Abstract
Study Objectives:
To address whether treatment of sleep apnea improves glucose tolerance.
Design:
Randomized, double-blind crossover study.
Setting:
Sleep clinic referrals.
Patients:
50 subjects with moderate to severe sleep apnea (AHI > 15) and impaired glucose tolerance.
Interventions:
Subjects were randomized to 8 weeks of CPAP or sham CPAP, followed by the alternate therapy after a one-month washout. After each treatment, subjects underwent 2-hour OGTT, polysomnography, actigraphy, and measurements of indices of glucose control.
Measurements and Results:
The primary outcome was normalization of the mean 2-h OGTT; a secondary outcome was improvement in the Insulin Sensitivity Index (ISI (0,120). Subjects were 42% men, mean age of 54 (10), BMI of 39 (8), and AHI of 44 (27). Baseline fasting glucose was 104 (12), and mean 2-h OGTT was 110 (57) mg/dL. Seven subjects normalized their mean 2-h OGTT after CPAP but not after sham CPAP, while 5 subjects normalized after sham CPAP but not after CPAP. Overall, there was no improvement in ISI (0,120) between CPAP and sham CPAP (3.6%; 95% CI: [-2.2%, 9.7%]; P = 0.22). However, in those subjects with baseline AHI ≥ 30 (n = 25), there was a 13.3% (95% CI: [5.2%, 22.1%]; P < 0.001) improvement in ISI (0,120) and a 28.7% (95%CI: [-46.5%, −10.9%], P = 0.002) reduction in the 2-h insulin level after CPAP compared to sham CPAP.
Conclusions:
This study did not show that IGT normalizes after CPAP in subjects with moderate sleep apnea and obesity. However, insulin sensitivity improved in those with AHI ≥ 30, suggesting beneficial metabolic effects of CPAP in severe sleep apnea.
Clinical Trials Information: ClinicalTrials.gov Identifier: NCT01385995.
Citation:
Weinstock TG; Wang X; Rueschman M; Ismail-Beigi F; Aylor J; Babineau DC; Mehra R; Redline S. A controlled trial of CPAP therapy on metabolic control in individuals with impaired glucose tolerance and sleep apnea. SLEEP 2012;35(5):617-625.
Keywords: Obstructive sleep apnea, diabetes mellitus
INTRODUCTION
Type 2 diabetes mellitus (T2DM) affects approximately 11% of U.S. adults.1 Its prevalence has tripled over the last 30 years,2 and if current trends continue, almost 20% of the adult population is projected to be afflicted with this chronic health problem over the next two decades.3 The rise in T2DM has been attributed to the growing prevalence of obesity and insulin resistance.4–6 Recent estimates indicate that diabetes contributes to $174 billion in annual direct and indirect health care costs and to almost 250,000 deaths per year,7 underscoring the need to identify novel targets for reducing diabetes incidence and morbidity.
The recent obesity epidemic also has likely contributed to an increase in prevalence of sleep disordered breathing (SDB), estimated to occur in approximately 17% of adults.8 SDB exposes affected individuals to recurrent episodes of hypoxemia, sleep fragmentation, and arousal. These adverse physiological exposures augment sympathetic nervous system activation and alter hypothalamic-pituitary-adrenal axis function, potentially reducing insulin sensitivity.9–15 Thus, SDB may directly contribute to the development of T2DM and exacerbate its clinical manifestations. Several longitudinal studies have provided evidence that snoring, a marker of SDB, increases the risk of incident T2DM.16,17
Numerous cross-sectional studies also have demonstrated associations between SDB with T2DM and/or impaired glucose tolerance.18–20 In contrast, there has been relatively limited research addressing the role of SDB treatment in improving insulin sensitivity, and no research on the role of the treatment of SDB in the prevention of T2DM.
To date, all studies that have examined the response of abnormal glucose metabolism to SDB treatment have been small and did not include rigorous control arms (reviewed in Punjabi et al.21). It is not surprising that the results are mixed, with some of these studies showing a relationship between treatment of SDB and improvement in insulin resistance22–29 and others showing no effect.30–35
We performed a randomized, crossover, controlled clinical trial comparing 8 weeks of continuous positive airway pressure (CPAP) to 8 weeks of sham CPAP in patients with moderate to severe SDB and impaired glucose tolerance (IGT). A rigorous assessment of metabolic responses to SDB treatment is of great clinical significance in this group because of their high risk for developing diabetes.36 We hypothesized that 8 weeks of CPAP would primarily normalize 2-hour glucose tolerance tests and secondarily lead to greater improvement in indices of insulin resistance when compared with sham CPAP.
METHODS
Study Sample
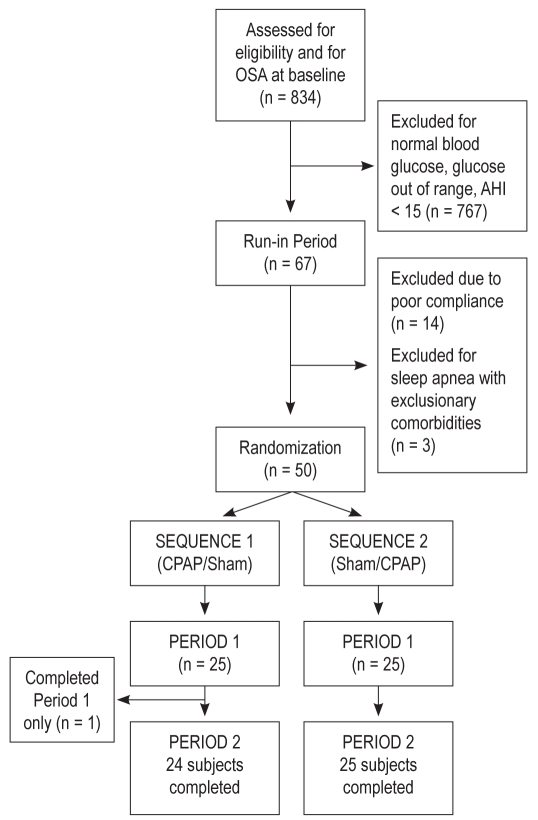
Subjects were eligible if they were 18 to 75 years old, had moderate to severe SDB defined by an apnea hypopnea index (AHI) ≥ 15, and had evidence of impaired glucose tolerance (IGT), defined by the mean 2-h oral glucose tolerance test (OGTT) glucose ≥ 140 mg/dL calculated from the two 2-h OGTTs performed within 3 days of each other during the screening period. Detailed exclusion criteria are provided in supplemental material following the article. Participant accrual occurred between April 2004 and October 2008. Potential subjects were identified through sleep laboratory referrals, rosters of individuals from prior research studies, and through local advertisements. Eight hundred thirty-four individuals were screened for sleep apnea and/or glucose metabolism. Of these, 67 were entered into the run-in period and 50 were randomized (Figure 1). Post-randomization, one subject was identified to have severe sleepiness after Period 1 and so was administratively withdrawn from the study prior to starting therapy in Period 2.
Figure 1.
Study schema showing key study design elements.
