Abstract
Study Objectives:
This study was designed to assess decision making and executive functions in patients with idiopathic REM sleep behavior disorder (iRBD). IRBD is often seen as an early sign of later evolving neurodegenerative disease, most importantly Parkinson disease (PD) and Lewy body dementia (DLB). It has been proposed that iRBD patients show a cognitive profile similar to patients with PD.
Design:
All participants performed an extensive test battery tapping executive functions as well as the IOWA gambling task, which measures decision making under ambiguity.
Setting:
University hospital sleep disorders center.
Participants:
16 iRBD patients and 45 age- and education-matched controls.
Intervention:
N.A.
Measurements and Results:
Compared with controls, iRBD patients showed disadvantageous decision making under ambiguity and did not learn by feedback over the task. IRBD patients' decision pattern was characterized by the lack of a consistent strategy, as indicated by frequent shifts between the single choices. A high proportion of iRBD patients (75%) showed random performance or worse even at the end of the task. No group differences were found in tasks assessing information sampling, flexibility and categorization, problem solving, and impulsivity.
Conclusions:
As suggested by the present investigation, iRBD patients may show difficulties in decision making under ambiguity in a stage when other cognitive functions are relatively well preserved. Whether this is driven by subgroups of patients prone to develop PD or DLB has to be assessed by follow-up investigations.
Citation:
Delazer M; Högl B; Zamarian L; Wenter J; Ehrmann L; Gschliesser V; Brandauer E; Poewe W; Frauscher B. Decision making and executive functions in REM sleep behavior disorder. SLEEP 2012;35(5):667-673.
Keywords: iRBD, IOWA gambling task, decision making, executive functions
INTRODUCTION
REM sleep behavior disorder (RBD) is a parasomnia characterized by loss of normal skeletal muscle atonia during REM sleep with prominent motor activity and dreaming.1 Typically, RBD patients show dream-enacting behaviors (e.g., shouting, punching) related to unpleasant and sometimes violent dreams. Clinical and pathological data suggest that iRBD may be the earliest manifestation of neurodegenerative disorders such as Parkinson disease (PD),2,3 Lewy body dementia (DLB),4 or multiple system atrophy (MSA),5 which may evolve in iRBD patients at a variable delay.6–8 Since both PD and DLB are associated with cognitive dysfunction, subtle cognitive changes might also be expected in subjects with iRBD.
So far, a few studies have assessed neuropsychological functions of iRBD patients. Ferini-Strambi et al.9 found impairments in tasks of visuo-constructive abilities and visuo-spatial learning in a group of 17 iRBD patients. A 2-year follow-up study in 24 cognitively asymptomatic iRBD patients revealed worsening in memory and visuo-constructive functions over time.10 Terzaghi et al.11 reported low performance of 23 iRBD patients in working memory, complex figure recall, and logical memory. Gagnon et al.12 found a high incidence of mild cognitive impairment (MCI) in 32 iRBD patients and 22 PD patients with RBD. The main subtype of MCI in iRBD patients was non-amnestic with impaired executive functions. As a group, iRBD patients performed lower than controls in tasks of working memory, set shifting, verbal fluency, and verbal memory.12,13 Massicotte-Marquez et al.14 reported reduced executive functions, attention, and verbal memory, as well as EEG slowing during wakefulness in 14 iRBD patients. A recent investigation15 found marked EEG slowing in iRBD patients with MCI and suggested that slowing of cortical EEG may indicate the short-term development of cognitive dysfunction. Summing up, neuropsychological investigations yielded only partially consistent results as regards a specific pattern of dysfunction. While three studies emphasized impairments in visuo-constructive abilities, visuo-spatial learning, or visuo-spatial memory,9–11 other investigations stressed deficits in executive functions.12–14 Despite these differences, all neuropsychological studies so far point to the similarities between the neuropsychological profile of RBD patients and the cognitive deficits typically associated with PD or DLB. They also agree that cognitive deficits might serve as early markers and could lead to presymptomatic identification of an underlying neurodegenerative disease in the future.9,12
Decision making is often found to be impaired in PD.16–21 Deficits in decision making under ambiguity have been attributed to a dysfunction of the limbic fronto-striatal loop,22 which is involved in risk and reward processing, learning from feedback, emotional regulation, and control. It is important to note, however, that performance in decision making is influenced by several factors including the stage of the disease, basal levels of dopamine function,23 dopaminergic treatment,23,24 as well as the presence or absence of executive function deficits.25 Moreover, the nature of the decision situation (decision under ambiguity versus decision under known risk) and the complexity of the given task may account for differences in performance.20,26
Based on reported similarities between iRBD and PD patients' cognitive profiles,9–12 we comprehensively assessed executive functions and decision making under ambiguity in iRBD patients.
