Abstract
Study Objectives:
Based on recent reports of the involvement of cyclic alternating pattern (CAP) in cognitive functioning in adults, we investigated the association between CAP parameters and cognitive performance in healthy children.
Design:
Polysomnographic assessment and standardized neurocognitive testing in healthy children.
Settings:
Sleep laboratory.
Participants:
Forty-two children aged 7.6 ± 2.7 years, with an even distribution of body mass percentile (58.5 ± 25.5) and SES reflective of national norms.
Measurements:
Analysis of sleep macrostructure following the R&K criteria and of cyclic alternating pattern (CAP). The neurocognitive tests were the Stanford Binet Intelligence Scale (5th edition) and a Neuropsychological Developmental Assessment (NEPSY)
Results:
Fluid reasoning ability was positively associated with CAP rate, particularly during SWS and with A1 total index and A1 index in SWS. Regression analysis, controlling for age and SES, showed that CAP rate in SWS and A1 index in SWS were significant predictors of nonverbal fluid reasoning, explaining 24% and 22% of the variance in test scores, respectively.
Conclusion:
This study shows that CAP analysis provides important insights on the role of EEG slow oscillations (CAP A1) in cognitive performance. Children with higher cognitive efficiency showed an increase of phase A1 in total sleep and in SWS
Citation:
Bruni O; Kohler M; Novelli L; Kennedy D; Lushington K; Martin J; Ferri R. The role of NREM sleep instability in child cognitive performance. SLEEP 2012;35(5):649-656.
Keywords: Children, sleep, cognitive performance, intelligence, cyclic alternating pattern
INTRODUCTION
In children, sleep is important for daytime functioning and neurocognitive performance.1 Of the various sleep parameters, a key role is thought to be played by sleep continuity/fragmentation (e.g., the frequency of stage shifts and time spent in individual sleep stages). However, despite clear evidence for an association, the correlation between traditional measures of sleep continuity/fragmentation and neurocognitive performance has typically been poor.
Therefore recent attention has focused on alternate measures, especially the microstructure of sleep and its association with neurocognitive performance. An emerging method for assessing sleep microstructure is cyclic alternating pattern (CAP) analysis.2 CAP is defined as a periodic EEG activity of NREM sleep and is thought to reflect the processes of sleep maintenance and arousability. CAP reflects periodic EEG activity during NREM sleep, which is characterized by repeated spontaneous sequences of transient events (phase A) with some characteristic features. These include a distinct pattern that is different from the background rhythm of the underlying sleep stage, an abrupt variation in amplitude, and a pattern that recurs at intervals up to 2 min. The return to background activity identifies the interval that separates the repetitive elements (i.e., phase B). The alternation between the transient events and the background electrical activity is most likely an expression of arousal instability/stability. The relative proportions of SWA and faster EEG rhythms allow the subdivision of the A-phase of CAP into A1, A2, and A3 subtypes. The A1 subtype is characterized by a predominance of high-voltage slow waves (EEG synchrony), whereas the A3 subtype has a predominance of fast low-amplitude rhythms (EEG desynchrony). The A2 subtype is a mixture of slow and fast EEG rhythms. A1 is the most common subtype of CAP, normally accounting for the majority of all CAP A-phases during normal sleep, occurring approximately 200-400 times per night.2
In adults, CAP variables have been associated with neurocognitive performance. Aricò et al.,3 in 8 healthy adults, reported that higher CAP A1 rate was correlated with higher verbal fluency, working memory, and both delayed recall and recognition of words; while higher CAP A2 and A3 were associated with worse visual/nonverbal performance. Ferri et al.,4 in 15 healthy adults, investigated the impact of sleep micro-fragmentation on spatial attention, selective attention cognitive flexibility, visuospatial processing, and inhibition/perceptuo-attentional processing. Ferri's group reported few significant relationships, but a trend for higher CAP A1 to be associated with better neurocognitive performance, and higher CAP A3 and (to lesser extent) CAP A2 to be associated with worse neurocognitive performance. In 10 healthy adults, increased CAP A1 following a learning task has been associated with improved motor learning,5 and a higher CAP rate has been reported in one young adult with superior memory.6
In healthy children, who are undergoing a rapid and critical phase of neurocognitive development, the association between CAP and cognition is less clear because of an absence of studies. CAP A1 has been positively associated with intelligence (IQ) in children with developmental disabilities but without mental retardation (e.g., Asperger syndrome and dyslexia),7,8 while children with developmental disabilities and mental retardation (e.g., Fragile X and Down syndrome) have a lower percentage of A1 and a higher percentage of A2 and A3, compared to normals.9 Similarly, a decrease of CAP rate has been reported in children with autistic spectrum disorder, this effect being most evident during SWS and mainly due to a reduction of A1 CAP subtypes.10
To date, however, our understanding of the relationship between CAP and neurocognitive performance is limited to children with developmental disorders, and the relationship in otherwise healthy children is unknown. Investigations of the association between CAP and cognitive performance in healthy children will provide great insight into the significance of sleep in development.
