Abstract
Study Objectives:
To examine whether sleep duration modifies genetic and environmental influences on body mass index (BMI).
Design:
Genotype-environment interaction twin study.
Setting:
University of Washington Twin Registry.
Patients or Participants:
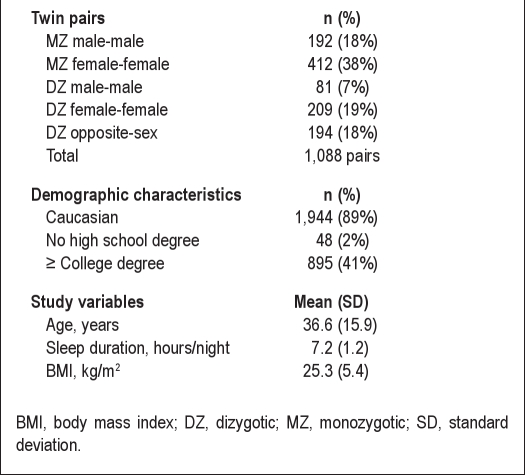
A population-based sample of US twins (1,088 pairs, 604 monozygotic, 484 dizygotic; 66% female; mean age = 36.6 yr, standard deviation (SD) = 15.9 yr).
Interventions:
N/A.
Measurements and Results:
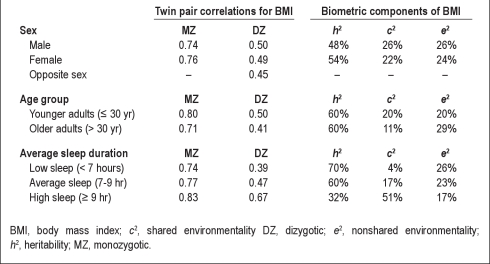
Participants self-reported information on height, weight, and sleep. Mean BMI was calculated as 25.3 kg/m2 (SD = 5.4) and mean habitual sleep duration was 7.2 hr/night (SD = 1.2). Data were analyzed using biometric genetic interaction models. Overall the heritability of sleep duration was 34%. Longer sleep duration was associated with decreased BMI (P < 0.05). The heritability of BMI when sleep duration was < 7 hr (h2 = 70%) was more than twice as large as the heritability of BMI when sleep duration was ≥ 9 hr (h2 = 32%); this interaction was significant (P < 0.05).
Conclusions:
Shorter sleep duration is associated with increased BMI and increased genetic influences on BMI, suggesting that shorter sleep duration increases expression of genetic risks for high body weight. At the same time, longer sleep duration may suppress genetic influences on body weight. Future research aiming to identify specific genotypes for BMI may benefit by considering the moderating role of sleep duration.
Citation:
Watson NF; Harden KP; Buchwald D; Vitiello MV; Pack AI; Weigle DS; Goldberg J. Sleep duration and body mass index in twins: a gene-environment interaction. SLEEP 2012;35(5):597-603.
Keywords: Sleep duration, twins, monozygotic, dizygotic, body mass index
INTRODUCTION
The optimal amount of sleep needed to maintain physiologic homeostasis is individualized and influenced by both genetic and environmental factors. The physiologically normal “sleep fraction” in humans is between 29% and 33% of the sleep-wake cycle, or 7 to 7.9 hr under conditions of environmental and temporal isolation.1,2 The heritability of sleep duration is between 31% and 55%, suggesting a substantial amount of sleep need is genetically determined.3–5 However, modern society with its ubiquitous technology often can cause misalignment between sleep need and sleep actualization.6 This frequently has adverse consequences for cognitive function and metabolic, cardiovascular, and immunologic health.7–13 Indeed, over the past century habitual sleep duration has dropped 1.5 hr per night and since 2001 the percentage of US adults getting at least 8 hr of sleep per night on weeknights has fallen from 38% to 27%.14,15
As sleep duration has declined, obesity rates, defined as a body mass index (BMI) ≥ 30 kg/m2, have increased. In 2009, more than one-fourth of the US population was obese, and every state had an obesity prevalence > 10%, compared with just 8 states in 1985.16,17 If current trends continue, more than 50% of adults in the United States will be considered clinically obese by 2030.18 Evidence is mounting that chronically reduced sleep times are associated with obesity.19–24 Experimental human studies show sleep curtailment influences the neuroendocrine control of appetite;25 population-based research shows a U-shaped nonlinear relationship between nightly sleep duration and BMI.26,27 Compared with those sleeping 7 to 8 hr per night, individuals sleeping ≤ 6 hr are at greater risk of being obese.27 Prospective family and cohort studies have found short sleep duration is associated with the development of obesity.28–30
Twins, if reared together, are identical in age and typically well matched for shared family background and numerous childhood and adolescent exposures. As such, twin comparisons can be used to control for third-variable confounders that typically differ among unrelated individuals. Previous work from our group in a subset of this twin sample has shown that monozygotic (MZ) twin differences in habitual sleep duration were associated with MZ-twin differences in BMI, an association that controls for the potential confounding influence of familial factors such as genetics and shared environment (e.g., in utero exposures, early life diet, and living conditions).5 This approach is particularly informative because many subjective and objective aspects of sleep are genetically influenced, and it was previously unknown whether associations between sleep duration and BMI were due to common genetic vulnerabilities influencing both phenotypes.4,31–37 Thus, our previous study established an environmentally mediated main effect of sleep duration on BMI. The current study extends this line of research by testing whether sleep duration additionally interacts with genetic influences on BMI; a gene x environment interaction effect (GxE). Analytically, we used a biometrical genetic model to assess this potential interaction effect. If sleep duration modifies the heritability of BMI, this suggests that expression of genetic risks for body weight is influenced by individually chosen sleeping behavior.
