Abstract
Humans are consciously aware of some memories and can make verbal reports about these memories. Other memories cannot be brought to consciousness, even though they influence behavior. This conspicuous difference in access to memories is central in taxonomies of human memory systems but has been difficult to document in animal studies, suggesting that some forms of memory may be unique to humans. Here I show that rhesus macaque monkeys can report the presence or absence of memory. Although it is probably impossible to document subjective, conscious properties of memory in nonverbal animals, this result objectively demonstrates an important functional parallel with human conscious memory. Animals able to discern the presence and absence of memory should improve accuracy if allowed to decline memory tests when they have forgotten, and should decline tests most frequently when memory is attenuated experimentally. One of two monkeys examined unequivocally met these criteria under all test conditions, whereas the second monkey met them in all but one case. Probe tests were used to rule out “cueing” by a wide variety of environmental and behavioral stimuli, leaving detection of the absence of memory per se as the most likely mechanism underlying the monkeys' abilities to selectively decline memory tests when they had forgotten.
The ability to consciously access stored information is a conspicuous feature of human cognition. Such access is linked to many forms of learning, complex thinking, and planning for the future (1–3). As well as demonstrating knowledge through competence, humans evidence memory by talking about remembered information (4, 5). Because animals cannot provide such verbal reports, students of animal cognition are forced to depend entirely on the assessment of competence in various tests to characterize animal memory (6, 7). Animals clearly remember many things, but there is little evidence that they know that they remember them (8, 9), encouraging the view that “higher forms of human memory … cannot be meaningfully modeled by animals” (10). Although modeling putative subjective experiences associated with memory in animals is probably not possible, it is routine to empirically study the functional properties of animal memory. Observed functional similarities can then serve as the basis for drawing parallels between human and animal memory. An important functional property of human conscious memory, one that distinguishes it from other forms of memory (11, 12), is the ability to discern the presence and absence of such memories. For instance, humans can judge whether a telephone number is remembered without having to place a call to determine whether the intended recipient answers. Humans can distinguish between remembering and forgetting. The objective of this work is to determine whether rhesus monkeys manifest this important functional property of human conscious memory. Determination of whether any animals possess this form of self-knowledge will inform us about the evolution of the human mind, and is critical in evaluating the psychological validity of animal models of human memory.
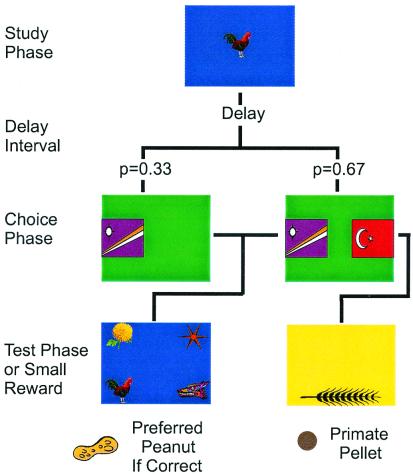
In the present study, recently described behavioral techniques (8, 13) were adapted to give monkeys the opportunity to report the presence or absence of memory for recently seen images (Fig. 1). In each trial, subjects briefly studied an image displayed on a video monitor. The image then disappeared for a delay interval during which the animals often forgot the studied image. At the end of two-thirds of the delay periods but before they saw the test, monkeys were allowed to choose between progressing to a memory test and declining the test. On the remaining trials, animals were not given the option of declining the memory test. Accurate performance on tests resulted in a favored reward, whereas failure resulted in no reward. Declining tests yielded a less preferred but guaranteed reward. Under these contingencies of reinforcement, an animal that knows whether it remembers the recently seen image should choose to take tests when it remembers and decline tests when it has forgotten. The present experiments differ from previous work (13) in that in the present experiments, monkeys were required to decide whether to take or decline the memory test before being presented with the test. Monkeys therefore were forced to base their judgments on the quality of self-generated memory retrieval, unsupported by repeated presentation of the studied image or direct experience with the difficulty of a given test. Previous studies using pigeons as subjects showed that this distinction is critical (8). Pigeons offered the option of declining tests concurrently with presentation of the test display behaved as if they knew when they remembered. However, when these same birds were required to judge memory before presentation of the memory test, they were unable to discriminate trials in which they remembered from those in which they had forgotten the sample.
