular effects antagonizing AD-associated phenotypes, including reduced Aβ and neuroprotection against Aβ-induced synaptotoxicity 127. While the exact cellular mechanisms underlying these actions are unclear, competition with an endogenous substrate, such as arachidonic acid (AA), may give rise to profound downstream changes in AA and AA-associated signaling pathways which have also been implicated in AD 102. Alternatively, DHA metabolites, such as the docosanoid neuroprotectin D1, may mediate some of the protective effects by counteracting pro-apoptotic as well as neuro-inflammatory signaling associated with AD 128. Recent work showed that DHA can also reduce Aβ levels by inhibiting both β-and γ-secretase activities while potentiating α-secretase cleavage, thus offering a novel perspective on how this lipid may exert neuroprotection 129. While polyunsaturated fatty acids, such as DHA, are generally viewed as neuroprotective, they are also primary substrates for lipid peroxidation, a phenomenon that is prominent in AD and other neurodegenerative disorders (Box 3). Thus, there long-term use should be considered with caution. Finally, recent genome-wide association studies (GWAS) have identified new risk genes in AD 130, some of which encode proteins that are linked to lipid metabolism (e.g., clusterin/Apolipoprotein J, cholesteryl ester transfer protein) 131-133. Despite the plausibility of the biological and pathological relevance of these gene products in AD, further validation studies are needed to better understand their role in AD pathogenesis.
TEXT BOX 3: The old theory of lipid peroxidation in AD.
Oxidative stress is a hallmark of many degenerative disorders. The brain is particularly vulnerable to this phenomenon due to high oxygen consumption, enrichment in polyunsaturated fatty acids (PUFAs) and high levels in redox metal ions 147. Lipid peroxidation products (LPPs) have been found in brain, cerebrospinal fluid and plasma from patients with AD 147. Primary substrates for lipid peroxidation are PUFAs and include ω-6 fatty acids (e.g., linoleic, arachidonic acid) as well as ω-3 fatty acids (e.g., DHA). Reactive oxygen species (ROS) are responsible for starting the chain by the production of an unstable lipid radical which is converted to a lipid peroxyl radical, leading to the peroxidation of other fatty acids (‘propagation’). This chain reaction stops (‘termination’) when two radicals react to produce a non-radical species or due to antioxidants (e.g., vitamin C and E) and enzymes of the superoxide dismutase, catalase, and peroxidase families 148. Oxidized PUFAs are further degraded to toxic products, such as 4-hydroxy-2-nonenal (HNE), acrolein and other short-chain aldehydes. Importantly, Aβ has been shown to cause oxidative stress through its interaction with transition metal ions, such as Cu2+ and Zn2+, which are enriched in senile plaques 149. Aβ can reduce these metal ions, thus producing hydrogen peroxide. During this process, Aβ becomes oxidized, thereby leading to the crosslinking of some of its residues’ side-chains and the formation of aggregate-prone adducts. Alternatively, hydrogen peroxide can be generated catalytically from Cu2+- or Zn2+-bound Aβ using other electron donors (e.g., PUFAs, cholesterol), a process leading to the generation of toxic LPPs, such as oxysterol and HNE. Finally, Aβ itself can be crosslinked by HNE. Key challenges in the field are to understand the role of LPP accumulation in the progression of AD-associated manifestations.
Conclusion and perspectives
One of the most outstanding questions in the field of AD and lipid research is the identification of the precise lipid-based molecular mechanisms governing AD-relevant processes that are intimately linked to the physicochemical and signaling properties of membranes. Specifically, what lipids modulate the targeting, trafficking, specificity and activity of the relevant secretases? Do specific lipid signatures predispose for or protect against AD? Are there common denominators in the lipid profiles of FAD and SAD-affected individuals that may help to explain or provide new perspectives on the convergence of these etiologically-distinct disorders toward AD pathological features? What lipid changes are causal for AD-linked pathogenicity and what changes are simple epiphenomena? Finally, which lipids and lipid metabolizing enzymes can be targeted or exploited for therapeutics?