Based on recent reports of the involvement of CAP in cognitive functioning in young adults, and the reported association in clinical groups of children, the aims of this study were to investigate the association between CAP parameters and cognitive performance in healthy children and to observe whether the different CAP subtypes (A1, A2 and A3) show a similar pattern of association with cognitive performance with those observed in adults.
METHODS
Subjects
Healthy nonsnoring children, aged 3-12 years, underwent standard polysomnography and neurocognitive assessment after recruitment via advertisements in local health clinics and newspapers. All children spoke English as a first language, had no prior diagnosis of a sleep, learning, or psychiatric disorder, and were free from other illness or upper respiratory infection during assessment. Socioeconomic status (SES) was determined using the Australian Bureau of Statistics' Index of Relative Socio-economic Advantage/Disadvantage 2006 national census data. A higher score on this index indicates increased income and occupational skills and/or training within the geographical area of residence (collection district), with a national population mean of 1000 and standard deviation of 100. Established growth charts, corrected for age and gender, were used to determine body mass index (BMI) percentile.11
Polysomnographic Measures
The PSG montage included ≥ 4 EEG channels (C3, C4, O1, O2) referenced to the contralateral mastoid, left and right electrooculogram (EOG), chin electromyogram (submental EMG), left and right tibialis EMG, electrocardiogram (ECG). Also, thoracic and abdominal movements were recorded, and air flow pressure was measured by nasal cannula and thermistor. Oxygen saturation was recorded continuously from a transcutaneous sensor (pulse oximetry). All recordings started at the patients' usual bedtime and continued until spontaneous awakening.
Neurocognitive Assessment
The following neurocognitive tests were administered by a psychologist blinded to child status during a single session on a weekday: the Stanford Binet Intelligence Scale, 5th edition,12 and a Neuropsychological Developmental Assessment (NEPSY).13 Both tests are normed and well-validated instruments with robust validity and reliability, with standardized scores being used in all analyses. The Stanford Binet provides measures of intellectual capacity in 5 distinct domains. Each domain includes a task of a verbal nature and task of a nonverbal nature. Fluid Reasoning examines the ability to reason from the specific to the general (inductive reasoning) or, given more general information, infer a specific conclusion or implication (deductive reasoning), using information that is novel to the individual. Knowledge refers to a person's accumulated fund of general information acquired from their surrounding environment and experiences, and thus in contrast to Fluid Reasoning, represents the crystallized dimension of intelligence. An individual's numerical problem solving skills, facility with numbers, and understanding of mathematical concepts is determined by the Quantitative Reasoning domain. The assessment of Visual-Spatial processing measures the ability to determine patterns and relationships from visual objects, describe spatial orientations, and apply inductive type reasoning skills to visual information. Finally, Working Memory assesses the facility in which information stored in short-term memory is inspected, organized, and manipulated to produce a solution to a given problem. The Verbal (VIQ) and Nonverbal IQ (NVIQ) scores from the Stanford Binet are composites of the skills required to solve tasks in the 5 verbal and 5 nonverbal subtests, respectively. A global measure of intellectual ability is provided by the Full Scale IQ (FSIQ) score, which is derived from the sum of all tasks.
The NEPSY was used to obtain estimates of skill in 4 key areas of neuropsychological function: executive attention assesses inhibition, planning, and general attention; language development assesses phonological processing, receptive language comprehension, and ability to name and accomplish speeded naming; sensorimotor ability assesses visuomotor skill and fine motor coordination; and memory assesses immediate and delayed memory for verbal and nonverbal stimuli.
The level of intelligence of the mothers was assessed by using the National Adult Reading Test (NART), a widely used method in clinical settings for estimating intelligence levels of English-speaking patients.14
Sleep Architecture
Sleep stages were scored according to the standard criteria by Rechtschaffen and Kales,15 and periodic leg movements in sleep (PLMS) were scored according to standard World Association of Sleep Medicine (WASM) criteria.16 Spontaneous and respiratory cortical arousals were scored according to the criteria of the American Sleep Disorders Task Force,17 and respiratory events scored according to pediatric ventilatory criteria.18
Cyclic Alternating Pattern
CAP was scored following the criteria by Terzano et al.,19 and the following CAP parameters were measured:
CAP rate (percentage of total NREM sleep time occupied by CAP sequences);
percentage of each A phase subtype;
A1 index (number of phases A1 per hour of NREM sleep, and of S1, S2, and SWS sleep stages);
A2 index (number of phases A2 per hour of NREM sleep, and of S1, S2, and SWS sleep stages);
A3 index (number of phases A3 per hour of NREM sleep, and of S1, S2 and SWS sleep stages);
All these variables were analyzed by means of the Hypnolab 1.2 sleep software analysis (SWS Soft, Italy); all recordings were visually scored by one of the investigators, and the sleep parameters derived were tabulated for statistical analysis.