Figure 1.
Method for assessing whether monkeys know when they remember. Each colored panel represents what monkeys saw on a touch-sensitive computer monitor at a given stage in a trial. At the start of each trial, monkeys studied a randomly selected image. A delay period followed over which monkeys often forgot the studied image. In two-thirds of trials, animals chose between taking a memory test (Right, left-hand stimulus) and declining the test (Right, right-hand stimulus). In one-third of trials, monkeys were forced to take the test (Left). Better accuracy on chosen than on forced tests indicates that monkeys know when they remember and decline tests when they have forgotten, if given the option.
General Methods
Two male rhesus monkeys (Macaca mulatta) that were 7.5 years old at the beginning of data collection were used. Subjects were fed a regulated diet of food and fruit when being tested. Animals were weighed weekly and allowed to gain weight gradually. All procedures and animal care were conducted according to National Institutes of Health guidelines. Training was accomplished by using a computer-controlled apparatus. Monkeys were positioned in front of a touch-sensitive video monitor inside a ventilated, sound-attenuating chamber. Food cups at the animals' right and left permitted delivery of peanuts and 190-mg primate pellet rewards, each accompanied by distinctive sounds. At the beginning of data collection, the animals had already been trained to perform memory tests with a restricted set of stimuli, and were familiar with the contingencies associated with the various trial stages. Monkeys were tested in a match to sample paradigm in which a recently seen image must be selected from among a set of distracter images. A novel set of four images was used each day. Each trial began when a randomly chosen, color clip-art image (200 × 160 pixels) appeared in the center of the monitor. Animals touched the image three times during the study phase of each trial. The screen then cleared and a delay period elapsed over which monkeys had to remember the studied image. In the choice phase, two distinctive stimuli appeared on the left and right halves of the monitor in two-thirds of the trials (see Fig. 1 Right). Touching the left-hand stimulus caused the screen to clear and a memory test to follow. In the test phase, the recently studied image and three distracter images were assigned randomly to the four corners of the computer screen. Selecting the recently studied image was rewarded with peanuts, whereas errors resulted in a 15-s timeout period. Touching the right-hand stimulus in the choice phase cleared the screen and resulted in presentation of the image of a wheat ear (Fig. 1 Bottom Right), which when touched was followed by a less preferred pellet reward. In the remaining third of the trials, monkeys were forced to take the memory test, because this option was offered only in the choice phase in these trials. Free- and forced-choice test trials were intermixed randomly within sessions so that the monkeys could not determine the trial type until the choice phase of that trial. Therefore, trial type could not affect the behavior of the monkeys during the study phase or during the delay period. Thirty seconds intervened between successive trials, during which the screen was black. Animals normally completed 96 trials in each daily session, 5 to 7 days a week. Six preference trials, which occurred at the beginning and end of each session, were used to ensure that animals indeed preferred the peanut reward to the pellet reward.
Experiment 1.
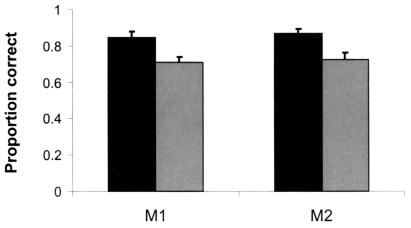
Monkeys M1 and M2 were tested in 10 sessions with fixed delay periods (M1, 34 s; M2, 38 s) selected to be long enough to ensure forgetting of the studied image in some trials. Both monkeys were more accurate in trials in which they freely chose memory tests than they were in forced-choice test trials where they were not given the option of declining the test (Fig. 2; paired t tests for each monkey: M1, t9 = 3.91, P < 0.01; M2, t9 = 4.51, P < 0.01). Accuracy on forced tests is a combination of accuracy on tests subjects would have declined, given the choice, and those they would freely have chosen to take. It is therefore not a direct measure of memory in trials in which the monkeys declined the memory test and underestimates the utility of declining tests. Because accuracy on freely chosen tests is known, as is the proportion of tests taken and declined in free-choice trials, the expected accuracy in trials in which the monkeys declined the memory test can be calculated. Using the proportions of tests monkeys declined when given the opportunity (M1, 0.51; M2, 0.36), and the accuracies shown in Fig. 2, expected accuracies on the trials monkeys declined are determined to be 58.1% and 46.8%, respectively. Thus, the accuracy of memory in trials in which monkeys declined the memory tests is substantially lower than it is in other trials. These results indicate that when given the opportunity, monkeys chose adaptively to decline memory tests when memory for the sample image was relatively poor.
