Abstract
We have previously reported that a modified Stone T-maze (STM), using escape from water as motivation, was effective in evaluating learning and memory ability in young C57/BL6 mice. Here we report on the effectiveness and sensitivity of the STM in the assessment of age-related learning and memory deficits in mice using either escape from foot shock or water as the motivational manipulations. C57BL/6Nia mice 7-, 12-, 20- and 24-mo old received 15 massed trials in the escape from foot shock motivated STM while C57BL/6Nia mice 5-, 12-, and 25-mo old were tested in the escape from water STM. Analysis of errors, the main performance variable, revealed similar results in both versions of the task with younger mice making fewer errors. Notably mice of all ages in the water-motivated version moved quickly through the maze, while all ages of mice in the shock-motivated version tended to wait for shock to be initiated to move forward. Overall, both versions of the STM appear to be sensitive to age-related changes in learning and memory and provide an alternative to other testing paradigms such as the Morris water waze which are susceptible to performance confounds which can lead to uninterpretable results.
Keywords: Aging, mice, learning, memory, maze
1. Introduction
Behavioral paradigms that reliably evaluate age-related learning and memory deficits in mice have been difficult to establish. This situation can impede progress to develop therapeutics for the treatment of age-associated neurodegenerative disorders affecting learning and memory. Several behavioral tasks have been the mainstay for this research field, specifically, the Morris water maze (MWM) or versions of the MWM (Ashe, 2001; Chacan et al., 2004; Fisher et al., 2003; Van Dam et al., 2008). However, the MWM was designed initially for assessment of learning and memory in the rat. Assessment of learning and memory in mice using the MWM has proven to be problematic, as it is often unclear if decrements are associated with swimming ability or spatial learning capabilities (Ikeda et al., 2005; Whishaw and Tomie, 1997). Although mice can be trained to perform in MWM paradigms, these paradigms can present possible performance confounds, such as shallow learning curves, refusal to swim or stay on the goal platform, thigmotaxic behavior, and motor confounds resulting from swimming ability, fatigue and thermoregulatory difficulties (Hartman et al., 2001; Iivonen et al., 2003; Rogers et al., 1999). Moreover, when using aged mice, additional potential confounds arise due to the possibility of age-associated declines in visual acuity and motor function (Spencer et al., 1995). Performance deficits in this task then can be falsely interpreted as impaired learning when comparing young mice to aged mice.
Over the course of many years, our laboratory has used the Stone 14-unit T-maze (STM) as a tool for assessing the neurobiology of age-associated cognitive impairment in rats (Ingram, 1988; Ingram et al., 1996; Ingram et al., 1994). Having been introduced to the literature by Calvin Stone in 1929, this maze paradigm is one of the earliest used to examine rodent learning (Stone, 1929). Charles Goodrick at the National Institute on Aging (NIA) was one of the first to use this paradigm to study age-related memory impairment in rats (Goodrick, 1968). In our hands, the STM has proven valuable for drug discovery and development. Data from the STM was used to obtain patents on novel anticholinesterases for the treatment of Alzheimer’s disease and advance them to clinical trials (Greig et al., 2000; Kadir et al., 2008; Klein, 2007). The major advantages of the STM are twofold: (1) visual ability requirements are minimized compared to other maze paradigms that heavily involve vision, and (2) motivation to perform can be equilibrated across age groups (Ingram, 1988).
Other classes of drugs have also shown efficacy in enhancing learning performance of old rats in the STM including the nitric oxide donor, molsidomine (Meyer et al., 1998a), while the nootropic drug, codergocrine (Walovitch et al., 1987), and the mitochondrial energy enhancer, acetyl-L-carnitine, were not effective (Barnes et al., 1990). Many other studies have been conducted in young rats to demonstrate that STM learning is impaired by inhibition of signaling in muscarinic cholinergic (Spangler et al., 1986), NMDA glutamatergic (Ingram et al., 1992), D2 dopaminergic (Umegaki et al., 2001), and nitric oxidergic (Meyer et al., 1998a; Meyer et al., 1998b) systems. Impaired learning in the STM is also observed in rats with lesions to the septo-hippocampal system (Kametani et al., 1989; Kametani et al., 1993), hippocampus (Duffy et al., 2008), striatum (Pistell et al., 2009), and temporal-parietal, but not to striate cortex (Jucker et al., 1990; Spangler et al., 1994) nor the ascending noradrenergic system (Spangler et al., 1990). The lack of effects following the lesions to the striate cortex is again a demonstration that visual processing is not a major performance requirement for this maze. In early versions of the STM, motivation to perform was manipulated by food deprivation (Goodrick, 1968). The rat version that we have used extensively was equipped to deliver scrambled footshock as aversive motivation, and the rat has 10 sec to move through each of 5 maze segments in order to avoid shock onset (contingency reset after each segment).
Over a number of years, we have attempted to either utilize existing tasks (Brooks et al., 2000), or develop new tasks, capable of reliably assessing age-associated declines in learning and memory in mice. All of these tasks proved to be unreliable as they failed to maintain the proper motivational drive required to keep the mice fully engaged in the task, resulting in inaccurate assessment of learning and memory. However, it was noted that in all these failures, the mice appeared to be driven primarily to escape the apparatus.
