Abstract
Purpose
The overactive bladder (OAB) syndrome is characterized by urgency usually with frequency and nocturia. Tamsulosin, α1-adrenergic receptor antagonist, is widely used to reduce symptoms of urinary obstruction and prostatic hyperplasia. Tamsulosin can across the blood-brain barrier. We investigated the effects of tamsulosin on the symptoms of OAB in relation to neuronal activity using rats.
Methods
Adult female Sprague-Dawley rats, weighing 250±10 g (9 weeks old), were used in this study. The animals were divided into five groups (n=8 in each group): control group, OAB-induced group, OAB-induced and 0.01 mg/kg tamsulosin-treated group, OAB-induced and 0.1 mg/kg tamsulosin-treated group, and OAB-induced and 1 mg/kg tamsulosin-treated group. OAB was induced by intraperitoneal injection of cyclophosphamide (75 mg/kg) every third day for 10 days. The rats in the tamsulosin-treated groups orally received tamsulosin once a day for 14 consecutive days at the respective dose of the groups, starting 1 day after the induction of OAB. Cystometry for bladder pressure determination, immunohistochemistry for c-Fos, nicotinamide adenine dinucleotide phosphate-diaphorase histochemistry for nitric oxide synthase (NOS) in the neuronal voiding centers and western blot for inducible NOS in the bladder were conducted.
Results
Cyclophosphamide injection enhanced contraction pressure and time, representing the induction of OAB. Contraction pressure and time were significantly suppressed by tamsulosin treatment. c-Fos and NOS expressions in the neuronal voiding centers were enhanced by induction of OAB. OAB-induced c-Fos and NOS expressions were suppressed by tamsulosin treatment.
Conclusions
Tamsulosin exerts inhibitory effect on neuronal activation in the neuronal voiding centers of OAB. The present results suggest the possibility that tamsulosin is effective therapeutic modality for ameliorating the symptoms of OAB.
Keywords: Overactive bladder, Cyclophosphamide, Tamsulosin, Rats
INTRODUCTION
The overactive bladder (OAB) syndrome is characterized by urgency usually with frequency and nocturia [1]. OAB represents a substantial problem in clinical practice, with an overall prevalence of 16 to 17% of adults worldwide. OAB is a symptom complex affecting both women and men, and it is more common in elder, however young person also have OAB symptoms as well [2]. The cost of managing OAB in the United States has been estimated to be as high as 6 billion United States Dollars [3]. In addition, patients with OAB tend to restrict their activities and many patients experience sleep disruption, depression, and poor life quality [2].
Normally, the detrusor muscle contracts and relaxes in response to the volume of urine in the bladder and the initiation of urination. In OAB patients, however, bladder smooth muscle contracts spastically, sometimes as unknown cause, induces sustained high bladder pressure, and causes urgent urination [3]. Cystometry measures the pressure and capacity of the bladder, and thereby can be used to evaluate the function of detrusor muscle and bladder in OAB patients [4].
Micturition involves processes within urinary tract and brain. Neuroanatomical tracing studies revealed that bladder and external urethral sphincter are innervated both directly and indirectly by many central nervous system regions, including the pontine micturition center (PMC), locus coeruleus, hypothalamus, preoptic area, and spinal cord [5]. Using a positron emission tomography scanner, Blok and Holstege [6] demonstrated that blood flow to the dorsomedial pons, periaqueductal gray (PAG), hypothalamus, and cerebral cortex was increased during micturition. Moreover, bladder hyperactivity was shown to induce chemical changes in the spinal cord [7].
The transcription factor c-Fos is encoded by the immediately early gene c-Fos, and c-Fos expression has been used as a marker of neuronal activity [8]. Stimulation of the bladder increased c-Fos-immunoreactive neurons in the PAG, PMC, and spinal cord [9,10].
Nitric oxide (NO) plays important physiologic roles in various organs such as regulation of vascular smooth muscle tone and neurotransmission of the peripheral nervous system and central nervous system [11]. Research on pathological bladder activity has shown the involvement of the NO pathway [12]. NO is synthesized from L-arginine by nitric oxide synthase (NOS). There are multiple forms of NOS, which are classified according to physical and chemical properties into three groups, neuronal NOS (nNOS), inducible NOS (iNOS), and endothelial NOS (eNOS). NOS is demonstrated in the lower urinary tract in animals and humans, and inducible NOS (iNOS) expression is associated with inflammation in the bladder [11]. Expression of iNOS was increased during bladder injury [13], thus NO plays an important role in non-adrenergic and non-cholinergic neurotransmission in a variety of organ systems, including lower urinary tract and voiding centers [13,14].
The most common drugs for the treatment of OAB are antimuscarinic drugs, however, limited efficacy and side effects such as dry mouth, somnolence, constipation, and blurred vision have been reported [2]. Tamsulosin is in a class of medications classified in α1-adrenergic receptor antagonists, and this drug is widely used to reduce urinary obstruction and to relieve associated manifestations in hypertensive or normotensive patients with symptomatic benign prostatic hyperplasia [15]. In addition, the crucial aspect for tamsulosin is its ability to cross the blood-brain barrier [16]. Several studies showed that antimuscarinic drugs treatment with α1-adrenergic receptor antagonists improved lower urinary tract symptoms in humans [17,18].