Figure 2.
Accuracy on freely chosen and forced tests. Dark bars represent accuracy on tests the monkeys chose to take. Light bars represent performance on trials where the animals were not given the choice of declining tests. Scores for the two monkeys are the means of 10 daily sessions. Error bars are standard errors. Subjects would be correct 25% of the time if guessing.
Experiment 2.
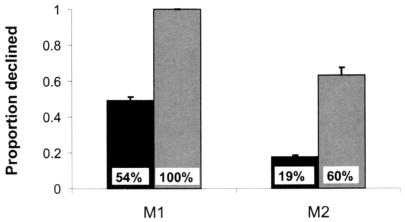
A critical concern in evaluating these results is that some factor, other than the absence of memory per se, might control the choice to decline memory tests. Events that might occur during the delay interval, such as noises, bouts of grooming, or changes in motivation might reliably result in forgetting. Such events could therefore cue animals to decline tests, giving a false impression that the animals attend to their own memory states. To rule out the possibility that monkeys' decisions to decline tests were controlled by such cues, monkeys were presented with 10 randomly distributed probe trials in each of six 96-trial test sessions. The unpredictable probe trials were identical to normal trials in every way except that no image was presented for study. In this way, the absence of memory was controlled experimentally, allowing a priori prediction of which tests animals should choose to decline. After an intertrial interval, and a delay period equivalent to that in normal trials, animals were given the choice of declining or taking a memory test, just as would occur in normal trials. If the absence of memory causes the monkeys to decline tests, they should decline tests in these no-sample probe trials, treating them like trials in which they have forgotten the studied image. If, however, the decision to decline tests is controlled by some environmental or behavioral event, subjects should decline normal and probe trials with equal probability, because such events are distributed evenly among the randomly intermixed normal trials and the no-sample probe trials. In the six test sessions with no-sample probe trials, both monkeys were much more likely to decline tests if no image was presented for study than they were to decline tests in normal trials (Fig. 3; M1, t5 = 24.34, P < 0.01; M2, t5 = 10.19, P < 0.01). These results, from the experimental manipulation of memory, provide compelling support for the hypothesis that the choice to decline tests is based on the absence of memory per se.
Figure 3.
Probability of declining tests on normal trials and on probe trials lacking an image to study. Dark bars show the proportion of normal trials in which monkeys declined tests; the light bars represent this proportion in probe trials. Error bars are standard errors. Inset in each bar is the percent of each test type declined only in the first session of testing. These results indicate that it is the absence of a memory that causes the monkeys to decline tests. If some factor other than the absence of memory per se, such as distracting noises, variation in motivation, or fatigue, controlled the decision to decline tests, normal and probe trials would be affected equally.
Monkeys might have learned gradually to base the decision to
decline tests on some distinguishing feature of probe trials, rather
than on the absence of a memory per se. The first session of
probe trials therefore was analyzed separately. Both monkeys declined
probe tests from the first session of testing (Fig. 3 inset
percentages; M1, χ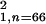 = 7.66,
P < 0.01; M2,
χ
= 7.66,
P < 0.01; M2,
χ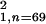 = 7.88, P
< 0.01), indicating that learning did not take place after the onset
of probe trials. Additionally, M1 declined every probe trial presented,
and could never have learned the negative consequences of choosing a
memory test in such trials. The high probability with which monkeys
declined the no-sample probe trials therefore reflects spontaneous
generalization to these probes as equivalent with trials in which
monkeys forgot the sample.
= 7.88, P
< 0.01), indicating that learning did not take place after the onset
of probe trials. Additionally, M1 declined every probe trial presented,
and could never have learned the negative consequences of choosing a
memory test in such trials. The high probability with which monkeys
declined the no-sample probe trials therefore reflects spontaneous
generalization to these probes as equivalent with trials in which
monkeys forgot the sample.
Experiment 3.