Based on our observations that escape appeared to be a primary motivational factor in our previous studies of mice in a number of behavioral tasks, we developed a modified version of the STM for mice. An initial study demonstrated that the mouse STM reliably measures learning and memory in young mice, and that they consistently perform in the task (Pistell and Ingram, 2010). In the studies reported here, we demonstrate the ability of the mouse STM to detect age-associated declines in learning and memory using escape from water and footshock as motivational manipulations in two separate versions of the task. Two independent studies were conducted in mice of various ages in separate laboratories. In one laboratory (Nutritional Neuroscience and Aging Laboratory at the Pennington Biomedical Research Center - PBRC), motivation to maintain task performance was established by requiring the mice to wade, not swim, through water to reach a dark and dry goal box allowing the mice to escape out of the water. In the other laboratory (Laboratory of Experimental Gerontology at the National Institute on Aging - NIA), motivation to keep moving was established by using footshock as the negative reinforcement.
2. Experimental Procedures
2.1 Animals
For all experiments virgin, male C57BL/6Nia mice were obtained from the aging rodent colonies maintained at Charles River Laboratories (Wilmington, MA) under contract from the NIA. Mice at NIA were 7- (N=8), 12- (N=10), 20- (N=5) and 24-months old (N=9) at testing, while mice at the PBRC were 5- (N=9), 12- (N=12) and 25-months old (N=12) at testing. At both the PBRC and NIA, mice were housed in vivaria under controlled environmental conditions (PBRC 22±2 °C, 70±10% humidity ; NIA 21±2 °C, 70% humidity) with a 12-h light/dark cycle. Mice at both facilities were group housed (PBRC 4/cage; NIA 5/cage) and had ad libitum access to both standard chow (PBRC: Lab Diets, 5001; NIA: NIH-31) and water. Both facilities had sentinel procedures in place and were determined to be free of specific pathogens at the time of the studies. All procedures were approved by the respective Institutional Animal Care and Use Committees of the PBRC and NIA Intramural Research Program (NIA IRP), and followed the NIH guidelines for the Care and Use of Laboratory Animals.
2.2 Apparatus
Both mazes were constructed by the instrument and fabrication shop maintained by the Intramural Research Program at NIA (Baltimore, MD), The stainless steel grid floor and wiring for the NIA maze were purchased from Med Associates (St Albans, VT) and scrambled foot shock was delivered by a grid floor shocker (Coulbourn Instruments E13-08, Lehigh Valley, PA).
2.2.1 Water Motivated Mouse STM (PBRC)
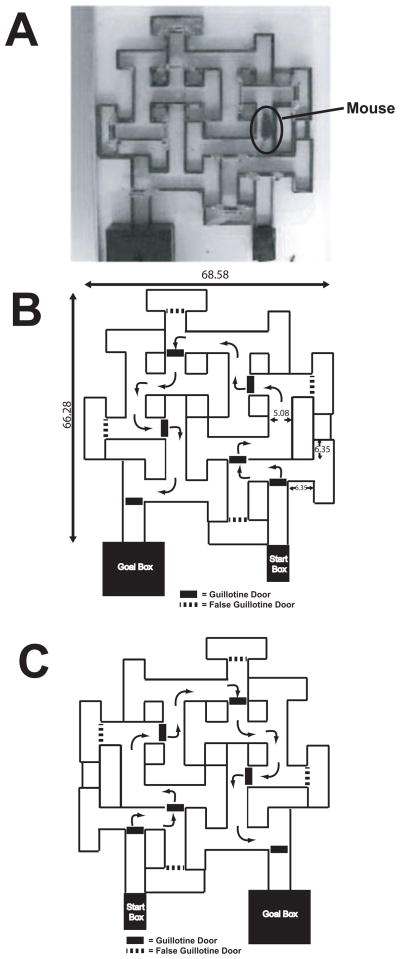
Additional details on the water-motivated version of the STM maze have previously been reported (Pistell and Ingram, 2010). A straight run (68.58 cm) was used for pretraining conducted on the day before training in the STM. Briefly, the straight run and maze were constructed from clear or black acrylic. Both the straight run and maze were placed into a steel pan filled with water to a depth of approximately 2.22 cm and which covered half the height of the interior walls of the maze. The water was maintained at 20–24 ° C. The walls of both the straight run and maze were constructed using black opaque acrylic 3.81 cm high with feet 0.64 cm in height placed at various locations under the bottom of walls to allow for circulation of water under the walls. The ceiling of the straight run and maze were covered with clear acrylic to prevent mice from rearing out of the water. These dimensions created a situation where the mice were capable of remaining in contact with the floor while maintaining their head above water, but the height prevented them from rearing up. The straight run consisted of an alley 68.58 cm long and the alley through which the mice traversed was 5.08 cm wide. At the beginning of each trial, the mouse was placed into a start box constructed completely out of opaque acrylic with a removable top and sliding door that was raised by hand at the start of a trial. Inside the back wall of the start box, there was a sliding panel that could be moved using a rod extending out the rear wall to push the mice from the start box if needed. The start box was 11.43 cm in length, with the height and width matching the dimensions of the alleys in the straight run and maze. An overhead view of the maze is shown in Figure 1A, and a schematic of the maze with dimensions is shown in Figure 1B.
Figure 1.