METHODS
Participants
The University of Washington Twin Registry is a community-based sample of twins constructed using data from the Washington State Department of Licensing. The minimum age for participation is 18 yr. As of July 2011, the Registry consisted of nearly 4,500 pairs. Zygosity is determined using previously validated self-report methods that are correct at least 95% of the time.38,39 Every twin enrolled in the Registry completes a recruitment survey. Since 2008, this survey has included questions about sleep duration and body habitus. In 2006 and 2008, a health survey was mailed to more than 4,000 enrolled twins that included the same sleep duration and body habitus questions. The data collection procedures were approved by the University of Washington Institutional Review Board and the State of Washington Attorney General's office. All twins were raised together.
The study sample includes 2,176 individuals from 1,088 complete twin pairs (604 MZ, 484 dizygotic [DZ]), expanding on the sample from our previous study.5 Sample characteristics are summarized in Table 1. Overall, the sample was young (mean 36.6 yr; standard deviation (SD) = 15.9), well educated (41% with a college degree or higher), predominantly Caucasian (89%), and female (66%). The most common twin relationship was female MZ pairs (38%). The sample mean sleep duration was 7.2 hr (SD = 1.2).
Table 1.
Sample characteristics
Measures and Covariates
Sleep Duration
Habitual sleep duration was obtained from responses to the question, “On average, how long do you sleep per night?” reported in hr and min. We categorized sleep duration into 3 groups. Normal sleep duration was considered 7-8.9 hr because this range encompasses the physiologically normal sleep fraction in humans1,2 and the sleep duration considered normal in previous studies.27,40,41
Body mass index
Self-reported height and weight were obtained from the following questions: “How tall are you without your shoes,” and “How much do you weigh without clothes or shoes?” With these data we calculated BMI as kg/m2. BMI was treated as a continuous variable for analytic purposes.42
Sociodemographics
Age, sex, and race were self-reported. Race was dichotomized into white and nonwhite (American Indian, Alaska Native, Native Hawaiian, Pacific Islander, Asian, Black or African American, or other) categories. Education was ascertained by the question, “What is the highest level of school you have completed?” A total of 7 responses were possible, ranging from “eighth grade or less” to “graduate or professional degree.” The midpoint was “some college, but no degree or certificate.”
Statistical Analyses
Initial analysis
We calculated the mean sleep duration in hr in each twin pair and divided the sample into 3 sleep duration groups: (1) “Short Sleep”, in which the mean sleep duration for the pair was < 7.0 hr/night (n = 317 pairs; 70.0% female; mean sleep = 6.06 hr [SD = 0.62]; mean age = 39.1 yr [SD = 15.2]); (2) “Normal Sleep”, in which the mean sleep duration for the pair was 7.0 to 8.9 hr/night (n = 724 pairs; 60.4% female; mean sleep = 7.53 hr [SD = 0.49]; mean age = 35.7 yr [SD = 16.1]), and (3) “Long Sleep”, in which the mean sleep duration for the pair was ' 9.0 hr/night (n = 47 pairs; 73.4% female; mean sleep = 9.31 hr [SD = 0.42]; mean age = 33.3 years [SD = 16.7]).
