Abstract
Study Objectives:
Aging is accompanied by changes in cognitive function, and changes in rest-activity patterns. Previous work has demonstrated associations between global rest-activity measures and cognitive performance on a number of tasks. Recently, we demonstrated that aging is associated with changes in the minute-to-minute fragmentation of rest-activity patterns in addition to changes in amounts of rest and activity. Given the body of experimental evidence linking sleep fragmentation with decrements in cognitive function in animals and humans, we hypothesized that increased fragmentation of rest-activity patterns would be associated with decreased cognitive function in older individuals.
Design:
Cross-sectional.
Participants:
700 community-dwelling individuals from the Rush Memory and Aging Project.
Measurements and Results:
We obtained up to 11 days of actigraphic recordings in subjects' home environments and quantified the fragmentation of rest and activity using a recently developed state transition metric. We tested the associations between this metric and performance in 5 cognitive domains. Greater fragmentation of both rest and activity were associated with lower levels of cognitive performance, and this association was independent of total amounts of rest or activity. There was a characteristic pattern of cognitive deficits associated with rest and activity fragmentation, with preferential involvement of perceptual speed, semantic memory, working memory, and visuospatial abilities, and relative sparing of episodic memory.
Conclusions:
The fragmentation of periods of rest and activity is a clinically important characteristic of rest-activity patterns that correlates with cognitive performance in older individuals.
Citation:
Lim ASP; Yu L; Costa MD; Leurgans SE; Buchman AS; Bennett DA; Saper CB. Increased fragmentation of rest-activity patterns is associated with a characteristic pattern of cognitive impairment in older individuals. SLEEP 2012;35(5):633-640.
Keywords: Actigraphy, sleep fragmentation, cognition, aging
INTRODUCTION
Aging is accompanied by a number of functional and biological changes, among which are changes in patterns of rest and activity, and deterioration in cognitive performance. For example, analyses of actigraphic data in older individuals have demonstrated age-related changes in actigraphically inferred nocturnal sleep time,1 total daily activity,2 hour-to-hour variability,1 day-to-day stability,1 and the fragmentation of rest and activity.3 Meanwhile, deterioration of cognitive performance in a number of domains is known to correlate with age.4
Given their co-occurrence in old age, it is reasonable to ask whether alterations in rest-activity patterns and changes in cognitive function might be related. Indeed, we and others have reported associations between cognitive performance and actigraphically inferred nocturnal sleep efficiency,5 diurnal daily activity,2 and hour-to-hour variability6 in older individuals.
What these studies have not addressed is whether differences in the local fragmentation of rest-activity patterns, as opposed to percentages of rest or activity, also are associated with cognitive performance. This question is of interest because there is increasing evidence that sleep fragmentation can have important effects on cognitive performance above and beyond those accounted for by changes in sleep duration. In rodents, experimental sleep fragmentation is associated with deficits in attentional set-shifting7 and visuospatial learning,8 despite only modest changes in the quantities of sleep stages. Similarly, experimental sleep fragmentation in humans is associated with decreased cognitive processing speed, and impaired reaction time independent of effects attributable to changes in the quantities of sleep states.9,10 Given this experimental evidence, it is reasonable to ask whether rest or activity fragmentation may also be associated with variation in cognitive performance in the real world.
To answer this question, we studied 700 subjects participating in the Rush Memory and Aging Project, a community-based study of aging and cognition. We obtained up to 11 days of activity recordings in subjects' home environments, and quantified the local fragmentation of rest and activity using a recently developed state-transition analysis yielding metrics of rest fragmentation (kRA) and activity fragmentation (kAR) distinct from more conventional analyses of actigraphic data.3 We then tested for associations between these measures and performance in 5 cognitive domains.
METHODS
Subjects
The Rush Memory and Aging Project (MAP) is an ongoing community-based cohort study of aging that began recruitment in 1997.11 More than 1400 participants have completed their baseline evaluation. Participants agreed to annual detailed clinical evaluations. All evaluations were performed in participants' own residences to reduce burden and enhance follow-up. Because the actigraphy data were not collected until 2005, actigraphy was not available for all Memory and Aging Project participants. The present study examined the first actigraphic recording for all participants. Records with < 2 full days of data were excluded. At the time of these analyses, actigraphy data had been collected from 732 individuals. Of these, we excluded 10 subjects with < 2 days of recording and 22 who had incomplete cognitive testing at the time of recording. This left 700 subjects available for analysis. Participants wore the actigraphs for a mean (SD) of 9.0 (1.2) days.
Statement of Ethics Approval
The study was conducted in accordance with the latest version of the Declaration of Helsinki and was approved by the Institutional Review Board of Rush University Medical Center. Written informed consent was obtained from all subjects.