Study Design
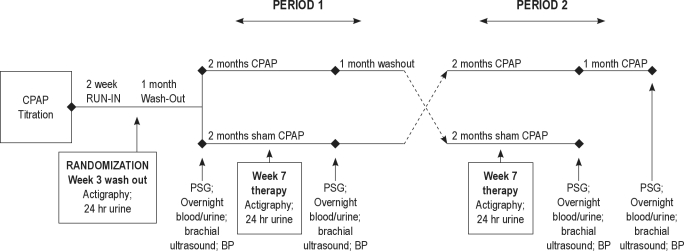
Details of the protocol are provided in the supplemental material. This was a randomized, double-blind, 2×2 crossover trial in patients with SDB and IGT. A schema of the study design is depicted in Figure 2. In brief, if subjects met initial study eligibility criteria, they underwent a polysomnography titration study. Each subject was then enrolled in a 2-week run-in period where they were asked to use CPAP at home for 2 weeks at the pressure settings identified by titration to resolve SDB events. Those subjects meeting minimal CPAP adherence during the 2-week run-in period received a one-month washout period and were then randomized to receive 8 weeks of CPAP or 8 weeks of sham CPAP (Period 1). Following Period 1, patients again received a one-month washout period and then crossed over to the alternate 8 weeks of therapy (Period 2). Throughout the study, each patient attended a series of research visits which included 4 overnight examinations: polysomnography with CPAP titration prior to the run-in period; polysomnography without CPAP therapy before the start of Period 1 (baseline visit); and polysomnography at the end of Period 1 and 2 (follow-up visits). Sequence order (Sequence 1: CPAP/sham CPAP; Sequence 2: sham CPAP/CPAP) was determined by a computerized program that generated random numbers.
Figure 2.
Consort diagram showing flow of participants from screening through study completion.
The sample size was chosen to have 80% power to detect differences in glucose tolerance status (impaired/normal) after treatment, assuming that between 20% and 35% of the CPAP group improved to normal, compared to 0% to 10% of the sham group. The study also had more than 80% power to detect effect sizes of 0.40 for differences in continuously measured outcomes.
Measurements
A detailed summary of the various measurements, including sleep measurements, biochemical outcomes, anthropometry, and blood pressure is included in the supplemental material.
Biochemical Outcomes
Following each polysomnography study and a 12-h fast, venipuncture was performed at a standardized time and again, 2 h after ingestion of 75 g of anhydrase glucose (the oral glucose tolerance test [OGTT]), measuring glucose and insulin. To identify potential early changes in glucose metabolism, fasting blood and 2-h OGTT were also obtained 5-7 days after the start of Period 1 and 2. Fasting blood and 2-h OGTT were also measured within 3 days of the baseline visit and at each of the 2 follow-up visits so that at each time point, duplicate measurements were available to enhance the reliability. All measurements were collected in the same research setting.
Interventions
Sleep hygiene, dietary counseling, and CPAP support
All subjects met with a sleep technician and research nutritionist prior to CPAP titration and received approximately 30 min of instruction on sleep hygiene and diet as described in the supplemental material.
Active CPAP:
CPAP titration was conducted prior to the run-in period. Each participant was provided a Philips-Respironics RemStar Pro CPAP machine, equipped with humidification and expiratory pressure relief as needed.
Sham CPAP:
Sham therapy was delivered using a customized Philips-Respironics device and masks with increased expiratory ports, configured to deliver a marginal pressure (0.4 ± 0.1 SD cm H2O). CPAP use was objectively monitored using data exported from the CPAP units, quantifying effective duration of treatment (time spent at prescribed pressure).
Statistical Analysis
Because all participants at screening had IGT, the primary outcome was identified a priori as “normalization of IGT” by the end of each Period. Normalization occurred if the patient had a 2-h OGTT glucose < 140 mg/dL by the end of each Period. To enhance the reliability of this outcome, determination of IGT at each time point (screening, post-CPAP follow-up visit, post-sham CPAP follow-up visit) was based on the mean value of two 2-h OGTT glucose measurements obtained within 3 days of each other. Secondary outcomes included continuously measured indices of glucose and insulin resistance from a venous sample obtained after fasting (“F”) and 2 h after the OGTT (“OGTT 2h”), with each value representing the average of 2 measurements. Similarly, insulin levels were obtained after fasting (“F”) and 2 h after the OGTT (“Insulin 2h”). The insulin sensitivity index was calculated using the Gutt Index,37,38 a clinically applicable method of calculating insulin sensitivity, derived from the fasting and 2-h postprandial plasma glucose and insulin level; the index has been validated against the euglycemic hyperinsulinemic clamp.37 Insulin resistance was also estimated using the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR).39 HOMA-B (β-cell function), a measurement used to assess pancreatic β-cell function (which we postulated would not vary with CPAP treatment) also was calculated.38 The ISI (0,120), HOMA-IR, and HOMA-B were log transformed to achieve approximate normality.
Preliminary Analyses
Indices of sleep, anthropometry, and CPAP adherence at each follow-up visit were summarized for each therapy in each of the 2 sequences. A paired 2-sample t-test was used to compare these indices between CPAP and sham CPAP. Indices of anthropometry included BMI and CT visceral abdominal tissue fat. CPAP adherence was expressed as average usage and percentage of days when therapy was used > 4 hours.
Primary Analyses
Based on an intent-to-treat approach, a generalized estimating equation (GEE) approach was used to estimate the effect of therapy (CPAP or sham CPAP) on the odds of normalization of IGT. Such a model provides an estimate of the odds ratio of normalizing the 2-h OGTT with CPAP compared with sham CPAP, while controlling for period and baseline 2-h OGTT glucose level. Models were also extended to include additional adjustment for baseline BMI, baseline AHI, gender, and race.
Secondary Analyses
Secondary outcomes included continuously measured indices of glucose and insulin resistance that were obtained after fasting and 2 h after the OGTT, as well as the ISI (0,120), HOMA-IR, and HOMA-B. Based on an intent-to-treat approach, a linear mixed effect model was used to estimate the association between therapy and each secondary outcome. Models estimated the adjusted geometric mean ratio of the outcome between CPAP and sham CPAP. Models were extended to include baseline BMI, AHI, gender, and race as additional covariates. Carryover effect was estimated for each secondary outcome by testing an interaction between therapy and period, although separate analyses were also conducted for each period.
Post Hoc Analyses
Post hoc stratified analyses were performed to assess whether differences in the effect of CPAP on outcomes were observed according to baseline SDB severity (defined by an AHI ≥ 30), gender, race, and baseline weight (dichotomized using the sample median BMI of 32). An interaction between treatment and each baseline factor was incorporated into each model and the effect of therapy on each outcome was estimated at each level of the baseline factor. Using only the data obtained when subjects received CPAP, we also estimated the association between the average hours of use of CPAP and changes in outcomes.
This study was reviewed and approved by the institutional review boards of University Hospitals Case Medical Center and Brigham and Women's Hospital. Written and informed consent was obtained from all subjects.
