Abstract
Background:
Self-reported long habitual sleep durations (≥ 9 h per night) consistently predict increased mortality. We compared objective sleep parameters of self-reported long versus normal duration sleepers to determine whether long sleepers truly sleep more or have an underlying sleep abnormality.
Methods:
Older men participating in the Osteoporotic Fractures in Men Study (MrOS) were recruited for a comprehensive sleep assessment, which included wrist actigraphy, overnight polysomnography (PSG), and a question about usual nocturnal sleep duration.
Results:
Of the 3134 participants (mean age 76.4 ± 5.6; 89.9% Caucasian), 1888 (60.2%) reported sleeping 7-8 h (normal sleepers) and 174 (5.6%) reported ≥ 9 h (long sleepers). On actigraphy, long sleepers spent on average 63.0 min more per night in bed (P < 0.001), slept 42.8 min longer (P < 0.001), and spent 6.8 min more per day napping (P = 0.01). Based on PSG, the apnea hypopnea index, periodic limb movement index, arousal index, and sleep stage distribution did not differ. After adjusting for differences in demographics, comorbidities, and medication usage, self-reported long sleepers continued to spend more time in bed and sleep more, based on both actigraphy and PSG. Each additional 30 min in bed or asleep as measured by actigraphy increased the odds of being a self-reported long-sleeper 1.74-fold and 1.33-fold, respectively (P < 0.001 for both).
Conclusions:
On objective assessment, self-reported long sleepers spend more time in bed and more time asleep than normal duration sleepers. This is not explained by differences in comorbidity or sleep disorders.
Citation:
Patel SR; Blackwell T; Ancoli-Israel S; Stone KL. Sleep characteristics of self-reported long sleepers. SLEEP 2012;35(5):641-648.
Keywords: Sleep duration, long sleeper, actigraphy, polysomnography
INTRODUCTION
Epidemiologic studies have consistently reported a U-shaped association between self-reported habitual sleep duration and adverse health outcomes such as mortality, incident heart disease, diabetes, and obesity.1,–6 While a large body of experimental work with short-term sleep deprivation has helped provide understanding of the mechanisms by which short habitual sleep times may adversely impact health,7,–9 few experimental studies have provided insight into how long habitual sleep times are related to disease. In fact, several studies using objective measures of sleep quantity have failed to find an association between long sleep and adverse health,10,11 suggesting the adverse effect attributed to self-reported long sleep may not be due to increased sleep duration per se. The poor correlation between self-reported sleep duration and objectively measured sleep has lent further support to this contention.11,12 It is therefore unclear what long self-reported sleep time represents. One possibility is that it may represent the individual's perception of an increased time in bed secondary to sleep disorders such as sleep apnea without an actual increase in sleep time. Another possibility is that a report of long habitual sleep duration may reflect a general lack of well-being not specific to sleep.13 While prior work has sought to identify demographic characteristics and comorbidities that predicted long self-reported sleep,14 little research has been done to investigate whether there are abnormalities in the objective measures of sleep in self-reported long sleepers. In this work, we analyzed data from a large cohort of men participating in the Osteoporotic Fractures in Men Study (MrOS) who had undergone detailed sleep phenotyping to characterize differences in the sleep characteristics of self-reported long and normal duration sleepers.
METHODS
Study Population
During the baseline examination (2000-02) for the Osteoporotic Fractures in Men Study (MrOS), 5994 community-dwelling men ≥ 65 years were enrolled at 6 clinical centers in the United States: Birmingham, Alabama; Minneapolis, Minnesota; Palo Alto, California; Pittsburgh, Pennsylvania; Portland, Oregon; and San Diego, California. Men were not eligible to participate if they reported bilateral hip replacement or required the assistance of another person in ambulation at the baseline examination. Further details on the MrOS cohort have been previously published.15,16
An ancillary study, the MrOS Sleep Study, conducted between December 2003 and March 2005, recruited 3135 of the MrOS participants for a comprehensive sleep assessment, of whom 3058 underwent actigraphy and 2911 underwent in-home overnight polysomnography (PSG). Of the 2859 participants who did not participate in the sleep study; 1997 were unwilling, 332 were not screened because recruitment goals were met, 344 died before the sleep study visit, 150 were ineligible due to exclusion criteria, and 36 withdrew from the study before the sleep visit. The protocols for the MrOS and MrOS Sleep studies were approved by the institutional review boards at all of the participating institutions. All participants provided written informed consent.