Actigraphic Recordings
The actigraph used in this study was the Actical (Phillips Respironics, Bend, OR). The Actical is a wristwatch-like accelerometer that continuously measures acceleration primarily in an axis parallel to the face of the device, rectifies this signal, summates the resultant signal across time, and then digitally samples and records this sum as a “count” for each 15-sec period—referred to hereafter as one “epoch.” The actigraphs were placed on subjects' non-dominant wrists by study staff. Participants were instructed to leave the device on their wrist until study staff returned to remove them in about 10 days.
Quantification of the Local Fragmentation of Rest and Activity
Actigraphic data were downloaded onto a PC, and analyses were performed using algorithms implemented in the MATLAB language (Mathworks, Natick, MA).
MAP participants were asked to keep the actigraphs on during the entire recording period, irrespective of bathing, swimming, exercise, or outings. To decrease participant burden, and to allow inclusion of persons with cognitive impairment in the analyses, they were not asked to keep a sleep diary or diary of device removals. To guard against analyzing periods during which the actigraph had been inadvertently removed, we flagged periods with > 4 consecutive h of immobility, and excluded the entire 24-h period surrounding this from the primary analysis; 1.9% of the total available person-days of recording were excluded for this reason, leaving 700 records with 5697 person-days of recording available for analysis. We also repeated the primary analyses after excluding periods of immobility > 45 min, to account for even shorter periods of potential inadvertent actigraph removal.
We quantified the fragmentation of rest and activity in each record as described previously.3 Briefly, we conceptualized each actigraphic record as a time-series of the binary states of rest and activity, and characterized the probabilities of transitions between these states. Operationally, we classified each 15-sec epoch as either rest (R), if the number of counts in the epoch was 0, or as active (A), if the number of counts in the epoch was > 0. We defined pAR(t) = P(R|At) as the conditional probability that an individual would be resting at time (t+1), given that the individual had been continuously active for the preceding t epochs. Similarly, we defined pRA(t) = P(A|Rt) as the conditional probability that a given individual would be active at time (t+1), given that the individual had been continuously resting. pRA(t) and pAR(t) were estimated from each record as described previously. In order to better identify temporal trends, estimates of pRA(t) and pAR(t) from each record were smoothed using nonparametric local regression (LOWESS regression), as previously described.3 We divided each plot into 3 regions—falling, constant, an rising regions, operationally defining the constant region as the longest stretch within which the LOWESS curve varied by no more than one standard deviation of the corresponding pAR(t) or pRA(t) curve.
We defined kRA as a weighted mean of the transition probability estimates in the constant region of the pRA(t) curve, and kAR as the weighted mean of the mean transition probability estimates in the constant region of the pAR(t) curve. Weights were proportional to the square root of the number of runs contributing to each probability estimate.
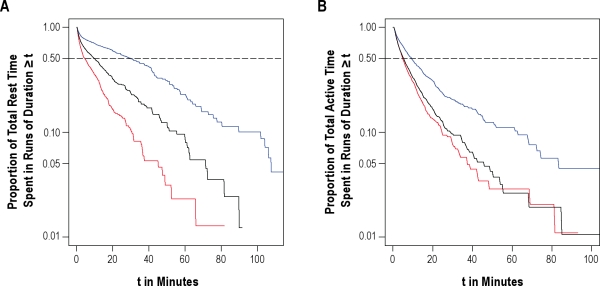
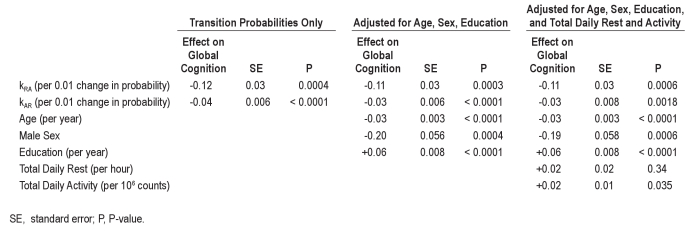
As defined above, kRA is a metric of the fragmentation of periods of sustained rest, and kAR is a metric of the fragmentation of periods of sustained activity. The lower the kRA or kAR, the greater the proportion of total rest or activity time spent in longer runs of rest or activity. As an illustration of this, in Figure 1A, we plot data from 3 representative individuals with kRA = 0.016, kRA = 0.026 (the median kRA in our study population), and kRA = 0.036. This figure depicts the proportion of total rest time spent in runs of duration t or greater, for different values of t. The value t50 refers to the run duration beyond which 50% of total rest time occurs—i.e., a t50 of 31 min indicates that 50% of total rest time occurs in runs of rest of duration ≥ 31 min. As illustrated in Figure 1A, as kRA increases from 0.016 to 0.036, the proportion of total rest time spent in longer runs of rest decreases substantially, and the t50 decreases from 31 min to 5 min. Figure 1B shows a similar plot illustrating the effect of kAR on the distribution of runs of activity, with 3 individuals with kAR = 0.047, kAR = 0.057 (the median kAR in our study population), and kAR = 0.067.