A) Photograph of the mouse STM at the Pennington Biomedical Research Center with a mouse navigating the maze. B) Schematic diagram of the mouse STM at the Nutritional Neuroscience and Aging Laboratory, Pennington Biomedical Research Center. C) Schematic diagram of the mouse STM at the Laboratory of Experimental Gerontology, National Institute on Aging.
Overall the maze measured 68.58 cm X 66.68 cm. Throughout the entire maze, the alleys through which the mice traveled were 5.08 cm wide. In general, the arms forming the stem of a T-junction were 6.35 cm long, and the two dead-end arms of the T were 5.72–6.35 cm long. The dimensions of the T’s were relatively consistent, but minor variations occurred when necessary to fit within the overall design and construction of the apparatus. Five sliding guillotine doors were placed at various locations throughout the maze (see Figure 1C). Once a mouse successfully navigated a section of the maze, the door was lowered to prevent the mouse from potentially backtracking after an error resulting in increased potential for a failed trial. These doors were located within a supporting framework attached to the ceiling of the maze, and were opened and closed manually by the experimenter using a fishing line with a weight at the end. To prevent the mice from utilizing the guillotine doors as navigation cues, false guillotine doors were placed at specific points in the maze over the top of alleys that were cul-de-sacs or dead-end T-junctions
The enclosed goal box was constructed from opaque black acrylic and measured 20.32 cm wide X 20.32 cm in length and was 11.76 cm high. An open door the same height and dimensions as the alleys of the maze and straight run was located on one wall, leading to a ramp that led up to an elevated floor within the box that was 4.78 cm above the floor of the pan holding the water. This allowed the mice to escape above the water level when reaching the goal box. The ceiling of the goal box was removable to allow access to the mice. All of the trials were recorded via an overhead camera.
2.2.2 Shock-Motivated Mouse STM (NIA)
Dimensions of the maze at the NIA were identical to the maze at the PBRC, except that the maze at the NIA was a mirror version. Thus, the start box for the NIA maze was located on the left and the goal box on the right side. A straight runway identical to that used at the PBRC, but with a stainless steel grid floor, was utilized to train mice in one-way active avoidance. The maze was placed onto a shock grid wired in series to a Coulbourn Instruments;E13-08 grid floor shocker (Lehigh Valley, PA). Scrambled footshock (0.4 mA) was initiated manually by the experimenter if the mouse remained in one section of the maze for 10 sec before successfully navigating to the next section (a section was defined as an area prior to the animal moving into the next section separated by one of the moveable guillotine doors). Once the mouse had moved into the next section, the shock was terminated and the timer was reset. Testing in the shock-motivated STM was recorded via an overhead camera for scoring of errors and runtime following completion of 15 massed practice trials
2.3 Procedure
2.3.1 Water-Motivated Stone T-Maze
On day 1 the mice underwent straight-run training. The straight-run training was implemented to establish the contingency that moving forward would allow them to escape from the water into the goal box. Successful completion of this training phase required that the mice reach the goal box in 15 sec or less on 13/15 trials, with a maximum of 30 trials administered. Any mice that were unable to reach this criterion were excluded from further behavioral testing. For all experiments, maze training commenced the following day. For this experiment 6–8 mice received all 15 maze acquisition trials in a single day. However, the mice were run in a squad where every mouse received Trial 1 prior to the first mouse receiving Trial 2. This resulted in an ITI of approximately 5–12 minutes in duration. During the ITI mice were placed in a holding cage with a dry towel. Retention was assessed one week following acquisition training.
The primary measures of learning were the latency to reach the goal box and the number of errors committed. The errors were scored online by the experimenter, but all of the straight run training and maze trials were recorded using a video tracking system for later review (Viewpoint Lifesciences, Montreal, Quebec, Canada). An error was defined as complete entry of the mouse’s head into an incorrect path. During acquisition, if a mouse failed to reach the goal box within 6 min, the trial was terminated and was scored as a failure. Any mouse achieving three failures was removed from further analysis.
2.3.2 Shock-Motivated Mouse STM
For this version of the maze, pretraining in the straight runway required the mice to successfully avoid a foot shock, initiated 10 sec after their entry into the runway. Successful completion of a trial was defined as complete avoidance of the shock by moving down the runway from a start box and entering a goal box. To assure sufficient motor performance in the maze, the pretraining criterion was for each mouse was to successfully complete 13/15 active one-way avoidance trials (no shock received) in less than 10 sec within a maximum of 30 trials. Any mouse that failed to reach this criterion was not tested in the maze. Two hours after pretraining, each mouse received 15 trials in the maze. Mice were run so that each mouse completed all 15 trials before the next mouse received its 1st trial, with a 2 min ITI. The maximum trial duration was 6 min, and if the maximum was reached, the mouse was given a 5-min recovery period in its home cage before the next trial. Mice failing to reach the goal box within 6 min on any of 3 trials were removed from the study.
Testing in the shock-motivated STM was recorded via an overhead camera for scoring of errors and runtime following completion of 15 massed practice trials. The primary measures of learning were the latency to reach the goal box and the number of errors committed. In addition, the number of shocks administered was also recorded. The errors were scored online by the experimenter, but all of the straight run training and maze trials were recorded using a video tracking system for later review (ANY-maze, Stoelting, Wood Dale, IL). An error was defined as complete entry of the mouse’s head into an incorrect path.