Self-Reported Sleep
Self-reported habitual sleep duration was obtained by response to the question, “On most nights, how many hours do you sleep each night?” with responses rounded to the nearest hour. Responses categorized men into short duration (≤ 6 h), normal duration (7-8 h), and long duration (≥ 9 h) sleepers. Of note, defining 7- to 8-h sleepers as normal was not meant to imply the other groups are abnormal. All but one of the 3135 men responded to the question on sleep duration. Analyses were limited to a comparison between the 174 self-reported long duration and 1888 normal duration sleepers.
Self-reported information about sleepiness was assessed using the Epworth Sleepiness Scale (ESS).17 An ESS score > 10 defined excessive daytime sleepiness.18 The Pittsburgh Sleep Quality Index (PSQI) assessed sleep quality, with a PSQI score > 5 defining poor quality sleep.19 The Functional Outcomes of Sleep Questionnaire (FOSQ) assessed sleep-related quality of life.20
Actigraphy
Average nightly sleep duration was obtained using wrist actigraphy (Sleepwatch-O, Ambulatory Monitoring, Inc., Ardsley NY) in 3058 participants. Subjects were asked to wear the actigraph ≥ 5 nights. Average use (SD) was 5.2 (0.9) nights. Data were collected continuously and stored in 1-min epochs. The digital integration mode of analysis, which has been validated against polysomnography in this cohort,21 was used to distinguish sleep from wake. Action W-2 software (Ambulatory Monitoring, Inc.) was used to analyze the raw data,22 and the University of California San Diego (UCSD) scoring algorithm was used to determine sleep/wake status.23 Participants completed sleep diaries for the time period they wore the actigraph. The diaries included time into and time out of bed and times when the actigraph was removed. This information was used in editing the actigraphy data files to set intervals for when the participant was in bed trying to sleep (after “lights off”), and to delete time when the actigraph was removed. Inter-scorer reliability for editing the actigraphy data files has been previously found to be high in our group (intra-class coefficient = 0.95), and this measure has been shown to have good concordance with total sleep time from polysomnography.24 Sleep as computed by the automated UCSD sleep scoring algorithm that occurred outside of time in bed was scored as nap time.
Variables estimated from actigraphy included: (1) total sleep time (TST): the hours per night spent sleeping while in bed after “lights off”; (2) time in bed (TIB): the time from “lights off” to the time the participant got out of bed; (3) total nap time (TNT): the minutes scored as sleeping between the last time out of bed in the morning and time to bed at night; (4) sleep efficiency (SE): the percentage of time in bed after “lights off” spent sleeping; (5) wake after sleep onset (WASO): minutes of wake after sleep onset during the time in bed interval; (6) time of sleep onset defined as the first point at which the participant achieved a 20-min continuous block of sleep after “lights off”; (7) time of sleep offset: the time of the last minute scored as sleep during the time in bed interval; and (8) sleep period midpoint: the point halfway from sleep onset to sleep offset. All exposure variables from actigraphy reflect data averaged over all nights they wore the device in order to obtain a more representative characterization of usual sleep patterns.
Polysomnography
In-home sleep studies, using unattended polysomnography (Safiro unit; Compumedics, Melbourne, Australia), were performed in 2911 MrOS participants. The recording montage included C3/A2 and C4/A1 electroencephalography (EEG), bilateral electrooculography, bipolar submental electromyography, thoracic and abdominal respiratory effort, airflow (nasal-oral thermocouple and nasal pressure), finger pulse oximetry, electrocardiography, body position, and bilateral leg movements. Centrally trained and certified staff performed home visits to set up the unit, verify the values of the impedances for each channel, confirm calibration of position sensors, and note any problems encountered during set-up—similar to the protocol used in the Sleep Heart Health Study.25 Staff collected the equipment the next morning and transmitted the data to the Central Sleep Reading Center (Cleveland, OH) to be scored by certified research polysomnologists. PSG data quality was excellent, with a failure rate < 4%, and > 70% of studies graded as being of excellent or outstanding quality.