Statistical Analysis
Correlations between CAP parameters and neurocognitive task scores were assessed by means of Pearson correlation. Significant associations were explored further using hierarchical regression modeling, entering significant covariates in a first step. Data are presented as mean ± standard deviation unless otherwise indicated. Because of the relatively large number of correlations computed, effects were considered as statistically significant when P < 0.01.
RESULTS
Description of the Sample
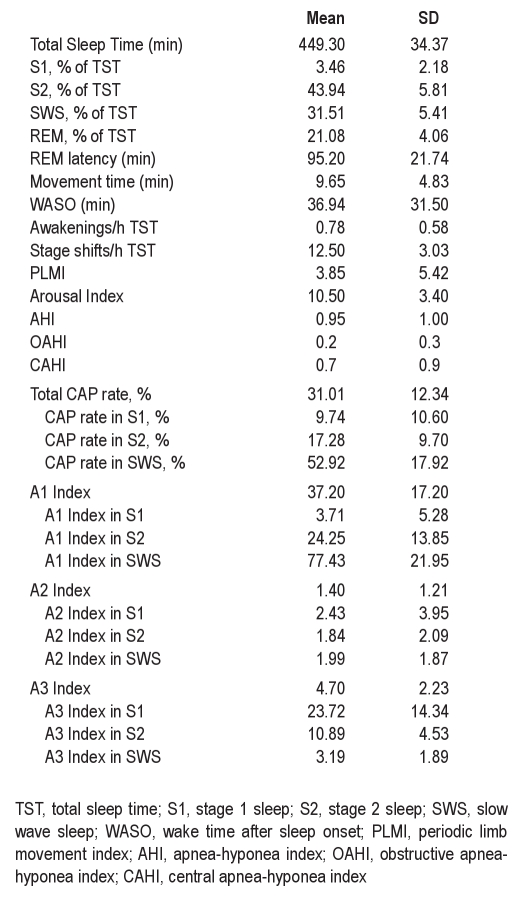
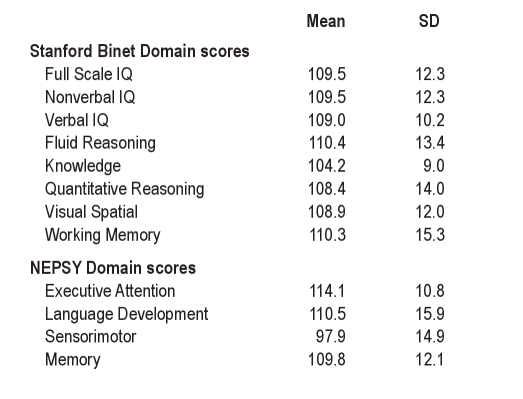
Forty-two children aged 7.6 ± 2.7 years, with an even distribution of body mass percentile (58.5 ± 25.5) and SES reflective of national norms (1007.8 ± 88.0) participated. Measures of maternal IQ were all in the normal range (range 100.8-125.6). Seventeen children were male (41%); all were Caucasian. Sleep architecture and CAP descriptive statistics are presented in Table 1. Domain scores from neurocognitive assessments were also consistent with standardized norms and are presented in Table 2.
Table 1.
Sleep macrostructure and CAP
Table 2.
Neurocognitive test scores
Correlations between Sleep Parameters and Cognitive Measures
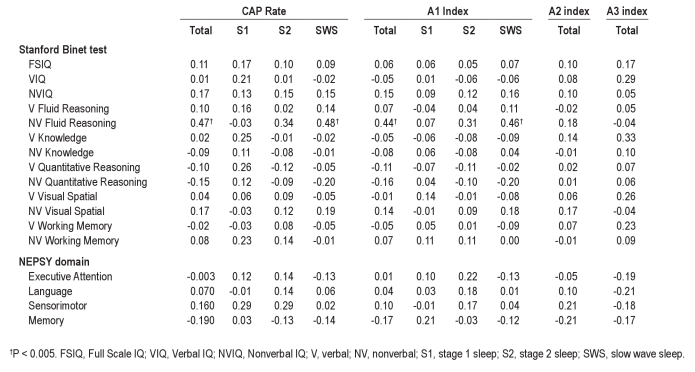
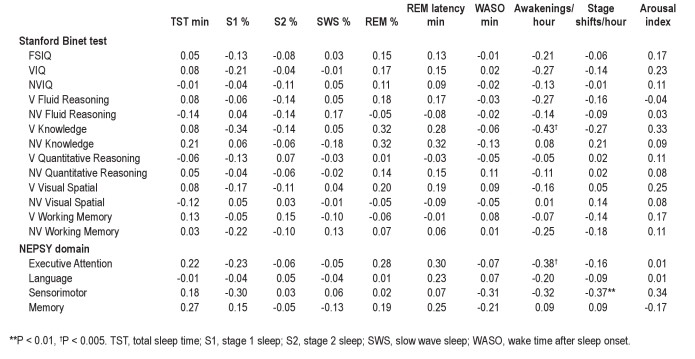
The association between CAP variables and neurocognitive scores and the association between standard PSG parameters and neurocognitive scores were explored using the Pearson correlation. Nonverbal fluid reasoning ability was significantly and positively associated with a number of CAP variables including CAP rate (total and in SWS) and A1 index (total and in SWS) (see Table 3 and Figure 1). Due to our strict criteria for significance, no other correlations were found reaching the P level of 0.01; however, other positive correlations (P < 0.05) were also seen for A2 index in S1 and a range of nonverbal scores including FSIQ, NVIQ, nonverbal visual spatial ability, and nonverbal working memory. Positive correlations (P < 0.05) were also shown between A2 index in S1 and sensorimotor function, and between verbal knowledge and both A3 index total and in SWS. In contrast, negative correlations (P < 0.05) were found between the A3 index in S2 and language development.