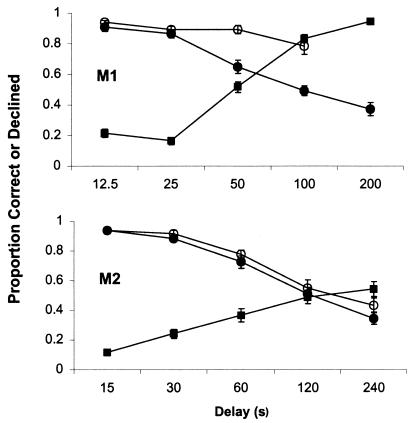
The delay over which monkeys had to remember the studied images was
manipulated in this final experiment. Because memory decays over time,
monkeys should decline tests infrequently shortly after studying an
image, and more often after long memory delays. As expected, accuracy
on forced tests was high for both monkeys at short delays but declined
as the delay interval increased (Fig. 4
filled circles; M1, F4,116 = 45.66,
P < 0.01; M2, F4,116
= 44.72, P < 0.01). Consistent with the decrease in
accuracy at longer delays, both monkeys declined tests more often after
long delays than after short delays (M1,
F4,116 = 196.02, P <
0.01; M2, F4,116 = 30.76,
P < 0.01). Again, to evaluate whether this pattern
emerged gradually or immediately, the first 100 free-choice trials with
variable delays were analyzed separately, comparing the probability of
declining tests in trials with delays less than the mean value to that
in trials with delays greater than the mean. M1 declined 85% of these
long-delay trials, compared with 43% of short-delay trials
(χ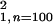 = 17.34, P <
0.01), whereas M2 declined 32% and 16% of these trials, respectively
(χ
= 17.34, P <
0.01), whereas M2 declined 32% and 16% of these trials, respectively
(χ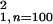 = 3.72;
P = .05).
= 3.72;
P = .05).
Figure 4.
Accuracy and the probability of declining tests after variable delay intervals. Filled squares depict the probability of declining tests. Open circles represent accuracy on freely chosen tests and filled circles represent accuracy on forced tests. Error bars are standard errors.
By selectively declining tests when they had forgotten the studied image, both monkeys were significantly more accurate overall on chosen tests than they were on forced tests (M1, 88% vs. 67%, F1,29 = 152.62, P < 0.01; M2, 78% vs. 69%, F1,29 = 21.20, P < 0.01). But in addition to improving overall accuracy, monkeys able to detect the absence of memory with high sensitivity should also be able to improve memory performance at each delay at which substantial forgetting occurs. No difference between chosen- and forced-test accuracy is expected at delays at which animals rarely forget, and accuracy is high, because there is little room for improvement. However, as the delay intervals increase and the forced-choice accuracies decline, a difference between accuracy in chosen and forced tests should emerge. M1 clearly showed this pattern (Fig. 4 Upper; F1,23 = 53.66, P < 0.01; only the first four delays were analyzed, because at the longest delay M1 declined nearly 100% of tests and did not choose any of these trials in over half of test sessions, thereby precluding an accurate estimate of accuracy). M2 was also more accurate on freely chosen tests at longer delays but this difference was not statistically significant (F1,25 = 3.17, P < 0.09).
Discussion
The ability of these monkeys to appropriately decline memory tests when they were unlikely to choose the correct image at test indicates that they know when they remember, a capacity associated with conscious cognition in humans. Like humans, monkeys can assess at least some of their own knowledge states. In striking contrast, pigeons tested under similar conditions appear to lack this capacity, suggesting that it may not be distributed evenly among species (8). A significant feature of the procedure used here is that monkeys were required to choose whether to take memory tests before viewing the test display. The decision to decline a memory test must therefore depend on the ability to assess the presence or absence of memory itself, and cannot be based on difficulty experienced in completing the test.