None of these outstanding questions could be adequately addressed without the technological advance in lipid analysis, namely, the use of ‘lipidomics’ 134,135. This systems-level analysis allows for the identification of a wide variety of lipid changes occurring as a result of experimental manipulations or a diseased state, with the anticipation that these lipid changes may instruct us on the biochemical pathways that are affected or causal in the disease. Similar to genome-wide and proteomics-based approaches, lipidomics can unmask crosstalk between biochemical pathways and provide essential mechanistic insights into the molecular basis of cellular or organismal changes. Additionally, lipidomics offers enormous potential for the identification of disease-linked body-fluid biomarkers, which can prove particularly helpful as diagnostic tools at early stages of dementia and in the diagnosis of AD-specific mild cognitive impairment. This rapidly growing area has already begun to address lipid changes in AD brains 108,136,137, in animal models of AD 102,136,138,139 and in Aβ-treated cells 108. The prediction is that the number of such studies will grow exponentially in the near future and touch upon multiple facets of AD and other neurodegenerative disorders.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Robin Chan, Tiago Gil Oliveira, Diego Berman and Laura Beth McIntire for critical reading of the manuscript. Work from the authors is supported by US National Institutes of Health grants NS056049, HD05547 and AG033212 (G.D.P.) and NS074536 and AG033199 (T.-W.K.), American Health Assistance Foundation (T.-W.K.), Cure Alzheimer’s Fund (T.-W.K.), Alzheimer’s Drug Discovery Foundation (T.-W.K.), the Alzheimer’s Association (G.D.P.) and the McKnight Foundation (G.D.P.).
Bibliography
- 1.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 2.Tanzi RE, Bertram L. Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci. 2007;8:663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 4.Small SA, Duff K. Linking Abeta and tau in late-onset Alzheimer’s disease: a dual pathway hypothesis. Neuron. 2008;60:534–542. doi: 10.1016/j.neuron.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foley P. Lipids in Alzheimer’s disease: A century-old story. Biochimica et biophysica acta. 2010;1801:750–753. doi: 10.1016/j.bbalip.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Corder EH, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 7.Bertram L, Tanzi RE. Thirty years of Alzheimer’s disease genetics: the implications of systematic meta-analyses. Nat Rev Neurosci. 2008;9:768–778. doi: 10.1038/nrn2494. [DOI] [PubMed] [Google Scholar]
- 8.Bu G. Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nat Rev Neurosci. 2009;10:333–344. doi: 10.1038/nrn2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartmann T, Kuchenbecker J, Grimm MO. Alzheimer’s disease: the lipid connection. J Neurochem. 2007;103(Suppl 1):159–170. doi: 10.1111/j.1471-4159.2007.04715.x. [DOI] [PubMed] [Google Scholar]
- 11.Dietschy JM, Turley SD. Cholesterol metabolism in the brain. Curr Opin Lipidol. 2001;12:105–112. doi: 10.1097/00041433-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Mesmin B, Maxfield FR. Intracellular sterol dynamics. Biochim Biophys Acta. 2009;1791:636–645. doi: 10.1016/j.bbalip.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vance JE, Hayashi H, Karten B. Cholesterol homeostasis in neurons and glial cells. Semin Cell Dev Biol. 2005;16:193–212. doi: 10.1016/j.semcdb.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Chang TY, Chang CC, Ohgami N, Yamauchi Y. Cholesterol sensing, trafficking, and esterification. Annu Rev Cell Dev Biol. 2006;22:129–157. doi: 10.1146/annurev.cellbio.22.010305.104656. [DOI] [PubMed] [Google Scholar]
- 15.Puglielli L, et al. Acyl-coenzyme A: cholesterol acyltransferase modulates the generation of the amyloid beta-peptide. Nat Cell Biol. 2001;3:905–912. doi: 10.1038/ncb1001-905. [DOI] [PubMed] [Google Scholar]
- 16.Bhattacharyya R, Kovacs DM. ACAT inhibition and amyloid beta reduction. Biochim Biophys Acta. 2010 doi: 10.1016/j.bbalip.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutter-Paier B, et al. The ACAT inhibitor CP-113,818 markedly reduces amyloid pathology in a mouse model of Alzheimer’s disease. Neuron. 2004;44:227–238. doi: 10.1016/j.neuron.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 18.Bryleva EY, et al. ACAT1 gene ablation increases 24(S)-hydroxycholesterol content in the brain and ameliorates amyloid pathology in mice with AD. Proc Natl Acad Sci U S A. 2010;107:3081–3086. doi: 10.1073/pnas.0913828107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirsch-Reinshagen V, Burgess BL, Wellington CL. Why lipids are important for Alzheimer disease? Mol Cell Biochem. 2009;326:121–129. doi: 10.1007/s11010-008-0012-2. [DOI] [PubMed] [Google Scholar]