Table 3.
Correlations between CAP parameters and Stanford Binet test and NEPSY domain scores
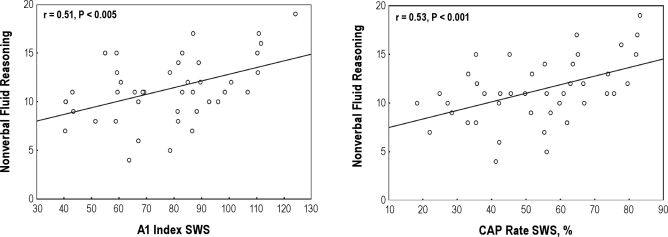
Figure 1.
Scatterplots of significant associations between CAP parameters in SWS and nonverbal fluid reasoning skill after controlling for child age and SES.
When considering standard PSG parameters, and with the exception of frequency of awakenings and stage shifts, none of the correlations reached a significance level of P < 0.01; however, 4 neurocognitive domains were less significantly (P < 0.05) associated with a range of values. Specifically, verbal knowledge was positively correlated with REM %, REM latency, and arousal index, and negatively correlated with frequency of awakenings and frequency of stage shifts. Nonverbal knowledge scores were also positively correlated with REM % and REM latency. Similarly, executive attention was positively correlated with REM % and REM latency and negatively correlated with frequency of awakenings (Table 4). Finally, sensorimotor function was negatively correlated with percent of stage 1 sleep, wake time during the night (WASO), frequency of awakenings, and frequency of stage shifts. A positive correlation was found between sensorimotor function and arousal index.
Table 4.
Correlations between classical sleep scoring parameters and Stanford Binet test and NEPSY domain scores
Sleep Parameters as Predictors of Cognitive Measures
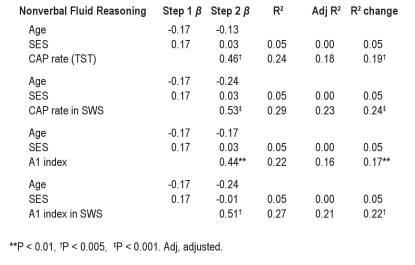
CAP associations were explored further using hierarchical regression modeling, entering significant covariates in a first step. The significant regression results are reported in Table 5. After entering age and SES in the first step due to significant correlations between these factors and a number of CAP variables, regression analysis revealed that CAP rate and CAP rate in SWS was a significant predictor of nonverbal fluid reasoning, explaining 19% to 24% of the variance in test score. Similarly, CAP A1 index (total and in SWS) was also a significant predictor of nonverbal fluid reasoning, explaining 17% to 22% of the variance in test score. This analysis was not performed for sleep scoring variables because of the low level of significance found in the previous analysis reported above.
Table 5.
Significant relationships between CAP parameters and neurocognitive test scores
DISCUSSION
To our knowledge, this is the first study that correlates the cognitive performance of healthy children with a more refined sleep analysis by means of the CAP method. Past studies on sleep and memory have relied on “macroscopic” estimates of sleep, i.e., amount of REM or slow wave sleep20–22 without specific findings. Our results suggest a more refined analysis of sleep might provide important insights on the role of sleep in cognitive performance in children.
Several studies have shown that sleep disruption impairs performance and memory retention in children with sleep disordered breathing23–27 and in normal children with short sleep duration.28–31 The few studies on CAP in these children (mainly with OSA) showed an alteration of sleep microstructure that could account for the reported cognitive and neurobehavioral dysfunction,32–34 although no studies have been conducted to specifically address this question. Recent reports in adults have highlighted the relationships between CAP and cognitive and memory performance, demonstrating that the CAP slow components (A1) are positively correlated to cognitive functioning: a learning task during the day preceding sleep increases the A1 percentage and the number of A1 per hour of sleep.5 Therefore, based on our previous research we hypothesized that a correlation might exist between some CAP parameters and cognitive measures in typically developing children.