The monkeys treated no-sample probe trials in Experiment 2 like trials in which they had forgotten the sample image. This result demonstrates that the choice to decline tests is not controlled by environmental or behavioral events that might distract animals or disrupt memory retention. Although these results rule out a variety of “trivial” accounts of the behavior, they do not specify the mental processes underlying the assessment of memory. Future work might discriminate among several possible mechanisms. One account requires a mental representation corresponding to memory strength. This representation might encode nothing about the study episode or the identity of the material being remembered but rather operates as a “flag” for the presence or absence of a memory. The monkey chooses to take a memory test when this flag is active. Because the flag reliably indicates the presence of a memory, the animal is able to accurately select the recently studied image when tested. This “simple” mechanism produces a pattern of overt behavior difficult to distinguish from what would be observed when humans attempt consciously to retrieve a memory and report the results in a nonverbal manner. Other possible mechanisms that might produce the patterns of behavior observed in the present experiments include decision processes based on recall of the specific study episodes or evaluation of the quality of a mental image of the recently studied material. By these accounts, monkeys choose to take the memory test when they can specifically remember studying the image in that trial or when they can produce a detailed mental image of the studied item.
Progress will be made in discriminating among the proposed mechanisms by which monkeys might discriminate between remembering and forgetting only when differential functional outcomes are specified clearly for each mechanism. The functions of various memory mechanisms, but not the experiences associated with remembering, can be determined experimentally in animals. Functional descriptions of memory systems can be applied equally well to human and nonhuman animals. In contrast, taxonomies of memory based on subjective experience, and various conscious states, create a rift between the study of human and nonhuman memory (e.g., ref. 14). Whether or not memory in monkeys is associated with subjective conscious states like those experienced by humans, this study documents in monkeys one important objective functional feature of human conscious cognition: the ability to make adaptive decisions about future behavior contingent on the current availability of knowledge.
Acknowledgments
I thank Mortimer Mishkin, Elisabeth Murray, Sara Shettleworth, and Cindy Buckmaster for helpful discussions and comments on a draft, and Endel Tulving for discussions and incitement. This work was supported by the National Institute of Mental Health Intramural Research Program.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 4833.
References
- 1.Clark R E, Squire L R. Science. 1998;280:77–81. doi: 10.1126/science.280.5360.77. [DOI] [PubMed] [Google Scholar]
- 2.Povinelli D J, Preuss T M. Trends Neurosci. 1995;18:418–424. doi: 10.1016/0166-2236(95)93939-u. [DOI] [PubMed] [Google Scholar]
- 3.Donald M. Neuropsychologia. 1985;33:1087–1102. doi: 10.1016/0028-3932(95)00050-d. [DOI] [PubMed] [Google Scholar]
- 4.Zola-Morgan S, Squire L R. Ann NY Acad Sci. 1990;608:434–456. doi: 10.1111/j.1749-6632.1990.tb48905.x. [DOI] [PubMed] [Google Scholar]
- 5.Rugg M D. Neuropsychologia. 1995;33:1131–1141. doi: 10.1016/0028-3932(95)00053-6. [DOI] [PubMed] [Google Scholar]
- 6.Ridley R M, Baker H F. Brain Res Rev. 1991;16:15–37. doi: 10.1016/0165-0173(91)90018-4. [DOI] [PubMed] [Google Scholar]
- 7.Shettleworth S J. Cognition, Evolution, and Behavior. New York : Oxford Univ. Press; 1998. pp. 6–9. [Google Scholar]
- 8.Inman A, Shettleworth S J. J Exp Psychol Anim Behav. 1999;25:389–395. [Google Scholar]
- 9.Cheney D L, Seyfarth R M. How Monkeys See the World. Chicago: Univ. of Chicago Press; 1990. pp. 305–308. [Google Scholar]
- 10.Tulving E, Markowitsch H J. Behav Brain Sci. 1994;17:498–499. [Google Scholar]
- 11.Shacter D L. Am Psychol. 1992;47:559–569. doi: 10.1037//0003-066x.47.4.559. [DOI] [PubMed] [Google Scholar]
- 12.Tulving E, Shacter D L. Science. 1990;247:301–306. doi: 10.1126/science.2296719. [DOI] [PubMed] [Google Scholar]
- 13.Smith J D, Shields W E, Washburn D A, Allendoerfer K R. J Exp Psychol Gen. 1998;127:227–250. doi: 10.1037//0096-3445.127.3.227. [DOI] [PubMed] [Google Scholar]
- 14.Griffiths D, Dickinson A, Clayton N S. Trends Cogn Sci. 1999;3:74–80. doi: 10.1016/s1364-6613(98)01272-8. [DOI] [PubMed] [Google Scholar]