- 20.Tall AR. Cholesterol efflux pathways and other potential mechanisms involved in the athero-protective effect of high density lipoproteins. J Intern Med. 2008;263:256–273. doi: 10.1111/j.1365-2796.2007.01898.x. [DOI] [PubMed] [Google Scholar]
- 21.Sun Y, Yao J, Kim TW, Tall AR. Expression of liver X receptor target genes decreases cellular amyloid beta peptide secretion. J Biol Chem. 2003;278:27688–27694. doi: 10.1074/jbc.M300760200. [DOI] [PubMed] [Google Scholar]
- 22.Hirsch-Reinshagen V, et al. The absence of ABCA1 decreases soluble ApoE levels but does not diminish amyloid deposition in two murine models of Alzheimer disease. The Journal of biological chemistry. 2005;280:43243–43256. doi: 10.1074/jbc.M508781200. [DOI] [PubMed] [Google Scholar]
- 23.Koldamova R, Staufenbiel M, Lefterov I. Lack of ABCA1 considerably decreases brain ApoE level and increases amyloid deposition in APP23 mice. J Biol Chem. 2005;280:43224–43235. doi: 10.1074/jbc.M504513200. [DOI] [PubMed] [Google Scholar]
- 24.Wahrle SE, et al. Deletion of Abca1 increases Abeta deposition in the PDAPP transgenic mouse model of Alzheimer disease. J Biol Chem. 2005;280:43236–43242. doi: 10.1074/jbc.M508780200. [DOI] [PubMed] [Google Scholar]
- 25.Simons M, et al. Cholesterol depletion inhibits the generation of beta-amyloid in hippocampal neurons. Proc Natl Acad Sci U S A. 1998;95:6460–6464. doi: 10.1073/pnas.95.11.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vetrivel KS, Thinakaran G. Membrane rafts in Alzheimer’s disease beta-amyloid production. Biochim Biophys Acta. 2010 doi: 10.1016/j.bbalip.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalvodova L, et al. Lipids as modulators of proteolytic activity of BACE: involvement of cholesterol, glycosphingolipids, and anionic phospholipids in vitro. J Biol Chem. 2005;280:36815–36823. doi: 10.1074/jbc.M504484200. [DOI] [PubMed] [Google Scholar]
- 28.Fassbender K, et al. Simvastatin strongly reduces levels of Alzheimer’s disease beta - amyloid peptides Abeta 42 and Abeta 40 in vitro and in vivo. Proc Natl Acad Sci U S A. 2001;98:5856–5861. doi: 10.1073/pnas.081620098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wahrle S, et al. Cholesterol-dependent gamma-secretase activity in buoyant cholesterol-rich membrane microdomains. Neurobiol Dis. 2002;9:11–23. doi: 10.1006/nbdi.2001.0470. [DOI] [PubMed] [Google Scholar]
- 30.Osenkowski P, Ye W, Wang R, Wolfe MS, Selkoe DJ. Direct and potent regulation of gamma-secretase by its lipid microenvironment. J Biol Chem. 2008;283:22529–22540. doi: 10.1074/jbc.M801925200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osawa S, et al. Phosphoinositides suppress gamma-secretase in both the detergent-soluble and -insoluble states. J Biol Chem. 2008;283:19283–19292. doi: 10.1074/jbc.M705954200. [DOI] [PubMed] [Google Scholar]
- 32.Riddell DR, Christie G, Hussain I, Dingwall C. Compartmentalization of beta-secretase (Asp2) into low-buoyant density, noncaveolar lipid rafts. Curr Biol. 2001;11:1288–1293. doi: 10.1016/s0960-9822(01)00394-3. [DOI] [PubMed] [Google Scholar]
- 33.Ehehalt R, Keller P, Haass C, Thiele C, Simons K. Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. J Cell Biol. 2003;160:113–123. doi: 10.1083/jcb.200207113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vetrivel KS, et al. Alzheimer disease Abeta production in the absence of S-palmitoylation-dependent targeting of BACE1 to lipid rafts. J Biol Chem. 2009;284:3793–3803. doi: 10.1074/jbc.M808920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benjannet S, et al. Post-translational processing of beta-secretase (beta-amyloid-converting enzyme) and its ectodomain shedding. The pro- and transmembrane/cytosolic domains affect its cellular activity and amyloid-beta production. J Biol Chem. 2001;276:10879–10887. doi: 10.1074/jbc.M009899200. [DOI] [PubMed] [Google Scholar]
- 36.Hattori C, et al. BACE1 interacts with lipid raft proteins. J Neurosci Res. 2006;84:912–917. doi: 10.1002/jnr.20981. [DOI] [PubMed] [Google Scholar]
- 37.Marquer C, et al. Local cholesterol increase triggers amyloid precursor protein-Bace1 clustering in lipid rafts and rapid endocytosis. The FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2011 doi: 10.1096/fj.10-168633. [DOI] [PubMed] [Google Scholar]