We then calculated the twin correlation for BMI, separately by zygosity and sleep group. The twin correlations were used to calculate 3 different quantities: (1) heritability (h2), the proportion of variance in the phenotype due to additive genes [h2 = 2*(rMZ – rDZ)]; (2) shared environmentality (c2), the proportion of variance in the phenotype due to environmental influences shared by twins raised in the same family, regardless of zygosity [c2 = 2*(rDZ – rMZ)]; and (3) nonshared environmental (e2), the proportion of variance in the phenotype due to environmental influences that make twins different from each other, plus measurement error [e2 = 1 – rMZ]. Because the sleep duration groups showed significant differences in mean age (F [2, 1085] = 5.94, P = 0.003, R2 = 0.01) and in sex (χ2 [4] = 9.75, P = 0.04), we also calculated the twin pair correlations for BMI stratified by sex and by age group.
Importantly, the initial analysis was primarily descriptive in nature. Our next, more rigorous analysis used individual sleep duration as a continuous variable, thereby avoiding potential bias introduced by categorizing sleep duration, and tested for the possible confounding role of age and sex.
Structural equation model analysis
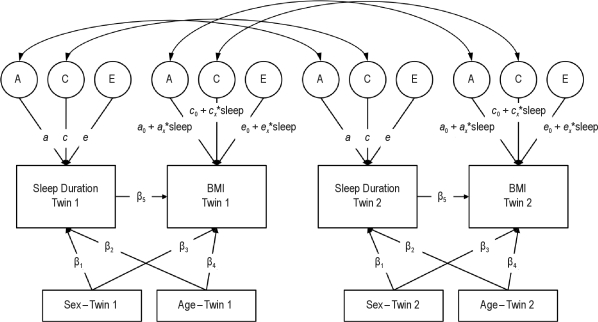
Next, we fit a more rigorous biometric genetic model for gene-environment interactions,43 using the software program Mplus (Muthén – Muthén, Los Angeles, CA). This model is shown in Figure 1. Total variance in each of the observed phenotypes (the boxes labeled “Sleep Duration” and “BMI”) is divided into 3 latent (i.e., unobserved) variables: additive genetic influences (the circle labeled A), shared environmental influences (i.e., environmental influences that make twins similar to each other, represented by the circle labeled C), and nonshared environmental influences (i.e., environmental influences that make twins different from each other, plus measurement error, represented by the circle labeled E). These latent variables are standardized (mean = 0, SD = 1), and the paths from the latent variables to the phenotypes are estimated (arrows labeled a, c, and e). Because no residual variance in the observed variables is estimated, the total amount of variation in each phenotype can be calculated as the sum of the squares of the a, c, and e paths. For sleep duration, the heritability (h2, i.e., the proportion of total variance due to genetic variation) of a given phenotype can thus be calculated as:
Figure 1.
Structural equation model of sleep duration and BMI in adult twins. A, additive genetic variance; C, shared environmental variance; E, nonshared environmental variance. A, C, and E components standardized (mean = 0, standard deviation = 1). Correlation between A components fixed at 1.0 in monozygotic twins and 0.5 in dizygotic twins. Correlation between C components fixed to 1.0 in all twins. Correlation between E components fixed to 0 in all twins.
For BMI, however, the paths from the A, C, and E variables are allowed to vary as a function of sleep duration. That is, rather than estimating a single value for the heritability of BMI, this model allows the heritability of BMI to vary according to the individual's sleep duration. In this case, the heritability of BMI at any value for sleep duration (x) can be calculated as:
The key values of interest here are the interaction parameters (ax, cx, and ex). Significant values for the interaction parameters indicate that the magnitude of genetic and environmental influences on BMI differ with sleep duration, with positive values indicating greater influence with greater sleep duration and negative values indicating less influence with greater sleep duration. All variables were standardized prior to the analysis; therefore, the values of a0, c0, and e0 represent the influence of genetic, shared environmental, and nonshared environmental influences, respectively, when sleep duration equals the sample mean.
The main effect of sleep duration on BMI was also estimated (the arrow labeled β5 in Figure 1). The main effect of age was estimated by the arrows labeled β2 and β4 and sex by the arrows labeled β1 and β3, respectively, for both twin 1 and twin 2 in Figure 1. In a series of simulation analyses, Purcell43 demonstrated that including the main effect of the moderator variable (in this case, sleep duration) on the outcome prevents estimates of gene-environment interaction from being biased by gene-environment correlation. In addition, the model controlled for participant age and sex.