The review of all the data from the studies on CAP in children with mental retardation (Down syndrome [DS], Fragile X syndrome [fraX], and autistic syndrome with mental retardation) showed a global decrease of CAP rate mainly represented by a reduction of percentage of A1, a decrease of A1 index either in stage 2 NREM or in SWS, and a higher percentage of A2 and A3 in all patient groups when compared to healthy controls.9,10 The most important alterations in CAP parameters found in fraX syndrome and DS might be in some way related to their level of mental retardation.35 The decline of EEG slow oscillations may represent a neurophysiological marker of mental retardation. On the other hand, the studies on CAP in children with neuropsychological disorders without mental retardation showed a different trend toward an increase of slow oscillations, or at least no particular differences with normal controls. For example, in children with dyslexia, we found a higher total CAP rate and A1 index in stage N3 compared to controls, which could be related to the compensatory overactivation of the ancillary frontal areas representing the generators of the CAP A1 subtypes.8 Moreover, dyslexic children showed a significant positive correlation between A1 index in NREM sleep stage 3 and verbal and full-scale IQ, while CAP rate in NREM sleep stage 3 was positively correlated with verbal IQ. The same positive correlation between IQ and slow CAP component was found previously in children and adolescents with Asperger syndrome.7
The same trend seems to be confirmed also in normal children: nonverbal tests of fluid reasoning ability were positively associated with CAP rate, mainly during SWS and with A1 total index and A1 index in SWS. It is reasonable to think that the amount and the distribution of the slow EEG oscillations might be considered a marker of intellectual ability. This finding has been corroborated by the regression analysis showing that only CAP rate in SWS and A1 index in SWS were associated with cognitive functioning; thus, they might be considered to be significant predictors of fluid reasoning and the nonverbal subtest of fluid reasoning in particular.
However, a recent study showed clusters of positive associations in the EEG alpha, sigma, and beta range for full scale IQ, fluid IQ, and working memory; in particular, the sigma power correlated with full scale and fluid IQ scores but not with verbal IQ scores. No correlation was found for frequencies in the delta and theta range.36 Accordingly, several adult-based studies have demonstrated a positive correlation between cognitive ability and spindle frequency activity.37,38 This association has not been reported in healthy children to date. As we described in a study on dyslexic children, sleep patterns like EEG slow oscillations (A1 phases) in combination with sleep spindles might reflect the re-expression of information acquired during active behavior prior to sleep and provide necessary properties important for plastic modifications underlying memory formation. The increase of both spindle activity and of CAP A1 subtypes could be part of the same process related to the learning disability that forces dyslexic subjects to continuously operate an activation of both frontal areas and thalamocortical loops. This is in agreement with recent studies showing that slow oscillations (SO), spindle activity, and hippocampal ripple activity are increased during sleep after a learning experience and positively related to the post-sleep improvement.39 Moreover, it has been demonstrated that the SO during the depolarizing state drives the generation of spindle activity via cortico-thalamic volleys, and that this temporal grouping of spindles by the SO is a key mechanism underlying sleep-dependent memory consolidation.40,41 Although we did not study the spindle activity in our subjects, we can speculate that the same process also takes place in our children that might present an increase of spindle activity in stage 2 paralleled by an increase of EEG SO in SWS. The explanation of the relations between EEG SO and cognitive scores can be easily linked to the neural efficiency theory42 and to the synaptic downscaling theory.43
Neural efficiency may derive from the disuse of many brain areas irrelevant for good task performance, meaning that the brains of brighter individuals use fewer energy resources to cope with task demands and focus energy on smaller brain areas.44 Therefore the reported negative relationship between IQ scores and physiological variables led to the conclusion that higher IQ scores were related to lower cortical activation. As stated by Geiger et al., children with higher cognitive efficiency, reflected by higher scores of cognitive measures, may also display higher nighttime efficiency (i.e., more efficient neuronal recovery) and, consequently, a shorter sleep duration.28 A merely neurophysiological explanation leads to the synaptic downscaling hypothesis to achieve greater neural efficiency. According to this hypothesis, plastic processes occurring during wakefulness result in a net increase in synaptic strength in many brain circuits. The role of sleep is to downscale synaptic strength to a baseline level that is energetically sustainable, makes efficient use of gray matter space, and is beneficial for learning and memory. Specifically, the higher the amount of synaptic potentiation in cortical circuits during wakefulness, the higher the increase in SWA during subsequent sleep, suggesting the process is mediated by SWA.