- 38.Yoon IS, et al. Low-density lipoprotein receptor-related protein promotes amyloid precursor protein trafficking to lipid rafts in the endocytic pathway. FASEB J. 2007;21:2742–2752. doi: 10.1096/fj.07-8114com. [DOI] [PubMed] [Google Scholar]
- 39.Schneider A, et al. Flotillin-dependent clustering of the amyloid precursor protein regulates its endocytosis and amyloidogenic processing in neurons. J Neurosci. 2008;28:2874–2882. doi: 10.1523/JNEUROSCI.5345-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Urano Y, et al. Association of active gamma-secretase complex with lipid rafts. J Lipid Res. 2005;46:904–912. doi: 10.1194/jlr.M400333-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Vetrivel KS, et al. Spatial segregation of gamma-secretase and substrates in distinct membrane domains. J Biol Chem. 2005;280:25892–25900. doi: 10.1074/jbc.M503570200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 43.Posse de Chaves E, Sipione S. Sphingolipids and gangliosides of the nervous system in membrane function and dysfunction. FEBS Lett. 2010;584:1748–1759. doi: 10.1016/j.febslet.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 44.He X, Huang Y, Li B, Gong CX, Schuchman EH. Deregulation of sphingolipid metabolism in Alzheimer’s disease. Neurobiol Aging. 2008;31:398–408. doi: 10.1016/j.neurobiolaging.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puglielli L, Ellis BC, Saunders AJ, Kovacs DM. Ceramide stabilizes beta-site amyloid precursor protein-cleaving enzyme 1 and promotes amyloid beta-peptide biogenesis. The Journal of biological chemistry. 2003;278:19777–19783. doi: 10.1074/jbc.M300466200. [DOI] [PubMed] [Google Scholar]
- 46.Castro BM, Silva LC, Fedorov A, de Almeida RF, Prieto M. Cholesterol-rich fluid membranes solubilize ceramide domains: implications for the structure and dynamics of mammalian intracellular and plasma membranes. J Biol Chem. 2009;284:22978–22987. doi: 10.1074/jbc.M109.026567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grimm MO, et al. Regulation of cholesterol and sphingomyelin metabolism by amyloid-beta and presenilin. Nat Cell Biol. 2005;7:1118–1123. doi: 10.1038/ncb1313. [DOI] [PubMed] [Google Scholar]
- 48.Sawamura N, et al. Modulation of amyloid precursor protein cleavage by cellular sphingolipids. J Biol Chem. 2004;279:11984–11991. doi: 10.1074/jbc.M309832200. [DOI] [PubMed] [Google Scholar]
- 49.Fantini J, Garmy N, Mahfoud R, Yahi N. Lipid rafts: structure, function and role in HIV, Alzheimer’s and prion diseases. Expert Rev Mol Med. 2002;4:1–22. doi: 10.1017/S1462399402005392. [DOI] [PubMed] [Google Scholar]
- 50.Zhang D, Manna M, Wohland T, Kraut R. Alternate raft pathways cooperate to mediate slow diffusion and efficient uptake of a sphingolipid tracer to degradative and recycling compartments. J Cell Sci. 2009;122:3715–3728. doi: 10.1242/jcs.051557. [DOI] [PubMed] [Google Scholar]
- 51.Hooff GP, Wood WG, Muller WE, Eckert GP. Isoprenoids, small GTPases and Alzheimer’s disease. Biochim Biophys Acta. 2010 doi: 10.1016/j.bbalip.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cole SL, Vassar R. Isoprenoids and Alzheimer’s disease: a complex relationship. Neurobiol Dis. 2006;22:209–222. doi: 10.1016/j.nbd.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 54.Edlund C, Soderberg M, Kristensson K, Dallner G. Ubiquinone, dolichol, and cholesterol metabolism in aging and Alzheimer’s disease. Biochem Cell Biol. 1992;70:422–428. doi: 10.1139/o92-065. [DOI] [PubMed] [Google Scholar]
- 55.Eckert GP, et al. Regulation of the brain isoprenoids farnesyl- and geranylgeranylpyrophosphate is altered in male Alzheimer patients. Neurobiol Dis. 2009;35:251–257. doi: 10.1016/j.nbd.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou Y, et al. Nonsteroidal anti-inflammatory drugs can lower amyloidogenic Abeta42 by inhibiting Rho. Science. 2003;302:1215–1217. doi: 10.1126/science.1090154. [DOI] [PubMed] [Google Scholar]
- 57.Weggen S, Rogers M, Eriksen J. NSAIDs: small molecules for prevention of Alzheimer’s disease or precursors for future drug development? Trends in pharmacological sciences. 2007;28:536–543. doi: 10.1016/j.tips.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 58.Kukar T, et al. Diverse compounds mimic Alzheimer disease-causing mutations by augmenting Abeta42 production. Nat Med. 2005;11:545–550. doi: 10.1038/nm1235. [DOI] [PubMed] [Google Scholar]
