Abstract
Background
To support pediatric study, a method to determine lumefantrine (LF) with small sample volume is needed. Matrix effect (ME) is a daunting issue in LF quantification in human plasma with LC MS/MS.
Results
Here we report an LC–MS/MS method with a deuterated LF as the internal standard (IS). Plasma sample (25 μl) was acidified with 5% formic acid prior to extraction with ethyl acetate. The recovery was over 80%. The absolute ME was within the range of 100 ± 8% for both LF and the IS, but cumulative ME was observed via large variation of IS as the IS. The linear signal. The cumulative ME and ionization saturation were overcome with the co-eluting LF-D9 range of calibration curve was 50–20,000 ng/ml.
Conclusion
ME and ionization saturation was overcome with a deuterated IS. The method utilized a small sample volume, suitable for pediatric study with capillary tube blood collection method.
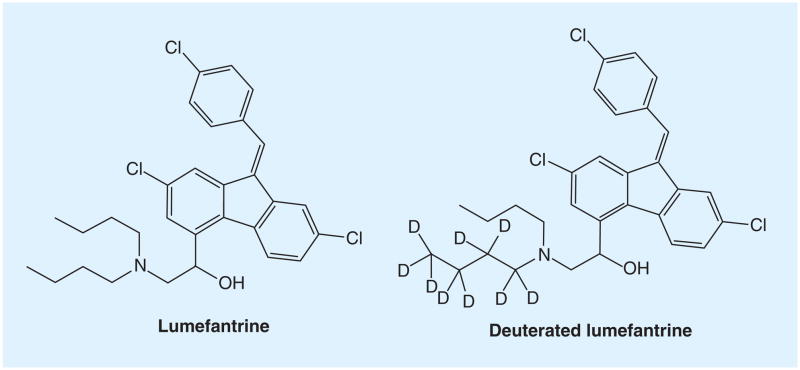
Lumefantrine (LF), previously named benflumetol, is a highly hydrophobic aromatic compound (Figure 1). It has a logP of 8.67 (25°C) and logD of 5.6 (pH 1–4, 25°C) and 7.0 (pH 7, 25°C), calculated using Advanced Chemistry Development Software V11.02. LF is used in combination with artemether under the brand name of Co-Artem® for antimalaria therapy [1]. The peak concentration of LF in patients is usually over 10,000 ng/ml reached 6–8 h after dosing [2]. Numerous methods have been reported for determination of LF in human plasma, most of these methods are based on HPLC-UV where a large plasma volume (≥200 μl) was used [3–8]. Methods for quantifying LF on dry blood spots were also reported with relative small sample volume (50–100 μl) but the LLOQ was high (130–150 ng/ml) [9,10]. For pediatric studies, a sensitive method with small sample volume and acceptable LLOQ is required. Early attempts on application of LC–MS method for determination of LF in human plasma failed due to matrix effect (ME) [11]. Recently, several LC–MS/MS methods were reported, but the issue of ME has not been addressed sufficiently [12–15]. Using protein precipitation for sample preparation, Hodel et al. reported the first LC–MS/MS method for LF, but the ME was significant: 22.7–29.1% (100% = no ME) [12], while César et al. claimed the ME in their method was within the 85 to 115% range, but the calibration curve required a weighted quadratic regression [15]. None of these methods utilized a co-eluting internal standard (IS), which is the most effective for overcoming ME. A stable isotope-labeled analyte is generally considered to be the best IS. Because the analyte and IS are generally co-eluted and ionized identically, ME will affect both analyte and IS to the same extent and ionization saturation occurring at high analyte concentrations will also affect the IS, which in turn, compensates for those effects on the analyte. Here we report an LC–MS/MS method using a deuterated LF (LF-D9) as the IS, with a focus on ME and ionization saturation. The method was validated according to AIDS Clinical Trials Group (ACTG) guidelines and crossvalidated with an HPLC-UV method.
Figure 1.
Lumefantrine and the deuterated lumefantrine.
Experimental
Chemicals
LF (Figure 1) was purchased from AK Scientific Inc. (CA, USA). LF-D9 was obtained as a gift from Novartis Pharma. Co. (NJ, USA). Acetonitrile (MeCN), water (HPLC grade) and ammonium formate were obtained from Fisher Scientific (NJ, USA). Ethyl acetate and formic acid (FA) was purchased from Sigma-Aldrich (MO, USA). All solvents were HPLC grade and chemicals were ACS reagents. Human plasma (EDTA as the anticoagulant) was purchased from Biological Specialty Co. (PA, USA).
LC–MS/MS conditions
The LC–MS/MS system consisted of twin PE 200 micro-LC pumps, PE 200 autosampler (Perkin-Elmer; CT, USA), and the API 2000 triple quadrupole MS system (AB Sciex, ON, Canada). The instrument was placed in a room controlled with an air conditioner. Chromatographic separation was achieved on a Zorbax C8 column (50 × 2.1 mm, 5 μm; Agilent Technologies Inc., CA, USA). The LC setting was as follows: solvent A was aqueous ammonium formate 10 mM at pH 4.0. Solvent B was MeCN with FA 0.1%. The gradient program consisted of linear segments with 50% B (0–1 min), 50–100% B (1–4 min), 100% B (4–6 min), 100–50% B (6–6.1 min) and 50% B (6.1–8 min). The flow rate was 0.4 ml/min. Injection volume was 10 μl. The retention times for LF and IS were both 3.5 min. ESI+ was used for ion source and multiple reaction monitoring mode was chosen for quantification. The precursor–product ion pairs were m/z 528→510 for LF and m/z 537→519 for the IS. The optimized acquisition parameters were as follows: Turbo (Heater) set at 400°C; curtain gas: 25 psi; nebulizer gas (gas 1): 40 psi; auxiliary (turbo) gas (gas 2): 70 psi; collision-activated dissociation gas: 4; IonSpray Voltage: 4000 V. All gas lines were supplied from a liquid nitrogen tank (industrial grade). The optimized parameters for LF and IS are as follows: declustering potential: 56; entrance potential: 9.5; focusing potential: 370; collision cell entrance potential: 22; collision energy: 29; collision cell exit potential: 24. The scan time was set at 250 ms for each transition and pause between mass ranges was set at 5 ms. Data were processed with Analyst 1.4.2. (Danaher Co., Washington, DC, USA).