Currently, treatment with α1-adrenergic receptor antagonists is suggested as the standard care for humans with lower urinary tract symptoms, because these drugs reduce smooth muscle tone in the prostate and bladder neck and also decrease bladder outlet resistance [18,19]. In many cases, OAB symptoms coexist with benign prostate hyperplasia or bladder outlet obstruction not caused by prostate origin, as a results pharmacotherapies targeting only prostate (and not bladder) may not sufficiently alleviate OAB symptoms [19]. The effects of α1-adrenergic receptor antagonists on voiding and storage symptoms have been suggested, however the mechanisms by which α1-adrenergic receptor antagonists may improve OAB remain controversial. In addition, there have been no reports on the efficacy of tamsulosin in producing changes in the neuronal activity of voiding centers in relation with OAB symptoms.
In the present study, we investigated the effects of tamsulosin on urinary bladder function, iNOS expression in the bladder, and c-Fos and NOS expressions in the neuronal voiding centers (medial preoptic nucleus [MPA], ventrolateral periaqueductal gray [vlPAG], PMC, and spinal cord [L4-L5]) following the induction of OAB in rats. For this study, cystometry, immunohistochemistry for c-Fos, histochemistry for nicotinamide adenine dinucleotide phosphate-diaphorase (NADPH-d), and western blot for iNOS were performed.
MATERIALS AND METHODS
Animals
Adult female Sprague-Dawley rats, weighing 250±10 g (9 weeks old), were obtained from a commercial breeder (Orient Co., Seoul, Korea) for the experiment. The experimental procedures were performed in accordance with the animal care guidelines of the National Institute of Health and the Korean Academy of Medical Sciences. Each animal was housed under controlled temperature (23±2℃) and lighting (8 A.M. to 8 P.M.) conditions with food and water made available ad libitum before and after surgery.
The animals were randomly divided into five groups (n=8 in each group): the control group, the OAB-induced group, the OAB-induced and 0.01 mg/kg tamsulosin-treated group, the OAB-induced and 0.1 mg/kg tamsulosin-treated group, and the OAB-induced and 1 mg/kg tamsulosin-treated group.
The rats in the tamsulosin-treated groups orally received tamsulosin (Harnal, Astellas Pharma Inc., Tokyo, Japan) once a day for consecutive 14 days at the respective doses of the groups, starting 1 day after the induction of OAB. The animals in the control group and in the OAB-induced group received the same amount of distilled water.
Induction of Overactive Bladder
OAB model was induced as the previously described method [20]. For the induction of chronic OAB model, 75 mg/kg cyclophosphamide (Sigma Chemical Co., St. Louis, MO, USA) was intraperiotneally injected every third day for 10 days. The control rats intraperitoneally received volume-matched saline.
Cystometry
Bladder function in rats was evaluated by cystometry 14 days after the induction of OAB. The rats were anesthetized with Zoletil 50 (10 mg/kg, i.p.; Vibac Laboratories, Carros, France). A sterile polyethylene catheter (PE50) was inserted into the urethra through the bladder dome. The catheter was connected to a pressure transducer (Havard Apparatus, Holliston, MA, USA) and syringe pump (Havard Apparatus) via a 3-way stopcock to record intravesical pressure and to infuse saline into the bladder. After the bladder was emptied, cystometry was performed with infusion of 0.5 mL saline. The contraction pressure and contraction time in the bladder were monitored using Labscribe (iWork System Inc., Dover, NH, USA).
Tissue Preparation
After tissue preparation of the bladder, the rats were transcardially perfused with 50 mM phosphate-buffered saline (PBS), followed by 4% paraformaldehyde in 100 mM sodium phosphate buffer (PB) at pH 7.4. The brain was removed, postfixed in the same fixative overnight, and transferred into a 30% sucrose solution for cryoprotection. Serial coronal sections of 40 µm thickness were made with a freezing microtome (Leica Biosystems Nussloch GmbH, Nussloch, Germany). The PMC was selected from the region spanning from Bregma -9.68 to -9.80 mm, the vlPAG was selected from the region spanning from Bregma -7.64 to -8.00 mm, the MPA was selected from the region spanning from Bregma -0.26 to 0.80 mm, and the spinal cord was selected from the L4-L5 regions. Ten sections on average in each region were collected from each rat.
c-Fos Immunohistochemistry
The central micturition areas were stained for c-Fos expression. Free-floating tissue sections were incubated overnight with rabbit anti-c-Fos antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) at a dilution of 1:1,000, and the sections were then incubated for 1 hour with biotinylated anti-rabbit secondary antibody (Vector Laboratories Inc., Burlingame, CA, USA). The sections were subsequently incubated with avidin-biotin-peroxidase complex (Vector Laboratories Inc.) for 1 hour at room temperature. Immunoreactivity was visualized by incubating the sections in a solution consisting of 0.05% 3,3-diaminobenzidine and 0.01% H2O2 in 50 mM Tris-buffer (pH 7.6) for approximately 3 minutes. The sections were then washed three times with PBS and mounted onto gelatine-coated slides. The slides were air-dried overnight at room temperature, and coverslips were mounted by using Permount mounting medium (Thermo Fisher Scientific Inc., Waltham, MA, USA).