- 59.Pedrini S, et al. Modulation of statin-activated shedding of Alzheimer APP ectodomain by ROCK. Plos Medicine. 2005;2:69–78. doi: 10.1371/journal.pmed.0020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cole SL, et al. Statins cause intracellular accumulation of amyloid precursor protein, beta-secretase-cleaved fragments, and amyloid beta-peptide via an isoprenoid-dependent mechanism. J Biol Chem. 2005;280:18755–18770. doi: 10.1074/jbc.M413895200. [DOI] [PubMed] [Google Scholar]
- 61.Stokes CE, Hawthorne JN. Reduced phosphoinositide concentrations in anterior temporal cortex of Alzheimer-diseased brains. J Neurochem. 1987;48:1018–1021. doi: 10.1111/j.1471-4159.1987.tb05619.x. [DOI] [PubMed] [Google Scholar]
- 62.Oliveira TG, Di Paolo G. Phospholipase D in brain function and Alzheimer’s disease. Biochim Biophys Acta. 2010;1801:799–905. doi: 10.1016/j.bbalip.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Petanceska SS, Gandy S. The phosphatidylinositol 3-kinase inhibitor wortmannin alters the metabolism of the Alzheimer’s amyloid precursor protein. Journal of Neurochemistry. 1999;73:2316–2320. doi: 10.1046/j.1471-4159.1999.0732316.x. [DOI] [PubMed] [Google Scholar]
- 64.Haugabook SJ, et al. Reduction of Abeta accumulation in the Tg2576 animal model of Alzheimer’s disease after oral administration of the phosphatidyl-inositol kinase inhibitor wortmannin. FASEB J. 2001;15:16–18. doi: 10.1096/fj.00-0528fje. [DOI] [PubMed] [Google Scholar]
- 65.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 66.Landman N, et al. Presenilin mutations linked to familial Alzheimer’s disease cause an imbalance in phosphatidylinositol 4,5-bisphosphate metabolism. Proc Natl Acad Sci U S A. 2006;103:19524–19529. doi: 10.1073/pnas.0604954103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoo AS, et al. Presenilin-mediated modulation of capacitative calcium entry. Neuron. 2000;27:561–572. doi: 10.1016/s0896-6273(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 68.Rossner S. New players in old amyloid precursor protein-processing pathways. Int J Dev Neurosci. 2004;22:467–474. doi: 10.1016/j.ijdevneu.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 69.Jenkins GM, Frohman MA. Phospholipase D: a lipid centric review. Cell Mol Life Sci. 2005;62:2305–2316. doi: 10.1007/s00018-005-5195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dall’armi C, et al. The phospholipase D1 pathway modulates macroautophagy. Nat Commun. 2010;1:142. doi: 10.1038/ncomms1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cai D, et al. Presenilin-1 uses phospholipase D1 as a negative regulator of beta-amyloid formation. Proc Natl Acad Sci U S A. 2006;103:1941–1946. doi: 10.1073/pnas.0510708103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cai D, et al. Phospholipase D1 corrects impaired betaAPP trafficking and neurite outgrowth in familial Alzheimer’s disease-linked presenilin-1 mutant neurons. Proc Natl Acad Sci U S A. 2006;103:1936–1940. doi: 10.1073/pnas.0510710103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu Y, et al. Intracellular trafficking of presenilin 1 is regulated by beta-amyloid precursor protein and phospholipase D1. J Biol Chem. 2009 doi: 10.1074/jbc.M808497200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ariga T, McDonald MP, Yu RK. Role of ganglioside metabolism in the pathogenesis of Alzheimer’s disease--a review. J Lipid Res. 2008;49:1157–1175. doi: 10.1194/jlr.R800007-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matsuzaki K, Kato K, Yanagisawa K. Abeta polymerization through interaction with membrane gangliosides. Biochim Biophys Acta. 2010 doi: 10.1016/j.bbalip.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 76.Kracun I, Kalanj S, Cosovic C, Talan-Hranilovic J. Brain gangliosides in Alzheimer’s disease. J Hirnforsch. 1990;31:789–793. [PubMed] [Google Scholar]
- 77.Kracun I, et al. Human brain gangliosides in development, aging and disease. Int J Dev Biol. 1991;35:289–295. [PubMed] [Google Scholar]
- 78.Yanagisawa K. Role of gangliosides in Alzheimer’s disease. Biochim Biophys Acta. 2007;1768:1943–1951. doi: 10.1016/j.bbamem.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 79.Yanagisawa K, McLaurin J, Michikawa M, Chakrabartty A, Ihara Y. Amyloid beta-protein (A beta) associated with lipid molecules: immunoreactivity distinct from that of soluble A beta. FEBS Lett. 1997;420:43–46. doi: 10.1016/s0014-5793(97)01484-1. [DOI] [PubMed] [Google Scholar]