Sleep period was the time from reported lights off to morning awakening. TST, TIB, SE, and WASO were defined similar to the actigraphy parameters. Sleep staging was performed using standard criteria.26 Sleep stages were expressed as percentage of sleep time in each stage. SE was defined as the time asleep divided by the sleep period time. Arousals were scored according to American Sleep Disorder Association criteria.27 The arousal index was defined as the number of EEG arousals per hour of sleep. Apneas were defined as a complete or almost complete cessation of airflow > 10 seconds. Hypopneas were defined as a > 30% reduction in amplitude of either respiratory effort or airflow > 10 sec associated with ≥ 4% oxygen desaturation. The apnea hypopnea index (AHI) was computed as the average number of apneas and hypopneas per hour of recorded sleep. Periodic leg movements were scored according to AASM criteria (≥ 4 consecutive 0.5- to 5-sec movements, each separated by 5-90 sec).28 Leg movements that occurred at the termination of respiratory events were not considered unless they were part of a cluster of ≥ 4 leg movements in which ≥ 2 leg movements occurred independently of respiratory event termination. Periodic leg movement with arousal (PLMA) was defined as a periodic leg movement in which an EEG arousal occurred within 3 sec of termination of the leg movement.
Covariates
Participants completed questionnaires, which included items about demographics, medical history, physical activity, smoking, and alcohol use. Caffeine consumption was estimated based on self-report of the average daily number of cups of caffeinated coffee and tea or cans of caffeinated soda consumed.29 Participants were asked to bring in all current medications used within the preceding 30 days. All prescription medications were entered into an electronic database, and each medication was matched to its ingredient(s) based on the Iowa Drug Information Service (IDIS) Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA).30 Cardiovascular disease was defined as a self-reported history of myocardial infarction, angina, congestive heart failure, coronary artery bypass surgery, coronary angioplasty, or cardiac pacemaker. The Geriatric Depression Scale (GDS) was used to assess depressive symptoms, and the standard cutoff of ≥ 6 symptoms was used to define depression.31 The level of physical activity was assessed using the Physical Activity Scale for the Elderly (PASE).32 Activity level was also measured objectively using activity count data from the actigraph. Objective activity level was defined as the median activity (counts/min) during the out of bed interval, averaged over all days the participant wore the actigraph. Cognitive functioning was assessed with the Modified Mini-Mental State (3MS) examination, with higher scores representing better cognitive functioning.33 Cognitive impairment was defined as 3MS < 80. This threshold has been previously demonstrated to predict dementia in an elderly population.33 During the home or clinic visits, body weight was measured with a standard balance beam or digital scale, height with a wall-mounted Harpenden stadiometer (Holtain, England); these measurements were used to calculate body mass index (BMI).
Statistical Analyses
Correlations between self-reported sleep time, total sleep time by actigraphy, and total sleep time by PSG were computed using Pearson correlation coefficients. Baseline characteristics were summarized by category of sleep duration at night and differences between the sleepers of normal duration (7-8 h) and long duration (≥ 9 h) were compared using t-tests for normally distributed continuous data, Wilcoxon rank-sum tests for continuous skewed data, and χ2 tests for categorical data.
Logistic regression models were used to identify independent predictors of reporting long sleep duration compared to those with normal sleep duration. Multivariable adjustment was done including all variables that were significantly associated with self-reported long sleep duration at a P-value < 0.10 in bivariate analyses. Secondary analyses were done simultaneously including time in bed and total sleep time as covariates in the same model to identify the strongest predictor of self-reported long sleep. In addition, linear regression was performed with self-reported sleep as a categorical predictor (long duration vs. normal duration) for each of the actigraphic and PSG sleep measures, with and without adjustment for covariates.
All analyses were performed using SAS statistical software (version 9.2, SAS Institute, Inc., Cary, North Carolina).
RESULTS
A total of 3134 men responded to the question on habitual sleep duration. The distribution of responses was 13 (0.4%) reporting ≤ 3 h; 84 (2.7%) reporting 4 h; 269 (8.6%) reporting 5 h; 706 (22.5%) reporting 6 h; 1074 (34.3%) reporting 7 h; 814 (26.0%) reporting 8 h; 132 (4.2%) reporting 9 h; 34 (1.1%) reporting 10 h; 6 (0.2%) reporting 11 h; and 2 (0.1%) reporting 12 h. Overall, self-reported sleep duration was modestly correlated with both total sleep time as assessed by actigraphy (r = 0.31, P < 0.001) and PSG (r = 0.20, P < 0.001).