Preparation of standard & validation (quality control) samples
Primary stock solutions (1 mg/ml) and working solutions of LF and IS were both prepared in MeCN:water (1:1) containing 0.5% FA. Calibration standards were prepared by spiking the LF working solutions into plasma to obtain concentrations of 50, 100, 500, 1000, 5000, 10,000, 15,000 and 20,000 ng/ml. Validation samples (also called quality control [QC]) were prepared at four different concentrations: 50 ng/ml (LLOQ), 120 ng/ml (low), 1500 ng/ml (medium) and 17,000 ng/ml (high). Calibration standards and validation samples were prepared from two different stock solutions made with separately weighted LF. The stock solutions, standards, QC samples and the IS solution were stored at −70°C before uses.
Sample preparation
A 25 μl aliquot of plasma sample was mixed with 25 μl 100 ng/ml LF-D9 and 100 μl 5% aqueous FA. The mixture was extracted with 900 μl ethyl acetate by vortex mixing for 20 s followed by rotating on a tube rotor for 30 min. After centrifugation at 15,000 × g for 2 min, the organic phase was transferred into a glass tube (12 × 75 mm) with a fine-tip disposable pipet, dried in 40°C water bath with purging of N2 and re-dissolved with 200 μl MeCN:water (1:1, 0.5% FA) by vortex mixing for 10 s. The reconstituted sample was transferred to an autosampler vial. Injection volume was 10 μl.
Method validation procedure
The method validation was conducted according to the ACTG guidelines [16], which were developed based on US FDA guidelines. The calibration curve should contain at least six nonzero points with back-calculated values within the range of 85 to 115% of nominal values, except for LLOQ in which 80–120% is allowed. Intra- and inter-day accuracy and precision for low-, medium- and high-validation samples should be within ±15%, but for LLOQ validation samples this can be ±20%. Intra-day accuracy and precision were determined with at least five replicates of validation sample at each concentration (LLOQ, low, medium and high) along with a set of calibrators. The same experiment was performed on at least five different days to determine inter-day accuracy and precision. Stability tests were performed at ambient temperature, −70°C, four freeze–thaw cycles, and in autosampler vials with low and high QC samples. The determined concentrations were compared with nominal values. Plasma samples at 60,000 ng/ml were diluted by four-, eight- and 12-fold to test sample integrity upon dilution. Stock solution in MeCN 50% containing FA 0.5% was diluted to 250 ng/ml and injected onto LC–MS/MS. The peak area was compared with that from freshly made stock solution. The following 12 potential concomitant drugs were tested for interference: nevirapine, lopinavir, ritonavir, zidovudine, lamivudine, efavirenz, chloroquine, sulfamethoxazole, trimethoprim, artemether, dihydroartemisinin and tenofovir, each drug spiked at 5000 ng/ml in medium validation sample (LF 1500 ng/ml). The ME was evaluated with three different approaches.
Infusion experiment
A 10 μg/ml solution of LF was infused constantly at a flow rate of 10 μl/min into the LC elute via a ‘T’ connector and the LC elute was directed into the MS source. A 10 μl aliquot of blank plasma extract was injected onto LC column and signal for LF was monitored for 60 min when LC ran in isocratic mode at 0.4 ml/min (65% solvent B). A 10 μl aliquot of 10 μg/ml LF in mobile phase solvent was injected as a reference. The LC was also operated manually according to the final gradient method and LF signal was monitored for 29 min. A 10 μl aliquot of mobile-phase solvent was injected as reference.
The approach established in ACTG guidelines
Six different lots of blank plasma were processed and injected into the LC–MS/MS. Medium QCs were prepared in triplicate with each of six plasma lots, processed and injected into LC–MS/MS, their concentrations were compared with nominal concentration (1500 ng/ml).
The approach recommended in ACTG guidelines
This approach is based on the work of Matuszewski and co-workers [17]. For recovery and ME, four sets of samples were prepared. Set 1a, aliquots (25 μl each) of LF solutions at low (120 ng/ml), medium (1500 ng/ml) and high (17,000 ng/ml) levels were diluted with 175 μl MeCN:water (1:1) containing 0.5% FA and analyzed in six replicates (n = 6). A 25 μl aliquot of IS (100 ng/ml) was also diluted with 175 μl mobile-phase solvent and analyzed in six replicates.
Set 1b, aliquots (25 μl each) of LF solutions at low, medium and high levels were mixed with 25 μl IS (100 ng/ml), diluted with 150 μl MeCN:water (1:1) containing 0.5% FA, and analyzed in six replicates (n = 6).
Set 2, 25 μl aliquots of blank plasma from six different lots were processed with liquid–liquid extraction (LLE) and reconstituted with 200 μl mobile phase solvent containing LF and IS to make the final concentration the same level as those of the set 1b samples.
Set 3, a 25 μl aliquot of each plasma sample at the low (120 ng/ml), medium (1500 ng/ml) and high (17000 ng/ml) levels was processed following the normal procedure. These samples were prepared in the six different lots of plasma used in set 2.
The recovery (RE), ME and process efficiency (PE) of LF and IS were assessed by comparing the peak areas of LF and IS with the following formulae:
Crossvalidation
Ninety-six clinical samples from a previous study were reanalyzed with this assay and the results were compared with those from an HPLC-UV method, based on the criteria as described by Fast et al. [18]. At least 67% of reanalysis should be within 20% compared with the reference value, which is the mean of the initial and reanalyzed value:
All clinical samples were from studies approved by the institutional review board at University of California, San Francisco, USA.
Clinical application to pediatric patients
The developed method was used to determine LF concentration from pediatric patients. Blood samples were collected with capillary tubes (200 μl capacity) and ~100 μl or less plasma was obtained. A total of 260 samples were analyzed with this method.