- 80.Bernardo A, et al. Elimination of GD3 synthase improves memory and reduces amyloid-beta plaque load in transgenic mice. Neurobiol Aging. 2009;30:1777–1791. doi: 10.1016/j.neurobiolaging.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 81.Matsuoka Y, et al. Novel therapeutic approach for the treatment of Alzheimer’s disease by peripheral administration of agents with an affinity to beta-amyloid. J Neurosci. 2003;23:29–33. doi: 10.1523/JNEUROSCI.23-01-00029.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Salminen A, Kaarniranta K. Siglec receptors and hiding plaques in Alzheimer’s disease. J Mol Med. 2009;87:697–701. doi: 10.1007/s00109-009-0472-1. [DOI] [PubMed] [Google Scholar]
- 83.Kamenetz F, et al. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 84.Cirrito JR, et al. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 85.Lacor PN, et al. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Palop JJ, Mucke L. Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat Neurosci. 2010;13:812–818. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Green KN, LaFerla FM. Linking calcium to Abeta and Alzheimer’s disease. Neuron. 2008;59:190–194. doi: 10.1016/j.neuron.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 88.Wallace MA. Effects of Alzheimer’s disease-related beta amyloid protein fragments on enzymes metabolizing phosphoinositides in brain. Biochim Biophys Acta. 1994;1227:183–187. doi: 10.1016/0925-4439(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 89.Cedazo-Minguez A, Popescu BO, Ankarcrona M, Nishimura T, Cowburn RF. The presenilin 1 deltaE9 mutation gives enhanced basal phospholipase C activity and a resultant increase in intracellular calcium concentrations. J Biol Chem. 2002;277:36646–36655. doi: 10.1074/jbc.M112117200. [DOI] [PubMed] [Google Scholar]
- 90.Berman DE, et al. Oligomeric amyloid-beta peptide disrupts phosphatidylinositol-4,5-bisphosphate metabolism. Nat Neurosci. 2008;11:547–554. doi: 10.1038/nn.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Irie F, Okuno M, Pasquale EB, Yamaguchi Y. EphrinB-EphB signalling regulates clathrin-mediated endocytosis through tyrosine phosphorylation of synaptojanin 1. Nat Cell Biol. 2005;7:501–509. doi: 10.1038/ncb1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gong LW, De Camilli P. Regulation of postsynaptic AMPA responses by synaptojanin 1. Proc Natl Acad Sci U S A. 2008;105:17561–17566. doi: 10.1073/pnas.0809221105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hsieh H, et al. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Snyder EM, et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 95.Voronov SV, et al. Synaptojanin 1-linked phosphoinositide dyshomeostasis and cognitive deficits in mouse models of Down’s syndrome. Proc Natl Acad Sci U S A. 2008;105:9415–9420. doi: 10.1073/pnas.0803756105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chiang HC, Wang L, Xie Z, Yau A, Zhong Y. PI3 kinase signaling is involved in Abeta-induced memory loss in Drosophila. Proc Natl Acad Sci U S A. 2010;107:7060–7065. doi: 10.1073/pnas.0909314107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stephenson DT, Lemere CA, Selkoe DJ, Clemens JA. Cytosolic phospholipase A2 (cPLA2) immunoreactivity is elevated in Alzheimer’s disease brain. Neurobiol Dis. 1996;3:51–63. doi: 10.1006/nbdi.1996.0005. [DOI] [PubMed] [Google Scholar]
- 99.Prasad MR, Lovell MA, Yatin M, Dhillon H, Markesbery WR. Regional membrane phospholipid alterations in Alzheimer’s disease. Neurochem Res. 1998;23:81–88. doi: 10.1023/a:1022457605436. [DOI] [PubMed] [Google Scholar]
- 100.Kriem B, et al. Cytosolic phospholipase A2 mediates neuronal apoptosis induced by soluble oligomers of the amyloid-beta peptide. FASEB J. 2005;19:85–87. doi: 10.1096/fj.04-1807fje. [DOI] [PubMed] [Google Scholar]
- 101.Malaplate-Armand C, et al. Soluble oligomers of amyloid-beta peptide induce neuronal apoptosis by activating a cPLA2-dependent sphingomyelinase-ceramide pathway. Neurobiol Dis. 2006;23:178–189. doi: 10.1016/j.nbd.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 102.Sanchez-Mejia RO, et al. Phospholipase A2 reduction ameliorates cognitive deficits in a mouse model of Alzheimer’s disease. Nat Neurosci. 2008;11:1311–1318. doi: 10.1038/nn.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sanchez-Mejia RO, Mucke L. Phospholipase A2 and arachidonic acid in Alzheimer’s disease. Biochim Biophys Acta. 2010;1801:784–790. doi: 10.1016/j.bbalip.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oliveira TG, et al. Phospholipase d2 ablation ameliorates Alzheimer’s disease-linked synaptic dysfunction and cognitive deficits. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:16419–16428. doi: 10.1523/JNEUROSCI.3317-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Raghu P, et al. Rhabdomere biogenesis in Drosophila photoreceptors is acutely sensitive to phosphatidic acid levels. Journal of Cell Biology. 