Figure 1.
Data from 6 example individuals illustrating the relationship between kRA or kAR and proportion of total rest or activity spent in runs of duration t or greater, for different values of t. (A) Runs of rest; blue line, kRA = 0.016, t50 = 31 min; black line, kRA = 0.026, t50 = 10 min; red line, kRA = 0.036, t50 = 5 min. (B) Runs of activity; blue line, kAR = 0.047, t50 = 5 min; black line, kAR = 0.057, t50 = 2.8 min; red line, kAR = 0.067, t50 = 2.5 min.
Other Actigraphic Metrics
In addition to the above analysis, for each record we also calculated the average total daily activity in counts2 and the number of hours of rest per day. Conventional algorithms for the inference of sleep/wake state from actigraphic data rely on determining whether a moving weighted average of counts within a certain window size does or does not exceed a predefined threshold. The optimal threshold and weights used are specific to each model of actigraph and to each particular study population and therefore need to be determined anew for each new device and each new sample; this has not been done for the Actical device when worn on the wrist or when used by individuals as old as those in the current study. Because the appropriate weights and threshold were thus unavailable, we did not use these algorithms in the present study.
Cognitive Evaluation and Diagnosis
Trained technicians administered 21 cognitive tests, 19 of which were used to document 5 cognitive domains as described previously12 and as listed in Table 1. Composite measures of 5 cognitive domains (episodic, semantic, and working memory, perceptual speed, and visuospatial ability) were created by converting each test into a z-score based on the mean and standard deviation for that test among all MAP participants at baseline, and averaging the z-scores for each of the tests within each domain. These measures were constructed such that 0 represents the mean score of all MAP participants at baseline, positive scores indicate better performance, and 1 unit represents approximately 1 standard deviation of performance. A composite measure of global cognition was made by averaging the z-scores of all 19 tests.
Table 1.
Characteristics of the study subjects
Individuals were classified as having dementia or not as previously described.13 Briefly, the results of cognitive tests were reviewed by a neuropsychologist to determine the presence or absence of cognitive impairment. A clinician then combined all available cognitive and clinical data to determine whether the subject was demented or non-demented according to the NINDS-ADRDA criteria requiring a history of cognitive decline and evidence of impairment in ≥ 2 cognitive domains.14
Measurement of Demographic and Clinical Variables
Age was computed from the self-reported date of birth and the date of actigraphy. Years of education was recorded at the time of the baseline interview. Sex was recorded at the time of the baseline interview.
We summarized vascular risk factors as a sum of hypertension, diabetes mellitus, and smoking, as assessed by clinical history. Vascular disease burden was the sum of myocardial infarction, congestive heart failure, claudication, and stroke, as assessed by clinical history.11 To assess joint pain, participants were asked whether or not they experienced any ache or pain in the joints most days in a month.
Depressive symptoms were assessed with a 10-item version of the Center for Epidemiologic Studies-Depression Scale.11
Subjects were classified by a clinician as having Parkinson disease or not using CAPIT criteria.15
The frequency of nighttime sleep disturbance was assessed using a single question: “How often are you troubled by waking up during the night?” The frequency of daytime sleepiness was assessed using a single question “How often do you get so sleepy during the day or evening that you have to take a nap?” For both questions, the possible answers were never, rarely, sometimes, often, or very often. For the purposes of the statistical analyses, both variables were dichotomized into rarely or never vs. sometimes, often, or very often.
Statistical Analyses
We tested for associations between the transition metrics kAR and kRA and the outcome of composite global cognition using multiple linear regression. This was performed in 3 stages. We first performed an unadjusted analysis for associations between the measures kRA and kAR, and the outcome of composite global cognition. We then augmented this model by adjusting for the effects of age, sex, and education. Next, we added total daily activity counts and total hours of rest per day as covariates to the model with age, sex, education, kRA, and kAR. These were our primary analyses. To examine the role of medical comorbidities, we further augmented this model to include the number of depressive symptoms, number of vascular risk factors, number of vascular diseases, and presence or absence of joint pain. Finally, to examine whether any observed effects are mediated by self-report nocturnal sleep disturbance or daytime sleepiness, the model was further augmented to include these variables as predictors. To allow for the possibility of nonlinear relationships, quadratic terms for kAR and kRA were initially included but removed if their partial F-test P-values were > 0.01. A threshold of 0.01 rather than 0.05 was used in order to favor parsimony in the construction of the model. Neither the quadratic term for kAR (P = 0.37) nor the quadratic term for kRA (P = 0.62) was significant by this criterion, so both were excluded. Interaction terms between sex and kRA or kAR were used in initial models but dropped if their partial F-test P-values were > 0.01. Neither interaction term was significant (P = 0.22 for the interaction term between kAR and sex; P = 0.42 for the interaction term between kRA and sex); so these interaction terms were excluded from the models, and men and women were pooled for all analyses.