Results & discussion
Method development
Co-eluting stable isotope-labeled analyte as the IS is considered as the best IS to compensate for signal variation due to ME. Here we used a LF-D9 as the IS. When a Waters symmetry® C18 column (150 × 4.6 mm, 5 μm) was used, LF-D9 was only partially co-eluted with LF. The ME on two compounds can be different if they are not co-eluted [19]. After switching to a Zorbax Eclipse C8 column (50 × 2.1 mm, 5 μm), the IS was co-eluted with LF (Supplementary Figure 1).
In reverse-phase LC–MS/MS methods, ME was commonly seen in the early elution phase due to polar substances such as salts, and late phase due to hydrophobic substances such as phospholipids. In some cases, the highly hydrophobic substances do not affect the immediate sample but affect the subsequent samples analysis, especially in isocratic elution method. Here LC conditions were optimized to elute LF with gradient elution and clean the column with 100% organic mobile phase so as to achieve optimal separation and reduce hydrophobic matrix carried over to subsequent analysis.
Sample preparation
Sample preparation is an important step in method development to overcome ME. A recently published LC–MS method utilized simple protein precipitation, ME reached 22.7% suggesting ion suppression >77% [12]. Combination of protein precipitation and SPE was also used for preparation of plasma sample of LF [6–7], but it is expensive and labor intensive. LLE is a superior method for sample preparation. It gives cleaner samples compared with protein precipitation and, in some cases, better samples than SPE.
To optimize sample preparation methods, SPE and LLE with different conditions were tested (Supplementary Table 1). SPE with a C8 column yielded higher RE than a HLB column, probably due to hydrophilic interaction with the charged amine group on LF during elution phase. LLE with ethyl acetate was better than methyl t-butyl ether. Acidification of the sample prior to extraction was important in order to achieve high RE. This is in consistence with the previous finding [3]: extraction with 1% acetic acid in ethyl acetate afforded much higher RE than ethyl acetate alone. It is also justified via the observation that LF is poorly soluble in water and most organic solvents, but soluble in acidified organic solvents. On the other hand, LF is highly bound to plasma protein (>99%) [20], disruption of protein binding during extraction is critical for high extraction efficiency. Previous methods required protein precipitation before SPE to release LF from plasma protein [5–7]. However, from this study, protein precipitation prior to SPE was not necessary if the plasma sample was acidified before SPE, suggesting that acidified LF was able to release easily from plasma protein. To acidify the plasma sample, FA was better than TFA and at least 0.5% FA in final solution was required to achieve high extraction RE. Munjal et al. used a simple protein precipitation method with 0.1% acetic acid in MeCN [14]; the RE ranged from 50–65%, probably due to insufficient acidification. The final conditions were as follows: plasma sample (25 μl) and the IS (25 μl) were acidified with 5% FA 100 μl and extracted with ethyl acetate 900 μl.
Calibration curve
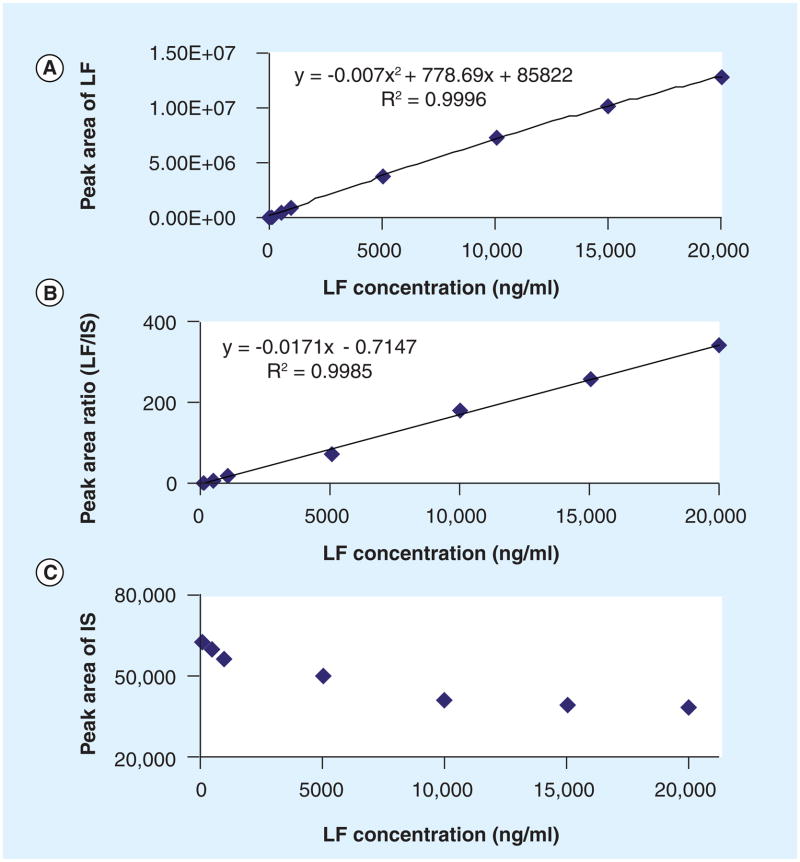
Without LF-D9 as the IS, the calibration curve was not linear in the range of 50 to 20,000 ng/ml. Quadratic fitting with 1/× weighting factor was required (Figure 2a). To check whether the signal saturation was caused by multiplier or ionization saturation, a lower abundant ion pair 528/291 was selected. Signal saturation was still observed with the weaker ion pair, suggesting ionization saturation occurred in the ion source. When using LF-D9 as the IS, the signal of the IS was decreased with the increase of LF concentration (Figure 2C). Since LF and LF-D9 were co-eluted and had the same affinity for protons, both signals were reduced at the saturated ion source and the ratio remained unaffected. This consequently extended the linear range for quantification of LF (Figure 2b).
Figure 2. Calibration standard curves with and without deuterated internal standard.
(A) No IS, calibration curve fitted with 1/× weighted quadratic regression. (B) Deuterated LF (LF-D9) as the IS, calibration curve fitted with 1/× weighted linear regression. (C) IS signal intensity at the different calibrator concentrations. The LF-D9 signal decreased at higher LF concentration, indicating signal saturation at the high end of calibrators. The ratio of LF/LF-D9 still increased in a linear mode.