METHODS
Participants
The study included 16 iRBD patients (13 men, 3 women) with a mean age of 65.2 ± 7.6 years. Mean RBD duration was 8.9 ± 7.1 years. Mean duration of education was 11.3 ± 2.8 years (range 8-17 years). Patients were compared to 45 healthy participants (22 men, 23 women) with a mean age of 63.9 ± 9.6 years and a mean duration of education of 11.8 ± 3.4 years (range 8-17 years). All iRBD patients and healthy controls > 60 years performed the Mini-Mental state examination (iRBD patients: 28.4 ± 1.4; controls: 28.7 ± 1.3). Groups were comparable in terms of age, education, and Mini-Mental state examination score (P values > 0.1). In all iRBD subjects, the diagnosis of RBD required (i) history of dream-enacting behaviors and (ii) nocturnal video-polysomnographic demonstration of prominent tonic and/or phasic EMG activity in the SINBAR EMG montage27,28 associated with abnormal behaviors and absence of electroencephalographic epileptiform activity during REM sleep.29 None of the patients fulfilled the criteria for dementia from Diagnostic and Statistical Manual of Mental Disorders, 4th edition or the UK Brain Bank criteria for Parkinson disease. Exclusion criteria were evidence of central nervous system comorbidities revealed by history (e.g., history of stroke) or clinical neurological examination, evidence of psychiatric comorbidity or untreated sleep apnea syndrome. Six patients received low doses of clonazepam (0.25-0.75 mg/day). No other medication possibly influencing cognitive processes was given.
In order to exclude major cognitive impairment, iRBD patients performed a short battery of neuropsychological background tests assessing naming to confrontation, verbal and figural episodic memory, visuo-constructive abilities, executive functions (CERAD Plus Battery [www.memoryclinic.ch],30 Frontal Assessment Battery,31,32 clock drawing) and a vocabulary test allowing the estimation of verbal intelligence.33 Patients also responded to a questionnaire on anxiety and depression (HADS-D).34 All median scores were in the average range of standardized norms, and no patient fulfilled the criteria of dementia.
The study was approved by the ethics committee of Innsbruck Medical University. Subjects' written informed consent was obtained according to the Declaration of Helsinki.
Experimental Tasks
Iowa Gambling Task computerized version (IGT)35
The IGT measures decision making under initial ambiguity. In the IGT, 4 decks of cards are presented, which are labelled in a row A, B, C, and D. Participants are required to select one card at the time through mouse click for a total of 100 card selections. The selection of a card from decks A and B results in large gains of money. These gains are, however, followed by a large penalty at certain unpredictable times, so that the accumulated penalties are larger than the accumulated gains. Decks A and B are therefore disadvantageous in the long run. The selection of a card from decks C and D produces small immediate gains of money. The unpredictable losses are also small for them, so that the accumulated penalties are smaller than the accumulated gains. Decks C and D are the advantageous decks in the long run. Following convention, performance is analyzed by dividing the 100 trials into 5 blocks of 20 card selections and calculating the difference (net score) between the number of selections from advantageous decks (C+D) and the number of selections from disadvantageous decks (A+B). For each block, we also calculate the number of shifts made between decks. An analysis by individual compares the distribution of advantageous and disadvantageous selections with a random distribution by means of binomial test. Since learning over the task is essential, we included only the last 2 blocks (i.e., 40 card selections) in this analysis. On the basis of this analysis, participants' performance in the last 2 blocks was classified as advantageous if they had a net score ≥ 14 ((C+D)-(A+B) ≥ +14)) in the last 2 blocks; as disadvantageous if they had a net score of ‒14 or less ((C+D)-(A+B) ≤ ‒14)); or as random (net score between ‒14 and +14).
Information Sampling Task (IST)36
The IST assesses information sampling before making a decision and reflection impulsivity. The task is described in detail in Clark et al.37 (for descriptions of all CANTAB tasks and interactive demos see also http://www.cantab.com/cantab-tests.asp). On each trial, participants are presented with a 5 × 5 matrix of gray boxes, with 2 larger colored panels below at the foot of the screen. Touching a gray box causes the box to reveal one of the 2 colors. Participants are instructed to decide the box color in the majority of boxes. There are 2 conditions (each 10 trials), with condition order counterbalanced across subjects. In the fixed win (FW) condition, the subject wins or loses 100 points on each trial, irrespective of the number of boxes opened. In the decreasing win (DW) condition, the win decreases from 250 points in 10 point steps with every box opened. In case of an incorrect decision, participants lose 100 points, regardless of the number of boxes opened. Performance on the IST is indexed by the number of boxes opened in each condition and by the probability (P) of the subject being correct at the point of decision. In the present study we also analyzed the number of discrimination errors. Discrimination errors are those trials in which the subject chooses a color that was not in the majority of boxes at the point of decision.