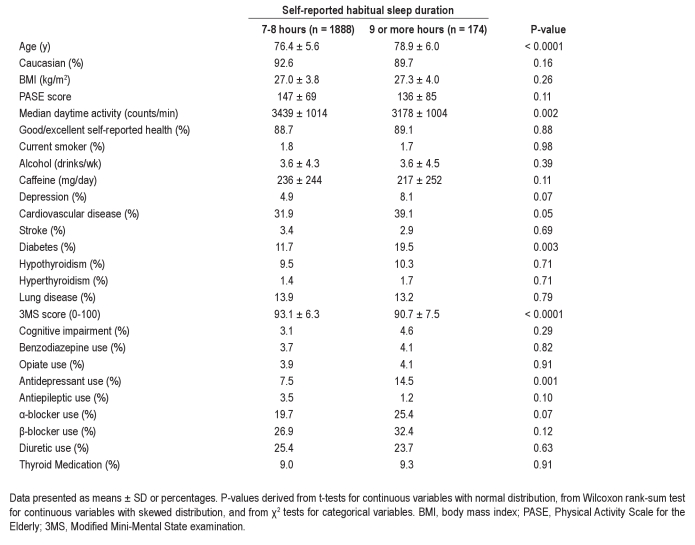
The analyses presented were limited to the 1888 men (60.2% of the overall cohort) reporting a habitual sleep duration of 7-8 h, and the 174 men (5.6%) reporting ≥ 9 h. Demographic and medical data on each group are shown in Table 1. Self-reported long sleepers were older, more likely to have diabetes, and had a lower score on the mental status examination. Although no difference was observed in self-reported activity levels, based on actigraphy, self-reported long sleepers were less physically active. There was also a trend towards more cardiovascular disease and depression among those reporting long sleep. Antidepressant use was nearly twice as common in self-reported long sleepers. In contrast, there were no significant differences in smoking, alcohol, caffeine, benzodiazepine, or opiate use.
Table 1.
Demographic characteristics by self-reported sleep duration
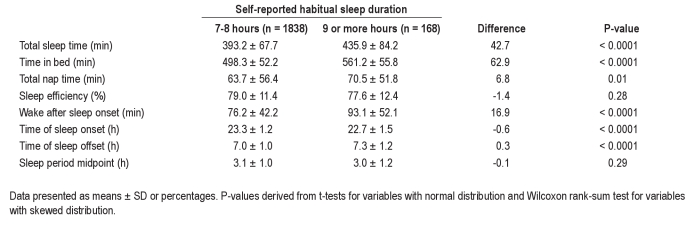
Actigraphy data are presented in Table 2. On average, self-reported long sleepers spent 43 more min asleep per night and 63 more min in bed (P < 0.0001 for both). In addition, they spent 6.8 more min per day napping (P = 0.01). Although SE did not substantially differ, WASO was 17 min greater (P < 0.0001). The increased TIB was accomplished by going to sleep earlier and awakening later, so that the midpoint of the sleep period did not differ.
Table 2.
Actigraphic sleep characteristics by reported sleep duration
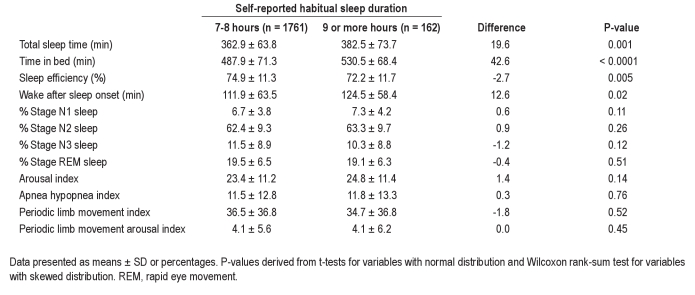
Data from the 2911 individuals with PSG are presented in Table 3. Again, self-reported long sleepers were found to spend more time asleep (P = 0.001) and more time in bed (P < 0.0001) than those reporting sleeping 7-8 h. The differences as assessed by PSG were 20 min for TST and 43 min for TIB, which were slightly smaller than those measured by actigraphy. Similar to the actigraphic assessment, WASO measured by PSG was 13 min greater in self-reported long sleepers (P = 0.02), while unlike actigraphy, SE was slightly worse (72.2% vs. 74.9%, P = 0.005). Sleep staging revealed no differences in the proportions of the various sleep stages between those reporting normal and long sleep durations. Similarly, no differences were found in terms of sleep disordered breathing, periodic limb movement severity, or the arousal index between the 2 groups.
Table 3.