NADPH-d Histochemistry
The central micturition areas were stained for NOS activity. In brief, free-floating sections were incubated at 37℃ for 60 minutes in 100 mM PBS (pH 7.4) containing 0.3% Triton X-100, 0.1 mg/mL nitroblue tetrazolium, and 0.1 mg/mL β-NADPH. The sections were then washed three times with PBS and mounted onto gelatin-coated slides. The slides were air dried overnight at room temperature, and coverslips were mounted by using Permount mounting medium (Thermo Fisher Scientific Inc.).
iNOS Western Blot Analysis
The bladder tissues were collected, and then immediately frozen at -80℃. The bladder tissues were homogenized on ice, and lysed in a lysis buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 0.5% deoxycholic acid, 1% Nonidet P40, 0.1% sodium dodecyl sulfate (SDS), 1 mM phenylmethylsulfonyl fluoride (PMSF), and 100 mg/mL leupeptin. Protein content was measured by using a Bio-Rad colorimetric protein assay kit (Bio-Rad Laboratories Inc., Hercules, CA, USA). Protein in amounts of 30 µg was separated on SDS-polyacrylamide gels and transferred onto a nitrocellulose membrane. Mouse actin antibody (1:500; Santa Cruz Biotechnology Inc.) and rabbit iNOS antibody (1:1,000; Santa Cruz Biotechnology Inc.) were used as the primary antibodies. Horseradish peroxidase-conjugated anti-rabbit antibody for iNOS (1:2,000; Vector Laboratories Inc.) was used as the secondary antibody. Experiments were performed under normal laboratory conditions and at room temperature except for membrane transfer. Membrane transfer was performed at 4℃ with a cold pack and prechilled buffer. Band detection was performed by use of an enhanced chemiluminescence detection kit (Santa Cruz Biotechnology Inc.).
Data Analysis
The number of c-Fos-positive and NADPH-d-positive cells in the MPA, PMC, vlPAG, and spinal cord (L4-L5) regions were counted hemilaterally through a light microscope (Olympus Co., Tokyo, Japan). The area of the MPA, PMC, vlPAG, and spinal cord (L4-L5) regions from each slice was measured by using an Image-Pro Plus computer-assisted image analysis system (Media Cybernetics Inc., Silver Spring, MD, USA) attached to a light microscope (Olympus Co.). To compare the relative expression of iNOS proteins, detected bands were calculated densitometrically by using Molecular Analyst ver. 1.4.1 (Bio-Rad Laboratories Inc.). Statistical analysis was performed by using one-way analysis of variance followed by Duncan's post-hoc test, and the results are expressed as the mean±standard error of the mean. Significance was set as P<0.05.
RESULTS
Effects of Tamsulosin on the Contraction Pressure and Time
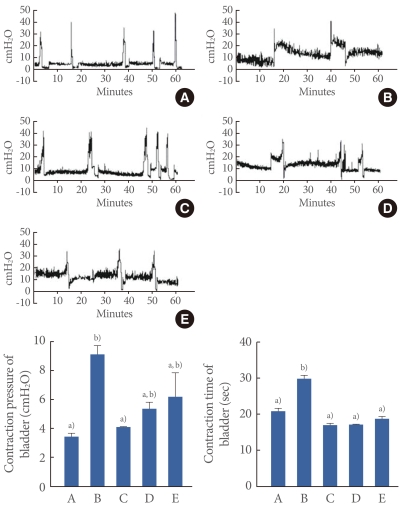
The contraction pressure and contraction time from the cystometry are presented Fig. 1. The contraction pressure was 3.40±0.27 cmH2O in the control group, 9.12±0.62 cmH2O in the OAB-induced group, 4.13±0.02 cmH2O in the OAB-induced and 0.01 mg/kg tamsulosin-treated group, 5.40±0.43 cmH2O in the OAB-induced and 0.1 mg/kg tamsulosin-treated group, and 6.20±1.61 cmH2O in the OAB-induced and 1 mg/kg tamsulosin-treated group.
Fig. 1.
Effect of tamsulosin on contraction pressure and time in the cystometry. Upper: Graph of cystometry in each group. (A) Control group, (B) overactive bladder (OAB)-induced group, (C) OAB-induced and 0.01 mg/kg tamsulosin-treated group, (D) OAB-induced and 0.1 mg/kg tamsulosin-treated group, (E) OAB-induced and 1 mg/kg tamsulosin-treated group. Lower: Analysis of contraction pressure (left) and contraction time (right) in each group. a,b)Statistically significant differences (P<0.05) after Duncan post-hoc test. For example, groups marked with different letters differ statistically.
These results showed that contraction pressure was increased by the induction of OAB (P<0.05), and that tamsulosin treatment significantly decreased the OAB-induced contraction pressure in a dose-dependent manner (P<0.05). The suppressing effect of tamsulosin on contraction pressure appeared most potent with the lowest dose of 0.01 mg/kg (P<0.05).
The contraction times was 20.80±0.87 seconds in the control group, 29.74±0.96 seconds in the OAB-induced group, 16.92±0.40 seconds in the OAB-induced and 0.01 mg/kg tamsulosin-treated group, 16.98±0.02 seconds in the OAB-induced and 0.1 mg/kg tamsulosin-treated group, and 18.79±0.56 seconds in the OAB-induced and 1 mg/kg tamsulosin-treated group.
These results showed that contraction time was increased by the induction of OAB (P<0.05) and that tamsulosin treatment significantly decreased the OAB-induced contraction time (P<0.05), although the improvement was not dose-dependent.