RESULTS
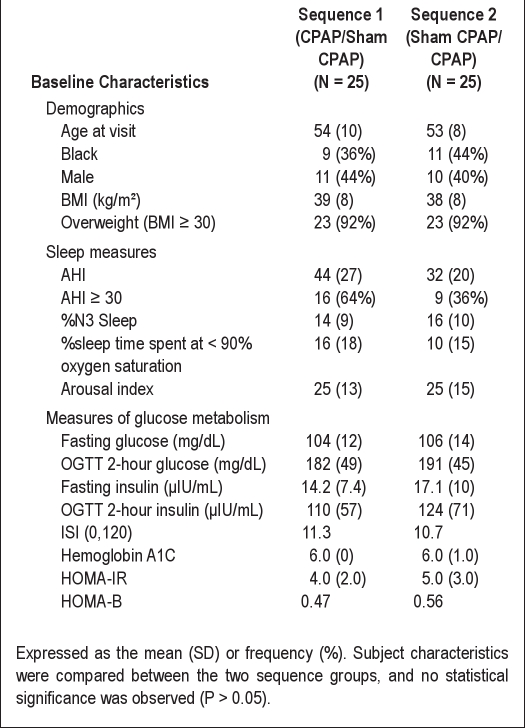
The baseline characteristics for all subjects by sequence are shown in Table 1. The sample consisted of 42% males and 50% with severe SDB (AHI ≥ 30); nearly all subjects were obese (BMI ≥ 30). Consistent with the presence of moderate-to-severe SDB, the sample had a relatively low percentage of Stage N3 sleep and spent a considerable amount of sleep time at low oxyhemoglobin saturation levels. Consistent with eligibility screening, the sample had, on average, a modest elevation of fasting plasma glucose (105 ± 13 mg/dL), abnormal 2-h OGTT (187 ± 47 mg/dL), and an average hemoglobin A1C level in the upper range of normal (6% ± 1%). The coefficients of variation for fasting and OGTT 2-h glucose measurements were 12% and 26%; for fasting and OGTT 2-h insulin measurements were each 56%. The Spearman correlation coefficient between baseline BMI and AHI was 0.26 (P = 0.08).There were no statistically significant differences in baseline characteristics of subjects within each of the 2 sequences.
Table 1.
Baseline participant characteristics by sequence
Primary Binary Outcome: Normalization of IGT
Among 47 participants with non-missing 2-h OGTT in both periods, 15% of subjects normalized their IGT after 8 weeks of CPAP but not after 8 weeks of sham CPAP. Conversely, 10.6% of subjects normalized their IGT after 8 weeks of sham CPAP but not after 8 weeks of CPAP. Consequently, there was no evidence of an association between therapy and normalization of IGT (odds ratio [OR]: 1.30; 95% CI: [0.52, 3.24]; P = 0.57), adjusting for period and baseline 2-h OGTT. Due to the higher adherence to CPAP in Period 1 (72% in Period 1 versus 52% in Period 2), the analysis was further restricted to Period 1 only. However, there remained no evidence of an association between therapy and normalization of IGT (OR: 1.82; 95% CI: [0.47, 7.11]; P = 0.39).
Secondary Continuous Outcomes of Glucose Homeostasis
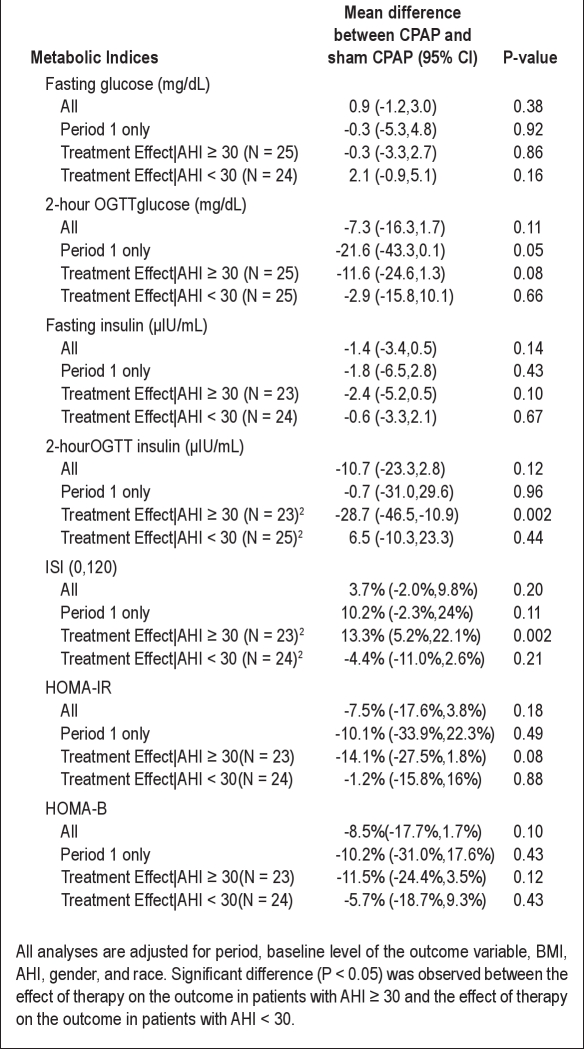
Table 2 shows the adjusted mean differences or geometric mean ratio (as well as 95%CI) of each secondary outcome using data from both periods, Period 1 only, as well as stratified by SDB severity. Using the data from both periods, there was no evidence of an effect of therapy on any of the outcomes. Restricting analyses to Period 1 only, the mean of 2-h OGTT at the end of therapy was 21.6 mg/dL lower after CPAP than sham CPAP (95% CI: [-43.3, 0.1]; P = 0.05), adjusting for baseline BMI, baseline AHI, gender, race, and baseline level of each outcome variable.
Table 2.
Mean change1 in metabolic indices between CPAP and sham CPAP for the entire sample, Period 1 only, and stratified by sleep apnea severity
Post Hoc Analyses
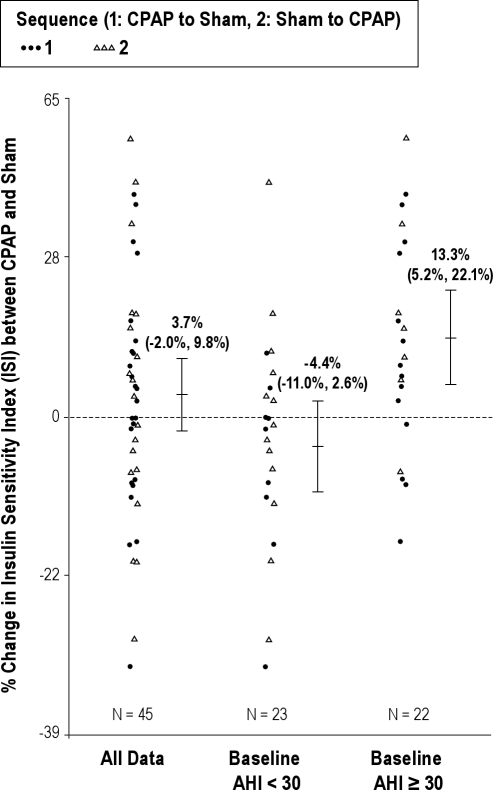
A test of the interaction between therapy and severe sleep apnea (defined by a baseline AHI ≥ 30) was significant for 2-h insulin level (P = 0.01) and ISI (0,120) (P = 0.002), suggesting that the effect of therapy on each outcome was significantly different between those subjects with severe sleep apnea compared to those patients without severe sleep apnea. Specifically, among subjects with severe SDB (defined as baseline AHI ≥ 30), the geometric mean of 2-h insulin was 28.7% lower (95%CI: [-46.5%, −10.9%], P = 0.002) and the geometric mean of ISI (0,120) was 13.3% higher (95%CI: [5.2%, 22.1%], P = 0.002) with CPAP compared to sham CPAP treatment (Figure 3). In contrast, among those without severe SDB, there was no evidence of an association between therapy and each of these indices. We did not identify additional differences in the effect of therapy on any of the outcomes when subgroups were defined by baseline BMI, race, gender, baseline statin use, and baseline percent sleep time with an oxygen saturation < 90%. Additional stratification by sleepiness (Epworth Sleepiness Scale score ≥ 16) suggested that the sleepier patients (n = 10) experience greater improvement in ISI (0,120) (12% vs 1.7%), fasting insulin (-4.5 vs −0.7), and 2-h insulin (-32.8 vs −4.5). However, in this small sample of sleepy patients, the interaction terms did not reach significance.