Polysomnographic sleep characteristics by reported sleep duration
An interesting finding in comparing self-reported sleep duration with TST was that long sleepers tended to overestimate their sleep duration to a greater extent than normal sleepers. Relative to actigraphic TST, long sleepers overestimated sleep duration by 2.0 h vs. 0.9 h for normal sleepers (P < 0.0001). Similarly, the difference between self-reported sleep and PSG TST was 2.9 h in long sleepers vs. 1.4 h in normal sleepers (P < 0.0001).
Sleep quality as assessed by questionnaire is presented in Table 4. No differences in sleepiness (assessed by the ESS) or sleep-related quality of life (assessed by the FOSQ) were found between the 2 groups. In contrast, self-reported long sleepers reported a better sleep quality, as assessed by a lower score on the PSQI (4.6 vs. 3.8, P < 0.0001), and a lower proportion with poor sleep quality (20.1% vs. 31.9%, P = 0.001). As expected, the majority of the difference in PSQI was due to better scores on the sleep duration component of this index. However, self-reported long sleepers also scored significantly better on the sleep quality and sleep efficiency components.
Table 4.
Subjective sleep characteristics by reported sleep duration
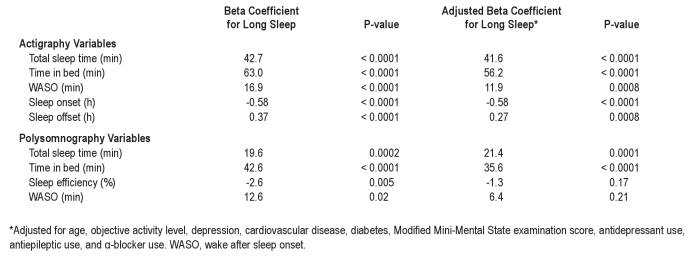
In analyses adjusted for age, activity level, history of depression, cardiovascular disease, diabetes, Mini-Mental Status score, antidepressant, antiepileptic, and α-adrenergic blocker use, self-reported long sleep continued to be associated with actigraphically measured greater TST, TIB, and WASO, as well as an earlier sleep onset and later sleep offset (Tables 5 and 6). Among the PSG sleep measures, a greater TST and a greater TIB were the only significant predictors of self-reported long sleep in adjusted analyses.
Table 5.
Predictors of long reported sleep duration
Table 6.
Long reported sleep duration predicting sleep outcomes
For both actigraphy and PSG, TST and TIB were the strongest predictors of self-reported long sleep, and these 2 measures were moderately correlated (Spearman correlation ρ = 0.55 for actigraphy and ρ = 0.57 for PSG). Additional analyses were done modeling both variables simultaneously as predictors to better understand which was most strongly associated with being a self-reported long sleeper. In the actigraphy analysis, TIB remained significant (OR = 1.71 per 30-min increase; 95% CI [1.53-1.91], P < 0.001) while TST was no longer significant (OR = 1.03 per 30-min increase; 95% CI [0.94-1.11], P = 0.57). Similarly, using the PSG measures, TIB remained a significant predictor (OR = 1.21 per 30-min increase; 95% CI [1.11-1.31], P < 0.001) but not TST (OR = 1.04 per 30-min increase; 95% CI [0.96-1.14], P = 0.34).
Additional analyses assessed the independent effect of WASO. In models including TST and WASO (either by actigraphy or PSG), both were independently associated with being a self-reported long sleeper. In models with both TIB and WASO (either by actigraphy or PSG), TIB remained an independent predictor of self-reported long sleep. In contrast, greater WASO was no longer associated with an increased likelihood of reporting being a long sleeper after adjusting for TIB.
In order to assess the robustness of findings, analyses were repeated limiting self-reported long sleepers to the 42 men reporting ≥ 10 h of sleep. Overall, the results were very similar, with TIB and TST from both actigraphy and PSG representing the 2 strongest predictors of reporting sleeping ≥ 10 h per day (P < 0.001 in adjusted analyses).
DISCUSSION
In this cohort of older men who underwent both 5 days of actigraphy and 1 night of overnight polysomnography, individuals who reported sleeping 9 hours or more, a level of sleep that has been consistently associated with adverse health outcomes, did in fact sleep more than their counterparts who reported lesser amounts of sleep. In addition, these self-reported long sleepers spent more time in bed, resulting in no difference in sleep efficiency as assessed by actigraphy but a slight reduction as assessed by PSG, although the clinical significance of this difference (72.2% vs. 74.9%) is questionable. Furthermore, after adjusting for differences in demographic and medical factors, PSG-assessed sleep efficiency no longer predicted self-reported long sleep duration. Similarly, other measures of sleep quality including arousal index and proportion of slow wave or REM sleep time did not differ between those reporting normal and long sleep durations. The diurnal phase of long sleepers also did not substantially differ—the increased amount of sleep obtained was a result of going to sleep earlier and waking up later. Finally, measures of sleep disorders such as sleep apnea and periodic limb movement disorder did not differ.