RESULTS
Initial Analysis
The twin correlations for BMI in MZ and dizygotic (DZ) twins across twin pair average sleep duration is shown in the bottom section of Table 2. For twin pairs averaging < 7 hr of sleep per night, additive genetic influences accounted for 70% of the variance in BMI, whereas shared environmental factors accounted for just 4%. In contrast, for twin pairs averaging ≥ 9 hr of sleep per night, additive genetic factors accounted for just 32% and shared environmental influences accounted for 51%. This pattern of results constitutes preliminary support for our hypothesis that genetic influences on BMI are moderated by habitual sleep duration. In contrast, the twin pair correlations and resulting heritability coefficients were generally consistent for both sexes (shown in the top portion of Table 2) and across age groups (shown in the middle portion of Table 2). This suggests that the differences in heritability evident across sleep groups are not an artifact of gene-by-age or gene-by-sex interaction effects.
Table 2.
Twin pair correlations and calculated biometric components by sex, age group, and twin pair average sleep duration
Structural Equation Model
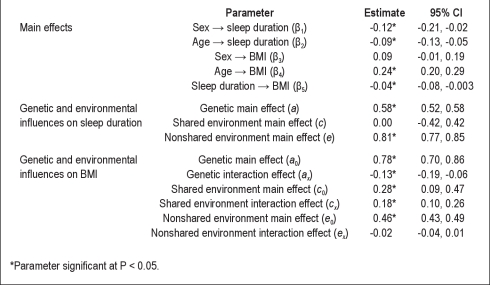
Table 3 shows the standardized parameter estimates from the biometric model. The top segment of the table shows the main effects of age, sex, and sleep duration on BMI. Males and older adults reported significantly shorter sleep duration, and older adults had significantly greater BMI. Also, as reported in our previous study using a subpopulation of this dataset,5 there was a modest but significant main effect of sleep duration on BMI, such that individuals who slept for longer periods had slightly lower BMIs.
Table 3.
Standardized parameter estimates for biometrical genetic interaction model examining the relationship between BMI and sleep duration
The middle segment of Table 3 shows the estimates for the genetic and environmental influences on sleep duration. The percentage of variation in sleep duration (controlling for age and sex) that can be accounted for by additive genetic, shared environmental, and nonshared environmental influences can be calculated by squaring the a, c, and e parameters, respectively. Of the total variance in sleep duration, 34% was due to additive genetic influences and the remaining 66% to nonshared environmental influences; shared environmental influences on sleep duration were negligible.
Finally, the bottom segment of Table 3 shows the estimates for the genetic and environmental influences on BMI, including the interaction effects that are the key parameters of interest in this study. We observed significant genetic (a0 = 0.78), shared environmental (c0 = 0.28), and nonshared environmental (e0 = 0.46) influences on BMI (all P < 0.05) after controlling for the main effects of sleep duration, age, and sex. As described previously, the study variables were standardized prior to structural equation modeling; thus these values represent the relative influence of genetic and shared environmental influence when sleep duration equals the sample mean (7.2 hr). In addition, a significant negative interaction (ax = −0.13; P < 0.05) between genes and sleep duration was noted, indicating that genetic influences on BMI decrease with increasing sleep duration. At the same time, there was a significant positive interaction (cx = 0.18; P < 0.05) between shared environmental influences and sleep duration, indicating that shared environmental influences on BMI increase with increasing sleep duration. Finally, the interaction between sleep duration and the nonshared environment was not significantly different than zero (ex = −0.02).
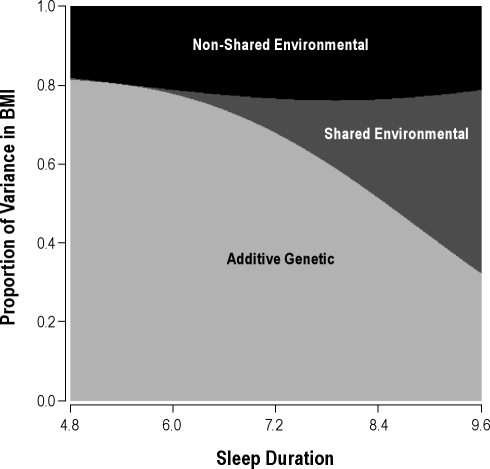
These interaction results are illustrated in Figure 2, which shows how the predicted proportions of variance in BMI due to additive genetic, shared environmental, and nonshared environmental influences change as a function of sleep duration. The range of the x-axis represents 2 SD below and above the sample mean for sleep duration. Consistent with the initial analysis, at short sleep duration, genetic influences on BMI were predominant, whereas at long sleep duration, shared environmental influences were predominant and genetic influences were suppressed.
Figure 2.