To ensure that any observed associations were not artificially driven by inclusion in our data of brief periods of actigraph removal, we then repeated our primary analyses after excluding periods of immobility ≥ 45 minutes.
To ensure that any observed associations were not solely driven by subjects with Parkinson Disease (n = 8), Dementia (n = 63), or both (n = 1), we then repeated our primary analyses excluding subjects with these conditions.
Next we tested for associations between kRA, kAR, and performance in specific cognitive domains to explore the possibility that there was a characteristic pattern of cognitive performance. For each of the 5 cognitive domains, we constructed a multiple linear regression model with kRA, kAR, age, sex, and years of education as predictors, and the cognitive domain composite score as outcome. We then added total daily activity and hours of rest per day as covariates to each of these models.
For all regression models, residual plots were examined to confirm the assumption of homogeneous variance, and partial regression plots were visually examined to assess for the qualitative adequacy of the shape of the proposed models. Cook's distance was calculated for each subject in each regression model to identify data points exerting a disproportionate influence on the models, and points with a Cook's distance > 0.05 were manually examined to be excluded where appropriate. No point had a Cook's distance > 0.05, indicating that no individual outliers unduly influenced the models.
All statistical analyses were performed using the R programming language16 or SAS 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
Descriptive Measures
Data from 700 participants were included in this study. On average, the duration of these recordings was 9.0 (1.2) days. The average age of the participants was 82.4 years, and 25% were male. The clinical, actigraphic, and cognitive characteristics of the participants are shown in Table 1.
Associations between Rest-Activity Fragmentation and Composite Global Cognition
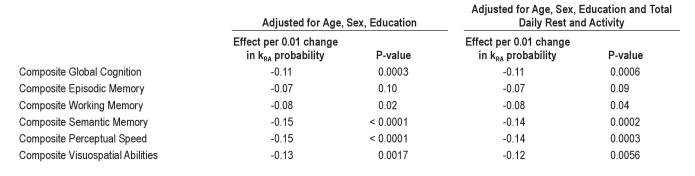
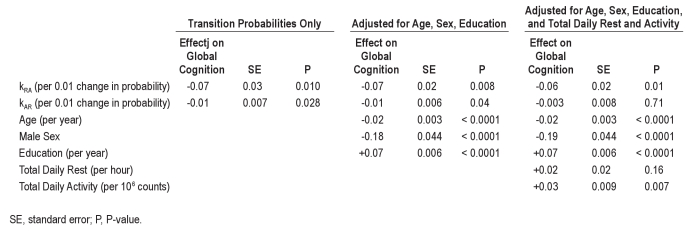
In the unadjusted analysis (Table 2, Columns 2-4), increased fragmentation of both rest and activity, as measured by kRA and kAR, were significantly associated with poorer composite global cognition.
Table 2.
Associations between kRA, kAR, and global cognitive composite score
In the analysis adjusted for age, sex, and education (Table 2, Columns 5-7), both kRA and kAR remained significant predictors of composite global cognition, as were age, sex, and education. Higher age, male sex, and lower education were all associated with lower composite global cognition (P < 0.001 for each of these). A 0.01 increment in the rest fragmentation metric kRA, roughly corresponding to 1 standard deviation of kRA and resulting in an effect on run distribution similar to that depicted in Figure 1A, was similar to 3.6 additional years of age (SE = 1.0) in its effect on global cognition. A 0.03 increment in the activity fragmentation metric kAR, roughly corresponding to 1 standard deviation of kAR and resulting in an effect on run distribution similar to that depicted in Figure 1B, was similar to 3.0 additional years of age (SE = 0.6) in its effect on global cognition.