Effects of Tamsulosin on the c-Fos Expressions in the Neuronal Voiding Centers
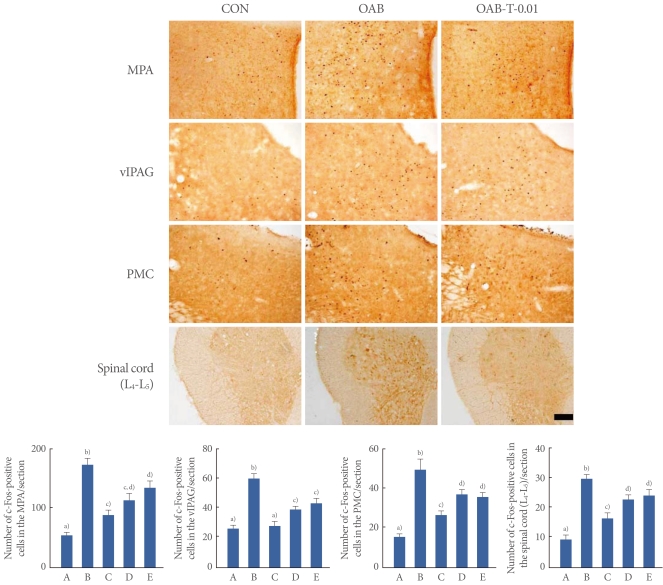
Photomicrographs of c-Fos-positive cells in the neuronal voiding centers are presented in Fig. 2. In the MPA region, the number of c-Fos-positive cells was 73.12±7.53/section in the control group, 142.00±8.17/section in the OAB-induced group, 93.75±18.22/section in the OAB-induced and 0.01 mg/kg tamsulosin-treated group, 115.33±11.98/section in the OAB-induced and 0.1 mg/kg tamsulosin-treated group, and 136.75±10.79/section in the OAB-induced and 1 mg/kg tamsulosin-treated group.
Fig. 2.
Effect of tamsulosin on c-Fos expressions in the neuronal voiding centers (medial preoptic nucleus [MPA], ventrolateral periaqueductal gray [vlPAG], pontine micturition center [PMC], spinal cord [L4-L5] regions) after the induction of overactive bladder (OAB). Upper: Photomicrographs of c-Fos-positive cells in the neuronal voiding centers. The sections were stained for c-Fos immunoreactivity (brown). The scale bar represents 200 µm. Control group (CON), OAB-induced group (OAB), OAB-induced and 0.01 mg/kg tamsulosin-treated group (OAB-T-0.01). Lower: Number of c-Fos-positive cells in each group. (A) Control group, (B) OAB-induced group, (C) OAB-induced and 0.01 mg/kg tamsulosin-treated group, (D) OAB-induced and 0.1 mg/kg tamsulosin-treated group, (E) OAB-induced and 1 mg/kg tamsulosin-treated group. a-d)Statistically significant differences (P<0.05) after Duncan post-hoc test. For example, groups marked with different letters differ statistically.
In the vlPAG region, the number of c-Fos-positive cells was 26.46±2.59/section in the control group, 61.07±3.60/section in the OAB-induced group, 28.42±3.20/section in the OAB-induced and 0.01 mg/kg tamsulosin-treated group, 39.68±2.36/section in the OAB-induced and 0.1 mg/kg tamsulosin-treated group, and 44.26±3.27/section in the OAB-induced and 1 mg/kg tamsulosin-treated group.
In the PMC region, the number of c-Fos-positive cells was 15.60±1.70/section in the control group, 49.84±5.59/section in the OAB-induced group, 26.71±2.15/section in the OAB-induced and 0.01 mg/kg tamsulosin-treated group, 37.23±2.43/section in the OAB-induced and 0.1 mg/kg tamsulosin-treated group, and 36.00±2.12/section in the OAB-induced and 1 mg/kg tamsulosin-treated group.
In the spinal cord L4-L5 regions, the number of c-Fos-positive cells was 9.30±1.48/section in the control group, 30.00±1.48/section in the OAB-induced group, 16.62±1.81/section in the OAB-induced and 0.01 mg/kg tamsulosin-treated group, 22.84±1.70/section in the OAB-induced and 0.1 mg/kg tamsulosin-treated group, and 24.4±1.97/section in the OAB-induced and 1 mg/kg tamsulosin-treated group.
These results showed that c-Fos expressions in the neuronal voiding centers (MPA, vlPAG, PMC, spinal cord [L4-L5]) were increased by the induction of OAB (P<0.05) and that tamsulosin treatment significantly decreased the OAB-induced c-Fos expressions in the neuronal voiding centers dose-dependently (P<0.05). The suppressing effect of tamsulosin on c-Fos expressions appeared most potent at the dose of 0.01 mg/kg (P<0.05).
Effects of Tamsulosin on the NOS Expressions in the Neuronal Voiding Centers
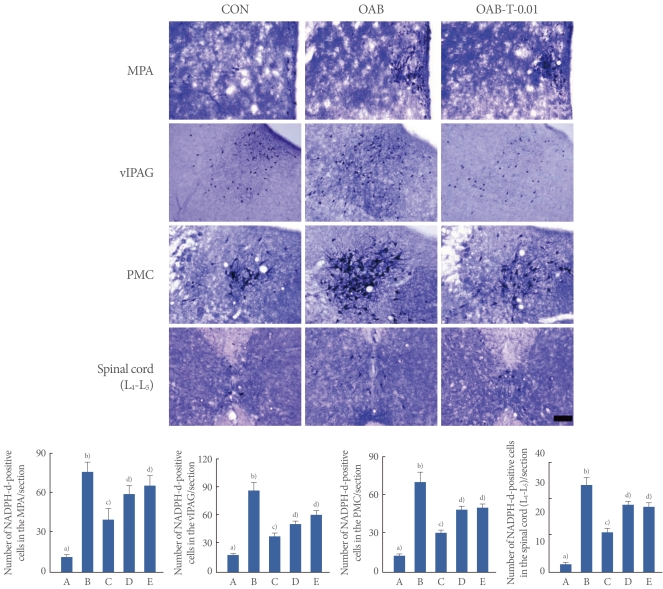
Photomicrographs of NADPH-d-positive cells in the neuronal voiding centers are presented in Fig. 3. In the MPA region, the number of NADPH-d-positive cells was 10.99±1.95/section in the control group, 76.34±7.95/section in the OAB-indcuced group, 40.11±8.55/section in the OAB-induced and 0.01 mg/kg tamsulosin-treated group, 59.66±6.45/section in the OAB-induced and 0.1 mg/kg tamsulosin-treated group, and 66.15±7.97/section in the OAB-induced and 1 mg/kg tamsulosin-treated group.