Figure 3.
Scatter plot demonstrating the percent change in ISI (0,120) after CPAP and after sham CPAP in the overall sample and the sample stratified by sleep apnea severity (AHI > 30). The smaller sample size reflects the number of subjects with complete data for the ISI assessments.
Table 3 summarizes indices of sleep, anthropometry, and adherence during follow-up for each therapy in each period. As expected, AHI, arousal index, and percentage oxygen saturation < 90% were significantly lower in CPAP than sham CPAP. No significant differences between CPAP and sham CPAP were observed for BMI or %N3 sleep. In sequence 1 only, sleep duration was higher during CPAP than sham CPAP, and visceral fat was lower during sham CPAP. To further explore potential effects of changes in visceral fat or sleep duration (measured by actigraphy or PSG) on the study outcomes, additional models were fit. Incorporating these variables did not change the observed associations between treatment group and ISI (0,120).
Table 3.
Indices of sleep, body fat, and adherence by sequence and period
Using data obtained when all patients were on CPAP, we found no association between changes in AHI and changes in HOMA-IR from baseline (2.04%; 95% CI: [-1.83%, 6.07%]; P = 0.30), changes in HOMA-B from baseline (2.72%; 95% CI: [-3.96%; 9.86%]; P = 0.43), or changes in ISI (0,120) from baseline (0.89%; 95% CI: [-2.63%, 4.52%]; P = 0.62).
Adherence to Treatment
The mean (interquartile range) time on CPAP was significantly higher than that on sham CPAP by 1.4 h per day (4.8 h/day on CPAP versus 3.4 h/day on sham CPAP; P < 0.001; Table 3). Subjects who received CPAP in Period 1 spent significantly more time on CPAP than on sham CPAP in Period 2 (P < 0.01). Differences in adherence to CPAP and sham CPAP were less for subjects who were assigned CPAP in Period 2 (P = 0.11). Using data obtained when all patients were on CPAP indicated that for each hour of CPAP use, mean change in ISI (0, 120) from baseline increased by 4.2% (95%CI: [0.4%, 8%]; P = 0.03). There was no evidence of an association between CPAP use and change in fasting glucose from baseline (-1.3 mg/dL; 95% CI: [-2.7, 0.2]; P = 0.09). Adherence was not associated with AHI level or sleepiness.
Three-Month Follow-Up Studies
Among 25 subjects who were assigned to CPAP treatment in Period 2, 17 elected to continue in the trial on CPAP for an additional 4 weeks after the primary study end date. There was no evidence of a significant difference in subject characteristics between those who continued on and those who stopped. At the end of the additional 4 weeks, there were no additional significant changes in measures of glucose or ISI (0,120) with CPAP compared to levels on sham CPAP.
DISCUSSION
To our knowledge, this study provides the first data from a controlled evaluation of the effect of CPAP therapy on direct measures of glucose metabolism as compared with a CPAP placebo in patients with sleep apnea and impaired glucose tolerance, but not overt T2DM. Across the entire sample, the majority of whom were obese, there was no evidence of a significant improvement in any index of glucose/insulin homeostasis observed after 8 weeks of CPAP therapy compared with 8 weeks of sham CPAP. However, among subjects with more severe sleep apnea (AHI ≥ 30), there was evidence of a large decrease in the 2-hour insulin levels (29%) as well as an improvement in a measure of insulin resistance, the ISI (0,120) (13%). Furthermore, we showed that each hour of active CPAP use was associated with a significant improvement in ISI (0,120) from baseline. The exploratory analysis also suggested greater improvement in the sleepier participants, who may be good targets for early intervention.
These results suggest that on a background of obesity and only mild-to-moderate SDB, intervening with CPAP for 8 weeks is inadequate for improving indices of glucose metabolism. In contrast, our data suggest that CPAP therapy improves measures of glucose tolerance (although does not result in normalization of IGT) in patients with more severe sleep apnea irrespective of elevated BMI. Further, the stability of the HOMA-B across treatment periods suggests that the changes observed in the measures of glucose metabolism are mainly secondary to improvements in peripheral insulin resistance rather than pancreatic β-cell function.
The biological plausibility of our findings showing improved insulin sensitivity among individuals with moderate to severe SDB treated with active CPAP is consistent with prior research implicating SDB-induced physiological stresses, notably hypoxia and oxidative stress, in the release of a number of cytokines, adhesion factors, and other hormones as contributors to insulin resistance.19,40–43 Hypoxia and arousal also stimulate sympathetic overactivity, leading to a cascade of inflammatory and hormonal responses, which may augment baseline sympathetic tone, leading to insulin resistance. Animal studies also have shown that insulin resistance may be induced or exacerbated by overnight sympathoexcitation and/or hypoxemia.9 Several clinic-based studies have demonstrated a relationship between SDB and insulin resistance.15,22,44–48 In one case-control study, HOMA-IR was significantly higher in a sleep apnea sample than a BMI-matched control group.49 The Sleep Heart Health Study demonstrated that AHI and average oxygen saturation were independently associated with higher fasting glucose and OGTT 2 h results, with stratified analyses showing associations persisted even in normal weight participants.50,51 In the current study, active CPAP was, as expected, associated with improved nocturnal oxygenation and reduced arousals, which may reflect a reduction in sympathetic activity.
In contrast to the studies demonstrating cross-sectional associations between SDB and glucose metabolism, there are a paucity of intervention studies evaluating the impact of CPAP on metabolic control. Several small studies have shown that acute or short periods of CPAP use improve levels of inflammatory or oxidative stress markers52–55—effects that may parallel changes in insulin. One small study did not demonstrate improved glucose control after about 5 months of nCPAP in patients with moderate to severe sleep apnea with an average baseline fasting glucose of 110 mg/dL, including 3 subjects on hypoglycemic agents, implying significant variability in the range of existing insulin resistance in this study population.30 Intervention results also have reported variable responses according to the underlying levels of obesity or comorbidities of the samples studied. Four uncontrolled studies of patients with diabetes and SDB suggested improved metabolic control after CPAP therapy. One study of 10 subjects with both T2DM and SDB showed improved insulin resistance following CPAP treatment.18 Another uncontrolled study of diabetic patients demonstrated improved glucose control after long-term CPAP use,56 and a third study showed improved glucose control during sleep.57 Another uncontrolled study demonstrated a correlation between HbA1c levels and degree of hypoxemia and reported that the latter improved after 3-5 months of CPAP.22
Two studies have examined insulin sensitivity after CPAP in patients without diabetes. The earlier study of predominantly men (only a small proportion of whom had IGT), reported an early improvement in measures of insulin sensitivity with CPAP therapy in the group as a whole, with the largest improvements occurring in the patients with BMI < 30. However, no improvement in insulin resistance could be demonstrated in those patients with BMI > 30.24 The later study conducted in a Chinese population examined the effect of nasal CPAP in 61 non-diabetic subjects with BMI ≤ 35 kg/m2 with moderate-to-severe SDB who were randomized to either CPAP or sham CPAP for 1 week, with continued observation over 12 weeks in the active CPAP group.29 Insulin sensitivity, measured by the short insulin tolerance test (SITT), was shown to be significantly improved after 1 week of CPAP, and the effect was sustained at 12 weeks in only a subgroup of obese patients. It is unclear from that report if the more obese subjects had more severe sleep apnea. In our controlled study of predominantly obese patients with impaired glucose tolerance, we did not observe differences in responses to therapy by BMI level, but rather by SDB severity. Our sample was selected explicitly to address changes in insulin levels in a sample with IGT and was thus mostly obese, limiting our ability to address changes in a non-obese subgroup. Secondary analyses did suggest that even in a setting of obesity and IGT, CPAP therapy may improve insulin resistance in patients with severe SDB.