This represents one of the first studies to compare the sleep of reported long sleepers with reported normal duration sleepers. However, prior work has compared the sleep of documented long and short sleepers.34,–36 In those studies, long sleepers were also found to have a greater WASO and lower sleep efficiency. In addition, the timing of sleep onset was earlier and sleep offset was later suggesting no consistent difference in circadian phase. These results are similar to our findings comparing long sleepers with 7-8 hour self-reported sleepers.
The amount of time in slow wave sleep has been found to be similar between documented long and short sleepers suggesting the percentage of slow wave sleep goes down with increased sleep time in contrast to our findings that the percentage of slow wave sleep is similar across self-reported sleep duration. There are several possible reasons for this difference. First, our analyses were based on self-reported sleep duration as opposed to documented sleep duration. Second, the difference in PSG-measured sleep between groups was much smaller in our study, making it less likely to detect differences in sleep stages. Third, our study, in the hopes of being generalizable included all participants while prior studies only selected individuals with proven long sleep durations who had regular sleep schedules, no sleep disorders, no comorbidities, and no medication use. In addition, our study focused on an older population, as opposed to prior studies focusing on individuals in their late teens and early twenties.
Overall, our results suggest that both more time in bed and more time asleep characterize self-reported long sleepers as compared to normal duration sleepers. In fact, in models where both sleep duration and time in bed (as assessed by either actigraphy or PSG) were simultaneously included as predictors of self-reported sleep, only time in bed was found to be an independent predictor. Thus, our data suggest that long sleepers do not have grossly abnormal sleep. There are no discernible differences in sleep stage distribution, sleep fragmentation, sleep-disordered breathing, or periodic limb movement disorder between those who report long versus normal sleep durations. In addition, long sleepers had better sleep quality as assessed by the PSQI. These findings are supported by results from Aeschbach et al. which have found no underlying differences in homeostatic sleep drive between long and short sleepers.36 Their finding that long sleepers may have a circadian rhythm-driven longer biological night without difference in phase is also consistent with our results.37
Although our analyses demonstrate that self-reported sleep time is predictive of actual sleep time, our data do indicate substantial measurement error. While one would expect the difference in total sleep time between those reporting 9 or more hours of sleep and those reporting 7-8 hours to be at least an hour, the measured differences were only 43 minutes by actigraphy and 20 minutes by PSG. This overestimate of true sleep differences would tend to cause studies that relied on self-reported sleep measures to underestimate the true impact of long sleep duration on adverse outcomes. Thus, a 20-minute difference in PSG sleep duration may have important clinical ramifications on risk of obesity, diabetes, and other health outcomes associated with self-reported long sleep. Further research is needed to confirm this finding.
One explanation for the underestimate is that individuals are actually basing their self-reported sleep on time in bed rather than time asleep. In fact, the measured differences in time in bed—63 minutes as assessed by actigraphy and 43 minutes as assessed by PSG—are much more similar to the magnitude of differences in self-reported sleep time.
Another issue apparent from our findings is that self-reported sleep duration is poorly calibrated with sleep time measured by either actigraphy or PSG. Our 7-8 hour sleepers had a mean sleep duration of only 6.0 h by PSG and 6.6 h by actigraphy, and this systematic difference has been reported in other studies.12,38 Thus, the amount of sleep that should be considered a normal or long duration may need to vary depending on the measurement technique used.
Overall, our results support the contention that the adverse health impact associated with long self-reported sleep duration likely reflects an association with altered sleep habits—either an increased time spent asleep or an increased time spent in bed. Because these variables are highly correlated, it is difficult to definitively identify the most important factor with an observational study design. However, to the extent that conclusions can be made based on our multivariable models simultaneously including both variables as predictors, time in bed appeared to be the strongest predictor of self-reported sleep duration. This is supported by the finding that increased WASO predicted being a self-reported long sleeper independent of total sleep time.