Sleep duration modifies the proportion of variance in body mass index (BMI) due to genetic, shared environmental, and nonshared environmental influences. Predicted from parameter estimates in Table 3. Sleep duration in hr, BMI in kg/m2.
DISCUSSION
We found a complex relationship whereby the genetic influences on BMI are moderated by habitual sleep duration. As sleep duration increased, genetic influences on BMI dissipated whereas shared environmental influences predominated and nonshared environmental contributions remained mostly static. This likely represents progressive phenotypic disparity in BMI among DZ twins compared with MZ twins as sleep duration decreased. Taken together, these findings show a robust gene-environment interaction between sleep duration and BMI.
Shared environment represents all environmental exposures that are not unique to an individual twin. Common examples include in utero exposures, birth history, diet, and childhood living conditions and location. We hypothesize that as sleep duration increases, the permissive environment for obesity-related gene expression is decreased, allowing behaviors learned earlier in life (such as meal timing and composition, lifestyle, and physical activity levels) to surface and drive body weight. Weight loss intervention studies corroborate this finding. In 1 study, sleep duration predicted success in a standardized weight loss program, with participants sleeping ≤ 6 or > 8 hr in a 24-hr period less likely to lose weight than those sleeping > 6 to ≤ 8 hr.44 Similarly, investigators controlled sleep duration and caloric intake over 2 separate 14-day periods and found a 55% reduction in the proportion of weight loss as fat in the sleep-deprived condition (5.5 hr/night) compared with the sleep satiated condition (8.5 hr/night).45 Although additional studies are needed to confirm these findings, preliminary results indicate that behavioral weight loss measures are most effective when genetic drivers of body weight are mitigated through sleep extension.
Contextually, this work builds on previous research by our group showing a direct environmental effect of sleep duration on BMI.5 This association was present after careful adjustment for familial factors (e.g., genetics and shared environment) and exhibited within twin pairs, even when restricting to MZ twins, suggesting the effect was solely due to nonshared environment. The gene-environment interaction in the current study expands this understanding by showing that sleep duration alters genetic contributions to BMI. This finding suggests causality, but this conclusion awaits carefully controlled experimental studies showing alterations in gene expression, epigenetic factors, and BMI phenotypic characteristics after careful tracking or manipulation of sleep duration over time.
BMI is highly heritable,46 runs in families,47 and epidemiologic studies indicate that sleep curtailment is a risk factor for obesity.24,26,28 Alterations in hormonal factors,25,26 glucose metabolism,48 and inflammation10 are implicated in this association, but the molecular pathways have not been elucidated. Beyond monogenic and syndromic forms of obesity, candidate gene association studies have identified more than 20 genes associated with obesity, including LEPR and PPARG, which are involved with satiety, fatty acid storage, energy use, and glucose metabolism.49,50 Genome-wide association studies have revealed 20 obesity-related single nucleotide polymorphisms either in or near 16 different genes such as FTO and MAF, which regulate energy intake and insulin gene transcription.50 These are but a few of the obesity- related genes and pathways potentially activated by sleep curtailment. Our work suggests latent genetic variability in susceptibility to obesity requires activation by sleep curtailment. Deducing exactly which pathways are involved will require epigenetic and transcriptomic assessments and use of a systems biology analytic approach.
Although the most parsimonious interpretation of our data is that sleep curtailment activates obesity-related genes, it may be that sleep extension is protective by suppressing expression of obesity genes. Contrary to this notion, published reports show that long sleep duration is associated with cardiovascular disease,12,51 insulin resistance,51,52 and mortality53 and may be a surrogate measure for poor health. We did not observe this in our sample, but our sample is much younger than those used in studies that established these adverse associations. Population-based studies demonstrate a significant U-shaped nonlinear relationship between nightly sleep duration and BMI, with higher body weights observed at both extremes of sleep duration.5,26,27 All told, an individual likely benefits from sleep extension until sleep need and sleep actualization are balanced, after which confounding factors related to chronic disease and sleep prolongation likely characterize the association. Assessment of obesity-related gene expression patterns over a range of sleep durations in the same individual over time, or between MZ twins, would help to confirm these notions. Previous twin studies by our group ruled out genetic pleiotropy as a contributing factor to the relationship between sleep duration and BMI, with bivariate analysis showing no shared genetics between these phenotypes.5
The use of twins to study BMI and sleep duration was advantageous for several reasons. Sleep duration is a heritable trait3–5,31 and sleep duration studies in unrelated individuals are confounded by the population-based variability in sleep need. Some individuals are more resistant to the effects of sleep deprivation than others. MZ twins theoretically have the same genetic sleep need, and thus the differential effect of sleep curtailment should be observed within twin pairs even if both twins are relatively sleep deprived or sleep satiated from a population standpoint.