Intra/Extra Dimensional Shift (IED)
The IED taps mental flexibility, categorization, and set-shifting. The IED36,38,39 requires the participants to learn a series of 2 alternative forced-choice discriminations using feedback provided by the computer. After 6 correct responses, the stimuli and/or rules are changed. There are 9 stages in fixed order, requiring intra- and extra-dimensional set shifting as well as reversal learning. We analyze the number of total errors adjusted for the number of stages completed, the number of Pre-ED errors (errors made prior to the extra-dimensional shift), of EDS errors (errors in the extra-dimensional stage), and of reversal errors (sum of errors committed in reversal stages 2, 5, 7, and 9).
One Touch Stockings of Cambridge (OTS)36,40
The OTS is a variant of the Tower of London task and measures complex problem solving. In the OTS subjects are not asked to execute the appropriate moves in order to achieve a solution, but to solve the problem mentally and to indicate the number of necessary moves. Performance in the OTS is indexed by problems solved on first choice, by the mean number of choices to a correct response, the mean latency to the first choice, and the mean latency to the correct choice.
Go-NoGo Task
The task measures response inhibition (adapted from Fox et al.41). In this task, different colored letters are presented on the screen (N, J, W, O, and E). Go stimuli consist of letters N, J, and W presented in blue or the letter O presented in red, green, or yellow. The NoGo stimuli include O presented in blue and E presented in pink. Subjects are instructed to press as fast as possible a button following the Go stimuli and to withhold a response to the NoGo stimuli. In the present study we analyzed the proportion of correctly answered Go trials and the proportion of correctly answered NoGo trials. We also analyzed the proportion of participants in each group scoring below a cutoff of 90% correct responses in the Go trials and the NoGo trials.
Statistical Analysis
Data were analyzed with parametric statistics where normality assumptions were met. Otherwise, nonparametric tests were used. Mean scores and standard deviations are reported for the tasks where parametric statistics were applied; median scores and interquartile ranges are reported for the tasks where nonparametric statistics were used. Greenhouse-Geisser correction was applied for repeated-measures ANOVA where assumption of sphericity was violated. One-way ANOVA was corrected where a violation of the assumption of homogeneity of variance in the data was detected. Frequency distributions were investigated by binomial test or Pearson χ2 test where appropriate. A Spearman rank-order correlation analysis between decision making tasks (net score in block 5 of the IGT, mean P(correct) in FW condition of the IST, mean P(correct) in DW condition of the IST), and executive function tasks (accuracy rate with NoGo stimuli, reversal errors in the IED, ED errors in the IED, number of problems solved on first choice in the OTS) was carried out for the iRBD patient group. Applying Bonferroni correction, significance level was set at P = 0.004.
RESULTS
Decisions under Ambiguity: Iowa Gambling Task (IGT)
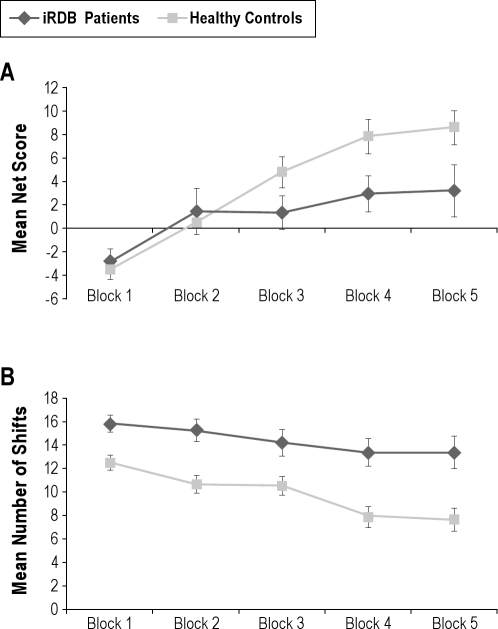
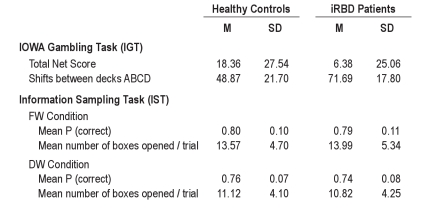
Overall, healthy controls showed learning over the task, while iRBD patients remained at random level. A mixed ANOVA of the net score indicated a significant main effect of block, F3.0,175.9 = 15.55, MSE = 867.54, P < 0.001, and a significant interaction of block with group, F3.0,175.9 = 2.66, MSE = 148.52, P = 0.05. The main effect of group was not significant, though iRBD patients had lower scores (iRBD patients: mean net score 6.38 ± 25.06; healthy controls: mean net score 18.36 ± 27.54). As shown in Figure 1A, healthy controls made significantly more advantageous choices than iRBD patients in the last block of the task (block 5, F1,59 = 4.00, MSE = 340.65, P = 0.050), but not in the first block (P = 0.601) (see footnote 1 following article).
Figure 1.