It has recently been suggested that sleep SWA may reflect the net strength of synapses in cortical circuits, which would therefore increase with wakefulness and decrease with sleep.43,45 Slow waves may also be causally involved in producing a sleep-dependent, progressive downscaling of synaptic strength, which would lead to several benefits in terms of both cellular function and network performance43,46,49; some studies appear to confirm this hypothesis.42,47,48 In our study, this is reflected by an increase of EEG slow oscillations (A1) in total sleep and in SWS in children with higher cognitive efficiency. Interestingly, based on the fact that fluid reasoning represents the inductive and deductive reasoning (or fluid) dimension of intelligence, and the component of intellectual ability that is more closely related to biological factors directly affected by brain injury,50 it is not surprising that we found it to be the most consistently related to the slow components of CAP and was the variable with the largest variance explained by CAP rate in SWS and A1 index.
Some limitations of the study should be noted. The sample size is relatively small and was selected following stringent criteria; it is homogeneous for SES and maternal IQ. We are aware that from this study we cannot draw strong conclusions and we need to replicate the study on a larger sample. However, the direction of correlations between CAP and cognitive measures is in agreement with the findings reported in previous studies on developmentally disabled children and confirm the study hypotheses.
In our analysis we have found that CAP correlates with SES; although we cannot clearly explain in this context this relationship, it has been reported in population studies that sleep is disrupted in children of lower SES, especially in those who also have impaired cognitive functioning.51 This effect is also seen in adults for whom both household income and level of education were predictive of sleep latency and efficiency52; also, sleep quality seems to be related to social status.53 Furthermore, a recent study has demonstrated that lower childhood SES was associated with increased stage 2 sleep and reduced SWS in adulthood.54 Given the well-documented literature on the association between SES and cognitive performance, we suggest that lower SES is also disruptive for sleep microstructure (either directly due to increased environmental stresses or indirectly by the impact of these stressors on development of sleep structure), and that this disruption may in turn impact on cognitive development and performance. In this study we controlled the possible influence of SES to better observe the direct association between CAP and cognitive performance in children.
Our results could also be important in understanding findings in clinical settings: we showed that the alteration of CAP, especially in SWS, is significantly associated to cognitive impairment. Impaired brain bioelectrical activity in brain-damaged patients or sleep disordered patients might affect sleep microarchitecture, interfering with several functions important for the normal brain development. The analysis of CAP might allow evaluation of the optimal development of the child and its effect on cognitive functioning.
Further studies are needed in a larger sample and in different age ranges in order to confirm our findings. Namely, a deeper examination of the data analyzing the relationships between CAP A1 phases and spindles could lead to a more comprehensive view of the mechanisms underlying the correlation between the neurophysiological aspects of sleep and cognition and memory.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Ferri has consulted for Merck & Co., Sanofi-Aventis, and Sapio Life. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Kheirandish L, Gozal D. Neurocognitive dysfunction in children with sleep disorders. Dev Sci. 2006;9:388–99. doi: 10.1111/j.1467-7687.2006.00504.x. [DOI] [PubMed] [Google Scholar]
- 2.Parrino L, Ferri R, Bruni O, Terzano MG. Cyclic alternating pattern (CAP): The marker of sleep instability. Sleep Med Rev. 2011 doi: 10.1016/j.smrv.2011.02.003. in press. [DOI] [PubMed] [Google Scholar]
- 3.Aricò D, Drago V, Foster PS, Heilman KM, Williamson J, Ferri R. Effects of NREM sleep instability on cognitive processing. Sleep Med. 2010;11:791–8. doi: 10.1016/j.sleep.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Ferri R, Drago V, Aricò D, et al. The effects of experimental sleep fragmentation on cognitive processing. Sleep Med. 2010;11:378–85. doi: 10.1016/j.sleep.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferri R, Huber R, Aricò D, et al. The slow-wave components of the cyclic alternating pattern (CAP) have a role in sleep-related learning processes. Neurosci Lett. 2008;432:228–31. doi: 10.1016/j.neulet.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 6.Ferini-Strambi L, Ortelli P, Castronovo V, Cappa S. Increased periodic arousal fluctuations during non-REM sleep are associated to superior memory. Brain Res Bull. 2004;63:439–42. doi: 10.1016/j.brainresbull.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Bruni O, Ferri R, Vittori E, et al. Sleep architecture and NREM alterations in children and adolescents with Asperger syndrome. Sleep. 2007;30:1577–85. doi: 10.1093/sleep/30.11.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruni O, Ferri R, Novelli L, et al. Slow EEG amplitude oscillations during NREM sleep and reading disabilities in children with dyslexia. Dev Neuropsychol. 2009;34:539–51. doi: 10.1080/87565640903133418. [DOI] [PubMed] [Google Scholar]