Fig. 3.
Effects of tamsulosin on nitric oxide synthase (NOS) expressions in the neuronal voiding centers (medial preoptic nucleus [MPA], ventrolateral periaqueductal gray [vlPAG], pontine micturition center [PMC], spinal cord [L4-L5] regions) after the induction of overactive bladder (OAB). Upper: Photomicrographs of nicotinamide adenine dinucleotide phosphate-diaphorase (NADPH-d)-positive cells in the neuronal voiding centers. The sections were stained for NOS like immunoreactivity (blue). The scale bar represents 200 µm. Control group (CON), OAB-induced group (OAB), OAB-induced and 0.01 mg/kg tamsulosin-treated group (OAB-T-0.01). Lower: Number of NADPH-d-positive cells in each group. (A) Control group, (B) OAB-induced group, (C) OAB-induced and 0.01 mg/kg tamsulosin-treated group, (D) OAB-induced and 0.1 mg/kg tamsulosin-treated group, (E) OAB-induced and 1 mg/kg tamsulosin-treated group. a-d)Statistically significant differences (P<0.05) after Duncan post-hoc test. For example, groups marked with different letters differ statistically.
In the vlPAG region, the number of NADPH-d-positive cells was 17.35±2.01/section in the control group, 88.74±8.12/section in the OAB-induced group, 39.66±4.09/section in the OAB-induced and 0.01 mg/kg tamsulosin-treated group, 52.15±5.11/section in the OAB-induced and 0.1 mg/kg tamsulosin-treated group, and 60.02±8.35/section in the OAB-induced and 1 mg/kg tamsulosin-treated group.
In the PMC region, the number of NADPH-d-positive cells was 12.11±1.57/section in the control group, 68.91±9.98/section in the OAB-induced group, 29.45±3.15/section in the OAB-induced and 0.01 mg/kg tamsulosin-treated group, 48.71±4.65/section in the OAB-induced and 0.1 mg/kg tamsulosin-treated group, and 50.30±4.15/section in the OAB-induced and 1 mg/kg tamsulosin-treated group.
In the spinal cord L4-L5 regions, the number of NADPH-d-positive cells was 8.96±1.55/section in the control group, 29.14±2.77/section in the OAB-induced group, 10.09±1.35/section in the OAB-induced and 0.01 mg/kg tamsulosin-treated group, 23.97±3.75/section in the OAB-induced and 0.1 mg/kg tamsulosin-treated group, and 26.04±3.09/section in the OAB-induced and 1 mg/kg tamsulosin-treated group.
These results showed that NOS expressions in the neuronal voiding centers (MPA, vlPAG, PMC, spinal cord [L4-L5]) were increased by the induction of OAB (P<0.05) and that tamsulosin treatment significantly decreased the OAB-induced NOS expressions in the neuronal voiding centers dose-dependently (P<0.05). The suppressing effect of tamsulosin on NOS expressions appeared most potent at the dose of 0.01 mg/kg (P<0.05).
Effect of Tamsulosin on the iNOS Expression in the Bladder
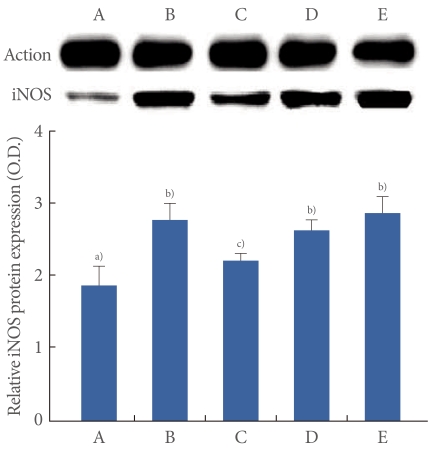
The iNOS (130 kDa) protein expression in the bladder is presented in Fig. 4. The expression of the iNOS protein was 1.87±0.27 in the control group, 2.79±0.22 in the OAB-induced group, 2.24±0.09 in the OAB-induced and 0.01 mg/kg tamsulosin-treated group, 2.64±0.14 in the OAB-induced and 0.1 mg/kg tamsulosin-treated group, and 2.89±0.21 in the OAB-induced and 1 mg/kg tamsulosin-treated group.
Fig. 4.
Effects of tamsulosin on the expressions of inducible niric oxide synthase (iNOS) protein in the bladder after the induction of overactive bladder (OAB). Actin was used as an internal control (46 kDa). (A) Control group, (B) OAB-induced group, (C) OAB-induced and 0.01 mg/kg tamsulosin-treated group, (D) OAB-induced and 0.1 mg/kg tamsulosin-treated group, (E) OAB-induced and 1 mg/kg tamsulosin-treated group. a-b)Statistically significant differences (P<0.05) after Duncan post-hoc test. For example, groups marked with different letters differ statistically.
These results showed that iNOS expression in the bladder was increased by induction of OAB (P<0.05) and that tamsulosin treatment significantly decreased OAB-induced NOS expressions in the bladder (P<0.05). The suppressing effect of tamsulosin on NOS expressions appeared at the 0.01 mg/kg dose (P<0.05).
DISCUSSION
Cyclophosphamide is a chemotherapeutic drug that is effective in the treatment of various cancers. However, this drug induces severe chemical cystitis in animals and humans. It is well established that this cystitis induces bladder overactivity, characterized by decreased bladder capacity and increased micturition frequency [20]. Thus, we used cyclophosphamide to induce OAB model in rats.
