Abstract
Study Objectives:
Although excessive daytime sleepiness (EDS) is a common problem in children, with estimates of 15%; few studies have investigated the sequelae of EDS in young children. We investigated the association of EDS with objective neurocognitive measures and parent reported learning, attention/hyperactivity, and conduct problems in a large general population sample of children.
Design:
Cross-sectional.
Setting:
Population based.
Participants:
508 children from The Penn State Child Cohort.
Interventions:
N/A.
Measurements and Results:
Children underwent a 9-h polysomnogram, comprehensive neurocognitive testing, and parent rating scales. Children were divided into 2 groups: those with and without parent-reported EDS. Structural equation modeling was used to examine whether processing speed and working memory performance would mediate the relationship between EDS and learning, attention/hyperactivity, and conduct problems. Logistic regression models suggest that parent-reported learning, attention/hyperactivity, and conduct problems, as well as objective measurement of processing speed and working memory are significant sequelae of EDS, even when controlling for AHI and objective markers of sleep. Path analysis demonstrates that processing speed and working memory performance are strong mediators of the association of EDS with learning and attention/hyperactivity problems, while to a slightly lesser degree are mediators from EDS to conduct problems.
Conclusions:
This study suggests that in a large general population sample of young children, parent-reported EDS is associated with neurobehavioral (learning, attention/hyperactivity, conduct) problems and poorer performance in processing speed and working memory. Impairment due to EDS in daytime cognitive and behavioral functioning can have a significant impact on children's development.
Citation:
Calhoun SL; Fernandez-Mendoza J; Vgontzas AN; Mayes SD; Tsaoussoglou M; Rodriguez-Muñoz A; Bixler EO. Learning, attention/hyperactivity, and conduct problems as sequelae of excessive daytime sleepiness in a general population study of young children. SLEEP 2012;35(5):627-632.
Keywords: Children, excessive daytime sleepiness, attention, learning problems
INTRODUCTION
The high prevalence of parent reported excessive daytime sleepiness (EDS) in children has led to increased concern and speculation about the clinical and functional implications of daytime sleepiness. We recently reported a prevalence of 15% for EDS in a general population sample of young children, and that obesity was the most significant independent predictor of EDS.1
Although many studies have reported on the impact of moderate to severe snoring or sleep disordered breathing (SDB) on daytime functioning in children, fewer studies have reported on the effect of sleepiness on daytime behavior (i.e., attention, learning, behavior), and only one was a population-based study.2 Of these studies, evidence of impairment in daytime functioning as a direct consequence of daytime sleepiness has yielded inconsistent results.
Studies of children with neurodevelopmental disorders such as attention deficit hyperactivity disorder (ADHD) and autism have demonstrated increased parent report of sleep problems including daytime sleepiness compared to control children, although more objective (e.g. polysomnography, actigraphy) methods for assessing nighttime sleep of children with neurodevelopmental disorders have disclosed few differences in objective sleep relative to controls. In two studies,3,4 however, increased daytime sleepiness as measured by the multiple sleep latency test (MSLT) was reported in children with ADHD. Interestingly, two studies analyzing parent-reported EDS within ADHD subtypes suggests an association between EDS and ADHD inattentive subtype but not ADHD combined type.3,5 Other studies6,7,8,9,10,11–12 have reported inconsistent evidence of impairment in aspects of performance and learning including working memory, overall cognitive ability, and attention as a direct consequence of sleepiness in children.
One population-based study2 reports no association between parent-reported EDS and teacher-reported hyperactivity, conduct, and emotional problems. Another study6 reported that bedtime resistance was associated with conduct problems, restless sleep was associated with hyperactivity, and EDS was associated with emotional symptoms such as anxiety and depression. Conclusions based on the existing literature are difficult to make, as there are numerous methodological issues (e.g., small sample sizes, inadequate control procedures, multiple definitions of EDS, variability between subjective and objective report, clinical versus community samples). More data are needed to characterize impairment in daytime functioning as a predictable response to sleepiness among children.