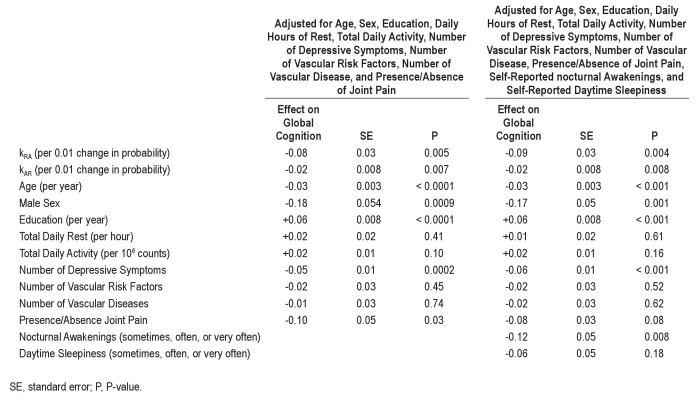
In the analysis model adjusted for total daily rest and activity in addition to age, sex, and education (Table 2, Columns 8-10), higher total daily activity was significantly associated with composite global cognition (P = 0.035), but the number of hours of rest per day was not (P = 0.34). The rest fragmentation metric kRA and the activity fragmentation metric kAR both remained significant independent predictors of composite global cognition (P = 0.00058 for kRA and P = 0.0018 for kAR), even after adjusting for total daily rest and activity. After further adjustment of the model for number of depressive symptoms, number of vascular risk factors, number of vascular diseases, and presence/absence of joint pain, the directions of the effects of kAR and kRA were unchanged, and both remained significant (P = 0.005 for kRA and P = 0.007 for kAR; Table S1 columns 2-4). Similarly, further adjustment for self-reported nocturnal awakenings and daytime sleepiness did not substantially change these results (P = 0.004 for kRA and P = 0.008 for kAR; Table S1 columns 5-7).
To ensure that any observed associations were not artificially driven by inclusion in our data of brief periods of actigraph removal, we then repeated our primary analyses after excluding periods of immobility ≥ 45 minutes. For both kAR and kRA, the directions of the effects were the same, and the P-values remained statistically significant (P = 0.0003 for kRA and P < 0.0001 for kAR).
To ensure that these associations were not driven solely by those with dementia or Parkinson Disease (which is known to be associated both with sleep-wake disturbances and with cognitive impairment), we repeated our primary analyses excluding individuals with Parkinson Disease, dementia, or both (63 participants with dementia, 8 with Parkinson Disease, and 1 with both). For both kAR and kRA, the directions of the effects were the same (Table S2), and the P-values remained statistically significant (P < 0.05), with the exception of the effect of kAR on composite global cognition in the model adjusted for total daily activity, whose P-value was nonsignificant (P = 0.71).
Associations between Rest-Activity Fragmentation and Different Cognitive Abilities
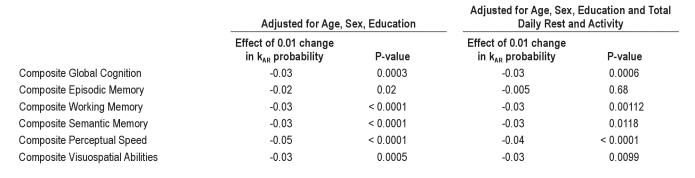
When we tested the associations between kRA and performance in a number of cognitive subdomains in analyses adjusted for age, sex, and education (Table 3, Columns 2 and 3), higher kRA was significantly associated with poorer performance in working memory, semantic memory, perceptual speed, and visuospatial abilities, and not significantly associated with performance in episodic memory. Both the effect sizes and statistical significance of these associations remained essentially unchanged after adjustment for hours of rest per day and total daily activity (Table 3, Columns 4 and 5), indicating that these associations are independent of any relationship between cognition and total daily rest or activity.
Table 3.
Associations between kRA and specific cognitive domains
When we tested the associations between the activity fragmentation metric kAR and performance in the 5 cognitive subdomains in analyses adjusted for age, sex, and education (Table 4, Columns 2 and 3), higher kAR was associated with poorer performance in all domains. The effect sizes and statistical significance of these associations remained essentially unchanged after adjustment for hours of rest per day and total daily activity (Table 4, Columns 4 and 5), with the exception of the association with episodic memory, for which the effect was attenuated and became nonsignificant.
Table 4.
Associations between kAR and specific cognitive domains
DISCUSSION
In this study of the activity-rest patterns of 700 community-dwelling older individuals, increased rest and activity fragmentation were associated with poorer cognitive function independent of demographic factors, and independent of daily hours of rest and total daily activity. Moreover, we identified a specific pattern of cognitive deficits associated with rest and activity fragmentation—namely diminished performance in perceptual speed, semantic memory, and visuospatial abilities, and working memory, with relative sparing of episodic memory.
Several studies have reported associations between a number of actigraphic metrics, and cognitive performance. We previously reported an association between actigraphically measured total daytime activity and performance on several of the same cognitive measures used in the present study,2 while others have reported associations between actigraphically inferred nocturnal wake/sleep time and performance on the Mini-Mental State Examination and the Trails B task,5 as well as associations between hour-to-hour actigraphic variability, and performance on measures of memory, executive function, and processing speed.6 Together, these results and others provide evidence for associations between global measures of rest and activity and cognitive performance. However these studies leave unanswered the question of whether the minute-to-minute fragmentation of rest and activity, as opposed to percentages or amounts of rest or activity, also are associated with cognitive performance.