A limitation of prior research on SDB and glucose metabolism has been the lack of data on factors that may confound potential treatment effects, including changes in visceral fat and sleep duration. Recent studies, however, have suggested that the amount of intra-abdominal fat, or visceral fat, is more tightly associated with metabolism and may not be adequately measured by BMI. As careful consideration of obesity and adiposity is particularly important in the setting of investigating parameters of metabolic syndrome, we assessed visceral fat after each treatment period using CT imaging. Similarly, because extreme sleep durations have been linked to insulin resistance,58,59 we obtained objective measurements of sleep duration using actigraphy prior to each re-assessment. We did not observe consistent differences in visceral fat or sleep duration across treatment arms and sequences, suggesting that changes in these factors were unlikely to have biased our assessments of treatment effects.
Prior literature has not consistently considered CPAP adherence on treatment outcomes. We objectively measured adherence to both CPAP and sham CPAP. Although our primary analyses were “intention to treat,” we explored the effect of CPAP adherence on outcomes and demonstrated incremental improvements in the change in ISI from baseline with each additional hour of CPAP use. This finding underscores the importance of efforts to maximize CPAP adherence in patients at risk for sleep apnea-related morbidities, such as those with moderate-severe sleep apnea and diabetes.
A unique feature of this study is its focus on a sample which had not yet developed overt T2DM, but rather had impaired glucose tolerance. Since this group is at high risk of developing T2DM,60 screening for and treatment of SDB could have significant clinical impact. Our findings suggest that more rigorous efforts to treat SDB, especially severe levels, may improve IGT. Future work is needed to address whether early treatment with CPAP may forestall or prevent the development of T2DM in vulnerable subgroups such as those with severe SDB and obesity.
This study's strengths include rigorous features designed to reduce misclassification of the outcome variables, minimize biases, and evaluate potential confounding. A run-in period was used to exclude those patients who would be noncompliant with CPAP treatment to optimize assessment of treatment efficacy. We also used a 2×2 crossover design to account for the expected small sample due to the very strict eligibility criteria. Although use of a run-in period and crossover design could bias individuals to correctly guess which CPAP arm they received in either period, a washout period was used to mitigate this effect. Indeed, the average sham CPAP use was comparable to that reported by the APPLES study, which used sham CPAP in a parallel design.61 CPAP adherence was objectively measured and its impact considered in secondary analyses. We also minimized variability in our outcome measures of glucose metabolism by performing fasting and OGTT in duplicate within 3 days of each other and averaging levels of glucose and insulin. Thus, each participant underwent 9-10 glucose tolerance tests over the length of the trial, in order to address the variability in OGTT and still permitting the use of this clinically relevant measure to assess insulin resistance. On the other hand, we did not use an intravenous glucose tolerance test or glucose clamp measurements, which more accurately measure insulin resistance. The ISI (0,120), calculated from the baseline and 120-minute insulin and glucose measurements (the measured values in this study), has been shown to a sensitive measure of insulin sensitivity in clinical application and epidemiologic studies37,38 as compared with other measures, such as those obtained from the invasive and costly euglycemic hyperinsulinemic clamp method. Additional strengths included the objective measurements of sleep duration and visceral fat, as well as high study retention, despite a rigorous protocol that lasted approximately 9 months.
A limitation of this study was its modest sample size. However, the crossover design enhanced study efficiency, and overall, was larger compared to other studies reported to date. We powered the study to demonstrate moderate improvements in glucose metabolism (normalization of impaired glucose tolerance) in a sample of 50 individuals. Perhaps because of the high levels of obesity in a sample with IGT, significant improvements were observed in only the 50% of the sample with severe SDB. Furthermore, we were unable to demonstrate normalization of IGT, our primary outcome. Although significant changes in several indices of insulin sensitivity were observed in the more severe SDB group, these observations were part of secondary analyses unadjusted for multiple comparisons and thus require replication.
In summary, this rigorous controlled trial shows that 8 weeks of CPAP is unlikely to improve glucose metabolism in individuals who are both obese and have only moderate levels of SDB. However, we show for the first time that moderately large improvements in insulin resistance may occur in obese patients with severe SDB and that changes are likely associated with improved peripheral insulin sensitivity. This group is at high risk for developing T2DM and cardiovascular disease and for increased mortality, and thus may particularly benefit from SDB treatment. Further research is needed to assess whether earlier diagnosis of SDB in the prediabetic and diabetic populations and dual-disease management may help reduce morbidity in this growing segment of the population.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Redline discloses receipt of CPAP units from Philips-Respironics and Resmed Inc. for use in NIH funded studies; receipt of a grant from Dymedix Inc. for conducting a sensor validation study; appointment as the first incumbent of an endowed professorship donated to the Harvard Medical School by Dr. P. Farrell, the founder and Board Chairman of Resmed, through a charitable trust instrument, with equal support equivalent to the endowment payout provided to HMS during Dr. Farrell's lifetime by the Resmed Co. through an irrevocable gift agreement. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Work for this study was supported by the following grants: NIH HL075077 and UL1-RR024989. This study was conducted at Case Western Reserve University, Cleveland, Ohio and Brigham and Women's Hospital, Boston, Massachusetts.
Footnotes
A commentary on this article appears in this issue on page 591.
SUPPLEMENTAL MATERIAL
Detailed Summary of Exclusion Criteria
The exclusion criteria included: current use of oral hypoglycemic medications or insulin; overt diabetes defined by a mean fasting plasma glucose (FPG) level ≥ 126; a mean 2-h OGTT glucose ≥ 250 mg/dL; a single abnormal FPG or 2-h OGTT glucose with diabetes symptoms or marked metabolic derangement (e.g., acidosis); use of supplemental oxygen; a primary sleep disorder other than SDB; severe chronic insomnia or circadian rhythm disorder with < 4 h of sleep per night; unstable medical conditions (e.g., new onset or changing angina, myocardial infarction, or congestive heart failure exacerbation documented within the previous 3 months, uncontrolled hypertension, etc.); daytime sleepiness with reports of sleepiness while driving or otherwise in situations which would present a risk for the subject or public (e.g., operating heavy equipment); alcohol abuse; or pregnancy.
Detailed Description of Study Design
This is a randomized, double-blind, 2×2 crossover trial in patients with sleep apnea and IGT. A schema of the study design is depicted in Figure 1. In brief, if subjects met initial study eligibility criteria, they underwent a polysomnography titration study. Each subject was then enrolled in a 2-week run-in period where they were asked to use CPAP at home for 2 weeks at the pressure settings identified by titration to resolve sleep apnea events. Those subjects meeting minimal CPAP adherence (5 h of use or 70% of sleep time) during the 2-week run-in period received a one-month washout period and were then randomized to receive 8 weeks of CPAP or 8 weeks of sham CPAP (Period 1). Following Period 1, patients again received a one-month washout period and then crossed over to the alternate 8 weeks of therapy (Period 2). Throughout the study, each patient attended a series of research visits at the Dahms Clinical Research Unit of University Hospitals Case Medical Center. These research visits included 4 overnight examinations: polysomnography with CPAP titration prior to the run-in period; polysomnography without CPAP therapy before the start of Period 1 (baseline visit); and polysomnography at the end of Period 1 and 2 (follow-up visits). The details of the measurements, which included anthropometry, venipuncture, actigraphy and abdominal CT imaging made in conjunction with each visit are summarized below. Sequence order (Sequence 1: CPAP/sham CPAP; Sequence 2: sham CPAP/CPAP) was determined by a computerized program that generated random numbers.