Our study is the first to report on the association between EDS and objective neurocognitive measures, as well as parent-reported learning, attention/hyperactivity, and conduct problems, while controlling for objectively measured sleep variables in a general population of sample of children. The purpose of this study was to identify possible sequelae of EDS.
METHODS
Sample
This study was designed in 2 phases, with the first phase designed for collecting general information from the parents about their child's sleep and behavioral patterns. In the first phase, elementary schools (kindergarten through grade 5) were selected each year, so that approximately 1500 students were enrolled. A screening questionnaire based on the survey published by Ali et al.,13 validated to identify children at high risk for SDB, was sent home to parents of every student in these school districts (n = 7,312), with a 78.5% response rate. In the second phase of this study, each year 200 children were selected from the questionnaires that were returned that year. Using a stratification of grade, sex, and risk for SDB, we randomly selected children from each stratum to maintain representativeness of the original sample. Seven hundred children completed phase 2, for a final response rate of 70%. We contrasted the 700 subjects who completed the PSG recordings with those who were selected and did not complete phase 2 (n = 6612). There were no significant differences between the 2 groups on grade, sex, or risk for SDB. All children from the second phase who completed the Pediatric Sleep Questionnaire and the majority of psychometric tests were included in this study. Children diagnosed with medical problems (36.0% allergies, 13.3% asthma, 1.2% juvenile diabetes), mental health disorders (11.0% ADHD, 1.7% depression/anxiety, 0.8% autism), or a learning disability (9.1%) were not excluded from the study, so that the sample is representative of the general population. Thus, our final sample for this study consisted of 508 children from the Penn State Child Cohort. This study was approved by the Institutional Review Board of Penn State College of Medicine. Informed consent was obtained from parents of all participants, and assent was obtained from all children prior to participation.
Procedures
Sleep laboratory
During their visit in the laboratory, all subjects underwent a series of subjective and objective measurements. A thorough medical assessment, including physical examination, and parent-completed questionnaires and rating scales (e.g., behavior, sleep, and child development) were completed for each subject. Height and weight were recorded for each child, and body mass index (BMI) was calculated.
All subjects were then evaluated for one night in sound-attenuated and temperature-controlled rooms. During this time, the child's sleep was continuously monitored for 9 h (24 analog channel and 10 dc channel TS amplifier using Gamma software, Grass-Telefactor Inc). A 4-channel electroencephalogram (EEG), a 2-channel electrooculogram (EOG), and a single-channel chin and anterior bilateral electromyogram (EMG) were recorded. Throughout the night, respiration was monitored by thermocouples at the nose and mouth (model TCT1R, Grass Instrument Co., Quincy, MA), nasal pressure (Validyne Engineering Corp), and thoracic and abdominal strain gauges (model 1312 Sleepmate Technologies Midlothian, VA). All-night recordings of hemoglobin oxygen saturation (SpO2) were obtained using a cardiorespiratory oximeter (model 8800, Nonin Medical, Inc., Plymouth, MN) attached to the finger. Snoring sounds were monitored by a sensor attached to the throat (Sleepmate model, 1250). Our records were screened for sleep apnea using criteria that are currently used clinically.14,15 An obstructive apnea was defined as cessation of airflow ≥ 5 sec and an out-of-phase strain gauge movement. A hypopnea was defined as reduction of airflow of approximately 50% with an associated decrease in oxygen saturation (SpO2) ≥ 3% or an associated arousal. Based on these data, an apnea-hypopnea index (AHI) was calculated [(apnea+hypopnea)/hours of sleep].