Bladder filling and storage processes require accommodation to increasing urine volumes at low intravesical pressure with appropriate sensation, still sphincter remains closed during increasing in intra-abdominal pressure, and involuntary bladder contraction not appear [21]. For these reasons, normal detrusor function allows bladder filling during the storage phase, with little or no change in bladder pressure [1]. In contrast, OAB is the observation of involuntary detrusor contraction, and both increased bladder pressure and time [21]. The present study showed that contraction pressure and time were significantly increased after cyclophosphamide injection, indicating that cyclophosphamide deteriorated bladder function and as a result, induced OAB.
The PMC play an important role in the control of urinary bladder function. Activation of PMC neurons induces bladder contractions and relaxation of the bladder neck and external urethral sphincter, which results in micturition. Two regions associated with the PMC are PAG and MPA of the hypothalamus [5]. The PAG-PMC projection is considered to take part in the micturition reflex. The vlPAG acts as a central sensorimotor integrative relay of the micturition reflex, via the reception of sensory information concerning bladder fullness and the direct projection to the PMC [6]. In addition, the PMC is densely innervated by the MPA [6]. The MPA sends projections to the PMC that synapse on neurons directly though projections to the spinal cord [5]. Bladder filling information from sensory interneurons in the lumbar spinal cord finally reaches to PMC in order to empty the bladder at appropriate time [22]. Electrical or chemical stimulation on the lower urinary tract was shown to cause changes in neuronal activity in the micturition centers, such as the PMC, PAG, MPA, and spinal cord [22,23]. Bon et al. [23] reported that injection of cyclophosphamide caused cystitis and increased number of c-Fos-positive cells in the PMC. Noxious stimulation of deep structures, such as muscle, joint, and viscera provoked a significant increase in the number of c-Fos-positive neurons in the vlPAG [24]. Chung et al. [25] indicated that chemical irritation on bladder provoked c-Fos expression in the PAG and MPA of the rats. Acute or chronic bladder irritation increased immediate early gene expression in the spinal neurons [7]. In the present results, expressions of c-Fos in the neuronal voiding centers (MPA, vlPAG, PMC, spinal cord [L4-L5] regions) were significantly increased after cyclophosphamide injection, indicating that induction of the OAB activated neurons in the voiding centers.
The exact mechanisms by which NO in the neuronal voiding centers modulates micturition are not fully understood. However, because NO regulates components of the lower urinary tract such as the bladder and urethra, NO levels in the neuronal voiding centers may also control the lower urinary tract status [26]. Gene expression and neurotransmitter synthesis in the brain can be altered by pain, peripheral irritation, and inflammation [26]. In the present results, expressions of NOS in the neuronal voiding centers (MPA, vlPAG, PMC, spinal cord [L4-L5] regions) were significantly increased after cyclophosphamide injection, indicating that induction of the OAB enhanced NO levels in the neuronal voiding centers.
As shown in this study, we suggest the relation of cyclophosphamide-induced expressions of c-Fos and NO in the neuronal voiding centers with cyclophosphamide-induced OAB symptoms. Enhanced expressions of c-Fos and NOS in the voiding centers activate neurons in the voiding centers and trigger OAB symptoms.
Increasing of NO production by iNOS exerts detrimental effect on bladder, such as overactive bladder [11,27]. Up-regulation of iNOS was detected after cyclophosphamide injection, lipopolysaccharide instillation, and during urinary obstruction [28]. iNOS expression is closely implicated in the bladder inflammation and injury [11,13]. Inhibition of iNOS activity in OAB animal model significantly attenuated the increase of bladder size and bladder contraction number and suppressed bladder fibrosis [29]. In the present results, iNOS expression in the bladder was significantly increased after cyclophosphamide injection, indicating that cyclophosphamide induced inflammation of bladder.
Change of α1-adrenoceptors expression in human blood vessels, spinal cord, ganglia, and nerve terminals has been postulated to contribute to the pathogenesis of lower urinary tract symptoms [30]. Activation of α1-adrenoceptors in bladder causes OAB symptoms, such as outflow obstruction, bladder instability, and frequent micturition [31]. Thus, the beneficial effect of α1-adrenergic receptor antagonists is a class effect in micturition function. Treatment with α1-adrenergic receptor antagonists relieves bladder obstruction, and may control voiding function by acting at bladder wall and spinal cord [32]. In human research, daytime frequency, nocturia, and incontinence episodes were significantly decreased with α1-adrenergic receptor antagonists [33]. Moreover, α1-adrenergic receptor antagonists significantly increased bladder capacity and decreased frequency [34]. In the present results, OAB-evoked increase of contraction pressure and time in the bladder was significantly suppressed by tamsulosin treatment. This suppressing effect of tamsulosin on contraction pressure and time can be ascribed to antagonizing effect of tamsulosin at α1-adrenergic receptors in the detrusor muscle or at prejunctional α1-adrenoceptors. This inhibition of tamsulosin may induce alteration of NO level in the lower urinary tract. The present study showed that iNOS protein expression in the bladder was significantly decreased by tamsulosin treatment. These results reveal that tamsulosin suppressed bladder inflammation, and as a result, alleviated the cyclophosphamide-induced OAB symptoms.