This question is important for a number of reasons. First, a body of evidence from the sleep literature supports the assertion that sleep fragmentation can have deleterious effects on cognitive function in both animals and humans, beyond those accounted for by changes in total sleep or wake time.7,8,17 Second, as we showed previously,3 age itself is associated with changes in the local fragmentation of rest-activity patterns. Third, several potentially treatable clinical conditions such as sleep apnea and narcolepsy feature prominent alterations in the local fragmentation of sleep, wake, and/or locomotor behavior—alterations that in some respects are more prominent than their effects on total sleep, wake, or movement time.
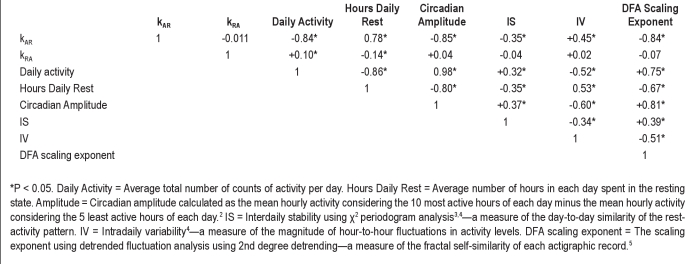
Recently, we developed a novel state-transition approach to quantifying fragmentation from actigraphic data, yielding metrics of rest fragmentation (kRA) and activity fragmentation (kAR). We showed that these metrics show characteristic associations with age, and sex, and that kRA in particular provides information distinct from a number of standard actigraphic metrics (Table S3).3
In the present study, we demonstrated that both fragmentation of rest and fragmentation activity, as measured by kRA and kAR, are indeed significantly correlated with cognitive performance. In an analysis adjusted for age, sex, and education, the effect on composite global cognition of an increase in the rest fragmentation metric kRA of 0.01 (roughly corresponding to 1 standard deviation of kRA) was similar in magnitude to that of 3.6 additional years of aging, while an increase in the activity fragmentation metric kAR of 0.03 (roughly corresponding to 1 standard deviation of kAR) was similar in magnitude to 3.0 additional years of aging. A change in kRA of this magnitude can have a considerable effect on the proportion of total rest time spent in bouts of longer duration as illustrated in Figure 1A—an important difference given the evidence from experimental sleep fragmentation that cognitive performance depends on the frequency of disruption on the experimental night.17 When we adjusted for total daily rest and activity, these associations remained significant, indicating that the relationship between kRA, kAR, and composite global cognition are above and beyond the effects of total daily rest and activity on global cognition. Further adjustment for a range of medical comorbidities did not substantially change the results, suggesting that kRA and kAR are not likely to be merely markers of underlying medical illness. Moreover, adjusting for self-reported nocturnal awakenings and daytime sleepiness also did not substantially alter these results, indicating that the effects of kRA and kAR on cognition are not mediated solely by effects on subjectively experienced sleep fragmentation or daytime sleepiness. With regard to kRA, these effects remained significant even after excluding subjects with dementia and PD, indicating that they were not driven solely by individuals with these conditions. For kAR, these effects remained significant after excluding subjects with dementia and PD in the model unadjusted for daily hours of rest and total daily activity. However, in the model excluding subjects with dementia and PD and adjusted for hours of daily rest and activity, the effect of kAR on cognition was attenuated. We think that this reflects our previous observation3 that in subjects without dementia or PD, kAR is quite strongly correlated with total daily activity (r = −0.84); this in turn reflects the likelihood that kAR, as a determinant of the duration of runs of activity, is also an important determinant of total daily activity. Thus, in subjects without PD and dementia, we suspect that total daily activity may mediate the effect of kAR on composite global cognition.
In experimental settings, the effects of sleep fragmentation on cognitive performance are not the same across cognitive domains, but rather preferentially involve specific domains. Experimental sleep fragmentation in rodents has been shown to result in deficits in spatial learning8 as well as attentional set shifting,7 while experimental sleep fragmentation in humans has been shown to result in deficits in reaction time and processing speed.9,10 Moreover, functional neuroimaging studies in humans following sleep deprivation show particular involvement of frontal and parietal structures.18 In the present study, the rest fragmentation metric kRA was associated with performance on tasks of working memory, perceptual speed, semantic memory, and visuospatial tasks, but not on episodic memory tasks. This pattern of cognitive performance overlaps substantially with that seen in experimental sleep fragmentation and is in keeping with the predilection of sleep deprivation to result in functional imaging changes in the frontal and parietal lobes, which are more associated with processing speed, working memory, visuospatial function, and semantic memory than with episodic memory. This suggests to us that that kRA may in part be a marker of sleep fragmentation and that its association with cognitive performance may in part be reflective of experimentally described associations between sleep fragmentation and cognitive performance. That adjustment for self-reported nocturnal awakenings did not significantly attenuate the effect of kRA on cognition is not out of keeping with this possibility. Rather, it may be that self-reported nocturnal awakenings and rest fragmentation as measured by kRA are measuring different aspects of nocturnal sleep fragmentation.