2.3.3 Rotarod
In addition to maze testing, the PBRC cohort of mice were also tested for motor function and coordination on an accelerating rotarod (Med-Associates, St. Albans, VT) to assess potential age-associated alterations in their behavioral function. Evaluation of rotarod performance occurred after completion of all maze testing. Testing on the rotarod consisted of three trials separated by an approximately 30 min ITI. For each trial, the mouse was placed onto the rotarod while it was stationary. The rotarod was then started at 4 rpm and accelerated to 40 rpm over 5 min. The maximum trial length was 5 min. The results from the 3 trials were then averaged.
2.4 Statistics
For analysis and display, the 15 acquisition trials were collapsed into 3 blocks of 5 trials. For all of the experiments, the maze data were analyzed using ANOVA with repeated measures, and the retention data were analyzed using within-subjects t-tests. Planned comparisons were made between the 5-month old group (water STM) or 7-month old (shock STM) and each of the older groups at each trial block. Statistical significance was accepted as p<0.05. We have found that assessment of learning in the mouse STM is most accurately measured by errors committed since, unlike latency, errors are unaffected by potential alterations in motor function. So while analysis of latency was conducted and reported, the analysis of errors is preferred to assess learning capacity. For the rotarod data, the data were analyzed with ANOVA followed by Tukey’s post-hoc comparisons with statistical significance accepted as p<0.05.
3. Results
3.1 Water-Motivated Mouse STM
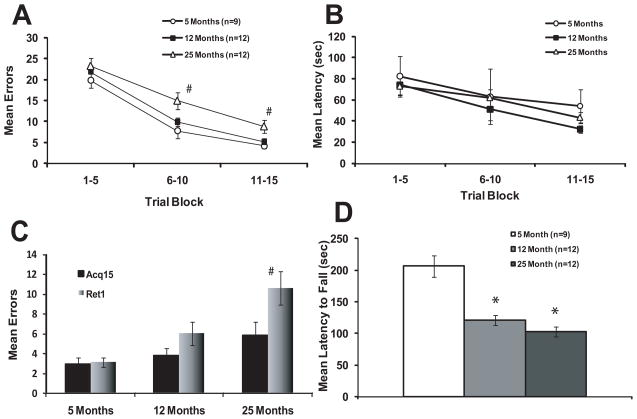
As observed in Figures 2A and 2B, in general, all age groups of mice exhibited significant learning in the STM indicated by reduced numbers of errors and runtimes across trials. The Group X Trial Block ANOVA for errors indicated a significant main effect for both Group (F(2,30) = 4.50, p = 0.02) and Trial Block (F(2,60) = 154.83, p < 0.001), but no significant interaction (F(4,60) = 1.02, p = 0.406). Planned comparisons of the 12-month old and 25-month old groups compared to the 5-month old group indicated that while none of the groups were significantly different at Trial Block 1, and the 12-month old group was not significantly different from the 5-month group on any of the Trial Blocks. The 25-month old group committed significantly more errors than the 5-month old group at Trial Block 2 (t(19) = 2.58, p = 0.018) and Trial Block 3 (t(19) = 2.60, p = 0.018) , indicating impaired acquisition.
Figure 2.
A) Mean errors (+/− sem) in 5 trial blocks during acquisition in the water-motivated mouse STM. # indicates p < 0.05 compared to 5-month old group. B) Mean latency (+/− sem) in 5 trial blocks during acquisition in the water-motivated mouse STM. C) Comparison of mean errors (+/− sem) for the last acquisition trial (15th trial) and first retention trial in the water-motivated mouse STM. # indicates p < 0.05 compared to performance on last acquisition trial. D) Mean latency (+/− sem) to fall from the accelerating rotarod. * indicates p < 0.01 in comparison to the 5-month old group.
The Group X Trial Block ANOVA for latency indicated a significant main effect for Trial Block (F(2,60)= 12.93, p < 0.001), but no significant effect of Group (F(2,30) = 0.44, p = 0.649) and no significant interaction (F(4,60) = 0.34, p = 0.849). Planned comparisons at each Trial Block indicated no significant differences between the 12-month old and 25-month old groups compared to the 5-month old group at any of the Trial Blocks. These results indicated the motor ability of the mice required to perform the task was not altered by age.
Retention performance was assessed by comparing performance on the last trial of acquisition with performance on the first trial of retention. The Trial X Block ANOVA for retention indicated a significant main effect for Group (F(2,29) = 6.00, p = 0.007) and Trial (F(1,29) = 10.57, p = 0.003), but no significant interaction (F(2,29) = 3.22, p = 0.055). Comparisons within each age group indicated only the 25-month old mice exhibited a significant increase in errors from the last acquisition trial compared to the first retention trial (t(11) = 4.07, p = 0.002).
We assessed motor performance in a rotarod task to further evaluate age effects on motor abilities. Contrasted to no differences in latency performance in the maze, the ANOVA for latency to fall from the rotarod indicated a significant main effect for Group (F(2,30) = 23.90, p < 0.001). A Tukey’s post hoc test indicated that while both the 12-month old and 25-month old groups exhibited a reduced latency to fall from the accelerating rotarod, compared to the 5-month old group (p < 0.001; see Figure 2D), the 12-month old and 25-month old groups exhibited similar performance (p > 0.05). Overall, these results indicate despite the detection of age-associated differences in motor function on the rotarod, mice of increasing age were capable of performing at performance levels similar to those of young mice in the water-motivated version of the mouse STM.