Parent rating scales
For the purposes of this study, the Pediatric Sleep Questionnaire developed by R.D. Chervin16 was completed by a parent to assess excessive daytime sleepiness (EDS) in our study population. Children were classified as having EDS when the parent reported “yes” for either “Does your child have a problem with sleepiness during the day?” and/or “Has a teacher or other supervisor commented that your child appears sleepy during the day?” Within those children with EDS, 42.1% endorsed parent only, 26.1% endorsed teacher only, and 31.9% endorsed both parent and teacher. In addition, a parent completed the Pediatric Behavior Scale (PBS),17 a 165-item rating scale developed to evaluate behavior and learning problems, and ADHD. Three scales were calculated: Attention/Hyperactivity (e.g., attention, impulsivity, and hyperactivity subscales), Conduct (e.g., disobedient, overreactive, explosive, irritable), and Learning problems (e.g., difficulty learning, failure to complete schoolwork, low grades, careless and disorganized schoolwork). Test scores were converted to T scores with a mean of 50 and a SD of 10.
Neurocognitive assessment
All children underwent a 2.5-h neurocognitive evaluation prior to their overnight stay in the sleep laboratory at approximately the same time each afternoon. The standardized tests were administered individually to each child by a trained psychometrist over one session. Tests in the neurocognitive battery were chosen because they measure intelligence and key neurocognitive functions including attention, executive functioning, memory, processing speed, and visual-motor skill.
Wechsler Abbreviated Scales of Intelligence (WASI):
The WASI18 consists of 4 subtests corresponding to the same subtests on the WISC-IV (Block Design, Matrix Reasoning, Vocabulary, and Similarities).
Digit Span:
WISC-III19 Digit Span has been used as a measure of attention and working memory in several neuropsychological studies.20
Developmental Test of Visual-Motor Integration (VMI):
The VMI21 has validity as a measure of dysgraphia or difficulty with handwriting.22
Coding:
The WISC-III Coding subtest is a measure of processing speed which is low in children with neurological disorders such as ADHD, autism, and learning disability.19
Symbol Search:
The Symbol Search is the second subtest from the WISC-III that measures processing speed.19,23
Stroop Color and Word Test Children's Version (Stroop):
The Stroop test is a measure of executive functioning.24 It is most often described as measuring ability to shift cognitive inhibition and ability to inhibit an overlearned dominant response in favor of an unusual one.25
Wisconsin Card Sorting Test-64 Card Version (WCST-64):
The WCST-6426 is a shortened version of the well-regarded 128-card version that measures executive functioning.
Statistical Analyses
The primary objective of the analysis was to evaluate the associations between parent-reported EDS and various sequelae in a general population of young children. Comparisons of the distribution of demographics and risk factors according to group membership (no EDS v. EDS) were made with independent t or χ2 tests. A series of univariate logistic regression analyses was performed to assess the relationship between each of the variables and each of the 2 groups. The initial unadjusted analysis was repeated 3 times: (1) adjusting for AHI and sleep efficiency; (2) adjusting for AHI, sleep duration, and sleep latency; and (3) adjusting for AHI, % REM and SWS, and sleep latency. Associations between sequelae and EDS are expressed as effect size (Cohen's d), P values, and odds ratios (ORs) ± 95% confidence intervals (CIs). A cutoff T-score of 65 was used for all psychological risk factors to create binary variables. The statistical confidence level selected for all analyses was P < 0.05. All analyses were performed using Predictive Analytics Software (PASW, Inc, Chicago, IL) Version 17.0.
In order to investigate the mediating effect of objective neurocognitive functioning, defined as a latent variable comprised of working memory and processing speed, we employed structural equation modelling (SEM) using AMOS 7.0.27 Observations with missing values were omitted from the sample. As EDS is a dummy variable and these kinds of variables are not normally distributed, an asymptotic distribution free (ADF) estimation was used.28
The fit of the structural models was assessed with various χ2 indices. Because this index is sensitive to sample size, we also used other goodness of fit indices: the root-mean square error of approximation (RMSEA), the Comparative Fit Index (CFI), and the goodness-of-fit index (GFI). If the RMSEA is < 0.05, it is indicative of a satisfactory approximate fit of the theoretical model.29 For the other indices (CFI and GFI), values > 0.90 (and preferably > 0.95) are considered to indicate a good fit.28 To compare the different models we followed a model comparison procedure where Δχ2 and Δdf between models were tested. Differences between models were also evaluated using the Akaike measure (AIC).30 Regarding this index, Burnham and Anderson31 have suggested specific guidelines for comparison of models. According to these authors, AIC differences of 0-2 show little difference between competing models, whereas differences > 4 show considerably more support for the model with the lowest AIC.