Mean net score (A) and mean number of shifts (B) as a function of group (healthy controls, iRBD patients) and block. Bars indicate the standard error of the mean.
We also analyzed the number of shifts between the 4 different decks. iRBD patients shifted overall more frequently between decks than healthy controls, thus showing less consistent behavior (iRBD patients: mean number of shifts 71.69 ± 17.80; healthy controls: mean number of shifts 48.87 ± 21.70). A mixed ANOVA revealed significant main effects of block, F3.2,188.7 = 10.77, MSE = 143.33, P < 0.001, and group, F1,59 = 14.24, MSE = 1229.41, P < 0.001, but no significant interaction, P = 0.282 (Figure 1B).
An analysis at the single subject level revealed significantly better performance of the control group. In the last 2 blocks of the task, the frequency of advantageous and disadvantageous selections did not differ from a random distribution for 10 iRBD patients (62.50%) and for 16 controls (35.56%). Four iRBD patients (25.00%) and 27 controls (60.00%) showed an advantageous decision pattern, while 2 iRBD patients (12.50%) and 2 controls (4.40%) showed a markedly disadvantageous decision pattern. The distribution of advantageous, disadvantageous and random performance significantly differed between iRBD patients and healthy controls, χ2 = 6.02, P = 0.049.
Information Sampling and Reflection Impulsivity: Information Sampling Task (IST)
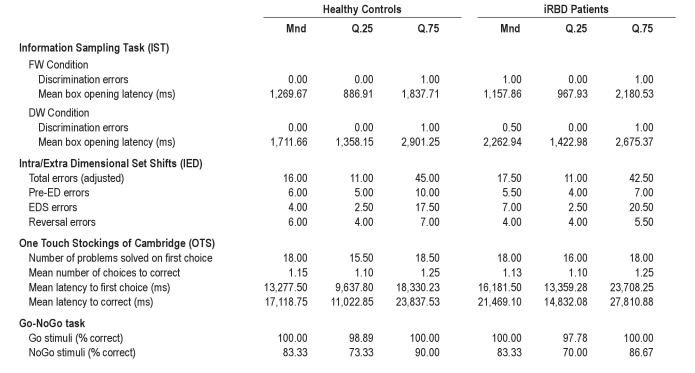
In this task we analyzed the probability of making a correct choice at the point of decision and did not find differences between iRBD and control group. A mixed ANOVA (see footnote 2 following article) on the mean P(correct) value indicated a significant main effect of condition (fixed win, decreasing win), F1,59 = 12.67, MSE = 0.05, P = 0.001, whereas the main effect of group and the 2-way interaction were not significant, P values > 0.1. The probability of making a correct choice at the point of decision was higher in the FW condition than in the DW condition (Table 1). The analysis of the mean number of open boxes per trial revealed the same pattern as the analysis of the mean P(correct) variable. Groups were also comparable in the number of discrimination errors (Mann-Whitney U-test, P values > 0.1; Table 2) and in terms of mean response latencies.
Table 1.
Means and standard deviations for healthy controls and iRBD patients in the IGT and the IST
Table 2.
Medians and interquartile ranges for healthy controls and iRBD patients in the IST, IED, OTS, and Go-NoGo Task
Flexibility and Categorization: Intra/Extra Dimensional Set Shift (IED)
In summary, iRBD patients and healthy controls performed the IED task comparably accurately. Results indicated that 12 iRBD patients (75.0%) and 35 controls (77.8%) completed all stages of the task (χ2 test, P > 0.1). There were no significant group differences in the number of trials needed to complete each single stage of the task, Mann-Whitney U-tests, Ps > 0.1. No significant group differences were found in the number of errors (Table 2), Mann-Whitney U-tests, Ps > 0.1.
Problem Solving: One Touch Stockings of Cambridge (OTS)
iRBD patients and healthy controls performed comparably on this task (number of problems solved on first choice, mean number of choices to correct, mean latency to first choice, mean latency to correct; Mann-Whitney U-tests, Ps > 0.1; Table 2).
Impulsivity: Go-NoGo Task
Groups' performance was comparable in this task.
Go stimuli
The proportion of iRBD patients performing without errors (8/15 (see footnote 3 following article), 53.3%) was comparable to that of healthy controls (30/44, 68.18%), χ2 test, P > 0.1. Only one iRBD patient and two controls obtained an accuracy rate < 90%.
NoGo stimuli
Two healthy participants and 2 iRBD patients performed without errors. Thirty controls (68.18%) and 12 iRBD patients (80.0%) obtained a score < 90%. The proportion of iRBD patients obtaining a score < 90% was comparable to that of healthy controls, χ2 test, P > 0.1. Analysis of accuracy scores by Mann-Whitney U-test confirmed comparable performance of the 2 groups (Table 2).