LF: Lumefantrine; IS: Internal standard.
Method validation
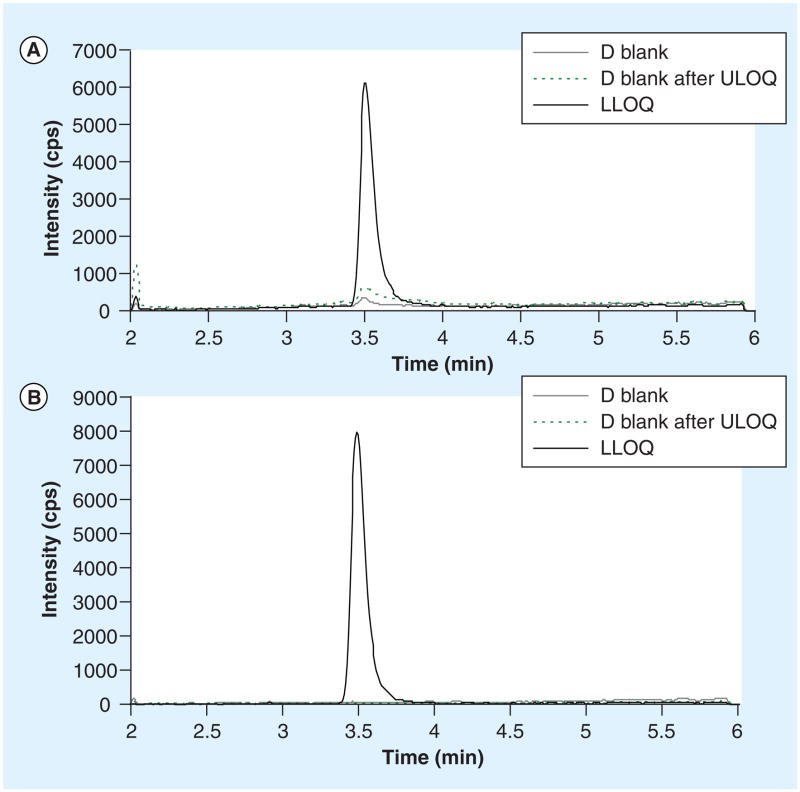
Using a 1/× weighted linear regression, the calibration range of this method was 50 to 20,000 ng/ml. The calibration equation over 6 days (mean ± SD) was y = (0.018 ± 0.008)× + (0.098 ± 0.109), r = 0.9984 ± 0.0014, where y represents peak area ratio (LF/LF-D9), × is LF concentration and r is the correlation coefficient. Based on the minimal (>50 ng/ml) and maximal (>10,000 ng/ml) concentrations of LF from previous pharmacokinetic studies [1,2], an LLOQ at 50 ng/ml would be sufficient. The S/N at 50 ng/ml (LLOQ) was 27. Representative multiple reaction monitoring ion chromatograms of blank sample, LLOQ and blank sample after ULOQ were shown in Figure 3. Notably, LF signal increased in the blank sample injected after ULOQ (dash line) when compared with the same blank injected in the beginning of assay (grey line), indicating carryover. But the carryover was generally less than 20% of LLOQ, which is not significant. Intra-day precisions (n = 5) over 6 days ranged from 1.3 to 10% and inter-day precision (n = 30) ranged from 5.3 to 6.1%. The intra- and inter-day accuracies were also within the acceptance limit (Table 1).
Figure 3.
Chromatograms of (A) lumefantrine and (B) the internal standard (deuterated lumefantrine) for blank plasma, LLOQ, and the blank plasma after ULOQ.
Table 1.
Intra- and inter-day precision and accuracy.
| Nominal (ng/ml) | Mean (ng/ml) | SD | RSD (%) | %dev | n |
|---|---|---|---|---|---|
| Intra-day | |||||
| 50 | 51.0–58.5 | 0.79–2.80 | 1.3–5.2 | 2.1–17 | 5 |
| 120 | 119–131 | 4.2–8.1 | 3.5–6.3 | −0.5–9.3 | 5 |
| 1500 | 1560–1646 | 35.6–160 | 2.2–10 | 4.0–9.7 | 5 |
| 17,000 | 16,660–18,220 | 709–1260 | 4.3–7.1 | −2.0–7.2 | 5 |
| Inter-day | |||||
| 50 | 54.16 | 3.21 | 5.9 | 8.3 | 30 |
| 120 | 127.2 | 6.7 | 5.3 | 6 | 30 |
| 1500 | 1607 | 88.7 | 5.5 | 7.1 | 30 |
| 17,000 | 17,593 | 1067 | 6.1 | 3.5 | 30 |
%dev: Deviation from nominal value.
RSD: Relative standard deviation.
LF is a very stable compound. There was no significant change of concentration for stock solution in 0.5% FA stored at −70°C for 18 months, and no significant degradation for plasma sample stored at −70°C for 1 year and at ambient temperature for 3 days (Table 2). In addition, sample dilution did not affect LF quantification, and the commonly concomitant drugs were not interfered with LF quantification either (Supplementary Tables 2 & 3).
Table 2.
Stability of lumefantrine (n = 3).
| Stability test conditions | Conc. (ng/ml) | % change | SD |
|---|---|---|---|
| Four freeze–thaw cycles, plasma | 120 | 1.4 | 7 |
| 170,000 | 1.2 | 1249 | |
|
| |||
| RT (22–28°C), 3 days, plasma | 120 | 11.9 | 4 |
| 170,000 | −1.6 | 404 | |
|
| |||
| RT (22°C), 72 h, autosampler | 120 | 4.7 | 3.0 |
| 170,000 | −3.3 | 814 | |
|
| |||
| −70°C, 1 year, plasma | 120 | 10 | 5 |
| 170,000 | 7.0 | 796 | |
| −70°C, 1.5 year stock in 0.5% FA, 50% MeCN | −0.53 | 0.1 | |
FA: Formic acid; MeCN: Acetonitrile; RT: Room temperature.