3.2 Shock-Motivated Mouse STM
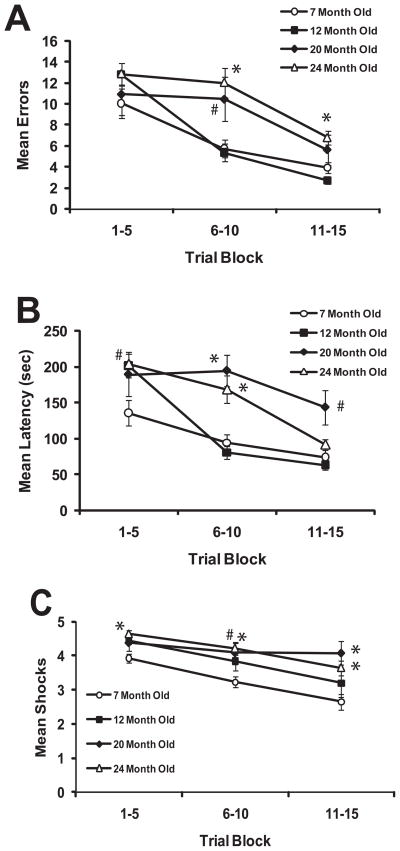
As presented in Figures 3A (errors), 3B (latency) & 3C (shock frequency), in general all groups of mice exhibited improved performance in the maze across Trial Blocks in all measures of maze performance. The Group X Trial Block ANOVA for errors indicated a main effect for Trial Block (F(2,56) = 51.27, p < 0.001), a main effect for Group (F(3,28) = 6.08, p = 0.003) as well as a Group X Trial Block interaction (F(6,56) = 3.66, p = 0.004). Planned comparisons indicated no differences between the 7-month old group and any of the other age groups at Trial Block 1, similar to the observation made in the water motivated version. At Trial Block 2 the 12-month old mice were still not different from 7-month old mice, but both the 20-month old (t(11) = 2.42, p = 0.034) and 24-month old mice (t(15) = 3.67, p = 0.002) exhibited significantly more errors compared to the 7-month group. However, at Trial Block 3, only the 24-month old group committed significantly more errors than the 7-month old group (t(15) = 3.49, p = 0.003).
Figure 3.
A) Mean errors (+/− sem) in 5 trial blocks during acquisition in the shock-motivated mouse STM. B) Mean latency (+/− sem) in 5 trial blocks during acquisition in the shock-motivated mouse STM. C) Mean shock frequency (+/− sem) in 5 trial blocks during acquisition in the shock-motivated mouse STM. # indicates p < 0.05 and * indicates p < 0.01 in comparison to 7-month old group.
The Group X Trial Block ANOVA for latency revealed both a main effect for Trial Block (F(2,56) = 40.08, p < 0.001) and Group (F(3,28) = 10.24, p , 0.001), as well as a Group X Trial Block interaction (F(6,56) = 4.81, p = 0.001). Planned comparisons indicated that the 7-month old mice completed the maze significantly faster than the 12-month old (t(16) = 2.73, p = 0.015) and 24-month old (t(15) = 2.84, p = 0.012) groups, but not the 20-month old group at Trial Block 1. At Trial Block 2, the 7-month old mice completed the maze significantly faster than the 20-month old (t(11) = 4.51, p = 0.001) and 24-month old (t(15) = 3.26, p = 0.005) groups, but not the 12-month old group. For Trial Block 3, only the 20-month old mice were significantly slower than the 7-month old mice at completing the maze (t(11) = 3.11, p = 0.01).
The Group X Trial Block for shock frequency confirmed significant main effects for Trial Block (F(2,56) = 27.63, p < 0.001) and Group (F(3,28) = 4.38, p = 0.012), but no Group X Trial Block interaction (F(6,56) = 1.27, p = 0.286). Planned comparisons indicated that, compared to 7-month old mice, the 12-month old (t(16) = 3.29, p = 0.005) and 24-month old (t(15) = 4.35, p = 0.001) mice received significantly more shock episodes at Trial Block 1. At Trial Block 2 the 20-month old (t(11) = 2.96, p = 0.013) and 24-month old (t(15) = 3.93, p = 0.001) groups received more shocks than the 7-month old group, and the same pattern occurred at Trial Block 3 (20-month old: t(11) = 3.56, p = 0.004; 24-month old: t(15) = 3.10, p = 0.007).
4. Discussion
Expanding from our initial report indicating the capability of the water-motivated mouse STM to maintain motivation and assess learning and memory in young mice (Pistell and Ingram, 2010), the current findings indicate the mouse STM is capable of detecting age-associated declines in learning and memory using both water escape and shock escape-avoidance as motivational manipulations. Error performance across blocks of 5 trials for both maze tasks demonstrates that learning occurs in all age groups. However, in both tasks aged mice (24–25 mo) exhibited deficits in learning compared to the young and middle –aged groups. More specifically, in comparison to 5-month old mice, the water-motivated version of the mouse STM was capable of detecting age-associated declines in 25-month old mice. The performance of 12-month old mice was not significantly different from the youngest group. The shock-motivated version of the mouse STM was capable of detecting age-associated declines between 7-month old mice and 24-month old mice. Overall, these results provide further support for the utilization of this task in evaluating learning and memory in mice.