Several issues about our study warrant discussion. Our twins were predominantly younger adult Caucasian women, and therefore our results should be applied to the general population with caution. However, our sample was derived from the community and not from a clinical population seeking healthcare. Self-reported sleep duration and BMI are commonly used in observational studies but can be problematic.24,27,28 Self-reported sleep duration approximates objective measures of sleep length,54,55 although recent studies suggest it may be biased by overestimation.56 BMI based on self-reported height and weight may be inaccurate, although validation studies indicate errors are unlikely to affect conclusions about associations between BMI and health variables.57–59 Future studies assessing gene-environment interactions between sleep duration and BMI should include objective measures of both.
In conclusion, we have shown, through sleep duration-stratified heritabilities and structural equation models, a negative association between sleep duration and genetic effects on obesity, with shorter sleep associated with greater genetic contribution to the proportion of variance in BMI. This work suggests that sleep curtailment provides a permissive environment for the expression of genes that promote obesity. The mechanism underlying this phenomenon is unknown but is likely to involve factors that influence gene expression, such as DNA and histone methylation, DNA and RNA transcription, and posttranslational protein modification. Future studies assessing long-term habitual sleep duration, as opposed to short-term, controlled, sleep deprivation paradigms will help illuminate the basis of the short sleep and obesity association while factoring in the potential influence of habituation to chronic sleep curtailment.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Watson serves on the Board of Directors of the American Academy of Sleep Medicine. Funds for the endowment of Allan I. Pack's professorship (John Miclot Professorship) are provided by the Phillips/Respironics Foundation. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the twins who participated in the University of Washington Twin Registry. We also thank Eric Strachan, Corinne Hunt, and Annemarie Succop for their work on the Registry. This work was supported by NIH grants NHLBI K23HL083350, NINR P30NR011400, and a University of Washington General Clinical Research Center Pilot and Feasibility Grant.
Footnotes
A commentary on this article appears in this issue on page 589.
REFERENCES
- 1.Weitzman ED, Czeisler CA, Zimmerman JC, Ronda JM. Timing of REM and stages 3 + 4 sleep during temporal isolation in man. Sleep. 1980;2:391–407. [PubMed] [Google Scholar]
- 2.Dijk DJ, Duffy JF, Czeisler CA. Age-related increase in awakenings: impaired consolidation of nonREM sleep at all circadian phases. Sleep. 2001;24:565–77. doi: 10.1093/sleep/24.5.565. [DOI] [PubMed] [Google Scholar]
- 3.Partinen M, Kaprio J, Koskenvuo M, Putkonen P, Langinvainio H. Genetic and environmental determination of human sleep. Sleep. 1983;6:179–85. doi: 10.1093/sleep/6.3.179. [DOI] [PubMed] [Google Scholar]
- 4.de Castro JM. The influence of heredity on self-reported sleep patterns in free-living humans. Physiol Behav. 2002;76:479–86. doi: 10.1016/s0031-9384(02)00699-6. [DOI] [PubMed] [Google Scholar]
- 5.Watson NF, Buchwald D, Vitiello MV, Noonan C, Goldberg J. A twin study of sleep duration and body mass index. J Clin Sleep Med. 2010;6:11–7. [PMC free article] [PubMed] [Google Scholar]
- 6.Basner M, Fomberstein KM, Razavi FM, et al. American time use survey: sleep time and its relationship to waking activities. Sleep. 2007;30:1085–95. doi: 10.1093/sleep/30.9.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayas NT, White DP, Al-Delaimy WK, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26:380–4. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 8.Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–9. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 9.Dawson D, Reid K. Fatigue, alcohol and performance impairment. Nature. 1997;388:235. doi: 10.1038/40775. [DOI] [PubMed] [Google Scholar]
- 10.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–62. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 11.De Jonghe S, Van Overmeire I, Poulton S, et al. Structure-activity relationship of short-chain sphingoid bases as inhibitors of sphingosine kinase. Bioorg Med Chem Lett. 1999;9:3175–80. doi: 10.1016/s0960-894x(99)00554-5. [DOI] [PubMed] [Google Scholar]
- 12.Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29:1009–14. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 13.Watson NF. Stroke and sleep specialists: an opportunity to intervene? J Clin Sleep Med. 6:138–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Webb W, Agnew H. Are we chronically sleep deprived? Bull Psychon Soc. 1975;6:47–8. [Google Scholar]
- 15.National Sleep Foundation. Washington, DC: National Sleep Foundation; 2002-2011. Sleep in America Poll. [Google Scholar]
- 16.Center for Disease Control and Prevention. Obesity Trends Among US Adults Between 1985 and 2009. http://www.cdc.gov/obesity/downloads/obesity_trends_2009.pdf.