ME & RE
Evaluation of ME in LC–MS/MS methods is complicated, since in most cases the ME is not displayed through an ‘observable’ peak, but indirectly affects analyte peak through changing its ionization. ACTG guidelines recommend evaluating ME with a complicated approach, but the criteria have not yet been finalized. Here, ME was evaluated with four different approaches.
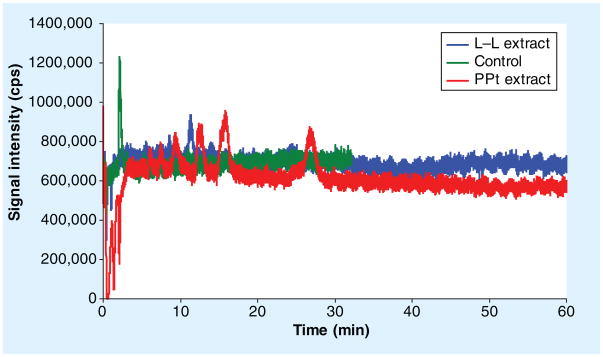
Firstly, an infusion experiment was performed by infusion of LF 10 μg/ml constantly while the LC ran at 0.4 ml/min at isocratic mode (65%B) (Figure 4). LF eluted at 2.1min (green line). When 10 μl of blank plasma extract from LLE was injected, no significant signal change was observed at the retention time of LF (2.1 min) and, thereafter, there is only one ion enhancement peak for 1 h (blue line). However, injection of blank plasma extract from protein precipitation with MeCN resulted in ion suppression at the retention time of LF and significant signal changes observed thereafter (red line), suggesting sample preparation with simple protein precipitation presented ME in the injected sample analysis and ME may also carryover to the following sample analysis. This also justified the need for column wash after each injection. Following this, an infusion experiment was also done with the final gradient elution method implemented with a wash phase. No significant signal change occurred at the retention time of LF when the blank plasma extract from LLE was injected (Supplementary Figure 2).
Figure 4. Matrix effect of plasma extracts from different sample preparations evaluated via an infusion experiment.
The peak at 2.1 min was lumefantrine. A 10 μg/ml of lumefantrine infused constantly into the LC elute via a ‘T’ joint and directed into MS source while the LC ran at 0.4 ml/min at isocratic mode (65%B). Signal was recorded after injected 10 μl of blank plasma extract from L–L extraction (blue line) and PPt (red line). A 10 μl of 10 μg/ml lumefantrine in mobile phase solvent injected as reference (green line). L–L: Liquid–liquid extraction; PPt: Protein precipitation.
Second, six different sources of blank plasma were spiked with LF at medium QC level (1500 ng/ml), and then processed and analyzed in triplicate. The deviation from nominal value (dev%) and variation from the mean (CV%) are all within 15% (Supplementary Table 4).
Third, comprehensive evaluation of ME was performed according to ACTG guidelines based on the strategies proposed by Matuszewski et al. [16,17]. Six different lots of human plasma were used and the raw data were provided in supplemental materials (Supplementary Table 5). The most important issue during method validation is estimation of relative ME, which can be performed through comparison of %CV in repetitive injections of neat standard (set 1b) and post-extraction spiked samples from different lots of the matrix (set 2). When comparing the %CV from sets 1b, 2 and 3, the difference between sets 1b and 2 is 0, −0.1 and 0.4% for LF at low, medium and high concentration levels, respectively. These values are 1.0, 0.1 and −0.4% for IS, respectively. All are less than 5%, which indicates no significant relative ME presents (Tables 3 & 4). Alternatively, the variability of slopes of standard line fitted through low, medium and high validation samples from at least five different lots of the matrix can be used as a good indicator of relative ME. Slopes of lines connecting low, medium and high LF samples from each lot of plasma were calculated and these are presented in Table 5. The CV% from set 3 is 4.8% (<5%), confirming that no significant ME on quantification was presented. Notably, ACTG guidelines recommend CV% of slopes should be <5%. Based on our experience with other methods, this value over 5% does not necessarily make the method invalid. Absolute ME was evaluated with mean peak area values from sets 1b and 2. A value of 100% means no ME. At low, medium and high LF concentration levels, the MEs were 97.9–100.4% for LF and 92.1–97.1% for IS, respectively (Table 6). These results indicate that ME in the method is negligible; however, effect of co-eluting substance was significant depending on concentration of LF and co-eluting matrix. High-QC concentration of LF significantly decreased the signal of LF-D9 (−30.4%), indicating ionization saturation. Surprisingly, the presence of LF-D9 reduced LF signal significantly at the low QC concentration while ionization saturation was not expected. Probably due to low water solubility of LF, equilibrium existed on the droplet surface during ionization even at low LF concentration [21] . Presence of LF-D9 shifted the equilibration point. It would be interesting to test non-aqueous mobile phase elution. We also noticed detection signal varied significantly from day to day, as demonstrated by IS levels, suggesting thatr matrix accumulated in the column affected subsequent analysis. Use of co-eluting LF-D9 as the IS was critical to compensate effectively for the cumulative ME.
Table 3.
Precision (%CV) of peak areas and peak area ratios in set 1–3.
| Low QC (conc.: 120 ng/ml) | Med. QC (conc.: 1500 ng/ml) | High QC (conc.: 17,000 ng/ml) | Column | |
|---|---|---|---|---|
| Lumefantrine peak area | ||||
| Set 1b | 2.8 | 0.8 | 1 | A |
| Set 2 | 2.8 | 0.7 | 1.4 | B |
| Set 3 | 4.3 | 3.3 | 2.5 | C |
| IS peak area | ||||
| Set 1b | 1.5 | 1.2 | 2.6 | D |
| Set 2 | 2.5 | 1.3 | 2.2 | E |
| Set 3 | 4.3 | 2 | 5.1 | F |
| Peak area ratio | ||||
| Set 1b | 2.2 | 1.7 | 2.4 | G |
| Set 2 | 4.1 | 1.7 | 2.2 | H |
| Set 3 | 5.4 | 2.1 | 4.8 | I |
n = 6.