Although the ages of the mice evaluated in the two different mazes were not exactly identical, a similar pattern of results was obtained regarding the errors committed. Despite the similarity of the overall conclusions, there were some differences between the two studies. In the shock-motivated version, the mice consistently received shock indicating that some form of motivation was required to keep the mice moving towards the goal box. Unlike healthy unimpaired rats in the shock-motivated STM, who frequently reach zero shocks during the later trial blocks (Pistell et al., 2007; Spangler et al., 1986), the mice in the current study never got below a mean of 3 (with the highest number possible being 5). This indicates the mice in the shock-motivated version of the mouse STM pause more frequently than those run in the water-motivated version of the mouse STM. The protocol for the shock-motivated version allowed the mice 10 sec to move through each section and past a door to the next section of the maze before shock was initiated until they escaped past the door to the next section and the avoidance-escape contingency was reset. Therefore, the mice were frequently taking longer than 10 sec in over half of the sections. This difference probably arises from the fact that in the water-motivated version the mice are in the water from the beginning so they are constantly escaping. In the shock-motivated version, the shock is not constantly present, and the mice are under an avoidance-escape contingency. Therefore, the constant presence of the water may result in increased motivation to reach the goal box. However, despite these minor motivational differences, the primary dependent measure of learning in the mouse STM, errors, was similar between the two studies.
Another advantage of the mouse STM, especially the water-motivated version, is that the physical demands of the task are not nearly as great as those required for the Morris water maze. This factor is critical for studies comparing mice of different ages because of the potential confound of motor function decline in the aged mice. Further, the physical demands of swimming may interact with an inability to effectively thermoregulate in older animals, i.e., loss of body temperature, that impact on their ability to swim or stay afloat in the MWM. Compared to the rat, the mouse has a greater challenge in thermoregulation due to its small body surface area, so this can be a problem even in young mice that is further accentuated with age (Shefer & Talan, 1997). When using latency as a measure of learning in the Morris water maze, it is often difficult to interpret increased latencies in aged mice as learning rather than impaired motor function. The current experiments indicate that despite learning acquisition deficits, as indicated by errors, mice of all ages (except for the 20-month old group in the shock-motivated study) exhibited similar latencies across all trial blocks. In fact, as indicated by rotarod testing in the Pennington cohort of mice, both of the older groups of mice exhibited declines in motor function. However, these deficits did not impact their ability to complete the maze with similar latencies to the youngest group of mice.
Overall, we believe the mouse STM, whether motivated by shock or escape from water, offers several advantages over existing paradigms used to measure learning and memory in aged mice. This task is capable of measuring learning independent of potential confounds arising from alterations in motor, visual and other physiological changes in function that occur in aged mice. Both the water and shock-motivated tasks can be partially automated using available tracking and scoring systems, e.g., Any-Maze (Stoelting, Kiel, WI). One of the major advantages of both of these tasks is the shortened testing period that allows for testing of aged mice in a short window of time, as little as a day. Both the MWM and appetitive tasks require more extensive testing, which can result in loss of subjects due to death or illness when they approach or exceed the mean lifespan. We are currently using the tasks to evaluate a number of interventions that will further demonstrate the utility of this paradigm.
Acknowledgments
Special thanks to Richard Zichos in the instrument shop at the NIA intramural program for construction of the mazes. This research was supported, in part, by the Intramural Research Program at the National Institute on Aging. Partial support was provided through the Animal Phenotyping Core supported through NORC Center Grant #1P30 DK072476 entitled ‘Nutritional Programming: Environmental and Molecular Interactions’ sponsored by NIDDK.