Detailed Description of Diet, Nutrition and CPAP Education and CPAP Interventions
Sleep hygiene, dietary counseling, and CPAP support:
All subjects met with a sleep technician and research nutritionist prior to CPAP titration and received approximately 30 min of instruction on sleep hygiene (including written materials) and diet. The nutritionist used a standardized instrument to guide a problem-specific approach to dietary counseling. A designated, unblinded research assistant oversaw the research titration studies and provided participant support for CPAP use across the duration of each intervention. The assistant was responsible for fitting masks, educating participants on CPAP use and for providing ongoing troubleshooting of problems with mask fit, pressure sensations, leak, nasal congestion, and behavioral resistance to use of CPAP. The assistant met with each participant at the time of titration and then periodically in person or by phone throughout the 8-month study period.
Active CPAP:
Prior to the run-in period, a trained research technician titrated the pressure during an overnight sleep study with the goals of eliminating obstructive apneas and reducing hypopneas to an AHI < 5, reducing respiratory-related arousals, maintaining oxygen saturation > 90%, and eliminating snoring. Each participant was provided a Philips-Respironics RemStar Pro CPAP machine, equipped with humidification and expiratory relief as needed.
Sham-CPAP:
Sham therapy was delivered using a customized Philips-Respironics device and masks with increased expiratory ports, configured to deliver a marginal pressure (0.4 ± 0.1 SD cm H2O). CPAP use was objectively monitored using data exported from the CPAP units, quantifying effective duration of treatment (time spent at prescribed pressure). Data were exported at the end of the run-in period and Period 1 and 2.
Detailed description of sleep measurements, biochemical outcomes, anthropometry and blood pressure measurements.
Biochemical outcomes:
Following each polysomnography study and a 12-h fast, venipuncture was performed and again after ingestion of 75 grams of anhydrase glucose (OGTT). To identify potential early changes in glucose metabolism, fasting blood and 2-h OGTT were also obtained 5-7 days after the start of Period 1 and 2. Fasting blood and 2-h OGTT were also measured within 3 days of the baseline visit and at each of the 2 follow-up visits so that at each time point, duplicate measurements were available to enhance the reliability. Whole blood was assayed for hemoglobin A1C using a BioRad VARIANT instrument based on ion-exchange high performance liquid chromatography. Insulin was determined using Coat-A-Count radioimmunoassay kits (Siemens Healthcare Diagnostics, Deerfield, IL). Glucose was determined using a Beckman Glucose Analyzer 2 (Beckman Instruments, Fullerton, CA) or a YSI 2300 StatPlus analyzer (YSI Life Sciences, Yellow Springs, OH). Lipids were measured by enzymatic methods.33
The insulin sensitivity index was calculated using the Gutt Index (ISI (0,120) ([75 000 mg + (fasting glucose – 2 h glucose) × 0.19 × body weight] / 120 min35). Insulin resistance was also estimated using the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR: Ins0 (μU/mL) × Glc0 (mmol/L)) / 22.5; “0” represents the fasting value).HOMA-B (β-cell function), a measurement used to assess pancreatic β-cell function (which we postulated would not vary with CPAP treatment) was calculated as: (fasting insulin in μU/mL) × 3.33 / (fasting glucose in mg/dL - 3.5). The ISI (0,120), HOMA-IR, and HOMA-B were log transformed to achieve approximate normality.
Anthropometry:
The following were measured in duplicate at the baseline visit and at the 2 follow-up visits by staff blinded to the interventions: height; weight; neck, waist, and hip circumferences; and skin-fold thickness. At the 2 follow-up visits, abdominal computed tomography (CT) was obtained to quantify abdominal total, visceral, and subcutaneous fat with 2 slices taken at lumbar 4-5 level (Siemens Sensation 16 scanner).
Blood pressure:
At each of the overnight examinations, blood pressure was measured supine at approximately 22:00 and then at 07:00 in triplicate.
Detailed Description of Statistical Analysis
Preliminary analyses:
Baseline characteristics were summarized by randomized sequence group (Sequence 1: CPAP then sham CPAP; Sequence 2: sham CPAP then CPAP) using the mean and standard deviation (SD) for quantitative variables and a frequency and percentage for categorical variables. A Wilcoxon-Mann-Whitney test, 2-sample t-test, or Fisher exact test was used as appropriate to compare baseline characteristics between the 2 sequence groups.
Indices of sleep, anthropometry, and CPAP adherence at each follow-up visit were summarized for each therapy in each of the 2 sequences. A paired 2-sample t-test was used to compare these indices between CPAP and sham CPAP. Indices of sleep included AHI, arousal index, oxygen saturation, %N3 (slow wave) sleep, and sleep duration. Indices of anthropometry included BMI and CT visceral abdominal tissue fat. Indices of adherence included average usage and percentage of days when therapy was used for > 4 hours.
Primary analyses:
Based on an intent-to-treat approach, a generalized estimating equation (GEE) approach was used to estimate the effect of therapy (CPAP or sham-CPAP) on the odds of normalization of IGT. The model treated subjects as clusters and included fixed effects for period, therapy, and baseline 2-h OGTT. Such a model provides an estimate of the odds ratio of normalizing the 2-h OGTT with CPAP compared with sham-CPAP, while controlling for period and baseline 2-h OGTT glucose level. Models were also extended to include additional adjustment for baseline BMI, baseline AHI, gender, and race.
The effect of carryover on normalization of IGT was also estimated. In this context of this 2×2 crossover study, carryover effects refer to when the effect of therapy in Period 1 persists into Period 2 and distorts the effect of the therapy in Period 2 on normalization of IGT. To assess this, we tested the interaction between therapy and period. Separate analyses were also conducted for each period to estimate the effect of therapy on normalization of IGT via a parallel group design. In this case, we adjusted for the baseline 2-h OGTT glucose, baseline BMI, baseline AHI, gender, and race.
Secondary analyses:
Secondary outcomes included continuously measured indices of glucose and insulin resistance that were obtained after fasting and 2 h after the OGTT, as well as the ISI (0,120), HOMA-IR, and HOMA-B. Based on an intent-to-treat approach, a linear mixed effect model was used to estimate the association between therapy and each secondary outcome. Each model included a random intercept to account for within subject correlation as well as fixed effects for period, therapy, and the baseline measure of each outcome. For normally distributed outcomes, these models estimated the adjusted mean difference in the outcome between CPAP and sham CPAP, while for log-normally distributed outcomes (such as ISI), these models estimated the adjusted geometric mean ratio of the outcome between CPAP and sham CPAP. Models were also extended to include baseline BMI, AHI, gender and race as additional covariates. As with the primary analyses, the carryover effect was estimated for each secondary outcome by testing an interaction between therapy and period although separate analyses were conducted for each period as well.
Post hoc analyses:
Post hoc stratified analyses were also performed to assess whether differences in the effect of CPAP on outcomes were observed according to baseline sleep apnea severity (defined by an AHI > 30), gender, race, and baseline weight (dichotomized using the sample median BMI 32). In this case, an interaction between treatment and each baseline factor was incorporated into each model and the effect of therapy on each outcome was estimated at each level of the baseline factor. Using only the data obtained when subjects received CPAP, we also estimated the association between the average hours of use of CPAP and changes in outcomes.