RESULTS
Sample Description
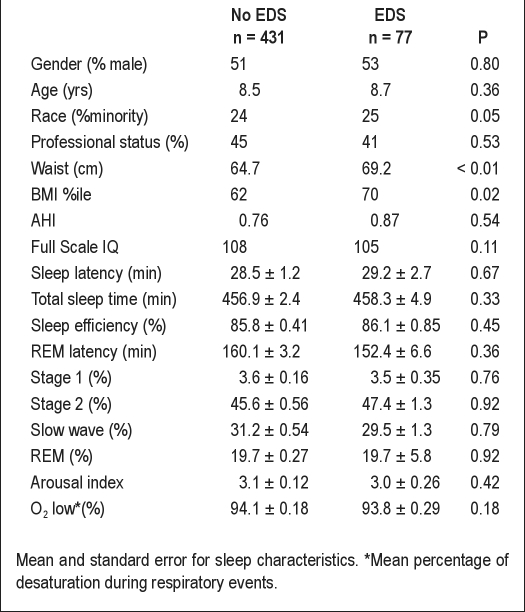
The final sample of 508 children consisted of 431 children without EDS and 77 children with EDS. The age range was 6-12 years, with an average age of 102.0 ± 0.08 months. Approximately one quarter of our sample was non-Caucasain; 51.8 % were boys, and 45% were from professional families (Table 1).
Table 1.
Sample characteristics
Primary Analyses
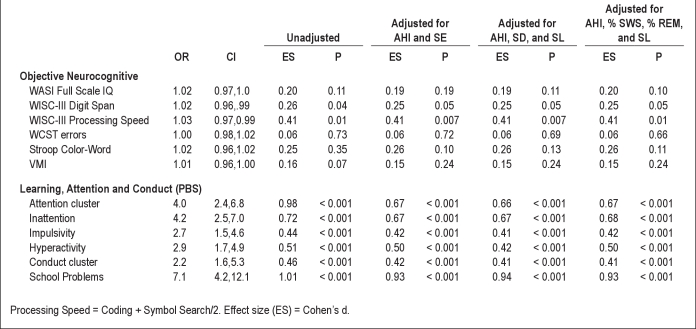
Most strikingly, parent-reported symptoms of learning problems had a 7 times increased odds of EDS. Parent-reported symptoms of attention/hyperactivity and conduct problems, as well as on objective neurocognitive measures of processing speed and working memory were also significantly associated with EDS. No association was found between EDS and Full Scale IQ, VMI, or on measures of executive functioning (e.g., WCST, Stroop). When we controlled for various objective sleep variables AHI, sleep efficiency, sleep duration, sleep latency, and % REM and %SWS, the pattern of associations did not change (Table 2).
Table 2.