IS: Internal standard; QC: Quality control.
Table 4.
Relative matrix effect.
| Low QC (conc.: 120 ng/ml) | Med. QC (conc.: 1500 ng/ml) | High QC (conc.: 17,000 ng/ml) | %CV dif. | |
|---|---|---|---|---|
| B–A | 0 | −0.1 | 0.4 | <5 |
| E–D | 1 | 0.1 | −0.4 | <5 |
| H–G | 1.9 | 0 | −0.2 | <5 |
| I–H | 1.3 | 0.4 | 2.6 | <5 |
n = 6.
IS: Internal standard; QC: Quality control.
Table 5.
Precision of slopes of lines fitted through low, medium and high quality control samples (n = 6).
| Plasma lot # | Slope | ||
|---|---|---|---|
| Set 1b | Set 2 | Set 3 | |
| 1 | 0.0079 | 0.0075 | 0.0067 |
| 2 | 0.0075 | 0.0078 | 0.0075 |
| 3 | 0.0074 | 0.0076 | 0.0073 |
| 4 | 0.0076 | 0.0078 | 0.0067 |
| 5 | 0.0073 | 0.0080 | 0.0071 |
| 6 | 0.0076 | 0.0076 | 0.0068 |
| Mean | 0.00755 | 0.00772 | 0.00702 |
| SD | 0.00021 | 0.00018 | 0.00034 |
| %CV | 2.75 | 2.38 | 4.80 |
Table 6.
Matrix effect, recovery and process efficiency (n = 6).
| Low conc. (120 ng/ml) | Med conc. (1500 ng/ml) | High conc. (17,000 ng/ml) | |
|---|---|---|---|
| LF mean peak area × 10e4 | |||
| Set 1b | 4.72 | 57 | 492 |
| Set 2 | 4.62 | 57.2 | 491 |
| Set 3 | 3.89 | 46.4 | 409 |
| IS mean peak area × 10e4 | |||
| Set 1 | 5.42 | 4.96 | 3.84 |
| Set 2 | 4.99 | 4.81 | 3.72 |
| Set 3 | 4.41 | 4.28 | 3.44 |
| ME | |||
| LF | 97.9 | 100.4 | 99.7 |
| IS | 92.1 | 96.9 | 97.1 |
| RE | |||
| LF | 84.3 | 81 | 83.4 |
| IS | 88.3 | 88.9 | 92.3 |
| PE | |||
| LF | 82.5 | 81.3 | 83.1 |
| IS | 81.4 | 86.2 | 89.6 |
IS: Internal standard; LF: Lumefantrine; ME: Matrix effect; PE: Process efficiency; RE: Recovery.
Crossvalidation
An HPLC-UV method for LF has been developed in our laboratory for a previous drug–drug interaction study on healthy subjects [7]. Here we reanalyzed 96 of the clinical samples. When the results were compared with those from the HPLC-UV method, only one of 96 samples has >20% deviation from the reference value (Table 7), suggesting the two methods are equivalent. Notably, the IS levels were varied significantly during the last batch of analysis (Supplementary Figure 3), but the results were still in good agreement with the reference values.
Table 7.
Comparison of lumefantrine concentrations in clinical samples using the HPLC-UV method (conc. 1) versus LC–MS/MS method (conc. 2).
| Sample # | Conc. 1 | Conc. 2 | %dev |
|---|---|---|---|
| S1 | 9303 | 10,900 | 15.8 |
| S2 | 9192 | 10,700 | 15.2 |
| S3 | 10,623 | 11,500 | 7.93 |
| S4 | 10,539 | 11,600 | 9.59 |
| S5 | 11,820 | 12,800 | 7.96 |
| S6 | 13,151 | 15,400 | 15.8 |
| S7 | 11,217 | 11,500 | 2.49 |
| S8 | 8236 | 8400 | 1.97 |
| S9 | 3826 | 4440 | 14.9 |
| S10 | 2289 | 1980 | −14.5 |
| S11 | 1356 | 1380 | 1.79 |
| S12 | 940 | 1000 | 6.17 |
| S13 | 699 | 735 | 5.09 |
| S14 | 437 | 451 | 3.15 |
| S15 | 352 | 351 | −0.28 |
| S16 | 310 | 308 | −0.65 |
| S17 | 5339 | 5800 | 8.28 |
| S18 | 4762 | 4860 | 2.04 |
| S19 | 5055 | 5410 | 6.79 |
| S20 | 5374 | 5820 | 7.97 |
| S21 | 4952 | 5340 | 7.54 |
| S22 | 5777 | 6280 | 8.34 |
| S23 | 4641 | 4750 | 2.33 |
| S24 | 3047 | 3210 | 5.22 |
| S25 | 1356 | 1230 | −9.74 |
| S26 | 632 | 613 | −2.99 |
| S27 | 311 | 272 | −13.3 |
| S28 | 247 | 254 | 2.98 |
| S29 | 184 | 203 | 9.78 |
| S30 | 119 | 111 | −6.74 |
| S31 | 100 | 89.4 | −11.1 |
| S32 | 103 | 99.4 | −3.31 |
| S33 | 13,893 | 14,900 | 7.00 |
| S34 | 13,306 | 15,700 | 16.5 |
| S35 | 13,359 | 14,700 | 9.56 |
| S36 | 12,457 | 13,800 | 10.2 |
| S37 | 14,193 | 15,800 | 10.7 |
| S38 | 18,966 | 21,300 | 11.6 |
| S39 | 16,825 | 18,300 | 8.40 |
| S40 | 11,167 | 12,900 | 14.4 |
| S41 | 8082 | 8660 | 6.91 |
| S42 | 4734 | 4910 | 3.64 |
| S43 | 2728 | 2820 | 3.30 |
| S44 | 1766 | 1900 | 7.34 |
| S45 | 1272 | 1420 | 11.0 |
| S46 | 808 | 841 | 3.98 |
| S47 | 623 | 665 | 6.52 |
| S48 | 524 | 540 | 3.00 |
| S49 | 17,221 | 18,900 | 9.29 |
| S50 | 16,701 | 16,400 | −1.82 |
| S51 | 16,471 | 17,000 | 3.16 |
| S52 | 15,334 | 15,900 | 3.63 |