- 9.Miano S, Bruni O, Elia M, et al. Sleep phenotypes of intellectual disability: a polysomnographic evaluation in subjects with Down syndrome and Fragile-X syndrome. Clin Neurophysiol. 2008;119:1242–7. doi: 10.1016/j.clinph.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Miano S, Bruni O, Elia M, et al. Sleep in children with autistic spectrum disorder: a questionnaire and polysomnographic study. Sleep Med. 2007;9:64–70. doi: 10.1016/j.sleep.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Bellizzi MC, Dietz WH. Workshop on childhood obesity: summary of the discussion. Am J Clin Nutr. 1999;70:173S–175S. doi: 10.1093/ajcn/70.1.173s. [DOI] [PubMed] [Google Scholar]
- 12.Roid GH. Stanford-Binet Intelligence Scales. Fifth Edition (SB:V) Itasca, IL: Riverside Publishing; 2003. [Google Scholar]
- 13.Korkman M, Kirk U, Kemp SL. Clinical and interpretative manual. San Antonio, TX: PsychCorp; 2007. NEPSY II. [Google Scholar]
- 14.Nelson HE. The National Adult Reading Test (NART): test manual. NFER-Nelson. 1982 [Google Scholar]
- 15.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. Washington: Washington Public Health service; US Government Printing Office; 1968. [Google Scholar]
- 16.Zucconi M, Ferri R, Allen R, et al. The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG) Sleep Med. 2006;7:175–83. doi: 10.1016/j.sleep.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 17.American Sleep Disorders Association. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 18.American Thoracic Society AT. Standards and indications for cardiopulmonary sleep studies in children. American Thoracic Society. Am J Respir Crit Care Med. 1996;153:866–78. doi: 10.1164/ajrccm.153.2.8564147. [DOI] [PubMed] [Google Scholar]
- 19.Terzano MG, Parrino L, Smerieri A, et al. Atlas, rules, and recording techniques for the scoring of cyclic alternating pattern (CAP) in human sleep. Sleep Med. 2001;2:537–53. doi: 10.1016/s1389-9457(01)00149-6. [DOI] [PubMed] [Google Scholar]
- 20.Busby K, Pivik RT. Sleep patterns in children of superior intelligence. J Child Psychol Psychiatry. 1983;24:587–600. doi: 10.1111/j.1469-7610.1983.tb00134.x. [DOI] [PubMed] [Google Scholar]
- 21.Plihal W, Born J. Effects of early and late nocturnal sleep on priming and spatial memory. Psychophysiology. 1999;36:571–82. [PubMed] [Google Scholar]
- 22.Schabus M, Gruber G, Parapatics S, et al. Sleep spindles and their significance for declarative memory consolidation. Sleep. 2004;27:1479–85. doi: 10.1093/sleep/27.7.1479. [DOI] [PubMed] [Google Scholar]
- 23.Beebe DW, Ris MD, Kramer ME, Long E, Amin R. The association between sleep disordered breathing, academic grades, and cognitive and behavioral functioning among overweight subjects during middle to late childhood. Sleep. 2010;33:1447–56. doi: 10.1093/sleep/33.11.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bourke R, Anderson V, Yang JS, et al. Cognitive and academic functions are impaired in children with all severities of sleep-disordered breathing. Sleep Med. 2011;12:489–96. doi: 10.1016/j.sleep.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Miano S, Paolino MC, Urbano A, et al. Neurocognitive assessment and sleep analysis in children with sleep-disordered breathing. Clin Neurophysiol. 2011;122:311–9. doi: 10.1016/j.clinph.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 26.Montgomery-Downs HE, Gozal D. Sleep-associated respiratory disorders and their psychobehavioral consequences in children. Handb Clin Neurol. 2011;98:489–99. doi: 10.1016/B978-0-444-52006-7.00032-0. [DOI] [PubMed] [Google Scholar]
- 27.Spruyt K, Capdevila OS, Kheirandish-Gozal L, Gozal D. Inefficient or insufficient encoding as potential primary deficit in neurodevelopmental performance among children with OSA. Dev Neuropsychol. 2009;34:601–14. doi: 10.1080/87565640903133566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geiger A, Achermann P, Jenni OG. Association between sleep duration and intelligence scores in healthy children. Dev Psychol. 2010;46:949–54. doi: 10.1037/a0019679. [DOI] [PubMed] [Google Scholar]