Models of the associations between children with and without EDS: (1) unadjusted; (2) adjusted for sleep efficiency and AHI; (3) adjusted for sleep duration, sleep latency, and AHI; and (4) adjusted for sleep latency, % REM, % slow wave sleep, and AHI
Secondary Analyses
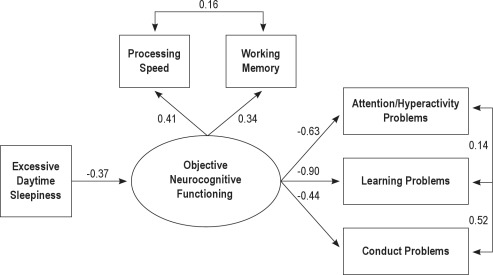
In the mediated model, EDS predicts processing speed and working memory, which in turn predicts attention/hyperactivity, learning and conduct problems. In this model, the relationship between EDS and different problems would be mediated by neurocognitive functioning. These analyses showed that this model fit very well to the observed data (χ2(6) = 8.32; GFI = 0.98, CFI = 0.98, RMSEA = 0.03, AIC = 38.32). As can be seen in Figure 1, EDS was significantly related to objective neurocognitive functioning (β = −0.37, P < 0.01). At the same time, objective neurocognitive functioning predicts attention/hyperactivity (β = −0.63, P < 0.01), learning (β = −0.90, P < 0.01), and conduct (β = −0.44, P < 0.01) problems. Our mediation prediction would be supported if the fit of the model would not be improved by the addition of direct paths from EDS to attention/hyperactivity, school, and conduct problems. This non-mediated alternative model, where both EDS and neurocognitive functioning directly predict problems, present as a worse fit to the data (χ2(4) = 14.36; GFI = 0.07, CFI = 0.96, RMSEA = 0.06, AIC = 48.20). Thus, the mediated model was supported since the fit was significantly better than the alternative model (Δχ2 = 6.04, Δdf = 2, P < 0.05).
Figure 1.
Structural Equation Model showing all significant and nonsignificant paths with standardized beta coefficients.
We also tested an alternative mediational model where EDS was the mediator of the relationship between neurocognitive deficits and the parent-reported problems. This mediational model showed a poor fit to the data (χ2(6) = 43.95; GFI = 0.83, CFI = 0.77, RMSEA = 0.11, AIC = 73.95) and was worse than the original model (Δχ2 = 35.69, ΔAIC = 35.63).
DISCUSSION
This study is the first to evaluate simultaneously a wide range of potential sequelae (e.g., parent report of learning, attention/hyperactivity, and conduct problems), objective measurement of working memory and processing speed, and association with EDS in a general population of young children (The Penn State Child Cohort). Similar to findings in adults in which sleepiness has been shown to be a strong predictor of certain aspects of neurobehavioral functioning, increased sleepiness in young children was associated with parent-reported learning, attention/hyperactivity, and conduct problems, and significantly lower scores on objective indices measuring processing speed and working memory. Thus, this study has three main findings. First, excessive daytime sleepiness in young children was associated with increased parent-reported learning, attention/hyperactivity, and conduct problems. Second, the data were consistent with a theoretical model in which EDS affects learning, attention/hyperactivity, and conduct problems via its impact on processing speed and working memory. The fact that this model shows the best fit as compared to other theoretical models further supports this paradigm. Third, poor objective sleep (PSG-measured AHI, sleep latency, sleep efficiency, sleep duration, % REM, or %SWS) was not associated with EDS and the potential effects on learning, attention/hyperactivity, and conduct problems.
Our data suggest that EDS impairs young school aged children's ability to pay attention (e.g., concentration, listening, distractibility) and level of activity (e.g., overactivity), and that this effect is large enough to be detected and reported by parents. In addition, EDS can make learning difficult.35 Learning problems were reported by 57% of the parents whose children had EDS, suggesting that the sleepier you are, the higher the risk for difficulty learning, incomplete and disorganized schoolwork, low grades, and trouble with reading, writing, and arithmetic. Our findings are supported by several studies suggesting that children with ADHD and/or learning disabilities have increased parent report of sleepiness.3,4–5 This study suggests that the dominance of processing speed (β = 0.41) may be the key mechanism by which EDS influences attention and learning outcomes, since the association between EDS and attention/hyperactivity and learning problems was mediated by processing speed. Sleepiness can slow down thought processes or ability to process information in a quick and efficient manner that can led to lower alertness and concentration. Working memory showed a slightly weaker effect on learning and attention outcomes as compared to processing speed, but was nevertheless significant. Indeed, previous research shows that both learning and attention depend on working memory. In addition to learning and attention/hyperactivity problems, conduct problems (e.g., irritability and aggression) are associated with EDS. The results are in keeping with a study by Chervin et al.36 and commonly reported in children with SDB.