An increased time in bed may be a reflection of depression or social isolation limiting opportunities to exchange sleep for other activities. In fact, each of these factors has been associated with being a long self-reported sleeper and in addition, has been associated with adverse health outcomes.14,39,40 Another possibility is that an increased time in bed may itself be the cause of behaviors such as reduced physical activity or limited socialization with others that may directly lead to poor health outcomes. Further detailed analyses of how decisions regarding time spent in bed are made and the extent to which these decisions are based on sleep need rather than non-sleep related cues will be important in better understanding whether interventions aimed at reducing time in bed might improve health outcomes, and if so, how such interventions might be designed to be maximally effective.
It should be noted that our results do not imply that long sleep duration or long time in bed are causally related to adverse health outcomes. These phenotypes may be a result of a circadian rhythm-driven longer biological night, for example, and this may be the true cause of the adverse health effects associated with long sleep. In such a case, interventions aimed at shortening sleep duration without impacting the underlying cause would not be expected to improve health outcomes and may in fact worsen them.
Given that long sleepers also overestimate their sleep duration to a greater extent than normal sleepers, another possibility is that the tendency to overestimate sleep time may be the underlying predictor of adverse health outcomes. However, the mechanism for such an effect is not clear.
Our study has many strengths, including the large sample size, objective measurement of sleep duration using both actigraphy and polysomnography, and collection of important covariates such as medication usage. Several limitations should also be recognized. Our cohort consisted exclusively of older men, and thus the extent to which these findings generalize to women or younger age groups is unknown. We cannot exclude the potential for selection bias, as the frailest subjects in the parent cohort were probably less likely to participate in the sleep assessments. In addition, we did not distinguish between weekday and weekend nights. However, given the age of the cohort, few participants were actively employed so sleep habits likely did not vary much across the week. It should be noted that actigraphic scoring of nap time has not been validated and so the differences noted in regards to napping may actually reflect differences in inactive wakefulness. Finally, most of the covariate data including medical history on comorbidities were obtained by self-report without validation.
In summary, our findings suggest that individuals who report a long habitual sleep time do not importantly differ in their sleep from those reporting normal sleep times other than the increased amount of time spent in bed and asleep. Thus, associations linking self-reported long sleep with adverse health outcomes are unlikely to be due to poor sleep quality, sleep disorders, or abnormal circadian phase. Instead, further research should be focused on understanding the causes of an increased time in bed.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Patel has accepted research support from HealthRight Products and Philips Respironics. He has been a paid consultant for SleepHealth Centers. Dr. Ancoli-Israel has been a paid consultant for Ferring Pharmaceuticals Inc., GlaxoSmithKline, Johnson – Johnson, Merck, NeuroVigil, Inc., Pfizer, Philips Respironics, and Purdue Pharma LP. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
In addition to the support below, this work was supported by National Institutes of Health grant HL081385 and AG08415. The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research under the following grant numbers: AR045580, AR045614, AR045632, AR045647, AR045654, AR045583, AG018197, AG027810, and RR024140. The National Heart, Lung, and Blood Institute (NHLBI) provides funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: HL071194, HL070848, HL070847, HL070842, HL070841, HL070837, HL070838, and HL070839.
REFERENCES
- 1.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 2.Patel SR, Ayas NT, Malhotra MR, et al. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27:440–4. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- 3.Ayas NT, White DP, Al-Delaimy WK, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26:380–4. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 4.Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–9. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 5.Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between reduced sleep and weight gain in women. Am J Epidemiol. 2006;164:947–54. doi: 10.1093/aje/kwj280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Youngstedt SD, Kripke DF. Long sleep and mortality: rationale for sleep restriction. Sleep Med Rev. 2004;8:159–74. doi: 10.1016/j.smrv.