- 17.Johnson KG, Johnson DC. Obstructive sleep apnea is a risk factor for stroke and atrial fibrillation. Chest. 2010;138:239. doi: 10.1378/chest.10-0513. author reply 239-40. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring) 2008;16:2323–30. doi: 10.1038/oby.2008.351. [DOI] [PubMed] [Google Scholar]
- 19.Patel SR, Redline S. Two epidemics: are we getting fatter as we sleep less? Sleep. 2004;27:602–3. [PubMed] [Google Scholar]
- 20.Vioque J, Torres A, Quiles J. Time spent watching television, sleep duration and obesity in adults living in Valencia, Spain. Int J Obes Relat Metab Disord. 2000;24:1683–8. doi: 10.1038/sj.ijo.0801434. [DOI] [PubMed] [Google Scholar]
- 21.Locard E, Mamelle N, Billette A, Miginiac M, Munoz F, Rey S. Risk factors of obesity in a five year old population. Parental versus environmental factors. Int J Obes Relat Metab Disord. 1992;16:721–9. [PubMed] [Google Scholar]
- 22.von Kries R, Toschke AM, Wurmser H, Sauerwald T, Koletzko B. Reduced risk for overweight and obesity in 5- and 6-y-old children by duration of sleep: a cross-sectional study. Int J Obes Relat Metab Disord. 2002;26:710–6. doi: 10.1038/sj.ijo.0801980. [DOI] [PubMed] [Google Scholar]
- 23.Sekine M, Yamagami T, Handa K, et al. A dose-response relationship between short sleeping hours and childhood obesity: results of the Toyama Birth Cohort Study. Child Care Health Dev. 2002;28:163–70. doi: 10.1046/j.1365-2214.2002.00260.x. [DOI] [PubMed] [Google Scholar]
- 24.Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 2005;28:1289–96. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- 25.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 26.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh M, Drake CL, Roehrs T, Hudgel DW, Roth T. The association between obesity and short sleep duration: a population-based study. J Clin Sleep Med. 2005;1:357–63. [PubMed] [Google Scholar]
- 28.Hasler G, Buysse DJ, Klaghofer R, et al. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep. 2004;27:661–6. doi: 10.1093/sleep/27.4.661. [DOI] [PubMed] [Google Scholar]
- 29.Chaput JP, Despres JP, Bouchard C, Tremblay A. The association between sleep duration and weight gain in adults: a 6-year prospective study from the Quebec Family Study. Sleep. 2008;31:517–23. doi: 10.1093/sleep/31.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between reduced sleep and weight gain in women. Am J Epidemiol. 2006;164:947–54. doi: 10.1093/aje/kwj280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heath AC, Kendler KS, Eaves LJ, Martin NG. Evidence for genetic influences on sleep disturbance and sleep pattern in twins. Sleep. 1990;13:318–35. doi: 10.1093/sleep/13.4.318. [DOI] [PubMed] [Google Scholar]
- 32.Hori A. Sleep characteristics in twins. Jap J Psychiatr Neurol. 1986;40:35–46. doi: 10.1111/j.1440-1819.1986.tb01610.x. [DOI] [PubMed] [Google Scholar]
- 33.Linkowski P. Genetic influences on EEG sleep and the human circadian clock: a twin study. Pharmacopsychiatry. 1994;27:7–10. doi: 10.1055/s-2007-1014266. [DOI] [PubMed] [Google Scholar]
- 34.Linkowski P. EEG sleep patterns in twins. J Sleep Res. 1999;8(Suppl 1):11–3. doi: 10.1046/j.1365-2869.1999.00002.x. [DOI] [PubMed] [Google Scholar]
- 35.Linkowski P, Kerkhofs M, Hauspie R, Mendlewicz J. Genetic determinants of EEG sleep: a study in twins living apart. Electroencephalogr Clin Neurophysiol. 1991;79:114–8. doi: 10.1016/0013-4694(91)90048-9. [DOI] [PubMed] [Google Scholar]
- 36.Linkowski P, Kerkhofs M, Hauspie R, Susanne C, Mendlewicz J. EEG sleep patterns in man: a twin study. Electroencephalogr Clin Neurophysiol. 1989;73:279–84. doi: 10.1016/0013-4694(89)90106-5. [DOI] [PubMed] [Google Scholar]
- 37.Franken P, Malafosse A, Tafti M. Genetic determinants of sleep regulation in inbred mice. Sleep. 1999;22:155–69. [PubMed] [Google Scholar]