Correlation Analysis
A Spearman rank-order correlation analysis indicated no significant correlation between the measures of decision making and the executive function measures in the iRBD group. However, this result may be biased by the small sample size.
DISCUSSION
To the best of our knowledge, this is the first study investigating decision making under initial ambiguity in iRBD. A high proportion of iRBD patients did not show learning over the task as healthy controls did and showed random performance even at the end of the task. IRBD patients' disadvantageous decision pattern was characterized by the lack of a consistent strategy as indicated by frequent shifts between the single choices. This result suggests that deficits in learning from feedback and in maintaining an advantageous strategy, rather than perseveration of risky choices, caused the disadvantageous outcome. Good performance in the information sampling task also suggests adequate risk processing in the iRBD group. Patients gathered information to the same extent as healthy controls and tolerated comparable levels of uncertainty when making a decision. That means simple risk processing was comparable between the iRBD and control group.
In line with previous studies emphasizing similarities between cognitive profiles in iRBD and PD,9–12 decision making under ambiguity as seen in iRBD patients in this study was comparable to what we observed in cognitively well-functioning PD patients.20 In PD patients, deficits in decision making under ambiguity have mostly been attributed to a dysfunction of the limbic fronto-striatal loop.22 The dopaminergic system is critically involved in reward experience and reward prediction42 and regulates learning from feedback as well as reversal learning.43,44 Both types of learning are essential in the Iowa Gambling Task—subjects have to adapt their choices to losses and gains and have to switch between choices in order to maximize their reward. Indeed, subtle structural or functional alterations of the dopaminergic system have been demonstrated even in the idiopathic form of RBD.45–52 Hypothetically, these alterations might account for iRBD patients' deficits in decision making. However, we in no way suggest that difficulties in decision making under ambiguity are specific for iRBD or PD. Decision making under ambiguity relies on several cognitive components and involves an extended network of fronto-striatal and limbic structures as well as neurotransmitter systems.
The present investigation suggests that iRBD patients may show difficulties in decision making under ambiguity in a stage when other cognitive functions are well preserved. The neuropsychological background testing evidenced no major cognitive impairment and the iRBD group overall performed well in the battery of computerized tasks (CANTAB)36 assessing several executive functions (information sampling and reflection impulsivity, set-shifting, working memory, and problem solving). We thus assume that the present patient sample indeed was cognitively relatively well performing. Whether difficulties in decision making have a predictive value for developing more pervasive cognitive deficits in the context of PD or DLB in later years has to be assessed by longitudinal investigations. Furthermore, it is open for investigation whether these difficulties have an impact on everyday decision making. Possibly, overall good cognitive functioning, intact reasoning, adequate information seeking, as well as risk processing allow patients to compensate for their deficits in implicit learning from feedback and in making decisions under ambiguous conditions.
FOOTNOTES
In order to exclude gender effects, an analysis comparing only male iRBD patients (n = 13) with age- and education-matched male subjects from the control group (n = 20) was performed. While the net scores did not differ between groups in block 1 (Mann-Whitney U-test, P > 0.1; median scores: controls −4, iRBD −2), controls performed significantly better in block 5 (Mann-Whitney U-test, P = 0.043; median scores: controls 11, iRBD 2). A further analysis on medication effects compared IGT performance between patients with clonazepam (n = 6) and patients without medication (n = 10). Net scores did not differ in any block (Mann-Whitney U-tests, Ps > 0.1; median scores in block 5: group with medication 4, group without medication 2).
A first analysis indicated that the effect of the presentation order (FW condition first, DW condition first) was not significant. Therefore, we did not further take into account this factor in the following analysis.
One control and one iRBD participant did not perform the Go-Nogo task.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Högl has received research support from UCB; she has received speaker honoraria from and/or serves as a consultant or on the advisory board of GSK, BI, UCB, Pfizer, Cephalon, Jazz, Sanofi, Lundbeck, Merz. Dr. Frauscher has received speaker honoraria from and/or serves as consultant for UCB, Pfizer, and Mundipharma. Dr. Poewe is the principal or site investigator of clinical trials sponsored by BI, Merz, and Merck-Serono. He has also received speaker honoraria from and/or serves as consultant for Astra Zeneca, Teva, Novartis, GSK, BI, UCB, Orion Pharma, Merck-Serono, and Solvay-Abbott. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The present research was supported by Leopold-Franzens University Innsbruck (Doktoratsstipendium to J.W.). L.Z. receives research support from FWF, Austrian Science Fund, Project-Nr. P21636-B18. The authors thank the patients and healthy control subjects for participation. The authors are grateful to A. Bechara for providing the IGT. Work for this study was performed at Department of Neurology, Innsbruck, Medical University, Innsbruck, Austria.