One previous study examined actigraphically inferred sleep in relation to cognitive performance in a cohort of older women.5 Although this study did not include direct measures of the local fragmentation of rest and activity, and it considered only the nocturnal component of the actigraphic records, it did find an association between actigraphically inferred sleep efficiency and cognitive performance. This fits well with our findings. One expected effect of having a higher kRA would be a relatively higher amount of activity during periods dominated by rest, which may be reflected by lower actigraphically inferred sleep efficiency and higher actigraphically inferred wake time after sleep onset. Thus, we suspect that the findings in this previous study of an association between sleep efficiency and cognitive performance reflects the fact that actigraphically inferred sleep efficiency is an indirect marker of kRA.
The causal direction of associations between the temporal organization of rest-activity patterns and cognitive performance remains unclear. There are three possible scenarios. In the first, increased fragmentation of rest and activity is a marker of underlying neurodegeneration, but does not cause it. In the second, increased fragmentation of rest and activity potentiate neurological deterioration, which in turn leads to cognitive impairment. In the third, increased fragmentation of rest and activity leads directly to cognitive impairment, without affecting neurodegenerative processes per se. Establishing the temporal relationships between changes in rest-activity fragmentation and changes in cognitive function may be helpful in distinguishing the relative contributions of these three processes. Meanwhile, careful clinico-pathological correlation studies will help to illuminate the pathological basis for changes in rest-activity fragmentation and their relationship with cognitive function.
A number of limitations to our analysis merit mention. The cross-sectional nature of the present analysis precludes conclusions about the direction of causation. In addition, although the nature of our cohort provides a window into a fast growing and important demographic group—primarily women aged 80 and over—it also means that our results strictly speaking apply only to older individuals, and similar analyses will need to be carried out in other subject populations for the results to be more generally applicable. Finally, our metrics shed little light on the underlying causes of changes in the fragmentation of rest and activity in older people. We cannot, for instance, distinguish fragmentation of rest and activity due to environmental factors such as environmental noise from alterations due to biological factors such as obstructive sleep apnea, depression, or neurodegenerative processes.
Notwithstanding these limitations, we conclude that both increased fragmentation of rest and increased fragmentation of activity are associated with poorer cognitive performance independent of total amounts of rest or activity and independent of demographic factors. Moreover, we identify a pattern of cognitive deficits associated with rest and activity fragmentation—namely, diminished performance in perceptual speed, semantic memory, and visuospatial abilities, with relative sparing of episodic memory. Taken as a whole, these results demonstrate that local rest and activity fragmentation are clinically important characteristics of rest-activity patterns that correlate with cognitive performance in older individuals, and may serve as potential biomarkers, predictors, or even therapeutic targets for cognitive performance in older individuals.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Bennett has received research support from Danone, Inc. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors acknowledge Dr. Ary Goldberger for his invaluable comments, Ms. Wenqing Fan for programming assistance, and Mr. Gregory Klein for assistance with data management. Funding Sources: Canadian Institutes of Health Research Bisby Fellowship; American Academy of Neurology Clinical Research Training Fellowship; Dana Foundation Clinical Neuroscience Grant; James S. McDonnell Foundation; NIH grants R01NS072337 R01AG17917, R01AG24480, K99AG030677, P01AG09975, and U01EB008577.
SUPPLEMENTAL MATERIAL
Table S1.
Associations between kAR, kRA, and global cognitive composite score adjusting for medical comorbidities and self-reported sleep complaints (n = 700; same study subjects as Table 1)
Table S2.
Associations between kAR, kRA, and global cognitive composite score in subjects without dementia or Parkinson Disease (n = 628)
Table S3.