A priori, for 80% power and an α level of 0.05, assuming a within-subject correlation of 0.5, we estimated that a final sample of 37 subjects would be needed to detect a treatment response of 25% if the CPAP group improved while 5% of the sham group improved. For analyses of group differences in levels of metabolic or vascular parameters, we estimated that an n = 50 provides > 90% power to detect effect sizes of > 0.50.
REFERENCES
- 1.Reis JP, Loria CM, Sorlie PD, Park Y, Hollenbeck A, Schatzkin A. Lifestyle factors and risk for new-onset diabetes: a population-based cohort study. Ann Intern Med. 2011;155:292–9. doi: 10.1059/0003-4819-155-5-201109060-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engelgau MM, Geiss LS, Saaddine JB, et al. The evolving diabetes burden in the United States. Ann Intern Med. 2004;140:945–50. doi: 10.7326/0003-4819-140-11-200406010-00035. [DOI] [PubMed] [Google Scholar]
- 3.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Kissebah AH, Vydelingum N, Murray R, et al. Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab. 1982;54:254–60. doi: 10.1210/jcem-54-2-254. [DOI] [PubMed] [Google Scholar]
- 5.DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–94. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- 6.Hirosumi J, Tuncman G, Chang L, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–6. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 7.Association AD. Economic Costs of Diabetes in the U.S. in 2007. Diabetes Care. 2008;31:596–615. doi: 10.2337/dc08-9017. [DOI] [PubMed] [Google Scholar]
- 8.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99:1592–9. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 9.Djovkar A. Horm Metaboll Res. 1983. Influence of intermittent hypoxia on intravemous glucose tolerance and insulin sensitivity in anaesthetized normal rats; pp. 254–5. [DOI] [PubMed] [Google Scholar]
- 10.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimsdale JE, Coy T, Ziegler MG, Ancoli-Israel S, Clausen J. The effect of sleep apnea on plasma and urinary catecholamines. Sleep. 1995;18:377–81. [PubMed] [Google Scholar]
- 12.Narkiewicz K, van de Borne PJ, Cooley RL, Dyken ME, Somers VK. Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation. 1998;98:772–6. doi: 10.1161/01.cir.98.8.772. [DOI] [PubMed] [Google Scholar]
- 13.Narkiewicz K, van de Borne PJ, Pesek CA, Dyken ME, Montano N, Somers VK. Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation. 1999;99:1183–9. doi: 10.1161/01.cir.99.9.1183. [DOI] [PubMed] [Google Scholar]
- 14.Hedner J, Ejnell H, Sellgren J, Hedner T, Wallin G. Is high and fluctuating muscle nerve sympathetic activity in the sleep apnoea syndrome of pathogenetic importance for the development of hypertension? J Hypertens Suppl. 1988;6:S529–31. doi: 10.1097/00004872-198812040-00166. [DOI] [PubMed] [Google Scholar]
- 15.Braun B, Rock PB, Zamudio S, et al. Women at altitude: short-term exposure to hypoxia and/or alpha(1)-adrenergic blockade reduces insulin sensitivity. J Appl Physiol. 2001;91:623–31. doi: 10.1152/jappl.2001.91.2.623. [DOI] [PubMed] [Google Scholar]
- 16.Elmasry A, Janson C, Lindberg E, Gislason T, Tageldin MA, Boman G. The role of habitual snoring and obesity in the development of diabetes: a 10-year follow-up study in a male population. J Intern Med. 2000;248:13–20. doi: 10.1046/j.1365-2796.2000.00683.x. [DOI] [PubMed] [Google Scholar]
- 17.Al-Delaimy WK, Manson JE, Willett WC, Stampfer MJ, Hu FB. Snoring as a risk factor for type II diabetes mellitus: a prospective study. Am J Epidemiol. 2002;155:387–93. doi: 10.1093/aje/155.5.387. [DOI] [PubMed] [Google Scholar]
- 18.Brooks B, Cistulli PA, Borkman M, et al. Obstructive sleep apnea in obese non-insulin-dependent diabetic patients: effect of continuous positive airway pressure treatment on insulin responsiveness. J Clin Endocrinol Metab. 1994;79:1681–5. doi: 10.1210/jcem.79.6.7989475. [DOI] [PubMed] [Google Scholar]
- 19.Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165:670–6. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 20.Davies RJO, Turner R, Crosby J, Stradling JR. Plasma insulin and lipid levels in untreated obstructive sleep apnea apnoea and snoring; their comparison with matched controls and response to treatment. J Sleep Res. 1994;3:180–5. doi: 10.1111/j.1365-2869.1994.tb00126.x. [DOI] [PubMed] [Google Scholar]
- 21.Punjabi NM, Ahmed MM, Polotsky VY, Beamer BA, O'Donnell CP. Sleep-disordered breathing, glucose intolerance, and insulin resistance. Respir Physiol Neurobiol. 2003;136:167–78. doi: 10.1016/s1569-9048(03)00079-x. [DOI] [PubMed] [Google Scholar]
- 22.Shpirer I, Rapoport MJ, Stav D, Elizur A. Normal and elevated HbA1C levels correlate with severity of hypoxemia in patients with obstructive sleep apnea and decrease following CPAP treatment. Sleep Breath. 2011 doi: 10.1007/s11325-011-0525-x. [DOI] [PubMed] [Google Scholar]
- 23.Brooks B, Cistulli PA, Borkman M, et al. Obstructive sleep apnea in obese non-insulin-dependent diabetic patients: effect of continuous positive airway pressure treatment on insulin responsiveness. The Journal of clinical endocrinology and metabolism. 1994;79:1681–5. doi: 10.1210/jcem.79.6.7989475. [DOI] [PubMed] [Google Scholar]
- 24.Harsch IA, Schahin SP, Radespiel-Troger M, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2004;169:156–62. doi: 10.1164/rccm.200302-206OC. [DOI] [PubMed] [Google Scholar]
- 25.Harsch IA, Schahin SP, Bruckner K, et al. The effect of continuous positive airway pressure treatment on insulin sensitivity in patients with obstructive sleep apnoea syndrome and type 2 diabetes. Respiration. 2004;71:252–9. doi: 10.1159/000077423. [DOI] [PubMed] [Google Scholar]
- 26.Lindberg E, Berne C, Elmasry A, Hedner J, Janson C. CPAP treatment of a population-based sample--what are the benefits and the treatment compliance? Sleep Med. 2006;7:553–60. doi: 10.1016/j.sleep.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Patruno V, Aiolfi S, Costantino G, et al. Fixed and autoadjusting continuous positive airway pressure treatments are not similar in reducing cardiovascular risk factors in patients with obstructive sleep apnea. Chest. 2007;131:1393–9. doi: 10.1378/chest.06-2192. [DOI] [PubMed] [Google Scholar]
- 28.Schahin SP, Nechanitzky T, Dittel C, et al. Long-term improvement of insulin sensitivity during CPAP therapy in the obstructive sleep apnoea syndrome. Med Sci Monit. 2008;14:CR117–21. [PubMed] [Google Scholar]
- 29.Lam JC, Lam B, Yao TJ, et al. A randomised controlled trial of nasal continuous positive airway pressure on insulin sensitivity in obstructive sleep apnoea. Eur Respir J. 2010;35:138–45. doi: 10.1183/09031936.00047709. [DOI] [PubMed] [Google Scholar]