ABBREVIATIONS
- DLB
dementia with Lewy bodies
- DW
decreasing win
- ED
extra-dimensional
- EDS
extra-dimensional stage
- FAB
frontal assessment battery
- FW
fixed win
- iRBD
idiopathic REM sleep behavior disorder
- IED
Intra/Extra Dimensional Set Shift
- IGT
IOWA gambling task
- IST
information sampling task
- MCI
mild cognitive impairment
- MSA
multiple system atrophy
- OTS
One Touch Stockings of Cambridge
- PD
Parkinson disease
- RBD
REM sleep behavior disorder
REFERENCES
- 1.Schenck CH, Bundlie SR, Ettinger MG, Mahowald MW. Chronic behavioral disorders of human REM sleep: a new category of parasomnia. Sleep. 1986;9:293–308. doi: 10.1093/sleep/9.2.293. [DOI] [PubMed] [Google Scholar]
- 2.Wetter TC, Trenkwalder C, Gershanik O, Högl B. Polysomnographic measures in Parkinson's disease: a comparison between patients with and without REM sleep disturbances. Wien Klin Wochenschr. 2001;113:249–53. [PubMed] [Google Scholar]
- 3.Gagnon JF, Bedard MA, Fantini ML, et al. REM sleep behavior disorder and REM sleep without atonia in Parkinson's disease. Neurology. 2002;59:585–9. doi: 10.1212/wnl.59.4.585. [DOI] [PubMed] [Google Scholar]
- 4.Boeve BF, Silber MH, Ferman TJ, et al. REM sleep behaviour disorder and degenerative dementia: an association likely reflecting Lewy body disease. Neurology. 1998;51:363–70. doi: 10.1212/wnl.51.2.363. [DOI] [PubMed] [Google Scholar]
- 5.Plazzi G, Corsini R, Povini F, et al. REM sleep behavior disorders in multiple system atrophy. Neurology. 1997;48:1094–7. doi: 10.1212/wnl.48.4.1094. [DOI] [PubMed] [Google Scholar]
- 6.Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder. Neurology. 1996;46:388–93. doi: 10.1212/wnl.46.2.388. [DOI] [PubMed] [Google Scholar]
- 7.Iranzo A, Molinuevo JL, Santamaria J, et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol. 2006;5:572–7. doi: 10.1016/S1474-4422(06)70476-8. [DOI] [PubMed] [Google Scholar]
- 8.Claassen DO, Josephs KA, Ahlskog JE, Silber MH, Tippmann-Peikert M, Boeve BF. REM sleep behavior disorder preceding other aspects of synucleinopathies by up to half a century. Neurology. 2010;75:494–9. doi: 10.1212/WNL.0b013e3181ec7fac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferini-Strambi L, Di Gioia MR, Castronovo V, Oldani A, Zucconi M, Cappa SF. Neuropsychological assessment in idiopathic REM sleep behavior disorder (RBD): does the idiopathic form of RBD really exist? Neurology. 2004;62:41–5. doi: 10.1212/01.wnl.0000101726.69701.fa. [DOI] [PubMed] [Google Scholar]
- 10.Fantini ML, Farini E, Ortelli P, et al. Longitudinal study of cognitive function in idiopathic REM sleep behavior disorder. Sleep. 2011;34:619–25. [PMC free article] [PubMed] [Google Scholar]
- 11.Terzaghi M, Sinforiani E, Zucchella C, et al. Cognitive performance in REM sleep behaviour disorder: a possible early marker of neurodegenerative disease? Sleep Med. 2008;9:343–51. doi: 10.1016/j.sleep.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Gagnon JF, Vendette M, Postuma RB, et al. Mild cognitive impairment in rapid eye movement sleep behavior disorder and Parkinson's disease. Ann Neurol. 2009;66:39–47. doi: 10.1002/ana.21680. [DOI] [PubMed] [Google Scholar]
- 13.Gagnon JF, Postuma RB, Joncas S, Desjardins C, Latreille V. The Montreal Cognitive Assessment: a screening tool for mild cognitive impairment in REM sleep behavior disorder. Mov Disord. 2010;25:936–40. doi: 10.1002/mds.23079. [DOI] [PubMed] [Google Scholar]
- 14.Massicotte-Marquez J, Decary A, Gagnon JF, et al. Executive dysfunction and memory impairment in idiopathic REM sleep behavior disorder. Neurology. 2008;70:1250–7. doi: 10.1212/01.wnl.0000286943.79593.a6. [DOI] [PubMed] [Google Scholar]
- 15.Iranzo A, Isetta V, Molinuevo JL, et al. Electroencephalographic slowing heralds mild cognitive impairment in idiopathic REM sleep behavior disorder. Sleep Med. 2010;11:534–9. doi: 10.1016/j.sleep.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Kobayakawa M, Koyama S, Mimura M, Kawamura M. Decision making in Parkinson's disease: Analysis of behavioral and physiological patterns in the Iowa gambling task. Mov Disord. 2008;23:547–52. doi: 10.1002/mds.21865. [DOI] [PubMed] [Google Scholar]