- 29.Gruber R, Laviolette R, Deluca P, Monson E, Cornish K, Carrier J. Short sleep duration is associated with poor performance on IQ measures in healthy school-age children. Sleep Med. 2010;11:289–94. doi: 10.1016/j.sleep.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Randazzo AC, Muehlbach MJ, Schweitzer PK, Walsh JK. Cognitive function following acute sleep restriction in children ages 10-14. Sleep. 1998;21:861–8. [PubMed] [Google Scholar]
- 31.Sadeh A, Gruber R, Raviv A. The effects of sleep restriction and extension on school-age children: what a difference an hour makes. Child Dev. 2003;74:444–55. doi: 10.1111/1467-8624.7402008. [DOI] [PubMed] [Google Scholar]
- 32.Kheirandish-Gozal L, Miano S, Bruni O, et al. Reduced NREM sleep instability in children with sleep disordered breathing. Sleep. 2007;30:450–7. doi: 10.1093/sleep/30.4.450. [DOI] [PubMed] [Google Scholar]
- 33.Lopes MC, Guilleminault C. Chronic snoring and sleep in children: a demonstration of sleep disruption. Pediatrics. 2006;118:e741–6. doi: 10.1542/peds.2005-3046. [DOI] [PubMed] [Google Scholar]
- 34.Miano S, Rizzoli A, Evangelisti M, et al. NREM sleep instability changes following rapid maxillary expansion in children with obstructive apnea sleep syndrome. Sleep Med. 2009;10:471–8. doi: 10.1016/j.sleep.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Bruni O, Novelli L, Miano S, Parrino L, Terzano MG, Ferri R. Cyclic alternating pattern: A window into pediatric sleep. Sleep Med. 2010;11:628–36. doi: 10.1016/j.sleep.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Geiger A, Huber R, Kurth S, Ringli M, Jenni OG, Achermann P. The sleep EEG as a marker of intellectual ability in school age children. Sleep. 2011;34:181–9. doi: 10.1093/sleep/34.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bódizs R, Kis T, Lázár AS, et al. Prediction of general mental ability based on neural oscillation measures of sleep. J Sleep Res. 2005;14:285–92. doi: 10.1111/j.1365-2869.2005.00472.x. [DOI] [PubMed] [Google Scholar]
- 38.Fogel SM, Nader R, Cote KA, Smith CT. Sleep spindles and learning potential. Behav Neurosci. 2007;121:1–10. doi: 10.1037/0735-7044.121.1.1. [DOI] [PubMed] [Google Scholar]
- 39.Eschenko O, Ramadan W, Moelle M, Born J, Sara SJ. Sustained increase in hippocampal sharp-wave ripple activity during slow-wave sleep after learning. Learn Mem. 2008;15:222–8. doi: 10.1101/lm.726008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–26. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 41.Mölle M, Bergmann TO, Marshall L, Born J. Fast and slow spindles during the sleep slow oscillation-disparate coalescence and engagement in memory processing. Sleep. 2011 doi: 10.5665/SLEEP.1290. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neubauer AC, Fink A. Intelligence and neural efficiency. Neurosci Biobehav Rev. 2009;33:1004–23. doi: 10.1016/j.neubiorev.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 43.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 44.Haier RJ, Siegel BV, Nuechterlein KH, et al. Cortical glucose metabolic rate correlates of abstract reasoning and attention studied with positron emission tomography. Intelligence. 1988;12:199–217. [Google Scholar]
- 45.Borbely AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythms. 1999;14:557–68. doi: 10.1177/074873099129000894. [DOI] [PubMed] [Google Scholar]
- 46.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 47.Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–3. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 48.Mölle M, Marshall L, Gais S, Born J. Learning increases human electroencephalographic coherence during subsequent slow sleep oscillations. Proc Natl Acad Sci. 2004;21(101):13963–8. doi: 10.1073/pnas.0402820101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stickgold R. Neuroscience: a memory boost while you sleep. Nature. 2006;444:559–60. doi: 10.1038/nature05309. [DOI] [PubMed] [Google Scholar]
- 50.Horn JL, Cattell RB. Refinement and test of the theory of fluid and crystallized general intelligences. J Educ Psychol. 1966;57:253–70. doi: 10.1037/h0023816. [DOI] [PubMed] [Google Scholar]
- 51.Buckhalt JA, El-Sheikh M, Keller P. Children's sleep and cognitive functioning: race and socioeconomic status as moderators of effects. Child Dev. 2007;78:213–31. doi: 10.1111/j.1467-8624.2007.00993.x. [DOI] [PubMed] [Google Scholar]
- 52.Friedman EM, Love GD, Rosenkranz MA, et al. Socioeconomic status predicts objective and subjective sleep quality in aging women. Psychosom Med. 2007;69:682–91. doi: 10.1097/PSY.0b013e31814ceada. [DOI] [PubMed] [Google Scholar]
- 53.Goodin BR, McGuire L, Smith MT. Ethnicity moderates the influence of perceived social status on subjective sleep quality. Behav Sleep Med. 2010;8:194–206. doi: 10.1080/15402002.2010.509193. [DOI] [PubMed] [Google Scholar]
- 54.Tomfohr LM, Ancoli-Israel S, Dimsdale JE. Childhood socioeconomic status and race are associated with adult sleep. Behav Sleep Med. 2010;8:219–30. doi: 10.1080/15402002.2010.509236. [DOI] [PMC free article] [PubMed] [Google Scholar]