Footnotes
Disclosure statement: None of the authors on this manuscript has any actual or potential conflict of interest pertinent to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashe KH. Learning and memory in transgenic mice modeling Alzheimer's disease. Learn Mem. 2001;8:301–308. doi: 10.1101/lm.43701. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Markowska AL, Ingram DK, Kametani H, Spangler EL, Lemken VJ, Olton DS. Acetyl-1-carnitine. 2: Effects on learning and memory performance of aged rats in simple and complex mazes. Neurobiol Aging. 1990;11:499–506. doi: 10.1016/0197-4580(90)90110-l. [DOI] [PubMed] [Google Scholar]
- Brooks AI, Cory-Slechta DA, Murg SL, Federoff HJ. Repeated acquisition and performance chamber for mice: a paradigm for assessment of spatial learning and memory. Neurobiol Learn Mem. 2000;74:241–258. doi: 10.1006/nlme.1999.3951. [DOI] [PubMed] [Google Scholar]
- Chacan MA, Barraa MI, Soto C, Inestrosa NC. Beta-sheet breaker peptide prevents Abeta-induced spatial memory impairments with partial reduction of amyloid deposits. Mol Psychiatry. 2004;9:953–961. doi: 10.1038/sj.mp.4001516. [DOI] [PubMed] [Google Scholar]
- Duffy KB, Spangler EL, Devan BD, Guo Z, Bowker JL, Janas AM, Hagepanos A, Minor RK, DeCabo R, Mouton PR, Shukitt-Hale B, Joseph JA, Ingram DK. A blueberry-enriched diet provides cellular protection against oxidative stress and reduces a kainate-induced learning impairment in rats. Neurobiol Aging. 2008;29:1680–1689. doi: 10.1016/j.neurobiolaging.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Fisher A, Pittel Z, Haring R, Bar-Ner N, Kliger-Spatz M, Natan N, Egozi I, Sonego H, Marcovitch I, Brandeis R. M1 muscarinic agonists can modulate some of the hallmarks in Alzheimer's disease: implications in future therapy. J Mol Neurosci. 2003:349–356. doi: 10.1385/JMN:20:3:349. [DOI] [PubMed] [Google Scholar]
- Goodrick CL. Learning, retention, and extinction of a complex maze habit for mature-young and senescent Wistar albino rats. J Gerontol. 1968;23:298–304. doi: 10.1093/geronj/23.3.298. [DOI] [PubMed] [Google Scholar]
- Greig NH, De Micheli E, Holloway HW, Yu QS, Utsuki T, Perry TA, Brossi A, Ingram DK, Deutsch J, Lahiri DK, Soncrant TT. The experimental Alzheimer drug phenserine: preclinical pharmacokinetics and pharmacodynamics. Acta Neurol Scand, Suppl. 2000;176:74–84. doi: 10.1034/j.1600-0404.2000.00311.x. [DOI] [PubMed] [Google Scholar]
- Hartman RE, Wozniak DF, Nardi A, Olney JW, Sartorius L, Holtzman DM. Behavioral phenotyping of GFAP-apoE3 and -apoE4 transgenic mice: apoE4 mice show profound working memory impairments in the absence of Alzheimer's-like neuropathology. Exp Neurol. 2001;170:326–344. doi: 10.1006/exnr.2001.7715. [DOI] [PubMed] [Google Scholar]
- Iivonen H, Nurminen L, Harri M, Tanila H, Puolivali J. Hypothermia in mice tested in Morris water maze. Behav Brain Res. 2003;141:207–213. doi: 10.1016/s0166-4328(02)00369-8. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Shoji M, Kawarai T, Kawarabayashi T, Matsubara E, Murakami T, Sasaki A, Tomidokoro Y, Ikarashi Y, Kuribara H, Ishiguro K, Hasegawa M, Yen SH, Chishti MA, Harigaya Y, Abe K, Okamoto K, St George-Hyslop P, Westaway D. Accumulation of filamentous tau in the cerebral cortex of human tau R406W transgenic mice. Am J Pathol. 2005;166:521–531. doi: 10.1016/S0002-9440(10)62274-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram DK. Complex maze learning in rodents as a model of age-related memory impairment. Neurobiol Aging. 1988;9:475–485. doi: 10.1016/s0197-4580(88)80101-5. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Garofalo P, Spangler EL, Mantione CR, Odano I, London ED. Reduced density of NMDA receptors and increased sensitivity to dizocilpine-induced learning impairment in aged rats. Brain Res. 1992;580:273–280. doi: 10.1016/0006-8993(92)90954-8. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Shimada A, Spangler EL, Ikari H, Hengemihle J, Kuo H, Greig N. Cognitive enhancement. New strategies for stimulating cholinergic, glutamatergic, and nitric oxide systems. Ann N Y Acad Sci. 1996;786:348–361. doi: 10.1111/j.1749-6632.1996.tb39076.x. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Spangler EL, Iijima S, Kuo H, Bresnahan EL, Greig NH, London ED. New pharmacological strategies for cognitive enhancement using a rat model of age-related memory impairment. Ann N Y Acad Sci. 1994;717:16–32. doi: 10.1111/j.1749-6632.1994.tb12070.x. [DOI] [PubMed] [Google Scholar]
- Jucker M, Kametani H, Bresnahan EL, Ingram DK. Parietal cortex lesions do not impair retention performance of rats in a 14-unit T-maze unless hippocampal damage is present. Physiol Behav. 1990;47:207–212. doi: 10.1016/0031-9384(90)90062-9. [DOI] [PubMed] [Google Scholar]
- Kadir A, Andreasen N, Almkvist O, Wall A, Forsberg A, Engler H, Hagman G, Larksater M, Winblad B, Zetterberg H, Blennow K, Langstrom B, Nordberg A. Effect of phenserine treatment on brain functional activity and amyloid in Alzheimer's disease. Ann Neurol. 2008;63:621–631. doi: 10.1002/ana.21345. [DOI] [PubMed] [Google Scholar]