Spearman correlation matrix of actigraphic metrics (modified with permission from1)
REFERENCES
- 1.Huang YL, Liu RY, Wang QS, Van Someren EJ, Xu H, Zhou JN. Age-associated difference in circadian sleep-wake and rest-activity rhythms. Physiol Behav. 2002;76:597–603. doi: 10.1016/s0031-9384(02)00733-3. [DOI] [PubMed] [Google Scholar]
- 2.Buchman AS, Wilson RS, Bennett DA. Total daily activity is associated with cognition in older persons. Am J Geriatr Psychiatry. 2008;16:697–701. doi: 10.1097/JGP.0b013e31817945f6. [DOI] [PubMed] [Google Scholar]
- 3.Lim AS, Yu L, Costa MD, et al. Quantification of the fragmentation rest-activity patterns in elderly individuals using a state transition analysis. Sleep. 2011;34:1569–81. doi: 10.5665/sleep.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson RS, Leurgans SE, Boyle PA, Schneider JA, Bennett DA. Neurodegenerative basis of age-related cognitive decline. Neurology. 2010;75:1070–8. doi: 10.1212/WNL.0b013e3181f39adc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006;61:405–10. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- 6.Oosterman JM, van Someren EJ, Vogels RL, Van Harten B, Scherder EJ. Fragmentation of the rest-activity rhythm correlates with age-related cognitive deficits. J Sleep Res. 2009;18:129–35. doi: 10.1111/j.1365-2869.2008.00704.x. [DOI] [PubMed] [Google Scholar]
- 7.McCoy JG, Tartar JL, Bebis AC, et al. Experimental sleep fragmentation impairs attentional set-shifting in rats. Sleep. 2007;30:52–60. doi: 10.1093/sleep/30.1.52. [DOI] [PubMed] [Google Scholar]
- 8.Tartar JL, Ward CP, McKenna JT, et al. Hippocampal synaptic plasticity and spatial learning are impaired in a rat model of sleep fragmentation. Eur J Neurosci. 2006;23:2739–48. doi: 10.1111/j.1460-9568.2006.04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonnet MH. Effect of sleep disruption on sleep, performance, and mood. Sleep. 1985;8:11–9. doi: 10.1093/sleep/8.1.11. [DOI] [PubMed] [Google Scholar]
- 10.Bonnet MH. Performance and sleepiness following moderate sleep disruption and slow wave sleep deprivation. Physiol Behav. 1986;37:915–8. [PubMed] [Google Scholar]
- 11.Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25:163–75. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- 12.Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. J Int Neuropsychol Soc. 2005;11:400–7. [PubMed] [Google Scholar]
- 13.Bennett DA, Schneider JA, Aggarwal NT, et al. Decision rules guiding the clinical diagnosis of Alzheimer's disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006;27:169–76. doi: 10.1159/000096129. [DOI] [PubMed] [Google Scholar]
- 14.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 15.Langston JW, Widner H, Goetz CG, et al. Core assessment program for intracerebral transplantations (CAPIT) Mov Disord. 1992;7:2–13. doi: 10.1002/mds.870070103. [DOI] [PubMed] [Google Scholar]
- 16.R Development Core Team. R: A language and environment for statistical computing. Vienne, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 17.Bonnet MH. Performance and sleepiness as a function of frequency and placement of sleep disruption. Psychophysiology. 1986;23:263–71. doi: 10.1111/j.1469-8986.1986.tb00630.x. [DOI] [PubMed] [Google Scholar]
- 18.Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9:335–52. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
SUPPLEMENTARY REFERENCES
- 1.Lim AS, Yu L, Costa MD, et al. Quantification of the fragmentation rest-activity patterns in elderly individuals using a state transition analysis. Sleep. 2011;34:1569–81. doi: 10.5665/sleep.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oosterman JM, van Someren EJ, Vogels RL, Van Harten B, Scherder EJ. Fragmentation of the rest-activity rhythm correlates with age-related cognitive deficits. J Sleep Res. 2009;18:129–35. doi: 10.1111/j.1365-2869.2008.00704.x. [DOI] [PubMed] [Google Scholar]
- 3.Sokolove PG, Bushell WN. The chi square periodogram: its utility for analysis of circadian rhythms. J Theor Biol. 1978;72:131–60. doi: 10.1016/0022-5193(78)90022-x. [DOI] [PubMed] [Google Scholar]
- 4.Witting W, Kwa IH, Eikelenboom P, Mirmiran M, Swaab DF. Alterations in the circadian rest-activity rhythm in aging and Alzheimer's disease. Biol Psychiatry. 1990;27:563–72. doi: 10.1016/0006-3223(90)90523-5. [DOI] [PubMed] [Google Scholar]
- 5.Hu K, Ivanov P, Chen Z, Hilton MF, Stanley HE, Shea SA. Non-random fluctuations and multi-scale dynamics regulation of human activity. Physica A. 2004;337:307–18. doi: 10.1016/j.physa.2004.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Associations between kAR, kRA, and global cognitive composite score adjusting for medical comorbidities and self-reported sleep complaints (n = 700; same study subjects as Table 1)
Table S2.
Associations between kAR, kRA, and global cognitive composite score in subjects without dementia or Parkinson Disease (n = 628)
Table S3.
Spearman correlation matrix of actigraphic metrics (modified with permission from1)