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 8.Spiegel K, Tasali E, Penev P, Van Cauter E. Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 9.Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–83. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 10.Lauderdale DS, Knutson KL, Rathouz PJ, Yan LL, Hulley SB, Liu K. Cross-sectional and longitudinal associations between objectively measured sleep duration and body mass index: The CARDIA Sleep Study. Am J Epidemiol. 2009;170:805–13. doi: 10.1093/aje/kwp230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van den Berg JF, Knvistingh Neven A, Tulen JH, et al. Actigraphic sleep duration and fragmentation are related to obesity in the elderly: the Rotterdam Study. Int J Obes (Lond) 2008 doi: 10.1038/ijo.2008.57. [DOI] [PubMed] [Google Scholar]
- 12.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19:838–45. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bliwise DL, Young TB. The parable of parabola: what the U-shaped curve can and cannot tell us about sleep. Sleep. 2007;30:1614–5. doi: 10.1093/sleep/30.12.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel SR, Malhotra A, Gottlieb DJ, White DP, Hu FB. Correlates of long sleep duration. Sleep. 2006;29:881–9. doi: 10.1093/sleep/29.7.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26:557–68. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–85. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 18.Johns MW. Sensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the Epworth sleepiness scale: Failure of the MSLT as a gold standard. J Sleep Res. 2000;9:5–11. doi: 10.1046/j.1365-2869.2000.00177.x. [DOI] [PubMed] [Google Scholar]
- 19.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 20.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–43. [PubMed] [Google Scholar]
- 21.Blackwell T, Redline S, Ancoli-Israel S, Stone K. Comparison of total sleep time from actigraphy and polysomnography in older men: The MrOS Sleep Study. Sleep. 2007;30:A346–A7. [Google Scholar]
- 22.Ardsley, NY: Ambulatory Monitoring, Inc.; Action-W User's Guide, Version 2.0. [Google Scholar]
- 23.Jean-Louis G, Kripke DF, Mason WJ, Elliot JA, Youngstedt SD. Sleep estimation from wrist movement quantified by different actigraphic modalities. J Neurosci Methods. 2001;105:185–91. doi: 10.1016/s0165-0270(00)00364-2. [DOI] [PubMed] [Google Scholar]
- 24.Blackwell T, Ancoli-Israel S, Gehrman PR, Schneider JL, Pedula KL, Stone KL. Actigraphy scoring reliability in the study of osteoporotic fractures. Sleep. 2005;28:1599–605. doi: 10.1093/sleep/28.12.1599. [DOI] [PubMed] [Google Scholar]
- 25.Redline S, Sanders MH, Lind BK, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21:759–67. [PubMed] [Google Scholar]
- 26.Rechtschaffen A, Kales A. Washington, DC: US Government Printing Office; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 27.American Sleep Disorders Association. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 28.American Academy of Sleep Medicine. Recording and scoring leg movements. The Atlas Task Force. Sleep. 1993;16:748–59. [PubMed] [Google Scholar]
- 29.Barone JJ, Roberts HR. Caffeine consumption. Food Chem Toxicol. 1996;34:119–29. doi: 10.1016/0278-6915(95)00093-3. [DOI] [PubMed] [Google Scholar]
- 30.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–11. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 31.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–73. [Google Scholar]
- 32.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 33.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–8. [PubMed] [Google Scholar]
- 34.Webb WB, Friel J. Sleep stage and personality characteristics of “natural” long and short sleepers. Science. 1971;171:587–8. doi: 10.1126/science.171.3971.587. [DOI] [PubMed] [Google Scholar]
- 35.Webb WB, Agnew HW., Jr Sleep stage characteristics of long and short sleepers. Science. 1970;168:146–7. doi: 10.1126/science.168.3927.146. [DOI] [PubMed] [Google Scholar]
- 36.Aeschbach D, Cajochen C, Landolt H, Borbely AA. Homeostatic sleep regulation in habitual short sleepers and long sleepers. Am J Physiol. 1996;270:R41–53. doi: 10.1152/ajpregu.1996.270.1.R41. [DOI] [PubMed] [Google Scholar]
- 37.Aeschbach D, Sher L, Postolache TT, Matthews JR, Jackson MA, Wehr TA. A longer biological night in long sleepers than in short sleepers. J Clin Endocrinol Metab. 2003;88:26–30. doi: 10.1210/jc.2002-020827. [DOI] [PubMed] [Google Scholar]
- 38.Silva GE, Goodwin JL, Sherrill DL, et al. Relationship between reported and measured sleep times: the Sleep Heart Health Study (SHHS) J Clin Sleep Med. 2007;3:622–30. [PMC free article] [PubMed] [Google Scholar]
- 39.Ferketich AK, Schwartzbaum JA, Frid DJ, Moeschberger ML. Depression as an antecedent to heart disease among women and men in the NHANES I study. National Health and Nutrition Examination Survey. Arch Intern Med. 2000;160:1261–8. doi: 10.1001/archinte.160.9.1261. [DOI] [PubMed] [Google Scholar]
- 40.Berkman LF, Syme SL. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am J Epidemiol. 1979;109:186–204. doi: 10.1093/oxfordjournals.aje.a112674. [DOI] [PubMed] [Google Scholar]