Another interesting finding of our study is that in young children, parent-reported EDS and subjective (learning, attention/hyperactivity, and conduct problems) and objective (processing speed and working memory) associations are not related to poor objective sleep (PSG measured AHI, sleep latency, sleep efficiency, sleep duration, % REM or %SWS). This is contrary to the commonly held assumption that EDS is always associated with objectively defined disturbances in the quality or quantity of nighttime sleep. Rather, as previously reported,1,5 it appears that EDS is a manifestation of obesity, ADHD-inattentive type, depression/anxiety symptoms, parent-reported trouble falling asleep, and asthma. Perhaps parents are better “identifiers” of sleepiness or possess “ecological validity” (child's actual functioning in daily life) or that PSG markers of sleep lack sensitivity. Thus, the use of a more sensitive measure of daytime sleepiness such as the multiple sleep latency test (MSLT) might yield different results and can be considered a limitation of our study. On a pragmatic level, such findings raise the question about the utility of conventional PSG indexes in diagnosing EDS in young children.
In secondary analysis, employing SEM, we tested the hypothesis that working memory and processing speed, objective markers of neurocognitive functioning, would have mediational and differential effects on the relationship between EDS and parent-reported learning, attention/hyperactivity, and conduct problems. We found that EDS affects learning, attention/hyperactivity, and conduct problems via its impact on processing speed and working memory. It appears that parents who reported increased sleepiness in their children, also “accurately” reported increased attention/hyperactivity, learning, and conduct problems. Interestingly, when we tested whether EDS was the mediator between neurocognitive functioning, learning, and neurobehavioral problems, the model was not supported.
These data add to the growing research literature on the neuropsychological effects of EDS. Results of our study suggest that impairment due to parent- and/or teacher-reported EDS in daytime cognitive and behavioral functioning is significantly related to development. From a clinical standpoint, professionals who evaluate and treat children should be cognizant of the role of metabolic factors such as obesity1,37 and inflammatory markers38,39 that contribute to the mechanism of EDS, the potential relevance of parent/teacher information regarding learning and attention/hyperactivity problems, and the mediating effects of processing speed and working memory on daytime sleepiness. Thus, when children are referred for neurobehavioral problems, they should be assessed for potential risk factors of EDS and objectively evaluated for neurocognitive weaknesses (low processing speed and working memory). The recognition and treatment of EDS can offer new strategies to address some of the most common neurobehavioral challenges in this age group.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants R01 HL063772, M01 RR010732, and C06 RR016499.
REFERENCES
- 1.Calhoun SL, Vgontzas AN, Fernandez-Mendoza J, et al. Prevalence and risk factors of excessive daytime sleepiness in a community sample of young children: The role of obesity, asthma, anxiety/depression, and sleep. Sleep. 2011;34:1–5. doi: 10.1093/sleep/34.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bos SC, Gomes A, Clemente V, et al. Sleep and behavioral problems in children: a population based study. Sleep Med. 2007;10:66–74. doi: 10.1016/j.sleep.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 3.LeBourgeois MK, Avis K, Mixon M, Olmi J, Harsh J. Snoring, sleep quality and sleepiness across Attention-Deficit Hyperactivity Disorder subtypes. Sleep. 2004;27:520–5. [PubMed] [Google Scholar]