During the storage phase, continence is maintained by inhibition of the parasympathetic system and by activation of the sympathetic system, through the acting of α1-adrenergic receptors, leads to compression of urethral sphincter and bladder. Conversely, during voiding, the PMC inhibits the sympathetic system and activates the parasympathetic system, resulting in a sustained relaxation of urethral sphincter and bladder [16]. In the present results, antagonizing effect of tamsulosin on α1-adrenergic receptors suppressed the OAB-induced expressions of c-Fos and NOS in the neuronal voiding centers. The inhibitory effects of tamsulosin on the expressions of c-Fos and NOS appeared most potently at the 0.01 mg/kg dose. These results show that tamsulosin may exert inhibitory effect on neuronal activation in the voiding centers and delay triggering of OAB symptoms.
From the present results, α1-adrenergic receptor antagonist tamsulosin may overcome OAB-induced micturition dysfunction through inhibition of neuronal voiding centers, thus we raise the possibility that tamsulosin is effective therapeutic modality for ameliorating the symptoms of OAB.
ACKNOWLEDGEMENTS
This study was supported by the National Research Foundation of Korean Government (2010-0003794), and by the Reasearh Fund, 2010 of Gachon University Gil Hospital.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Abrams P. Describing bladder storage function: overactive bladder syndrome and detrusor overactivity. Urology. 2003;62(5 Suppl 2):28–37. doi: 10.1016/j.urology.2003.09.050. [DOI] [PubMed] [Google Scholar]
- 2.Andersson KE. Pharmacotherapy of the overactive bladder. Discov Med. 2009;8:118–124. [PubMed] [Google Scholar]
- 3.Chu FM, Dmochowski R. Pathophysiology of overactive bladder. Am J Med. 2006;119(3 Suppl 1):3–8. doi: 10.1016/j.amjmed.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Gordon D, Groutz A. Evaluation of female lower urinary tract symptoms: overview and update. Curr Opin Obstet Gynecol. 2001;13:521–527. doi: 10.1097/00001703-200110000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Rickey LM, Sarkey S, DonCarlos LL. Estrogen-sensitive projections from the medial preoptic area to the dorsal pontine tegmentum, including Barrington's nucleus, in the rat. Neurourol Urodyn. 2008;27:440–445. doi: 10.1002/nau.20522. [DOI] [PubMed] [Google Scholar]
- 6.Blok BF, Holstege G. Direct projections from the periaqueductal gray to the pontine micturition center (M-region). An anterograde and retrograde tracing study in the cat. Neurosci Lett. 1994;166:93–96. doi: 10.1016/0304-3940(94)90848-6. [DOI] [PubMed] [Google Scholar]
- 7.de Groat WC, Yoshimura N. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Prog Brain Res. 2006;152:59–84. doi: 10.1016/S0079-6123(05)52005-3. [DOI] [PubMed] [Google Scholar]
- 8.Lee TH, Jang MH, Shin MC, Lim BV, Kim YP, Kim H, et al. Dependence of rat hippocampal c-Fos expression on intensity and duration of exercise. Life Sci. 2003;72:1421–1436. doi: 10.1016/s0024-3205(02)02406-2. [DOI] [PubMed] [Google Scholar]
- 9.Dinis P, Charrua A, Avelino A, Cruz F. Intravesical resiniferatoxin decreases spinal c-fos expression and increases bladder volume to reflex micturition in rats with chronic inflamed urinary bladders. BJU Int. 2004;94:153–157. doi: 10.1111/j.1464-4096.2004.04855.x. [DOI] [PubMed] [Google Scholar]
- 10.Kavia RB, Dasgupta R, Fowler CJ. Functional imaging and the central control of the bladder. J Comp Neurol. 2005;493:27–32. doi: 10.1002/cne.20753. [DOI] [PubMed] [Google Scholar]
- 11.Ho MH, Bhatia NN, Khorram O. Physiologic role of nitric oxide and nitric oxide synthase in female lower urinary tract. Curr Opin Obstet Gynecol. 2004;16:423–429. doi: 10.1097/00001703-200410000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Pandita RK, Persson K, Andersson KE. Capsaicin-induced bladder overactivity and nociceptive behaviour in conscious rats: involvement of spinal nitric oxide. J Auton Nerv Syst. 1997;67:184–191. doi: 10.1016/s0165-1838(97)00116-1. [DOI] [PubMed] [Google Scholar]
- 13.Mumtaz FH, Khan MA, Thompson CS, Morgan RJ, Mikhailidis DP. Nitric oxide in the lower urinary tract: physiological and pathological implications. BJU Int. 2000;85:567–578. doi: 10.1046/j.1464-410x.2000.00459.x. [DOI] [PubMed] [Google Scholar]
- 14.Mamas MA, Reynard JM, Brading AF. Nitric oxide and the lower urinary tract: current concepts, future prospects. Urology. 2003;61:1079–1085. doi: 10.1016/s0090-4295(03)00131-6. [DOI] [PubMed] [Google Scholar]
- 15.Barendrecht MM, Abrams P, Schumacher H, de la Rosette JJ, Michel MC. Do alpha1-adrenoceptor antagonists improve lower urinary tract symptoms by reducing bladder outlet resistance? Neurourol Urodyn. 2008;27:226–230. doi: 10.1002/nau.20481. [DOI] [PubMed] [Google Scholar]
- 16.de Groat WC, Yoshiyama M, Ramage AG, Yamamoto T, Somogyi GT. Modulation of voiding and storage reflexes by activation of alpha1-adrenoceptors. Eur Urol. 1999;36(Suppl 1):68–73. doi: 10.1159/000052324. [DOI] [PubMed] [Google Scholar]