- 38.Torgersen S. The determination of twin zygosity by means of a mailed questionnaire. Acta Genet Med Gemellol (Roma) 1979;28:225–36. doi: 10.1017/s0001566000009077. [DOI] [PubMed] [Google Scholar]
- 39.Eisen S, Neuman R, Goldberg J, Rice J, True W. Determining zygosity in the Vietnam Era Twin Registry: an approach using questionnaires. Clin Genet. 1989;35:423–32. doi: 10.1111/j.1399-0004.1989.tb02967.x. [DOI] [PubMed] [Google Scholar]
- 40.Steptoe A, Peacey V, Wardle J. Sleep duration and health in young adults. Arch Intern Med. 2006;166:1689–92. doi: 10.1001/archinte.166.16.1689. [DOI] [PubMed] [Google Scholar]
- 41.Cappuccio FP, Taggart FM, Kandala NB, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–26. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 43.Purcell S. Variance components models for gene-environment interaction in twin analysis. Twin Res. 2002;5:554–71. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- 44.Elder CR, Gullion CM, Funk KL, Debar LL, Lindberg NM, Stevens VJ. Impact of sleep, screen time, depression and stress on weight change in the intensive weight loss phase of the LIFE study. Int J Obes (Lond) doi: 10.1038/ijo.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nedeltcheva AV, Kilkus JM, Imperial J, Schoeller DA, Penev PD. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 153:435–41. doi: 10.1059/0003-4819-153-7-201010050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watson NF, Goldberg J, Arguelles L, Buchwald D. Genetic and environmental influences on insomnia, daytime sleepiness, and obesity in twins. Sleep. 2006;29:645–9. doi: 10.1093/sleep/29.5.645. [DOI] [PubMed] [Google Scholar]
- 47.Yang W, Kelly T, He J. Genetic epidemiology of obesity. Epidemiol Rev. 2007;29:49–61. doi: 10.1093/epirev/mxm004. [DOI] [PubMed] [Google Scholar]
- 48.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 49.Bell CG, Walley AJ, Froguel P. The genetics of human obesity. Nat Rev Genet. 2005;6:221–34. doi: 10.1038/nrg1556. [DOI] [PubMed] [Google Scholar]
- 50.Walley AJ, Asher JE, Froguel P. The genetic contribution to non-syndromic human obesity. Nat Rev Genet. 2009;10:431–42. doi: 10.1038/nrg2594. [DOI] [PubMed] [Google Scholar]
- 51.Nagai M, Hoshide S, Kario K. Sleep duration as a risk factor for cardiovascular disease-a review of the recent literature. Curr Cardiol Rev. 2010;6:54–61. doi: 10.2174/157340310790231635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep. 2007;30:1667–73. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. J Sleep Res. 2009;18:148–58. doi: 10.1111/j.1365-2869.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- 54.Lockley SW, Skene DJ, Arendt J. Comparison between subjective and actigraphic measurement of sleep and sleep rhythms. J Sleep Res. 1999;8:175–83. doi: 10.1046/j.1365-2869.1999.00155.x. [DOI] [PubMed] [Google Scholar]
- 55.Hauri PJ, Wisbey J. Wrist actigraphy in insomnia. Sleep. 1992;15:293–301. doi: 10.1093/sleep/15.4.293. [DOI] [PubMed] [Google Scholar]
- 56.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19:838–45. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stevens J, Keil JE, Waid LR, Gazes PC. Accuracy of current, 4-year, and 28-year self-reported body weight in an elderly population. Am J Epidemiol. 1990;132:1156–63. doi: 10.1093/oxfordjournals.aje.a115758. [DOI] [PubMed] [Google Scholar]
- 58.Stewart AL. The reliability and validity of self-reported weight and height. J Chronic Dis. 1982;35:295–309. doi: 10.1016/0021-9681(82)90085-6. [DOI] [PubMed] [Google Scholar]
- 59.Stunkard AJ, Albaum JM. The accuracy of self-reported weights. Am J Clin Nutr. 1981;34:1593–9. doi: 10.1093/ajcn/34.8.1593. [DOI] [PubMed] [Google Scholar]