- 4.Golan N, Shahar A, Ravid S, Pillar G. Sleep disorders and daytime sleepiness in children with attention-deficit/hyperactivity disorder. Sleep. 2004;27:261–6. doi: 10.1093/sleep/27.2.261. [DOI] [PubMed] [Google Scholar]
- 5.Mayes SD, Calhoun SL, Bixler EO, et al. ADHD subtypes and comorbid anxiety, depression, and oppositional defiant disorder: Differences in sleep problems. J Pediatr Psychol. 2008;12:1–10. doi: 10.1093/jpepsy/jsn083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smedje H, Broman J-E, Hetta J. Associations between disturbed sleep and behavioural difficulties in 635 children aged six to eight years: a study based on parents' perception. Eur Child Adolesc Psychiatry. 2001;10:1–9. doi: 10.1007/s007870170041. [DOI] [PubMed] [Google Scholar]
- 7.Sadeh A, Gruber R, Raviv A. Sleep, neurobehavioral functioning, and behavior problems in school age children. Child Dev. 2002;73:405–17. doi: 10.1111/1467-8624.00414. [DOI] [PubMed] [Google Scholar]
- 8.Gruber R, Laviolette R, Deluca P, Monson E, Cornish K, Carrier K. Short sleep duration is associated with poor performance on IQ measures in health children. Sleep Med. 2010;11:289–94. doi: 10.1016/j.sleep.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Lewin DS, England SJ, Rosen RC. Cognitive and behavioral sequelae of obstructive sleep apnea in children. Sleep. 1999;22:s126. doi: 10.1016/s1389-9457(01)00070-3. [DOI] [PubMed] [Google Scholar]
- 10.Lecendreux M, Konofal E, Bouvard M, et al. Sleep and alertness in children with ADHD. J Child Psychol Psychiatry. 2000;41:803–12. [PubMed] [Google Scholar]
- 11.Ali NJ, Pitson D, Stradling JR. Sleep disordered breathing: effects of adenotonsillectomy on behavior and psychological functioning. Eur J Pediatr. 1996;155:56–62. doi: 10.1007/BF02115629. [DOI] [PubMed] [Google Scholar]
- 12.Chervin RD, Weatherly RA, Ruzicka DL, et al. Subjective sleepiness and polysomnographic correlates in children scheduled for adenotonsillectomy vs other surgical care. Sleep. 2006;29:495–503. [PMC free article] [PubMed] [Google Scholar]
- 13.Ali NJ, Pitson D, Stradling JR. Sleep disordered breathing: effects of adenotonsillectomy on behavior and psychological functioning. Eur J Pediatr. 1996;155:56–62. doi: 10.1007/BF02115629. [DOI] [PubMed] [Google Scholar]
- 14.American Thoracic Society. Standards and indications for cardiopulmonary sleep studies in children. Am J Respir Crit Care Med. 1996;153:866–78. doi: 10.1164/ajrccm.153.2.8564147. [DOI] [PubMed] [Google Scholar]
- 15.American Academy of Pediatrics. Clinical practice guidelines: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109:704–12. doi: 10.1542/peds.109.4.704. [DOI] [PubMed] [Google Scholar]
- 16.Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000;1:21–32. doi: 10.1016/s1389-9457(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 17.Lindgren SD, Koeppl GK. Prinz RJ. Advances in behavioral assessment of children and families. Greenwich, CT: JAI; 1987. Assessing child behavior problems in a medical setting: development of the Pediatric Behavior Scale; pp. 57–90. [Google Scholar]
- 18.The Psychological Corporation. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- 19.Brocki KC, Bohlin G. Executive functions in children aged 6 to 13: a dimensional and developmental study. Dev Neuropsychol. 2004;26:571–93. doi: 10.1207/s15326942dn2602_3. [DOI] [PubMed] [Google Scholar]