- 17.Kobayakawa M, Tsuruya N, Kawamura M. Sensitivity to reward and punishment in Parkinson's disease: an analysis of behavioral patterns using a modified version of the Iowa gambling task. Parkinsonism Relat Disord. 2010;16:453–7. doi: 10.1016/j.parkreldis.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Pagonabarraga J, Garcia-Sanchez C, Llebaria G, Pascual-Sedano B, Gironell A, Kulisevsky J. Controlled study of decision-making and cognitive impairment in Parkinson's disease. Mov Disord. 2007;22:1430–5. doi: 10.1002/mds.21457. [DOI] [PubMed] [Google Scholar]
- 19.Perretta JG, Pari G, Beninger RJ. Effects of Parkinson disease on two putative nondeclarative learning tasks: probabilistic classification and gambling. Cogn Behav Neurol. 2005;18:185–92. doi: 10.1097/01.wnn.0000187939.81541.1d. [DOI] [PubMed] [Google Scholar]
- 20.Delazer M, Sinz H, Zamarian L, et al. Decision making under risk and under ambiguity in Parkinson's disease. Neuropsychologia. 2009;47:1901–8. doi: 10.1016/j.neuropsychologia.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 21.Ibarretxe-Bilbao N, Junque C, Tolosa E, et al. Neuroanatomical correlates of impaired decision-making and facial emotion recognition in early Parkinson's disease. Eur J Neurosci. 2009;30:1162–71. doi: 10.1111/j.1460-9568.2009.06892.x. [DOI] [PubMed] [Google Scholar]
- 22.Gleichgerrcht E, Ibanez A, Roca M, Torralva T, Manes F. Decision-making cognition in neurodegenerative diseases. Nat Rev Neurol. 2010;6:611–23. doi: 10.1038/nrneurol.2010.148. [DOI] [PubMed] [Google Scholar]
- 23.Cools R, Barker RA, Sahakian BJ, Robbins TW. L-Dopa medication remediates cognitive inflexibility, but increases impulsivity in patients with Parkinson's disease. Neuropsychologia. 2003;41:1431–41. doi: 10.1016/s0028-3932(03)00117-9. [DOI] [PubMed] [Google Scholar]
- 24.Poletti M, Frosini D, Lucetti C, Del Dotto P, Ceravolo R, Bonuccelli U. Decision making in de novo Parkinson's disease. Mov Disord. 2010;25:1432–6. doi: 10.1002/mds.23098. [DOI] [PubMed] [Google Scholar]
- 25.Brand M, Recknor EC, Grabenhorst F, Bechara A. Decisions under ambiguity and decisions under risk: correlations with executive functions and comparisons of two different gambling tasks with implicit and explicit rules. J Clin Exp Neuropsychol. 2007;29:86–99. doi: 10.1080/13803390500507196. [DOI] [PubMed] [Google Scholar]
- 26.Euteneuer F, Schaefer F, Stuermer R, et al. Dissociation of decision-making under ambiguity and decision-making under risk in patients with Parkinson's disease: a neuropsychological and psychophysiological study. Neuropsychologia. 2009;47:2882–90. doi: 10.1016/j.neuropsychologia.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 27.Frauscher B, Iranzo A, Högl B, et al. Quantification of electromyographic activity during REM sleep in multiple muscles in REM sleep behavior disorder. Sleep. 2008;31:724–31. doi: 10.1093/sleep/31.5.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iranzo A, Frauscher B, Santos H, et al. Usefulness of the SINBAR electromyographic montage to detect the motor and vocal manifestations occurring in REM sleep behavior disorder. Sleep Med. 2011;12:284–8. doi: 10.1016/j.sleep.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 29.American Academy of Sleep Medicine. International classification of sleep disorders. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. REM sleep behavior disorder; pp. 148–52. [Google Scholar]
- 30.Berres M, Monsch AU, Bernasconi F, Thalmann B, Stahelin HB. Normal ranges of neuropsychological tests for the diagnosis of Alzheimer's disease. Stud Health Technol Inform. 2000;77:195–9. [PubMed] [Google Scholar]
- 31.Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a Frontal Assessment Battery at bedside. Neurology. 2000;55:1621–6. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- 32.Hofmann GC. Fröuherkennung dementieller Syndrome - Zur Validitöat der Frontal Assessment Battery. In: Pantel J, editor. Psychosoziale Interventionen zur Pröavention und Therapie der Demenz. Berlin: Logos Verlag; 2008. [Google Scholar]
- 33.Lehrl S. Manual zum MWT-B. Balingen: Spitta; 1991. [Google Scholar]
- 34.Herrmann C, Buss U, Snaith RP. HADS-D Hospital Anxiety and Depression Scale-Deutsche Version. Bern: Verlag Hans Huber; 1995. [Google Scholar]