- Kametani H, Bresnahan EL, Chachich ME, Spangler EL, Ingram DK. Comparison of retention performance between young rats with fimbria-fornix lesions and aged rats in a 14-unit T-maze. Behav Brain Res. 1989;35:253–263. doi: 10.1016/s0166-4328(89)80145-7. [DOI] [PubMed] [Google Scholar]
- Kametani H, Spangler EL, Bresnahan EL, Kobayashi S, Long JM, Ingram DK. Impaired acquisition in a 14-unit T-maze following medial septal lesions in rats is correlated with lesion size and hippocampal acetylcholinesterase staining. Physiol Behav. 1993;53:221–228. doi: 10.1016/0031-9384(93)90197-n. [DOI] [PubMed] [Google Scholar]
- Klein J. Phenserine. Expert Opin Investig Drugs. 2007;16:1087–1097. doi: 10.1517/13543784.16.7.1087. [DOI] [PubMed] [Google Scholar]
- Meyer RC, Spangler EL, Kametani H, Ingram DK. Age-associated memory impairment. Assessing the role of nitric oxide. Ann N Y Acad Sci. 1998a;854:307–317. doi: 10.1111/j.1749-6632.1998.tb09911.x. [DOI] [PubMed] [Google Scholar]
- Meyer RC, Spangler EL, Patel N, London ED, Ingram DK. Impaired learning in rats in a 14-unit T-maze by 7-nitroindazole, a neuronal nitric oxide synthase inhibitor, is attenuated by the nitric oxide donor, molsidomine. Eur J Pharmacol. 1998b;341:17–22. doi: 10.1016/s0014-2999(97)01428-3. [DOI] [PubMed] [Google Scholar]
- Pistell P, Ingram D. Development of a water-escape motivated version of the Stone T-Maze for Mice. Neuroscience. 2010;166:61–72. doi: 10.1016/j.neuroscience.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistell PJ, Daffin LW, Jr, Nelson CM, Duffy KB, Bowker JL, Spangler EL, Ingram DK, Devan BD. Combined administration of subthreshold doses of the nitric oxide inhibitor, nitro-L-arginine, and muscarinic receptor antagonist, scopolamine, impairs complex maze learning in rats. Behav Pharmacol. 2007;18:801–805. doi: 10.1097/FBP.0b013e3282f18d2f. [DOI] [PubMed] [Google Scholar]
- Pistell PJ, Nelson CM, Miller MG, Spangler EL, Ingram DK, Devan BD. Striatal lesions interfere with acquisition of a complex maze task in rats. Behav Brain Res. 2009;197:138–143. doi: 10.1016/j.bbr.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers DC, Jones DNC, Nelson PR, Jones CM, Quilter CA, Robinson TL, Hagan JJ. Use of SHIRPA and discriminant analysis to characterise marked differences in the behavioural phenotype of six inbred mouse strains. Behav Brain Res. 1999;105:207–217. doi: 10.1016/s0166-4328(99)00072-8. [DOI] [PubMed] [Google Scholar]
- Shefer VI, Talan MI. The effect of exercise traininng in a cold environment on thermoregulation in asult and aged C57BL/6J mice. Exp Gerontol. 1997;32:695–705. doi: 10.1016/s0531-5565(97)00085-5. [DOI] [PubMed] [Google Scholar]
- Spangler EL, Heller B, Hengemihle J, Muth NJ, Jones BE, Garofalo P, Ingram DK. Thrombosis of parietal, but not striate, cortex impairs acquisition of a 14-unit T-maze in the rat. Physiol Behav. 1994;56:95–101. doi: 10.1016/0031-9384(94)90265-8. [DOI] [PubMed] [Google Scholar]
- Spangler EL, Rigby P, Ingram DK. Scopolamine impairs learning performance of rats in a 14-unit T-maze. Pharmacol Biochem Behav. 1986;25:673–679. doi: 10.1016/0091-3057(86)90158-9. [DOI] [PubMed] [Google Scholar]
- Spangler EL, Wenk GL, Chachich ME, Smith K, Ingram DK. Complex maze performance in rats: effects of noradrenergic depletion and cholinergic blockade. Behav Neurosci. 1990;104:410–417. doi: 10.1037//0735-7044.104.3.410. [DOI] [PubMed] [Google Scholar]
- Spencer RL, O'Steen WK, McEwen BS. Water maze performance of aged sprague-dawley rats in relation to retinal morphologic measures. Behav Brain Res. 1995;68:139–150. doi: 10.1016/0166-4328(94)00167-e. [DOI] [PubMed] [Google Scholar]
- Stone CP. The age factor in animal learning: I. Rats in the problem box and the maze. Genet Psychol Monogr. 1929;5:1–130. [Google Scholar]
- Umegaki H, Munoz J, Meyer RC, Spangler EL, Yoshimura J, Ikari H, Iguchi A, Ingram DK. Involvement of dopamine D(2) receptors in complex maze learning and acetylcholine release in ventral hippocampus of rats. Neuroscience. 2001;103:27–33. doi: 10.1016/s0306-4522(00)00542-x. [DOI] [PubMed] [Google Scholar]
- Van Dam D, Coen K, De Deyn PP. Cognitive evaluation of disease-modifying efficacy of donepezil in the APP23 mouse model for Alzheimer's disease. Psychopharmacology (Berl) 2008;197:37–43. doi: 10.1007/s00213-007-1010-x. [DOI] [PubMed] [Google Scholar]
- Walovitch RC, Ingram DK, Spangler EL, London ED. Co-dergocrine, cerebral glucose utilization and maze performance in middle-aged rats. Pharmacol Biochem Behav. 1987;26:95–101. doi: 10.1016/0091-3057(87)90540-5. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Tomie JA. Of Mice and Mazes: Similarities between mice and rats on dry land but not water mazes. Physiol Behav. 1997;60:1191–1197. doi: 10.1016/s0031-9384(96)00176-x. [DOI] [PubMed] [Google Scholar]