- 17.Athanasopoulos A, Perimenis P. Efficacy of the combination of an alpha1-blocker with an anticholinergic agent in the treatment of lower urinary tract symptoms associated with bladder outlet obstruction. Expert Opin Pharmacother. 2005;6:2429–2433. doi: 10.1517/14656566.6.14.2429. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan SA, Roehrborn CG, Chancellor M, Carlsson M, Bavendam T, Guan Z. Extended-release tolterodine with or without tamsulosin in men with lower urinary tract symptoms and overactive bladder: effects on urinary symptoms assessed by the International Prostate Symptom Score. BJU Int. 2008;102:1133–1139. doi: 10.1111/j.1464-410X.2008.07761.x. [DOI] [PubMed] [Google Scholar]
- 19.Chapple CR. A comparison of varying alpha-blockers and other pharmacotherapy options for lower urinary tract symptoms. Rev Urol. 2005;7(Suppl 4):S22–S30. [PMC free article] [PubMed] [Google Scholar]
- 20.LaBerge J, Malley SE, Zvarova K, Vizzard MA. Expression of corticotropin-releasing factor and CRF receptors in micturition pathways after cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol. 2006;291:R692–R703. doi: 10.1152/ajpregu.00086.2006. [DOI] [PubMed] [Google Scholar]
- 21.Fowler CJ. Integrated control of lower urinary tract: clinical perspective. Br J Pharmacol. 2006;147(Suppl 2):S14–S24. doi: 10.1038/sj.bjp.0706629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blok BF. Brain control of the lower urinary tract. Scand J Urol Nephrol Suppl. 2002;(210):11–15. doi: 10.1080/003655902320765908. [DOI] [PubMed] [Google Scholar]
- 23.Bon K, Lantéri-Minet M, de Pommery J, Michiels JF, Menétrey D. Cyclophosphamide cystitis as a model of visceral pain in rats. A survey of hindbrain structures involved in visceroception and nociception using the expression of c-Fos and Krox-24 proteins. Exp Brain Res. 1996;108:404–416. doi: 10.1007/BF00227263. [DOI] [PubMed] [Google Scholar]
- 24.Keay KA, Clement CI, Owler B, Depaulis A, Bandler R. Convergence of deep somatic and visceral nociceptive information onto a discrete ventrolateral midbrain periaqueductal gray region. Neuroscience. 1994;61:727–732. doi: 10.1016/0306-4522(94)90395-6. [DOI] [PubMed] [Google Scholar]
- 25.Chung IM, Kim YS, Sung YH, Kim SE, Ko IG, Shin MS, et al. Effects of acupuncture on abdominal leak point pressure and c-Fos expression in the brain of rats with stress urinary incontinence. Neurosci Lett. 2008;439:18–23. doi: 10.1016/j.neulet.2008.04.100. [DOI] [PubMed] [Google Scholar]
- 26.Nazif O, Teichman JM, Gebhart GF. Neural upregulation in interstitial cystitis. Urology. 2007;69(4 Suppl):24–33. doi: 10.1016/j.urology.2006.08.1108. [DOI] [PubMed] [Google Scholar]
- 27.Kim HS, Kim JC, Choo MS. Effects of nitric oxide synthases on detrusor overactivity after removal of bladder outlet obstruction in rats. Urol Int. 2008;81:107–112. doi: 10.1159/000137650. [DOI] [PubMed] [Google Scholar]
- 28.Jang J, Park EY, Seo SI, Hwang TK, Kim JC. Effects of intravesical instillation of cyclooxygenase-2 inhibitor on the expression of inducible nitric oxide synthase and nerve growth factor in cyclophosphamide-induced overactive bladder. BJU Int. 2006;98:435–439. doi: 10.1111/j.1464-410X.2006.06207.x. [DOI] [PubMed] [Google Scholar]
- 29.Felsen D, Dardashti K, Ostad M, Lemer ML, Gross SS, Chen J, et al. Inducible nitric oxide synthase promotes pathophysiological consequences of experimental bladder outlet obstruction. J Urol. 2003;169:1569–1572. doi: 10.1097/01.ju.0000054885.51858.99. [DOI] [PubMed] [Google Scholar]
- 30.Pinggera GM, Mitterberger M, Pallwein L, Schuster A, Herwig R, Frauscher F, et al. alpha-Blockers improve chronic ischaemia of the lower urinary tract in patients with lower urinary tract symptoms. BJU Int. 2008;101:319–324. doi: 10.1111/j.1464-410X.2007.07339.x. [DOI] [PubMed] [Google Scholar]
- 31.Andersson KE. Overactive bladder: pharmacological aspects. Scand J Urol Nephrol Suppl. 2002;(210):72–81. doi: 10.1080/003655902320766006. [DOI] [PubMed] [Google Scholar]
- 32.Michel MC, de la Rosette JJ. Efficacy and safety of tamsulosin in the treatment of urological diseases. Expert Opin Pharmacother. 2004;5:151–160. doi: 10.1517/14656566.5.1.151. [DOI] [PubMed] [Google Scholar]
- 33.Athanasopoulos A, Gyftopoulos K, Giannitsas K, Fisfis J, Perimenis P, Barbalias G. Combination treatment with an alpha-blocker plus an anticholinergic for bladder outlet obstruction: a prospective, randomized, controlled study. J Urol. 2003;169:2253–2256. doi: 10.1097/01.ju.0000067541.73285.eb. [DOI] [PubMed] [Google Scholar]
- 34.Jeong MS, Lee JG. The role of spinal and peripheral alpha1- and alpha2-adrenoceptors on bladder activity induced by bladder distension in anaesthetized rat. BJU Int. 2000;85:925–931. doi: 10.1046/j.1464-410x.2000.00572.x. [DOI] [PubMed] [Google Scholar]