- 30.Chung S, Yoon IY, Lee CH, Kim JW. The effects of nasal continuous positive airway pressure on vascular functions and serum cardiovascular risk factors in obstructive sleep apnea syndrome. Sleep Breath. 2011;15:71–6. doi: 10.1007/s11325-009-0323-x. [DOI] [PubMed] [Google Scholar]
- 31.Stoohs RA, Facchini FS, Philip P, Valencia-Flores M, Guilleminault C. Selected cardiovascular risk factors in patients with obstructive sleep apnea: effect of nasal continuous positive airway pressure (n-CPAP) Sleep. 1993;16:S141–2. doi: 10.1093/sleep/16.suppl_8.s141. [DOI] [PubMed] [Google Scholar]
- 32.Ip MS, Lam KS, Ho C, Tsang KW, Lam W. Serum leptin and vascular risk factors in obstructive sleep apnea. Chest. 2000;118:580–6. doi: 10.1378/chest.118.3.580. [DOI] [PubMed] [Google Scholar]
- 33.Trenell MI, Ward JA, Yee BJ, et al. Influence of constant positive airway pressure therapy on lipid storage, muscle metabolism and insulin action in obese patients with severe obstructive sleep apnoea syndrome. Diabetes Obes Metab. 2007;9:679–87. doi: 10.1111/j.1463-1326.2006.00649.x. [DOI] [PubMed] [Google Scholar]
- 34.West SD, Nicoll DJ, Wallace TM, Matthews DR, Stradling JR. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax. 2007;62:969–74. doi: 10.1136/thx.2006.074351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kohler M, Stoewhas AC, Ayers L, et al. The effects of CPAP therapy withdrawal in patients with obstructive sleep apnea: a randomised controlled trial. Am J Respir Crit Care Med. 2011 doi: 10.1164/rccm.201106-0964OC. [DOI] [PubMed] [Google Scholar]
- 36.Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab. 2004;89:2583–9. doi: 10.1210/jc.2004-0535. [DOI] [PubMed] [Google Scholar]
- 37.Gutt M, Davis CL, Spitzer SB, et al. Validation of the insulin sensitivity index (ISI(0,120)): comparison with other measures. Diabetes Res Clin Pract. 2000;47:177–84. doi: 10.1016/s0168-8227(99)00116-3. [DOI] [PubMed] [Google Scholar]
- 38.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 39.Bonora E, Targher G, Alberiche M, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 40.Ohga E, Nagase T, Tomita T, et al. Increased levels of circulating ICAM-1, VCAM-1, and L-selectin in obstructive sleep apnea syndrome. J Appl Physiol. 1999;87:10–4. doi: 10.1152/jappl.1999.87.1.10. [DOI] [PubMed] [Google Scholar]
- 41.Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: Role of sleep disturbance and obesity. J Clin Endocrinol Metab. 1997;82:1313–6. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- 42.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Circadian interleukin-6 secretion and quantity and depth of sleep. J Clin Endocrinol Metab. 1999;84:2603–7. doi: 10.1210/jcem.84.8.5894. [DOI] [PubMed] [Google Scholar]
- 43.Louis M, Punjabi NM. Effects of acute intermittent hypoxia on glucose metabolism in awake healthy volunteers. J Appl Physiol. 2009;106:1538–44. doi: 10.1152/japplphysiol.91523.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strohl KP, Novak RD, Singer W, et al. Insulin levels, blood pressure and sleep apnea. Sleep. 1994;17:614–8. doi: 10.1093/sleep/17.7.614. [DOI] [PubMed] [Google Scholar]
- 45.Van Cauter E, Blackman JD, Roland D, Spire JP, Refetoff S, Polonsky KS. Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J Clin Invest. 1991;88:934–42. doi: 10.1172/JCI115396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tijhonen M, Partinen M, Narvanen S. The severity of obstructive sleep apnea is associated with insulin resistance. J Sleep Res. 1993;2:56–61. doi: 10.1111/j.1365-2869.1993.tb00062.x. [DOI] [PubMed] [Google Scholar]
- 47.Jicker JH, Dertinger SH, Siegfried W, et al. Obstructive sleep apnoea and diabetes mellitus: the role of cardiovascular autonomic neuropathy. Eur Respir J. 1998;11:14–9. doi: 10.1183/09031936.98.11010014. [DOI] [PubMed] [Google Scholar]
- 48.Resnick HE, Redline S, Shahar E, et al. Diabetes and sleep disturbances: findings from the Sleep Heart Health Study. Diabetes Care. 2003;26:702–9. doi: 10.2337/diacare.26.3.702. [DOI] [PubMed] [Google Scholar]
- 49.McArdle N, Hillman D, Beilin L, Watts G. Metabolic risk factors for vascular disease in obstructive sleep apnea: a matched controlled study. Am J Respir Crit Care Med. 2007;175:190–5. doi: 10.1164/rccm.200602-270OC. [DOI] [PubMed] [Google Scholar]
- 50.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep disordered breathing, glucose intolerance, and insulin resistance: The Sleep Heart Health Study. Am J Epidemiol. 2004;160:521–30. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 51.Seicean S, Kirchner HL, Gottlieb DJ, et al. Sleep-disordered breathing and impaired glucose metabolism in normal-weight and overweight/obese individuals: the Sleep Heart Health Study. Diabetes Care. 2008;31:1001–6. doi: 10.2337/dc07-2003. [DOI] [PubMed] [Google Scholar]
- 52.Phillips BG, Narkiewicz K, Pesek CA, Haynes WG, Dyken ME, Somers VK. Effects of obstructive sleep apnea on endothelin-1 and blood pressure. J Hypertens. 1999;17:61–6. doi: 10.1097/00004872-199917010-00010. [DOI] [PubMed] [Google Scholar]
- 53.Kimura H, Niijima M, Abe Y, et al. Compensatory excretion of prostacyclin and thromboxane metabolites in obstructive sleep apnea syndrome. Intern Med. 1998;37:127–33. doi: 10.2169/internalmedicine.37.127. [DOI] [PubMed] [Google Scholar]
- 54.Dyugovskaya L, Lavie P, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med. 2002;165:934–9. doi: 10.1164/ajrccm.165.7.2104126. [DOI] [PubMed] [Google Scholar]
- 55.Ip MS, Lam B, Chan LY, et al. Circulating nitric oxide is suppressed in obstructive sleep apnea and is reversed by nasal continuous positive airway pressure. Am J Respir Crit Care Med. 2000;162:2166–71. doi: 10.1164/ajrccm.162.6.2002126. [DOI] [PubMed] [Google Scholar]
- 56.Babu AR, Herdegen J, Fogelfeld L, Shott S, Mazzone T. Type 2 Diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch Intern Med. 2005;165:447–52. doi: 10.1001/archinte.165.4.447. [DOI] [PubMed] [Google Scholar]
- 57.Dawson A, Abel SL, Loving RT, et al. CPAP therapy of obstructive sleep apnea in type 2 diabetics improves glycemic control during sleep. J Clin Sleep Med. 2008;4:538–42. [PMC free article] [PubMed] [Google Scholar]
- 58.Ayas NT, White DP, Al-Delaimy WK, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26:380–4. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 59.Gottlieb DJ, Punjabi NM, Newman AB, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165:863–7. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- 60.Nathan DM, Davidson MB, DeFronzo RA, et al. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 2007;30:753–9. doi: 10.2337/dc07-9920. [DOI] [PubMed] [Google Scholar]
- 61.Kushida CA, Nichols DA, Quan SF, et al. The Apnea Positive Pressure Long-term Efficacy Study (APPLES): rationale, design, methods, and procedures. J Clin Sleep Med. 2006;2:288–300. [PubMed] [Google Scholar]