| S53 | 14,836 | 15,200 | 2.42 |
| S54 | 14,949 | 15,200 | 1.66 |
| S55 | 12,217 | 15,100 | 21.1 |
| S56 | 9168 | 10,900 | 17.3 |
| S57 | 4512 | 4720 | 4.50 |
| S58 | 1810 | 1920 | 5.92 |
| S59 | 984 | 1060 | 7.43 |
| S60 | 700 | 762 | 8.52 |
| S61 | 498 | 539 | 7.91 |
| S62 | 328 | 336 | 2.41 |
| S63 | 272 | 288 | 5.86 |
| S64 | 223 | 219 | −1.91 |
| S65 | 9508 | 9490 | −0.19 |
| S66 | 9680 | 8430 | −13.8 |
| S67 | 9061 | 9200 | 1.53 |
| S68 | 8903 | 8800 | −1.16 |
| S69 | 8652 | 8550 | −1.19 |
| S70 | 15,601 | 14,900 | −4.60 |
| S71 | 14,278 | 14,400 | 0.85 |
| S72 | 10,467 | 10,600 | 1.26 |
| S73 | 4348 | 4380 | 0.74 |
| S74 | 2612 | 2550 | −2.40 |
| S75 | 1508 | 1640 | 8.39 |
| S76 | 1167 | 1230 | 5.22 |
| S77 | 910 | 951 | 4.44 |
| S78 | 516 | 535 | 3.65 |
| S79 | 491 | 476 | −3.10 |
| S80 | 270 | 268 | −0.91 |
| S81 | 10,022 | 10,600 | 5.61 |
| S82 | 9792 | 9760 | −0.33 |
| S83 | 9293 | 9850 | 5.82 |
| S84 | 8641 | 9200 | 6.27 |
| S85 | 8002 | 9120 | 13.1 |
| S86 | 19,647 | 16,900 | −15.0 |
| S87 | 15101 | 13,800 | −9.00 |
| S88 | 9589 | 8960 | −6.78 |
| S89 | 2484 | 2460 | −0.98 |
| S90 | 1250 | 1230 | −1.64 |
| S91 | 728 | 719 | −1.18 |
| S92 | 430 | 434 | 0.93 |
| S93 | 332 | 315 | −5.15 |
| S94 | 279 | 265 | −5.05 |
| S95 | 196 | 185 | −5.77 |
| S96 | 191 | 184 | −3.73 |
%dev: % deviation.
Clinical application
This method was applied to clinical samples from pediatric patients from whom ~200 μl blood was collected by capillary tubes. So far, 259 samples were analyzed; 27 samples, including a few predose samples were below LLOQ and eight samples were above 10,000 ng/ml with the highest concentration at 28,000 ng/ml. The precision of QC samples during the period of sample analysis was 9.35–10.8%. The detailed analysis of data will be reported elsewhere.
Conclusion
A robust LC–MS/MS method was developed for determination of LF in human plasma. With LF-D9 as the IS, this method was significantly improved on the issues of ME and signal saturation when compared with previously published methods. Through LLE of sample and optimization of LC conditions, absolute ME was minimized to 92–100%. Cumulative matrix was still observed, causing significant variation of signals over days of use. The co-eluting LF-D9 as the IS was able to compensate effectively for the cumulative ME. Furthermore, ionization saturation was also compensated by the deuterated IS, and this extended the linear range of calibration curve to 50–20,000 ng/ml. Only 25 μl plasma volume is required for analysis and is suitable for pharmacokinetic study of LF in pediatric patients.
Future perspective
In respect to the difficulty of blood sample collection and limited sample volume from pediatric patients, we believe the less the sample volume, the better the method. A quantification method using less than 50 μl plasma will facilitate blood collection with capillary tubes. In the future, simple finger pricking and blood collection with capillary tube combined with a small plasma volume quantification method or dried blood spot quantification method will likely be the standard for pediatric pharmacokinetic studies.
Supplementary Material
Executive summary.
Method development
Acidifying plasma samples prior to sample preparation is important to achieve a high recovery.
Matrix effect (ME) was reduced to minimal through sample preparation (liquid–liquid extraction) and LC separation (gradient elution followed with 100% organic washing phase).
Stable isotope-labeled lumefantrine used as the internal standard compensated for ME and ionization saturation.
Method validation
Cumulative ME observed from day to day was compensated by a co-eluting stable isotope-labeled internal standard.
Application
A small plasma sample volume (25 μl) used in the method was suitable for pediatric studies where blood sample collection with capillary tubes may be used.