- 20.Mayes SD, Calhoun SL. WISC-IV and WISC-III profiles in children with ADHD. J Atten Disord. 2006;9:486–93. doi: 10.1177/1087054705283616. [DOI] [PubMed] [Google Scholar]
- 21.Wechsler D. WISC-IV technical and interpretive manual. San Antonio, TX: Psychological Corporation; 2003. [Google Scholar]
- 22.Beery KE. The Beery-Buktenica Developmental Test of Visual-motor Integration (VMI) 4th ed., Rev. Parsippany, NJ: Modern Curriculum Press; 1997. [Google Scholar]
- 23.Maeland AF. Handwriting and perceptual-motor skills in clumsy, dysgraphic, and normal children. Percept Mot Skills. 1992;75:1207–17. doi: 10.2466/pms.1992.75.3f.1207. [DOI] [PubMed] [Google Scholar]
- 24.Calhoun SL, Mayes SD. Processing speed in children with clinical disorders. Psychol Sch. 2005;42:333–43. [Google Scholar]
- 25.Golden CJ, Freshwater SM, Golden GL. Stroop Color and Word Test Children's Version. Wood Dale, IL: Stoelting; 2003. [Google Scholar]
- 26.Jensen AR, Rohwer WD., Jr The Stroop color word test: a review. Acta Psychol (Amst) 1966;25:36–93. doi: 10.1016/0001-6918(66)90004-7. [DOI] [PubMed] [Google Scholar]
- 27.Kongs SK, Thompson LL, Iverson GL, Heaton RK. Wisconsin Card Sorting Test-64 Card Version. Lutz, FL: Psychological Assessment Resources; 2000. [Google Scholar]
- 28.Chase-Carmichael CA, Ris MD, Weber AM, Schefft BK. Neurologic validity of the Wisconsin Card Sorting Test with a pediatric population. Clin Neuropsychol. 1999;13:405–13. doi: 10.1076/1385-4046(199911)13:04;1-Y;FT405. [DOI] [PubMed] [Google Scholar]
- 29.Arbuckle JL. Amos (Version 7.0) Chicago: SPSS; 2006. [Google Scholar]
- 30.Weston R, Gore PA., Jr A brief guide to structural equation modeling. Couns Psychol. 2006;34:719–51. [Google Scholar]
- 31.Burnham KP, Anderson DR. Model section and multimodel inference: a practical information-theoretic approach. 2nd ed. New York: Springer-Verlag; 2002. [Google Scholar]
- 32.James LR, Mulaik SA, Brett JM. A tale of two methods. Organizational Research Methods. 2006;9:233–44. [Google Scholar]
- 33.Bentler PM, Bonnet DG. Significance test and goodness of fit in the analysis of covariance structures. Psychol Bull. 1980;88:588–606. [Google Scholar]
- 34.Akaike H. Factor analysis and AIC. Psychometrika. 1987;5:317–32. [Google Scholar]
- 35.Dewald JF, Meijer AM, Oort FJ, Kerkhof GA, Bogels SM. The influence of sleep quality, sleep duration and sleepiness on school performance in children and adolescents: a meta-analytic review. Sleep Med Rev. 2010;14:179–89. doi: 10.1016/j.smrv.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Chervin RD, Dillon JE, Archbold KH, Ruzicka DL. Conduct problems and symptoms in sleep disorders in children. J Am Acad Child Adolesc Psychiatry. 2003;42:201–8. doi: 10.1097/00004583-200302000-00014. [DOI] [PubMed] [Google Scholar]
- 37.Gozal D, Kheirandish-Gozal L. Obesity and excessive daytime sleepiness in prepubertal children with obstructive sleep apnea. Pediatrics. 2009;123:13–8. doi: 10.1542/peds.2008-0228. [DOI] [PubMed] [Google Scholar]
- 38.Tsaoussoglou M, Bixler EO, Calhoun S, Chrousos GP, Sauder K, Vgontzas A. Sleep disordered breathing in obese children with associated with prevalent excessive daytime sleepiness, inflammation, and metabolic abnormalities. J Clin Endocrinol Metab. 2010;95:143–50. doi: 10.1210/jc.2009-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gozal D, Crabtree VM, Sans Capdevilla O, Witcher LA, Kheirandish-Gozal L. C-reactive protein, obstructive sleep apnea, and cognitive dysfunction in school aged children. Am J Respir Crit Care Med. 2007;176:188–93. doi: 10.1164/rccm.200610-1519OC. [DOI] [PMC free article] [PubMed] [Google Scholar]