- 35.Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- 36.CANTAB. Test Administration Guide. Cambridge: Cambridge Cognition Ltd, ; 2006. [Google Scholar]
- 37.Clark L, Robbins TW, Ersche KD, Sahakian BJ. Reflection impulsivity in current and former substance users. Biol Psychiatry. 2006;60:515–22. doi: 10.1016/j.biopsych.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 38.Downes JJ, Roberts AC, Sahakian BJ, Evenden JL, Morris RG, Robbins TW. Impaired extra-dimensional shift performance in medicated and unmedicated Parkinson's disease: evidence for a specific attentional dysfunction. Neuropsychologia. 1989;27:1329–43. doi: 10.1016/0028-3932(89)90128-0. [DOI] [PubMed] [Google Scholar]
- 39.Lawrence AD, Sahakian BJ, Rogers RD, Hodge JR, Robbins TW. Discrimination, reversal, and shift learning in Huntington's disease: mechanisms of impaired response selection. Neuropsychologia. 1999;37:1359–74. doi: 10.1016/s0028-3932(99)00035-4. [DOI] [PubMed] [Google Scholar]
- 40.Robbins TW, James M, Owen AM, et al. A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers: implications for theories of executive functioning and cognitive aging. Cambridge Neuropsychological Test Automated Battery. J Int Neuropsychol Soc. 1998;4:474–90. doi: 10.1017/s1355617798455073. [DOI] [PubMed] [Google Scholar]
- 41.Fox AM, Michie PT, Wynne CD, Maybery MT. ERP correlates of response inhibition to elemental and configural stimuli in a negative patterning task. Clin Neurophysiol. 2000;111:1045–53. doi: 10.1016/s1388-2457(00)00257-1. [DOI] [PubMed] [Google Scholar]
- 42.Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–63. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- 43.Cools R. Role of dopamine in the motivational and cognitive control of behavior. Neuroscientist. 2008;14:381–95. doi: 10.1177/1073858408317009. [DOI] [PubMed] [Google Scholar]
- 44.Frank MJ, Seeberger LC, O'reilly RC. By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science. 2004;306:1940–3. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- 45.Iranzo A, Lomena F, Stockner H, et al. Decreased striatal dopamine transporter uptake and substantia nigra hyperechogenicity as risk markers of synucleinopathy in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study [corrected] Lancet Neurol. 2010;9:1070–7. doi: 10.1016/S1474-4422(10)70216-7. [DOI] [PubMed] [Google Scholar]
- 46.Eisensehr I, Linke R, Noachtar S, Schwarz J, Guildehaus FJ, Tatsch K. Reduced striatal dopamine transporters in idiopathic rapid eye movement sleep behavior disorder. Comparison with Parkinson's disease and controls. Brain. 2000;123:1155–60. doi: 10.1093/brain/123.6.1155. [DOI] [PubMed] [Google Scholar]
- 47.Albin RL, Koeppe RA, Chervin RD, et al. Decreased striatal dopaminergic innervation in REM sleep behavior disorder. Neurology. 2000;55:1410–2. doi: 10.1212/wnl.55.9.1410. [DOI] [PubMed] [Google Scholar]
- 48.Stiasny-Kolster K, Doerr Y, Möller JC, et al. Combination of “idiopathic” REM sleep behavior disorder and olfactory dysfunction as possible indicator of alpha-synucleinopathy demonstrated by dopamine transporter FP-CIT-SPECT. Brain. 2005;128:126–37. doi: 10.1093/brain/awh322. [DOI] [PubMed] [Google Scholar]
- 49.Unger MM, Möller JC, Stiasny-Kolster K, et al. Assessment of idiopathic REM sleep behavior disorder by transcranial sonography, olfactory function test and FP-CIT-SPECT. Mov Disord. 2008;23:596–9. doi: 10.1002/mds.21908. [DOI] [PubMed] [Google Scholar]
- 50.Kim YK, Yoon IY, Kim JM, et al. The implication of nigrostriatal degeneration in the pathogenesis of REM sleep behavior disorder. Eur J Neurol. 2010;17:487–92. doi: 10.1111/j.1468-1331.2009.02854.x. [DOI] [PubMed] [Google Scholar]
- 51.Scherfler C, Frauscher B, Schocke M, et al. White and gray matter abnormalities in idiopathic rapid eye movement sleep behavior disorder: a diffusion-tensor imaging and voxel-based morphometry study. Ann Neurol. 2011;69:400–7. doi: 10.1002/ana.22245. [DOI] [PubMed] [Google Scholar]
- 52.Unger M. Diffusion tensor imaging in idiopathic REM sleep behavior disorder reveals microstructural changes in the brainstem, substantia nigra, olfactory region, and other brain regions. Sleep. 2010;33:767–73. doi: 10.1093/sleep/33.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]