2009;185:129–145. doi: 10.1083/jcb.200807027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Grosgen S, Grimm MO, Friess P, Hartmann T. Role of amyloid beta in lipid homeostasis. Biochim Biophys Acta. 2010 doi: 10.1016/j.bbalip.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 107.Puzzo D, et al. Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. J Neurosci. 2008;28:14537–14545. doi: 10.1523/JNEUROSCI.2692-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ryan SD, et al. Amyloid-{beta}42 signals tau hyperphosphorylation and compromises neuronal viability by disrupting alkylacylglycerophosphocholine metabolism. Proc. Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0905654106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nicholson AM, Ferreira A. Increased membrane cholesterol might render mature hippocampal neurons more susceptible to beta-amyloid-induced calpain activation and tau toxicity. J Neurosci. 2009;29:4640–4651. doi: 10.1523/JNEUROSCI.0862-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Johnson GV. Tau phosphorylation and proteolysis: insights and perspectives. J Alzheimers Dis. 2006;9:243–250. doi: 10.3233/jad-2006-9s326. [DOI] [PubMed] [Google Scholar]
- 111.de Calignon A, et al. Caspase activation precedes and leads to tangles. Nature. 2010;464:1201–1204. doi: 10.1038/nature08890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kawarabayashi T, et al. Dimeric amyloid beta protein rapidly accumulates in lipid rafts followed by apolipoprotein E and phosphorylated tau accumulation in the Tg2576 mouse model of Alzheimer’s disease. J Neurosci. 2004;24:3801–3809. doi: 10.1523/JNEUROSCI.5543-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hernandez P, Lee G, Sjoberg M, Maccioni RB. Tau phosphorylation by cdk5 and Fyn in response to amyloid peptide Abeta (25-35): involvement of lipid rafts. J Alzheimers Dis. 2009;16:149–156. doi: 10.3233/JAD-2009-0933. [DOI] [PubMed] [Google Scholar]
- 114.Schenck A, et al. The endosomal protein Appl1 mediates Akt substrate specificity and cell survival in vertebrate development. Cell. 2008;133:486–497. doi: 10.1016/j.cell.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 115.Furuya T, et al. Negative regulation of Vps34 by Cdk mediated phosphorylation. Mol Cell. 38:500–511. doi: 10.1016/j.molcel.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Garcia-Arencibia M, Hochfeld WE, Toh PP, Rubinsztein DC. Autophagy, a guardian against neurodegeneration. Semin Cell Dev Biol. 2010 doi: 10.1016/j.semcdb.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Frost B, Diamond MI. Prion-like mechanisms in neurodegenerative diseases. Nat Rev Neurosci. 2010;11:155–159. doi: 10.1038/nrn2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nixon RA. Niemann-Pick Type C disease and Alzheimer’s disease: the APP-endosome connection fattens up. Am J Pathol. 2004;164:757–761. doi: 10.1016/S0002-9440(10)63163-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nixon RA. Endosome function and dysfunction in Alzheimer’s disease and other neurodegenerative diseases. Neurobiol Aging. 2005;26:373–382. doi: 10.1016/j.neurobiolaging.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 120.Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. Lancet. 2000;356:1627–1631. doi: 10.1016/s0140-6736(00)03155-x. [DOI] [PubMed] [Google Scholar]
- 121.Wolozin B, et al. Simvastatin is associated with a reduced incidence of dementia and Parkinson’s disease. BMC Med. 2007;5:20. doi: 10.1186/1741-7015-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Arvanitakis Z, et al. Statins, incident Alzheimer disease, change in cognitive function, and neuropathology. Neurology. 2008;70:1795–1802. doi: 10.1212/01.wnl.0000288181.00826.63. [DOI] [PubMed] [Google Scholar]
- 123.Feldman HH, et al. Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology. 2010;74:956–964. doi: 10.1212/WNL.0b013e3181d6476a. [DOI] [PubMed] [Google Scholar]
- 124.Abrahamson EE, Ikonomovic MD, Dixon CE, DeKosky ST. Simvastatin therapy prevents brain trauma-induced increases in beta-amyloid peptide levels. Ann Neurol. 2009;66:407–414. doi: 10.1002/ana.21731. [DOI] [PubMed] [Google Scholar]
- 125.Kandiah N, Feldman HH. Therapeutic potential of statins in Alzheimer’s disease. J Neurol Sci. 2009;283:230–234. doi: 10.1016/j.jns.2009.02.352. [DOI] [PubMed] [Google Scholar]