Key Terms
- Matrix effect
Concomitant presence of other substances in the ion source affects ionization of the analyte leading to enhanced or suppressed signal of the analyte
- Stable isotope-labeled analyte
Having the same chemical structure with the analyte except for certain atoms (usually H and/or C) substituted with corresponding stable isotopes (2H or/and 13C)
- Ionization saturation
Occurred when high concentration of compounds presented in ion source competing for ionizing agent (H+, NH4+)
Footnotes
For reprint orders, please contact reprints@future-science.com
Supplementary data accompanies this paper and can be found at www.future-science.com/doi/full/10.4155/BIO.11.303
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Financial & competing interests disclosure
This work was supported by NIH/NIAID International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Group (grant number 1U01AI068632), ACTG (1U01AI068636–02), UCSF-GIVI Center for AIDS Research (CFAR) and Novartis Pharm. Co. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.White NJ, van Vugt M, Ezzet F. Clinical pharmacokinetics and pharmacodynamics of artemether-lumefantrine. Clin Pharmacokinet. 1999;37:105–125. doi: 10.2165/00003088-199937020-00002. [DOI] [PubMed] [Google Scholar]
- 2.German P, Parikh P, Lawrence J, et al. Lopinavir/ritonavir affects pharmacokinetic exposure of artemether/lumefantrine in HIV-uninfected healthy volunteers. J Acquir Immune Defic Syndr. 2009;51:424–429. doi: 10.1097/QAI.0b013e3181acb4ff. [DOI] [PubMed] [Google Scholar]
- 3.Zeng M, Lu Z, Yang S, et al. Determination of benflumetol in human plasma by reversed-phase high-performance liquid chromatography with ultraviolet detection. J Chromatogr B. 1996;681:299–306. doi: 10.1016/0378-4347(95)00542-0. [DOI] [PubMed] [Google Scholar]
- 4.Khalil IF, Abildrup U, Alifrangis LH, et al. Measurement of lumefantrine and its metabolite in plasma by high performance liquid chromatography with ultraviolet detection. J Pharm Biomed Anal. 2011;54:168–172. doi: 10.1016/j.jpba.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Lindegardh N, Annerberg A, Blessborn D, Bergqvist Y, Day N, White NJ. Development and validation of a bioanalytical method using automated solid-phase extraction and LC–UV for the simultaneous determination of lumefantrine and its desbutyl metabolite in plasma. J Pharm Biomed Anal. 2005;37:1081–1088. doi: 10.1016/j.jpba.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 6.Annerberg A, Singtoroj T, Tipmanee P, White NJ, Day NP, Lindegårdh N. High-throughput assay for the determination of lumefantrine in plasma. J Chromatogr B. 2005;822:330–333. doi: 10.1016/j.jchromb.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 7▪▪.Huang L, Lizak PS, Jayewardene AL, Marzan F, Lee MT, Aweeka FT. A modified method for determination of lumefantrine in human plasma by HPLC-UV and combination of protein precipitation and solid-phase extraction: application to a pharmacokinetic study. Anal Chem Insights. 2010;5:15–23. doi: 10.4137/aci.s4431. Describes the reference method for crossvalidation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong RPM, Salman S, Ilett KF, Siba PM, Mueller I, Davis TME. Desbutyl-lumefantrine is a metabolite of lumefantrine with potent in vitro antimalarial activity that may influence artemether–lumefantrine treatment outcome. Antimicrob Agents Chemother. 2011;55:1194–1198. doi: 10.1128/AAC.01312-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blessborn D, Romsing S, Annerberg A, et al. Development and validation of an automated solid-phase extraction and liquid chromatographic method for determination of lumefantrine in capillary blood on sampling paper. J Pharm Biomed Anal. 2007;45:282–287. doi: 10.1016/j.jpba.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Ntale M, Ogwal-Okeng JW, Mahindi M, Gustafsson LL, Beck O. A field-adapted sampling and HPLC quantification method for lumefantrine and its desbutyl metabolite in whole blood spotted on filter paper. J Chromatogr B. 2008;876:261–265. doi: 10.1016/j.jchromb.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Ashley EA, Stepniewska K, Lindegardh N, et al. Pharmacokinetic study of artemether-lumefantrine giving once daily for the treatment of uncomplicated multidrug-resistant falciparum malaria. Trop Med Int Health. 2007;12:201–208. doi: 10.1111/j.1365-3156.2006.01785.x. [DOI] [PubMed] [Google Scholar]
- 12▪▪.Hodel EM, Zanolari B, Mercier T, et al. A single LC–tandem mass spectrometry method for the simultaneous determination of 14 antimalarial drugs and their metabolites in human plasma. J Chromatogr B. 2009;877:867–886. doi: 10.1016/j.jchromb.2009.02.006. First reported LC–MS/MS method for lumefantrine: protein precipitation was used and matrix effect was significant. [DOI] [PubMed] [Google Scholar]
- 13.Wahajuddin W, Singh SP, Jian GK. Determination of lumefantrine in rat plasma by liquid–liquid extraction using LC–MS/MS with electrospray ionization: assay development, validation and application to a pharmacokinetic study. J Chromatogr B. 2009;877:1133–1139. doi: 10.1016/j.jchromb.2009.02.058. [DOI] [PubMed] [Google Scholar]
- 14▪.Munjal V, Paliwal N, Chaursia BK, Varshney B, Ahmed T, Paliwal J. LC-tandem mass spectrometry method for quantification of lumefantrine in human plasma and its application to bioequivalence study. Chromatographia. 2010;71:505–510. Low recovery from protein precipitation. [Google Scholar]
- 15▪.César IC, de Aquino Ribeiro AJ, Teixeira LS, et al. Liquid chromatography-tandem mass spectrometry for the simultaneous quantitation of artemether and lumefantrine in human plasma: application for a pharmacokinetic study. J Pharm Biomed Anal. 2011;54:114–120. doi: 10.1016/j.jpba.2010.07.027. Quadratic fitting of calibration curve and protein precipitation. [DOI] [PubMed] [Google Scholar]
- 16.ACTG guidelines for method development and validation based on (and including) FDA guidelines dated by 2001. Version 2 (2005).
- 17.Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC–MS/MS. Anal Chem. 2003;75:3019–3030. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 18.Fast DM, Kelley M, Viswanathan CT, et al. 2010 White paper on recent issues in regulated bioanalysis and global harmonization of bioanalytical guidance. AAPS J. 2009;11(2):238. doi: 10.4155/bio.10.164. [DOI] [PubMed] [Google Scholar]
- 19.Lindegardh N, Annerberg A, White NJ, Day NP. Development and validation of a liquid chromatographic-tandem mass spectrometric method for determination of piperaquine in plasma: stable-isotope-labeled internal standard does not always compensate for matrix effects. J Chromatogr B. 2008;862:227–236. doi: 10.1016/j.jchromb.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Colussi D, Parisot C, Legay F, Lefevre G. Binding of artemether and lumefantrine to plasma proteins and erythrocytes. Eur J Pharm Sci. 1999;9:9–16. doi: 10.1016/s0928-0987(99)00037-8. [DOI] [PubMed] [Google Scholar]
- 21.Trufelli H, Palma P, Famiglini G, Cappiello A. An overview of matrix effects in liquid chromatography-mass spectrometry. Mass Spectrom Rev. 2011;30:491–509. doi: 10.1002/mas.20298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.