- 126.Durakoglugil MS, Chen Y, White CL, Kavalali ET, Herz J. Reelin signaling antagonizes beta-amyloid at the synapse. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15938–15943. doi: 10.1073/pnas.0908176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cole GM, Ma QL, Frautschy SA. Omega-3 fatty acids and dementia. Prostaglandins Leukot Essent Fatty Acids. 2009;81:213–221. doi: 10.1016/j.plefa.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Palacios-Pelaez R, Lukiw WJ, Bazan NG. Omega-3 essential Fatty acids modulate initiation and progression of neurodegenerative disease. Mol Neurobiol. 2010;41:367–374. doi: 10.1007/s12035-010-8139-z. [DOI] [PubMed] [Google Scholar]
- 129.Grimm MO, et al. Docosahexaenoic Acid reduces amyloid {beta} production via multiple, pleiotropic mechanism. The Journal of biological chemistry. 2011 doi: 10.1074/jbc.M110.182329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bertram L, Tanzi RE. Genome-wide association studies in Alzheimer’s disease. Hum Mol Genet. 2009;18:R137–145. doi: 10.1093/hmg/ddp406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Harold D, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lambert JC, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 133.Sanders AE, et al. Association of a functional polymorphism in the cholesteryl ester transfer protein (CETP) gene with memory decline and incidence of dementia. JAMA. 2010;303:150–158. doi: 10.1001/jama.2009.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wenk MR. The emerging field of lipidomics. Nat Rev Drug Discov. 2005;4:594–610. doi: 10.1038/nrd1776. [DOI] [PubMed] [Google Scholar]
- 135.Piomelli D, Astarita G, Rapaka R. A neuroscientist’s guide to lipidomics. Nat Rev Neurosci. 2007;8:743–754. doi: 10.1038/nrn2233. [DOI] [PubMed] [Google Scholar]
- 136.Han X, Holtzman DM, McKeel DW., Jr. Plasmalogen deficiency in early Alzheimer’s disease subjects and in animal models: molecular characterization using electrospray ionization mass spectrometry. J Neurochem. 2001;77:1168–1180. doi: 10.1046/j.1471-4159.2001.00332.x. [DOI] [PubMed] [Google Scholar]
- 137.Cheng H, Xu J, McKeel DW, Jr., Han X. Specificity and potential mechanism of sulfatide deficiency in Alzheimer’s disease: an electrospray ionization mass spectrometric study. Cell Mol Biol (Noisy-le-grand) 2003;49:809–818. [PubMed] [Google Scholar]
- 138.Cheng H, Zhou Y, Holtzman DM, Han X. Apolipoprotein E mediates sulfatide depletion in animal models of Alzheimer’s disease. Neurobiol Aging. 2010;31:1188–1196. doi: 10.1016/j.neurobiolaging.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sharman MJ, et al. Profiling brain and plasma lipids in human APOE epsilon2, epsilon3, and epsilon4 knock-in mice using electrospray ionization mass spectrometry. J Alzheimers Dis. 2010;20:105–111. doi: 10.3233/JAD-2010-1348. [DOI] [PubMed] [Google Scholar]
- 140.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 141.Lingwood D, Kaiser HJ, Levental I, Simons K. Lipid rafts as functional heterogeneity in cell membranes. Biochem Soc Trans. 2009;37:955–960. doi: 10.1042/BST0370955. [DOI] [PubMed] [Google Scholar]
- 142.Allen JA, Halverson-Tamboli RA, Rasenick MM. Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci. 2007;8:128–140. doi: 10.1038/nrn2059. [DOI] [PubMed] [Google Scholar]
- 143.Jacobson K, Mouritsen OG, Anderson RG. Lipid rafts: at a crossroad between cell biology and physics. Nat Cell Biol. 2007;9:7–14. doi: 10.1038/ncb0107-7. [DOI] [PubMed] [Google Scholar]
- 144.Ramstedt B, Slotte JP. Sphingolipids and the formation of sterol-enriched ordered membrane domains. Biochim Biophys Acta. 2006;1758:1945–1956. doi: 10.1016/j.bbamem.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 145.Ramstedt B, Slotte JP. Membrane properties of sphingomyelins. FEBS Lett. 2002;531:33–37. doi: 10.1016/s0014-5793(02)03406-3. [DOI] [PubMed] [Google Scholar]
- 146.Xu X, London E. The effect of sterol structure on membrane lipid domains reveals how cholesterol can induce lipid domain formation. Biochemistry. 2000;39:843–849. doi: 10.1021/bi992543v. [DOI] [PubMed] [Google Scholar]
- 147.Butterfield DA, et al. In vivo oxidative stress in brain of Alzheimer disease transgenic mice: Requirement for methionine 35 in amyloid beta-peptide of APP. Free Radic Biol Med. 2010;48:136–144. doi: 10.1016/j.freeradbiomed.2009.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schneider C, Porter NA, Brash AR. Routes to 4-hydroxynonenal: fundamental issues in the mechanisms of lipid peroxidation. J Biol Chem. 2008;283:15539–15543. doi: 10.1074/jbc.R800001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bush AI, Tanzi RE. Therapeutics for Alzheimer’s disease based on the metal hypothesis. Neurotherapeutics. 2008;5:421–432. doi: 10.1016/j.nurt.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.